Abstract
Inferences about late risk of end-stage renal disease (ESRD) in live kidney donors have been extrapolated from studies averaging <10 years of follow-up. Since early postdonation ESRD (<10 years postdonation) and late postdonation ESRD (10+ years postdonation) may differ by causal mechanism, it is possible that extrapolations are misleading. To better understand postdonation ESRD, we studied patterns of common etiologies including diabetes, hypertension, and glomerulonephritis (GN)(as reported by providers) using donor-registry data linked to ESRD-registry data. Overall, 125,427 donors were observed for a median of 11.0 years (interquartile range 5.3–15.7; maximum 25). The cumulative incidence of ESRD increased from 10 events per 10,000 at 10 years postdonation to 85 events per 10,000 at 25 years postdonation (incidence rate ratio [IRR] for late vs. early ESRD [adjusted for age, race, and sex]: 1.31.72.3 [subscripts are 95% confidence intervals]). Early postdonation ESRD was predominantly reported as GN-ESRD; however, late postdonation ESRD was more frequently reported as diabetic-ESRD and hypertensive-ESRD (IRR 2.37.725.2 and 1.42.64.6). These time-dependent patterns were not seen with GN-ESRD (IRR 0.40.71.2). Since ESRD in live kidney donors has traditionally been reported in studies averaging <10 years of follow-up, our findings suggest caution in extrapolating such results over much longer intervals.
INTRODUCTION
Recent reports suggest that there is an increase in the risk of end stage renal disease (ESRD) following live kidney donation (1–3). Estimates of this risk correlate with the duration of donor follow-up: 1.34 cases of donor ESRD per 10,000 person-years in a United States national study with a mean follow-up of 9.8 years (4) versus 3.02 cases per 10,000 person-years in a Norwegian national study with a median follow-up of 15.1 years (1). Ideally, donors seek information about lifetime risk of ESRD; meanwhile, observational data averaging less than 10 years of follow-up inform our understanding of early postdonation ESRD and extrapolations of these data have attempted to make inferences about late postdonation ESRD (2, 5). However, because the risk of ESRD may increase over time, it is plausible that extrapolations based on the first decade of follow-up substantially underestimate the proportion of donors who develop ESRD in subsequent decades (6, 7).
ESRD in the early postdonation period is unlikely to result from diabetes and hypertension since these conditions are absolute and relative contraindications to live kidney donation (8–10); however, glomerulonephritis (GN) may cause early ESRD (1, 4). GN may be associated with poorly characterized molecular risks in those with normal renal function (11, 12), and may also be associated with poorly characterized genetic risks in donors who are biologically related to live donor kidney transplant recipients with GN-ESRD (13, 14), making predonation screening challenging. Moreover, if proteinuria and persistent hematuria develop after donation as manifestations of GN, they may in some instances progress rapidly to ESRD even after interventions are instituted (13). By contrast, ESRD in the late postdonation period may result from de novo diabetes (15), and what providers commonly report as hypertensive nephrosclerosis (16), both systemic conditions associated with aging and arteriosclerosis.
To better understand the patterns of postdonation ESRD without extrapolation, we studied cause-specific cumulative incidence, exploring diabetes, hypertension, and GN as commonly reported etiologies with potentially different patterns of development. To inform extrapolations and risk prediction, we estimated the hazard rate of ESRD caused by these commonly reported diseases for each successive postdonation year.
METHODS
Data sources
This study used data from the Scientific Registry of Transplant Recipients (SRTR). The SRTR data system includes data on all donors, waitlisted candidates, and transplant recipients in the United States, submitted by the members of the Organ Procurement and Transplantation Network (OPTN) and has been described elsewhere (17). The Health Resources and Services Administration (HRSA), US Department of Health and Human Services, provides oversight to the activities of the OPTN and SRTR contractors.
Study population
All live kidney donors who underwent donor nephrectomy in the United States between October 1, 1987 and July 31, 2014 were included in this study. Those who donated after April 1, 1994 (the first available outcomes linkage) entered the analysis on the date of donation whereas those who donated before April 1, 1994 entered on this date as late-entries (i.e., data from the date of donation through April 1, 1994 were left censored).
Outcome ascertainment
Incident ESRD was defined as the initiation of maintenance dialysis or receipt of a living or deceased donor kidney transplant, whichever was identified first (2). For donors with incident ESRD, the cause of ESRD as reported by providers was ascertained from the CMS medical evidence form 2728 (a validated tool) (18, 19), and – because of small or no case counts for some categories, which we did not consider for this study – reclassified into 3 broad categories: diabetes, hypertension/large vessel disease, and GN. Diabetes includes type II, adult-onset type or unspecified type diabetes; and type I, juvenile type, ketosis prone diabetes. Hypertension includes renal disease due to hypertension (no primary renal disease), renal artery stenosis; renal artery occlusion; and, cholesterol emboli, renal emboli. GN includes GN (histology not examined); focal segmental GN; membranous nephropathy; membranoproliferative GN; dense deposit disease; IgA nephropathy; IgM nephropathy (proven by immunofluorescence); rapidly progressive GN; Goodpasture’s syndrome; post infectious GN; and other proliferative GN.
