Summary
Our aim was to investigate risk factors and outcome of Acute Respiratory Distress Syndrome (ARDS) in severe burn patients. A descriptive study was carried out on 159 adult burn patients with burn extent ≥ 20% total body surface area, treated at the Burn Intensive Care Unit, National Institute of Burns. ARDS was defined according to the 2012 Berlin definition. Risk factors for developing ARDS and outcome were recorded and analyzed. Results showed that 45 patients developed ARDS (28.3%). Severe ARDS was recorded in 30 of these patients, accounting for 66.7%. Inhalation injury, burn surface area over 40% and full thickness burn area over 20% TBSA were determined as risk factors for the development of ARDS. Mortality rate of patients with ARDS was extremely high (80%), especially for severe ARDS patients (p < 0.01), and deaths were mostly due to multiple organ failure.
Keywords: ARDS, severe burn, outcomes
Abstract
Notre de but était d’étudier les facteurs de risque de SDRA et le devenir des patients brûlés en souffrant. Une étude descriptive a été conduite sur 159 patients brûlés sur plus de 20% SCT traités dans l’Unité de Réanimation pour Brûlés de l’Institut National des Brûlés. La classification de Berlin 2012 a été utilisée pour définir le SDRA. Quarante cinq (28,3%) patients ont développé un SDRA, classé comme sévère pour les 2/3 d’entre eux. Une inhalation de fumée, une surface totale brûlée > 40% et une atteinte profonde > 20% sont des facteurs de risque. La mortalité de ces patients est majeure (80%), dans le cadre de défaillance multiviscérale, en particulier si le SDRA est sévère (p<0,01).
Introduction
Acute Respiratory Distress Syndrome (ARDS), which was first described by Ashbaugh et al. in 1967, is a common syndrome in the intensive care unit and is characterized by severe hypoxemia and non-hydrostatic pulmonary edema. In 1994, a formal definition and classification of ARDS was reported by the American-European Consensus Conference Committee on ARDS (AECC). In 2012, a new definition of ARDS was introduced at the Berlin meeting to overcome limitations of the 1994 AECC definition and to make diagnosis and classification of ARDS easier.1-3
Epidemiologic study indicated that there are about 190,000 cases of this clinical syndrome per year in the United States. Despite advances, mortality from ARDS has been reported to be as high as 60% in developed countries.4 According to previous reports, the incidence of ARDS in burn patients is about 20 - 56% and is one of the leading causes of death among severe burn patients.5 To date, not many reports have mentioned ARDS among burn patients in developing countries. In this study we determined risk factors and outcome of ARDS among adult severe burn patients treated at the National Institute of Burns, Hanoi, Vietnam.
Materials and methods
Patients and methods
Between April 2014 and October 2016, 159 adults with severe burns were admitted to the Burn Intensive Care Unit (BICU), National Institute of Burns, Hanoi. They were classified according to the following criteria: admission within 48 hours post burn; burn surface area ≥ 20% of total body surface area (TBSA). The Berlin definition was used to determine the development of ARDS as well as the severity of hypoxemia and oxygenation among patients with ARDS.2,3 In brief, ARDS is characterized by the following criteria:
lung injury of acute onset, within 1 week of an apparent clinical insult and with progression of respiratory symptoms;
bilateral opacities on chest imaging not explained by other lung pathology (e.g. pleural effusion, pneumothorax or nodules):
respiratory failure not explained by heart failure or volume overload;
severity classification of decreased arterial PaO2/FiO2 ratio:
mild ARDS: ratio is 201 - 300 mmHg
moderate ARDS: 101 - 200 mmHg
severe ARDS: ≤ 100 mmHg.
Inhalation injury was diagnosed by the circumstance of burn injury (closed space), clinical manifestations (facial burn, soot in mouth or pharynx, hoarseness and carbonaceous matter) and confirmation by flexible bronchoscopy performed during the first 3 days of admission.
Protective ventilation strategy with low tidal volume as guided by ARDS network was applied for patients who developed ARDS.6,7 Accordingly, an initial tidal volume of 6 mL/kg of ideal body weight was titrated to keep plateau pressures (alveolar pressure at the end of a paused inspiration) lower than 30 cmH 2O. Positive end expiratory pressure (PEEP) value and FiO2 value were adjusted as guided (Table I).
Table I. PEEP/FiO2 combinations for ventilation of patients with ARDS.