Cumulative incidence of cause-specific ESRD
We used the Kaplan-Meier method to generate 3 unadjusted cumulative incidence estimates, one each for diabetic-ESRD, hypertensive-ESRD, and GN-ESRD. Donors contributed follow-up time from the day they entered the study until they developed ESRD, died, or reached the end of study follow-up (July 31, 2014).
Incidence and hazard rate of cause-specific ESRD in the early vs. late postdonation period
We used Poisson regression to generate 3 adjusted incidence rate ratios (IRR) (adjusted for age, sex, and race), one each for diabetic-ESRD, hypertensive-ESRD, and GN-ESRD. Each adjusted IRR compared the incidence of cause-specific ESRD in the late postdonation period (10–25 years postdonation) with the early postdonation period (<10 years postdonation). For each cause-specific model, we divided the follow-up time into 10–25 years postdonation vs. <10 years postdonation and used these time periods as the primary exposures under investigation. However, we also reported the associations between risk of ESRD and age, race, and sex. We subsequently used a three-parameter generalized gamma regression (GG: β, σ, λ) to describe the hazard rate of ESRD for these common etiologies for every successive year postdonation (20).
Statistical analysis
Two types of absolute risks were estimated: cumulative incidences (using Kaplan-Meier and generalized gamma models) and hazard rates (using generalized gamma models). We also estimated one type of relative risk: the incidence rate ratio (using multivariable Poisson models) to compare follow-up beyond 10 years with the first 10 years of follow-up. Confidence intervals are reported as per the method of Louis and Zeger (21). All analyses were performed using Stata 14.0/MP for Linux (Stata Corp, College Station, TX). All hypothesis tests were 2 sided (α = .05).
RESULTS
Study Population
Among 125,427 live kidney donors, the median age at donation was 40 years, 58.9% were female, 74.8% were of white or other race, 37.1% were college graduates, and 31.3% were unrelated to their recipient. The median BMI was 27 kg/m2, median serum creatinine was 0.8 mg/dL, and median eGFR was 98 ml/min/1.73m2. Fifty nine percent of the donors in this study underwent nephrectomy between 1987 and 2005; the rest underwent nephrectomy between 2006 and 2014 (Table 1). There were no substantive differences in demographic and health characteristics between the pre-2005 and post-2005 donor populations.
Table 1.
Characteristics of live kidney donors at the time of donation, United States, October 1, 1987 – July 31, 2014a
| n=125,427 | |
|---|---|
| Age, median years [IQR]b | 40 [31–48] |
| 18–39 | 49 |
| 40–49 | 29 |
| 50–59 | 17 |
| 60+ | 4 |
|
| |
| Female (%): b | 59 |
|
| |
| Race/Ethnicity (%): b | |
| White/Other | 75 |
| Black | 13 |
| Hispanic | 12 |
|
| |
| Education (%):c | |
| High School or less | 35 |
| Attended College | 28 |
| Graduate or more | 37 |
|
| |
| Relationship (%): b | |
| Parent/Child | 30 |
| Sibling/Other related | 39 |
| Unrelated | 31 |
|
| |
| Body-mass Index (BMI), median kg/m2 d d[IQR]d | 27 [24–30] |
| <25 | 32 |
| 25–29 | 41 |
| 30–34 | 21 |
| 35+ | 5 |
|
| |
| Creatinine, median mg/dL [IQR]e | 0.8 [0.7–1.0] |
|
| |
| eGFR, median ml/min/1.73m2 [IQR] e,f | 98 [84–110] |
| <80 | 18 |
| 80–99 | 35 |
| 100+ | 47 |
|
| |
| Blood pressure, mmHgg | |
| SBP, median [IQR] | 120 [111–130] |
| <120 | 45 |
| 80–139 | 47 |
| 140+ | 8 |
| DBP, median [IQR] | 74 [68–80] |
| <80 | 69 |
| 80–89 | 26 |
| 90+ | 5 |
|
| |
| Year Of Transplant (%):b | |
| 1987–1993 | 11 |
| 1994–1997 | 11 |
| 1998–2001 | 16 |
| 2002–2005 | 21 |
| 2006–2009 | 20 |
| 2010–2014 | 21 |
Data before April 1, 1994 were left censored
Data on age, sex, race/ethnicity, donor/recipient relationship, and year of transplant were available throughout the study
Not available before 1999; 50% with missing values in 2000; 30% with missing values in 2005; and 10% with missing values in 2009
Not available before 1998; 57% with missing values in 1999; 20% with missing values in 2000; and 10% with missing values in 2005
Not available before 1999; 49% with missing values in 1999; 11% with missing values in 2000; and 6% with missing values in 2001
Estimated using the CKD-EPI equation (41)
Not available before 1999; 43% with missing values in 1999; 20–30% with missing values from 2000–2003; 9–16% with missing values from 2004–2007
Cumulative incidence of cause-specific ESRD
Donors were followed for a median of 11.0 years (interquartile range 5.3–15.7, maximum 25 years). Over 1,329,964 person-years, 257 donors developed ESRD; 158 (61%) of these were reported as diabetic-ESRD (n=33), hypertensive-ESRD (n=70), and GN-ESRD (n=55). Of these commonly reported etiologies, ESRD in the early postdonation period was predominantly GN-ESRD. By contrast, ESRD in the late postdonation was reported most frequently as diabetic-ESRD and hypertensive-ESRD (Table 2).