Burn management
All patients received initial fluid resuscitation using intravenous Ringer’s lactate solution, according to the Parkland formula (4ml x kg x %TBSA), with additional fluids given as necessary to obtain a minimum urine output of 0.5 ml/kg/h. Intubation and/or mechanical ventilation was provided if patients had compromised upper airway at admission due to deep burn injury on the face, inhalation injury or loss of consciousness. Early enteral nutrition was supplied through oral or enteral feeding. Burn wounds were dressed once a day using topical antimicrobials such as silver sulfadiazine. Surgical excision of burn eschar and early coverage of excised burn wounds with autografts and allografts were performed during the first 7 days from burn injury. Porcine dermis was used for temporary wound coverage in most cases.
Assessment criteria included rate and severity of ARDS according to the Berlin definition, relationship between demographic criteria, burn severity, inhalation injury and development of ARDS. Mortality rate, and the relationship between ARDS and death rate were also recorded.
Data analysis
Collected data were sub-classified as age, gender, burn surface area, full thickness area, inhalation injury, admission time from burn occurrence: change in assessment criteria was assessed using Chi square and Ttest with Stata intercool 11.0 software. P value ≤.05 was seen as a significant level.
Ethical issues
Ethical clearance was granted by the Committee of Human Research Ethics of the National Institute of Burns, Hanoi, Vietnam.
Results
Table II summarizes the characteristics of the studied patients. Males were predominant with 126 patients, and mean age was 34. Mean total burn surface area was 49.1% TBSA with 20% full-thickness area. Inhalation injury was diagnosed in 40 patients (25.1%) and the mean time from burn occurrence to admission to the National Institute of Burns was around 7h. ARDS developed in 45 of the 159 patients, accounting for 28.3%. The time to onset of ARDS from admission was 7 days, ranging from the 4th to the 22rd day post burn.
Table II. Demographic criteria of patients (n = 159).
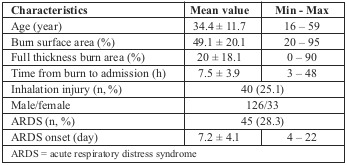
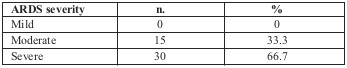
Table III shows ARDS severity according to the Berlin definition (value of PaO2/FiO2): 15 patients (33.3%) were defined as moderate; the remaining 30 patients (66.7%) were classified as having severe ARDS.
Table III. Severity classification of ARDS according to the Berlin definition (n = 45).
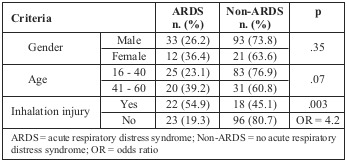
Distribution of ARDS by gender, age group and burn severity is described in Table IV. As can be seen, there was no significant difference between ARDS incidence in males and females. ARDS rate was higher in patients over 40 years of age, but the difference was not significant (39.2% vs. 23.1% respectively; p = .07). A higher incidence of ARDS developed among patients with inhalation injury (54.9% vs. 19.3% respectively; p: .003; OR: 4.2).
Table IV. Distribution of ARDS by gender, age and inhalation injury.
The incidence of ARDS increased along with burn surface area and full thickness burn area (Table V). Frequency of ARDS increased significantly from 7.8% to 31.5% then 73.9% when the burn surface area increased from 40% to 60% TBSA and over. Furthermore, ARDS incidence increased markedly from 15.2% to 26.8% then 72.7% when full thickness burn area increased from 20% to 40% TBSA and over.
Table V. Distribution of ARDS by burn extent.
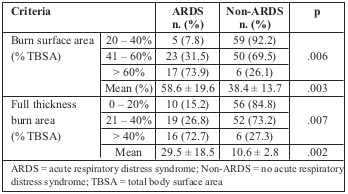
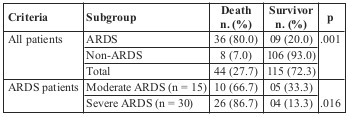
Table VI shows that there was no significant relationship between ARDS development and admission plasma glucose concentration (8.8 ± 3.2 mmol/l and 7.8 ± 3.2 mmol/l respectively; p > .05). Meanwhile, patients who developed ARDS were transfused a remarkable amount of fresh frozen plasma (FFP) and red blood cells (RBC) compared to the non-ARDS group. Analysis of the relationship between number of absolute risk factors, including burn surface area > 40% TBSA, full thickness burn area > 20%, and presence of inhalation injury, and the development of ARDS showed that ARDS incidence significantly increased along with the number of risk factors. ARDS incidence was 2.22% with no risk factors, 15.54% and 20.49% with one and two risk factors respectively, and up to 51.17% when three risk factors presented (Fig. 1).
Table VI. Relationship between ARDS development and other criteria.
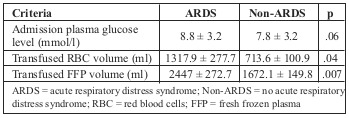
Fig. 1. Relationship between number of risk factors and ARDS development.