Table 2.
Cumulative Incidence of end stage renal disease (ESRD) following live kidney donation, United States, October 1, 1987 – July 31, 2014
| Year | Cumulative Incidence of ESRD per 10,000 donors By Cause of ESRDa |
||
|---|---|---|---|
|
| |||
| Diabetes (n=33)b | Hypertension (n=70)c | Glomerulonephritis (n=55)d | |
| 5 | 0.000.100.70 | 0.100.300.90 | 0.300.701.50 |
| 10 | 0.100.401.20 | 1.202.003.20 | 2.293.404.90 |
| 15 | 1.102.003.59 | 5.407.4010.1 | 4.596.308.60 |
| 20 | 5.808.9013.6 | 11.515.320.7 | 8.1011.115.3 |
| 25 | 9.6914.822.7 | 20.228.239.2 | 9.1912.817.8 |
Cause of ESRD as per the CMS form 2728 narrative (18)
Type II, adult-onset type or unspecified type diabetes; and Type I, juvenile type, ketosis prone diabetes
Renal disease due to hypertension (no primary renal disease); renal artery stenosis; renal artery occlusion; and, cholesterol emboli, renal emboli
Glomerulonephritis (GN) includes GN (histology not examined); focal segmental GN; membranous nephropathy; membranoproliferative GN; dense deposit disease; IgA nephropathy; IgM nephropathy (proven by immunofluorescence); rapidly progressive GN; Goodpasture’s syndrome; post infectious GN; and other proliferative GN.
Incidence rate of cause-specific ESRD in the early vs. late postdonation period
Overall, the cumulative incidence of ESRD increased from 10 ESRD cases per 10,000 at 10 years postdonation to 85 ESRD cases per 10,000 at 25 years postdonation (incidence rate ratio [IRR] for late vs. early ESRD [adjusted for age, race, and sex]: 1.31.72.3). More specifically, the incidence of diabetic-ESRD was: higher in the late compared with the early postdonation period (IRR 2.37.725.2), higher in older compared with younger donors (IRR 1.01.31.8 per 10 year increase in age), higher in black compared with white donors (IRR 1.94.08.5), and higher in male compared with female donors (IRR 2.55.010.0). Similarly, the incidence of what was reported as hypertensive-ESRD was: higher in the late compared with the early postdonation period (IRR 2.32.64.6), higher in older compared with younger donors (IRR 0.91.11.3 per 10 year increase in age), higher in black compared with white donors (IRR 2.33.96.7), and higher in male compared with female donors (IRR 1.32.03.3). By marked contrast, the incidence of GN-ESRD was: no higher in the late compared with the early postdonation period (IRR 0.40.71.2), no higher in older compared with younger donors (IRR 0.60.81.1 per 10 year increase in age), almost an order of magnitude higher in black compared with white donors (IRR 4.17.312.8), and only marginally higher in male compared with female donors (IRR 0.91.72.5) (Table 3).
Table 3.
Incidence of late postdonation end stage renal disease (ESRD) 10–25 years following live kidney donation compared with early postdonation ESRD 0–9 years following live kidney donation, United States, October 1, 1987 – July 31, 2014
| IRRs from Cause-specific ESRD Modelsa | |||
|---|---|---|---|
|
| |||
| Diabetes | Hypertension | Glomerulonephritis | |
| Timing of postdonation ESRD | |||
| <10 years (Early) | Reference | Reference | Reference |
| 10–25 years (Late) | 2.37.725.2 | 1.52.64.6 | 0.40.71.2 |
| Age, per 10 year increaseb | 1.01.31.8 | 0.91.11.3 | 0.60.81.1 |
| Race/Ethnicity | |||
| White/Other | Reference | Reference | Reference |
| Black | 1.94.08.5 | 2.33.96.7 | 4.17.312.8 |
| Hispanic | 0.20.83.4 | 1.02.14.1 | 0.20.82.6 |
| Sex | |||
| Female | Reference | Reference | Reference |
| Male | 2.55.010.0 | 1.32.03.3 | 0.91.72.5 |
Incidence Rate Ratios (IRR) from Poisson parametric models 1–3. The parametric assumption is that incidence rate was constant <10 years postdonation; and may change to another constant rate 10–25 postdonation IRR≠1
Incidence rate ratios were adjusted for age, sex, and race/ethnicity. Inferences were the same regardless of: how age was modeled (lagged or not lagged by 10 years in the postdonation period); and, how variance was quantified (clustered or not on the individual donor; since a donor might have contributed person-time to the early- and late-postdonation periods)
Hazard rate of cause-specific ESRD
The hazard rate of diabetic-ESRD increased exponentially from 0.10.20.3 ESRD cases per 10,000 donors per year at 10 years postdonation to 1.02.53.8 ESRD cases per 10,000 donors per year at 25 years postdonation. Similarly, the hazard rate of what providers reported as hypertensive-ESRD increased from 0.50.60.8 ESRD cases per 10,000 donors per year at 10 years postdonation to 1.72.94.1 ESRD cases per 10,000 donors per year at 25 years postdonation. By contrast, the hazard rate of GN-ESRD did not change significantly: from 0.50.610.8 ESRD cases per 10,000 donors per year at 10 years postdonation to 0.30.671.3 ESRD cases per 10,000 donors per year at 25 years postdonation (Table 4 & Figure 1).
Table 4.
Parametric models for risk of cause-specific end stage renal disease (ESRD) following live kidney donation, United States, October 1, 1987 – July 31, 2014
| ESRD-Modela | Generalized Gamma Parameters | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| β | P | σ | P | λ | P | |
| Diabetes | 2.755.117.46 | <0.01 | 0.020.419.35 | 0.6 | −1.600.632.88 | 0.6 |
| Hypertension | 2.675.428.16 | <0.01 | 0.000.4071.8 | 0.7 | −3.800.915.70 | 0.8 |
| Glomerulonephritis | 5.9111.216.6 | <0.01 | 4.9515.850.9 | <0.01 | −31.0−9.5012.2 | 0.4 |
Parametric models 1–3: Generalized gamma. For interpretation of these parameters see Discussion.
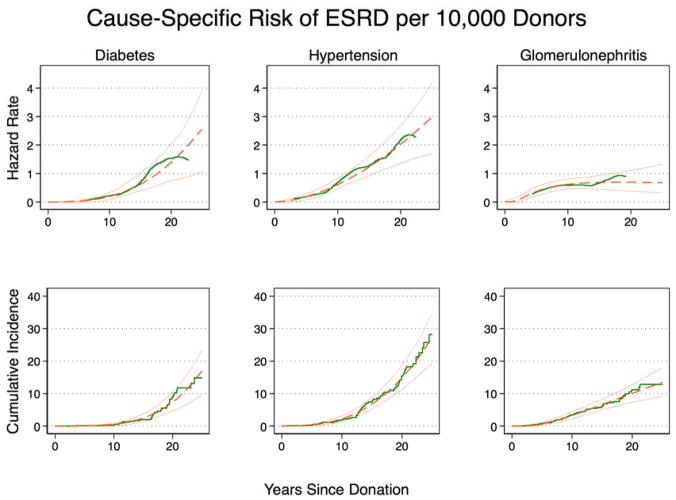
Figure 1. Hazard rate and cumulative incidence of cause-specific end stage renal disease (ESRD) in live kidney donors, United States, October 1, 1987 – July 31, 2014.
Kaplan-Meier (green, solid line) and generalized gamma model (GG) (orange, dashed line; 95% CI solid) illustrate, for diabetes: a low unadjusted hazard rate of ESRD <10 years postdonation (0.10.20.3 cases per 10,000 donors per year at 10 years) and a substantial increase in unadjusted hazard rate of ESRD 10–25 years postdonation (1.02.53.8 cases per 10,000 donors per year at 25 years); hypertension: a low unadjusted hazard rate of ESRD 0–10 years (0.50.60.8 cases per 10,000 donors per year) and a substantial increase in unadjusted hazard rate of ESRD 10–25 years (1.72.94.1 cases per 10,000 donors per year); and for glomerulonephritis (GN): a relatively constant unadjusted hazard rate of ESRD 0–25 years postdonation (0.50.610.8 per 10,000 donors per year at 10 years vs. 0.30.671.3 cases per 10,000 donors per year at 25 years. As per GG, the cumulative incidence of cause-specific ESRD at 25 years was 9.316.622.7, 19.026.833.7, and 9,113.417.7 per 10,000 donors for diabetes, hypertension, and GN (very closely approximating the Kaplan-Meier estimates)
DISCUSSION
In this national study of cause-specific ESRD in kidney donors, there was a 7.7-fold higher risk of late postdonation (10–25 years) compared with early postdonation (<10 years) diabetic-ESRD. Similarly, there was a 2.6-fold higher risk of late postdonation compared with early postdonation events reported by providers as hypertensive-ESRD. By contrast, there was no significant change over time in event rate for GN-ESRD. An emerging belief, supported by the relatively high incidence of GN-ESRD in the first 10 postdonation years in this study, is that kidney diseases that progress quickly enough to be enumerated within 10 years of nephrectomy are predominately glomerulonephritides (22, 23). As such, our findings support the view that extrapolations of postdonation risk of ESRD based on the first decade of follow-up substantially underestimate the proportion of donors who develop ESRD in subsequent decades (15), especially for ESRD cases with a long prodromal course. Methods that account for the development of diabetes, hypertension, and other risk factors are necessary for improved prediction of late postdonation risk of ESRD, particularly in selected cohorts such as kidney donors.
Unlike prior reports that have treated ESRD in donors as an all-encompassing clinical outcome (1–4, 16, 24), this study viewed ESRD in donors as an endpoint preceded by biologically distinct pathways: what providers reported as diabetic-ESRD, hypertensive-ESRD, and GN-ESRD. In showing that the three most commonly reported causes of ESRD in donors mirror those in the general population (though not necessarily in the same order of relative frequently) (25), our findings suggest that similar biological pathways lead to ESRD in these two populations. However, unlike the general population, donors were screened for diabetes (via blood tests for hyperglycemia), hypertension (via blood pressure measurements), and GN (via urine tests for hematuria and proteinuria), with confirmation of normal test results prior to donation (8–10, 26). This explains why donors had a very low risk of ESRD in the early postdonation period, but with an exponential increase in risk in the late postdonation period. Although donor nephrectomy represents a 50% reduction in nephron number and a 25 to 40% reduction in GFR (27, 28), and although for these reasons de novo renal disease may reach ESRD sooner in donors compared with their healthy nondonor counterparts (1–3), our study shows that the absolute risk of what providers reported as diabetic-ESRD, hypertensive-ESRD, and GN-ESRD were very low over a 25-year period. As such, our findings reaffirm the effectiveness of the current practices of donor evaluation (8–10).
Some primary glomerular disease remains undiagnosed and may be reported as hypertensive-ESRD (29–32). A very important limitation of our study is that we did not have available to us clinical data including serology and renal biopsy results to accurately define the cause of ESRD. Also, like other large population-based studies of ESRD in the general population (33–36), our kidney donor study was limited by the absence of baseline and longitudinal assessments of renal function and blood pressure. As such, like other studies, we were unable to evaluate the incidence of ESRD attributable to hypertension in the specific subgroup of individuals with benign essential hypertension, normal serum creatinine, and no albuminuria (i.e., the profile of an individual with hypertension who is cleared for donor nephrectomy). In other words, our data cannot quantify the proportion of cases that providers reported as hypertensive-ESRD that, in fact, resulted from benign essential hypertension.
That said, in a somewhat recent study of 316,675 individuals with eGFR>60 ml/min/1.73m2 and negative dipstick urinalysis for proteinuria or hematuria, a strong, direct, and graded association was observed between systolic blood pressure and the risk of ESRD over 8,210,431 person-years of follow-up; and this association was observed throughout the distribution of blood-pressure readings (37). For these reasons, there might be an important and potentially preemptive role for the monitoring and control of blood pressure throughout the postdonation period regardless of hypertension status at the time of donation. It is therefore important to discuss the second limitation that, although we studied one of the most important physiological consequences of donor nephrectomy, ESRD represents a subset of stage 5 of chronic kidney disease (38)—the tip of the iceberg, as it were (39)—and our study was not able to quantify the interim incidence and hazard rates for CKD; or to describe the postdonation patterns of risk factors including hyperglycemia, hypertension, and proteinuria. Since the earlier stages of CKD are more likely to lead to heart disease than to ESRD (40), our analysis was limited in not being able to characterize these very important non-renal outcomes of CKD.
Granted, our study has several strengths and these mainly lie in the generalizability of its inferences to all donors in the US, the large sample size permitting the study of rare outcomes, and the robustness of ESRD ascertainment. We identified all donors who developed ESRD by either initiation of maintenance dialysis treatment or receipt of a deceased or a living donor transplant. Using CMS and OPTN data, we ascertained the cause of ESRD as reported by care providers to the CMS (18). Our study is the first, to the best of our knowledge, to provide empirical support to the view that diabetic-ESRD (15), as well as what is commonly reported as hypertensive-ESRD, may be among the leading determinants of risk of ESRD over the lifetime of kidney donors despite being initially rare. Using parametric survival methods (20), our study characterized the risk of ESRD by reported underlying cause in each successive postdonation year. Since we also used non-parametric Kaplan-Meier methods to report our risk estimates, we were able to affirm that our parametric models fit that data well.
Our parametric models, fit using the three-parameter generalized gamma regression (GG: β, σ, λ), described the cause-specific hazard rate of ESRD for every successive postdonation year. Since β is directly correlated with the median time-to-ESRD for fixed values of σ and λ, it is difficult to interpret it because σ and λ varied by reported cause of ESRD in this study. However, the hazard rate of ESRD increased with time if σ had values 0 < σ < 1, as observed for what providers reported as diabetic-ESRD and hypertensive-ESRD. This reflects the biological characteristic of these diseases: that they evolved only after cumulative, chronic exposure to putative risk factors including high blood glucose and high blood pressure. By contrast, the hazard rate was relatively constant if σ had values σ > 1, as observed for GN-ESRD. This reflects the biological characteristic of glomerulonephritides: that in some instances they may evolve relatively rapidly in susceptible persons, regardless of the number of years postdonation; though, perhaps, dependent upon susceptibility factors that are poorly understood (11–14). Finally, the interquartile ratio of the parameters β and σ are described by λ, which, as such, describes how clustered ESRD events were in the postdonation years. For what providers reported as diabetic-ESRD and hypertension-ESRD, ESRD events were clustered much later in time than in the early years following donor nephrectomy. By marked contrast, GN-ESRD events were spread somewhat uniformly overtime.
In conclusion, since ESRD in live kidney donors has traditionally been reported in studies averaging less than 10 years of follow-up, our findings suggest caution in extrapolating such results over much longer intervals. Also, because the risk of what providers reported as diabetic-ESRD and hypertensive-ESRD increases exponentially over time, these findings emphasize the importance of follow-up and surveillance of kidney donors for hyperglycemia, blood pressure elevation, and renal function for many decades following nephrectomy.
Acknowledgments
This study was supported by Health Resources and Services Administration contract 234-2005-370011C and by grants R01DK096008 (Segev) and K24DK101828 (Segev) from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK).
Abbreviations
- ESRD
end stage renal disease
- GN
glomerulonephritis
- SRTR
scientific registry of transplant recipients
- CMS
Centers for Medicare and Medicaid Services
- OPTN
Organ Procurement and Transplantation Network
- HRSA
Health Resources and Services Administration
- GG
generalized gamma regression
- eGFR
estimated glomerular filtration rate
- CKD
chronic kidney disease
Footnotes
DISCLAIMER
The analyses described here are the responsibility of the authors alone and do not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products or organizations imply endorsement by the U.S.
The data reported here have been supplied by the Minneapolis Medical Research Foundation (MMRF) as the contractor for the Scientific Registry of Transplant Recipients (SRTR). The interpretation and reporting of these data are the responsibility of the authors and in no way should be seen as an official policy of or interpretation by the SRTR or the U.S. Government. Government.
DISCLOSURE
The authors of this manuscript have conflicts of interest to disclose as described by the American Journal of Transplantation. Dr. Amit Garg is supported by the Dr. Adam Linton Chair in Kidney Health Analytics, and he has received partnership grant funding from Astellas for a Canadian Institutes of Health Research grant on living kidney donor outcomes. The other authors have no conflicts of interest to disclose.
References
- 1.Mjoen G, Hallan S, Hartmann A, Foss A, Midtvedt K, Oyen O, et al. Long-term risks for kidney donors. Kidney international. 2014;86(1):162–7. doi: 10.1038/ki.2013.460. [DOI] [PubMed] [Google Scholar]
- 2.Muzaale AD, Massie AB, Wang MC, Montgomery RA, McBride MA, Wainright JL, et al. Risk of end-stage renal disease following live kidney donation. Jama. 2014;311(6):579–86. doi: 10.1001/jama.2013.285141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grams ME, Sang Y, Levey AS, Matsushita K, Ballew S, Chang AR, et al. Kidney-Failure Risk Projection for the Living Kidney-Donor Candidate. The New England journal of medicine. 2016;374(5):411–21. doi: 10.1056/NEJMoa1510491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cherikh WS, Young CJ, Kramer BF, Taranto SE, Randall HB, Fan PY. Ethnic and gender related differences in the risk of end-stage renal disease after living kidney donation. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2011;11(8):1650–5. doi: 10.1111/j.1600-6143.2011.03609.x. [DOI] [PubMed] [Google Scholar]
- 5.Kiberd BA. Estimating the long term impact of kidney donation on life expectancy and end stage renal disease. Transplantation research. 2013;2(1):2. doi: 10.1186/2047-1440-2-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kiberd B, Tennankore K. Kidney donation and risk of ESRD. Jama. 2014;312(1):93. doi: 10.1001/jama.2014.5518. [DOI] [PubMed] [Google Scholar]
- 7.Boudville N, Garg AX. End-stage renal disease in living kidney donors. Kidney international. 2014;86(1):20–2. doi: 10.1038/ki.2013.560. [DOI] [PubMed] [Google Scholar]
- 8.Bia MJ, Ramos EL, Danovitch GM, Gaston RS, Harmon WE, Leichtman AB, et al. Evaluation of living renal donors. The current practice of US transplant centers. Transplantation. 1995;60(4):322–7. doi: 10.1097/00007890-199508270-00003. [DOI] [PubMed] [Google Scholar]
- 9.Kasiske BL, Ravenscraft M, Ramos EL, Gaston RS, Bia MJ, Danovitch GM. The evaluation of living renal transplant donors: clinical practice guidelines. Ad Hoc Clinical Practice Guidelines Subcommittee of the Patient Care and Education Committee of the American Society of Transplant Physicians. Journal of the American Society of Nephrology : JASN. 1996;7(11):2288–313. doi: 10.1681/ASN.V7112288. [DOI] [PubMed] [Google Scholar]
- 10.Mandelbrot DA, Pavlakis M, Danovitch GM, Johnson SR, Karp SJ, Khwaja K, et al. The medical evaluation of living kidney donors: a survey of US transplant centers. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2007;7(10):2333–43. doi: 10.1111/j.1600-6143.2007.01932.x. [DOI] [PubMed] [Google Scholar]
- 11.Shankland SJ, Pollak MR. A suPAR circulating factor causes kidney disease. Nature medicine. 2011;17(8):926–7. doi: 10.1038/nm.2443. [DOI] [PubMed] [Google Scholar]
- 12.Hayek SS, Sever S, Ko YA, Trachtman H, Awad M, Wadhwani S, et al. Soluble Urokinase Receptor and Chronic Kidney Disease. The New England journal of medicine. 2015;373(20):1916–25. doi: 10.1056/NEJMoa1506362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kofman T, Audard V, Narjoz C, Gribouval O, Matignon M, Leibler C, et al. APOL1 polymorphisms and development of CKD in an identical twin donor and recipient pair. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2014;63(5):816–9. doi: 10.1053/j.ajkd.2013.12.014. [DOI] [PubMed] [Google Scholar]
- 14.Clark EG, Knoll G, Bugeja A, Burns KD, Scofield RH. Lupus after kidney donation to an affected male relative. Transplantation. 2015;99(4):e27–8. doi: 10.1097/TP.0000000000000643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Steiner RW, Ix JH, Rifkin DE, Gert B. Estimating risks of de novo kidney diseases after living kidney donation. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2014;14(3):538–44. doi: 10.1111/ajt.12625. [DOI] [PubMed] [Google Scholar]
- 16.Fehrman-Ekholm I, Norden G, Lennerling A, Rizell M, Mjornstedt L, Wramner L, et al. Incidence of end-stage renal disease among live kidney donors. Transplantation. 2006;82(12):1646–8. doi: 10.1097/01.tp.0000250728.73268.e3. [DOI] [PubMed] [Google Scholar]
- 17.Levine GN, McCullough KP, Rodgers AM, Dickinson DM, Ashby VB, Schaubel DE. Analytical methods and database design: implications for transplant researchers, 2005. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2006;6(5 Pt 2):1228–42. doi: 10.1111/j.1600-6143.2006.01277.x. [DOI] [PubMed] [Google Scholar]
- 18.Centers for Medicare and Medicaid Services website. [Accessed April 17, 2015]; http://www.cms.gov/Medicare/CMS-Forms/CMS-Forms/downloads/cms2728.pdf.
- 19.Longenecker JC, Coresh J, Klag MJ, Levey AS, Martin AA, Fink NE, et al. Validation of comorbid conditions on the end-stage renal disease medical evidence report: the CHOICE study. Choices for Healthy Outcomes in Caring for ESRD. Journal of the American Society of Nephrology : JASN. 2000;11(3):520–9. doi: 10.1681/ASN.V113520. [DOI] [PubMed] [Google Scholar]
- 20.Cox C, Chu H, Schneider MF, Munoz A. Parametric survival analysis and taxonomy of hazard functions for the generalized gamma distribution. Statistics in medicine. 2007;26(23):4352–74. doi: 10.1002/sim.2836. [DOI] [PubMed] [Google Scholar]
- 21.Louis TA, Zeger SL. Effective communication of standard errors and confidence intervals. Biostatistics. 2009;10(1):1–2. doi: 10.1093/biostatistics/kxn014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Steiner RW. Moving closer to understanding the risks of living kidney donation. Clinical transplantation. 2016;30(1):10–6. doi: 10.1111/ctr.12652. [DOI] [PubMed] [Google Scholar]
- 23.Steiner RW. The Risks of Living Kidney Donation. The New England journal of medicine. 2016;374(5):479–80. doi: 10.1056/NEJMe1513891. [DOI] [PubMed] [Google Scholar]
- 24.Ibrahim HN, Foley R, Tan L, Rogers T, Bailey RF, Guo H, et al. Long-term consequences of kidney donation. The New England journal of medicine. 2009;360(5):459–69. doi: 10.1056/NEJMoa0804883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.United States Renal Data System. [Accessed August 17, 2015];Annual Data Report Reference Tables. 2014 http://www.usrds.org/reference.aspx.
- 26.Delmonico F Council of the Transplantation S. A Report of the Amsterdam Forum On the Care of the Live Kidney Donor: Data and Medical Guidelines. Transplantation. 2005;79(6 Suppl):S53–66. [PubMed] [Google Scholar]
- 27.Krohn AG, Ogden DA, Holmes JH. Renal function in 29 healthy adults before and after nephrectomy. Jama. 1966;196(4):322–4. [PubMed] [Google Scholar]
- 28.Kasiske BL, Anderson-Haag T, Israni AK, Kalil RS, Kimmel PL, Kraus ES, et al. A Prospective Controlled Study of Living Kidney Donors: Three-Year Follow-up. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2015;66(1):114–24. doi: 10.1053/j.ajkd.2015.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roland AS, Hildreth EA, Sellers AM. Occult Primary Renal Disease in the Hypertensive Patient. Archives of internal medicine. 1964;113:101–10. doi: 10.1001/archinte.1964.00280070103017. [DOI] [PubMed] [Google Scholar]
- 30.Freedman BI, Iskandar SS, Appel RG. The link between hypertension and nephrosclerosis. American journal of kidney diseases : the official journal of the National Kidney Foundation. 1995;25(2):207–21. doi: 10.1016/0272-6386(95)90001-2. [DOI] [PubMed] [Google Scholar]
- 31.Hsu CY. Does non-malignant hypertension cause renal insufficiency? Evidence-based perspective. Current opinion in nephrology and hypertension. 2002;11(3):267–72. doi: 10.1097/00041552-200205000-00001. [DOI] [PubMed] [Google Scholar]
- 32.Hsu CY. Does treatment of non-malignant hypertension reduce the incidence of renal dysfunction? A meta-analysis of 10 randomised, controlled trials. Journal of human hypertension. 2001;15(2):99–106. doi: 10.1038/sj.jhh.1001128. [DOI] [PubMed] [Google Scholar]
- 33.Iseki K, Ikemiya Y, Fukiyama K. Blood pressure and risk of end-stage renal disease in a screened cohort. Kidney international. 1996:S69–S71. [PubMed] [Google Scholar]
- 34.Iseki K, Iseki C, Ikemiya Y, Fukiyama K. Risk of developing end-stage renal disease in a cohort of mass screening. Kidney international. 1996;49(3):800–5. doi: 10.1038/ki.1996.111. [DOI] [PubMed] [Google Scholar]
- 35.Klag MJ, Whelton PK, Randall BL, Neaton JD, Brancati FL, Ford CE, et al. Blood pressure and end-stage renal disease in men. The New England journal of medicine. 1996;334(1):13–8. doi: 10.1056/NEJM199601043340103. [DOI] [PubMed] [Google Scholar]
- 36.Marin R, Gorostidi M, Fernandez-Vega F, Alvarez-Navascues R. Systemic and glomerular hypertension and progression of chronic renal disease: the dilemma of nephrosclerosis. Kidney international Supplement. 2005;(99):S52–6. doi: 10.1111/j.1523-1755.2005.09910.x. [DOI] [PubMed] [Google Scholar]
- 37.Hsu CY, McCulloch CE, Darbinian J, Go AS, Iribarren C. Elevated blood pressure and risk of end-stage renal disease in subjects without baseline kidney disease. Archives of internal medicine. 2005;165(8):923–8. doi: 10.1001/archinte.165.8.923. [DOI] [PubMed] [Google Scholar]
- 38.Levey AS, Coresh J, Balk E, Kausz AT, Levin A, Steffes MW, et al. National Kidney Foundation practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Annals of internal medicine. 2003;139(2):137–47. doi: 10.7326/0003-4819-139-2-200307150-00013. [DOI] [PubMed] [Google Scholar]
- 39.Grams ME, Chow EK, Segev DL, Coresh J. Lifetime incidence of CKD stages 3–5 in the United States. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2013;62(2):245–52. doi: 10.1053/j.ajkd.2013.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sarnak MJ, Levey AS, Schoolwerth AC, Coresh J, Culleton B, Hamm LL, et al. Kidney disease as a risk factor for development of cardiovascular disease: a statement from the American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention. Circulation. 2003;108(17):2154–69. doi: 10.1161/01.CIR.0000095676.90936.80. [DOI] [PubMed] [Google Scholar]
- 41.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Annals of internal medicine. 2009;150(9):604–12. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]