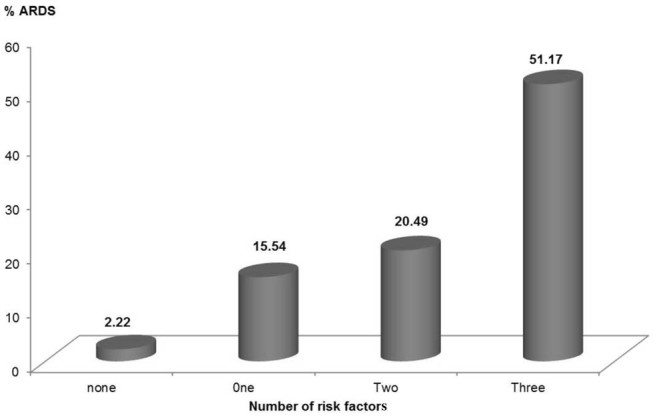
Table VII indicates that the overall mortality rate was 27.7% (44 patients). Only 9 of the 45 patients who developed ARDS survived, accounting for 20%. ARDS patients with severe oxygenation disorder had a mortality rate of 86.7%, which was significantly higher than the rate for those with moderate hypoxemia (66.7%), where P < 0.05. MOF contributed to 22 (61.1%) of the 36 deaths among the ARDS patients (data not shown).
Table VII.
Discussion
The term “Acute Respiratory Distress Syndrome” was first used in 1967 to describe a distinct clinical entity characterized by acute abnormality of both lungs. In 1994, the AECC established criteria for the diagnosis of ARDS.1 The AECC definition was widely adopted by clinical researchers and clinicians, and has advanced the knowledge of ARDS and led to improvements in the quality of care for ARDS patients. The Berlin Definition addresses some of the limitations of the AECC definition, including clarification of the exclusion of hydrostatic edema and adding minimum ventilator settings, and provides slight improvement in predictive validity.2 Despite substantial progress in understanding ARDS pathophysiology, ARDS remains a major clinical problem and mortality is still high. Our work is one of a number of studies to apply the Berlin definition to burn patients.
Over the last few decades, the outcome of burn patients has improved as a result of better management of burn shock, more effective topical antimicrobials, better systemic antibiotics, earlier excision, and alternative measures for wound closure. However, it has recently been claimed that ARDS is important in respiratory dysfunction in burns, and studies have indicated that ARDS is still one of the leading complications causing death among burn patients. According to previous reports, the incidence of ARDS in burn patients is about 20 - 56% depending on burn severity.5 A prospective study on ARDS characteristics in burned military casualties using the Berlin definition showed that the prevalence of ARDS was 32.6% of total patients.8 Recently, results from studies on mechanically ventilated burn patients revealed that the incidence of ARDS was 43% and 38.6% of total cases.9,10 Most studies agree that the onset of ARDS was around 6 to 7 days post burn.5,9,11 In our study, the prevalence of ARDS was 28.3% and the time point of ARDS was around the 7th day post burn. The role of age as risk factor for ARDS development is still controversial. Works by Johnston and colleagues concluded that increased age was associated with rising ARDS prevalence among trauma patients.12 A report by Dancey et al. showed that among 126 burn patients, ARDS significantly developed in the group of older patients (50.2 ± 16.9 vs. 43.2 ± 18.4 years old, respectively; p=0.03).5 In addition, Steinvall and colleagues reported that most of the ARDS complications developed in patients over 60 years old.13 Meanwhile, the results of a study by Liffner et al. on 91 burn patients with average burn surface area of 31% and average age of 41.1 years old showed that there was no relationship between age and ARDS development (p = 0.14).14 In this study, the age of the patients ranged from 16 to 60 years old, and we found that higher ARDS incidence developed among those aged over 40 compared to the under 40s (39.2% vs. 23.1% respectively), but the difference was not significant.
It is accepted world-wide that increased burn extent is one of the risk factors for post burn complications, including ARDS.5,8,9 Our results indicate that ARDS development increased along with the presence of risk factors including surface area > 40% TBSA and full thickness burn area > 20% TBSA. The contribution of inhalation injury to the development of ARDS was also investigated. Results from our study showed that inhalation injury increased the odds of ARDS development by more than fourfold (54.9% vs. 19.3%; OR = 4.2). Results from Silva et al.10 also confirm inhalation injury to be a risk factor for ARDS (OR = 9.75; p < .001). However, work by Cartotto et al. in 2016 did not find a relationship between inhalation injury and ARDS development among burn patients.9
Transfusion-related acute lung injury/ARDS (TRALI) was first introduced in 1983 and seen as a risk factor for ARDS in the intensive care unit. To date, there are not many reports on this complication in burn patients. In 2007, Higgins and colleagues reported worsening chest X ray and PaO2/FiO2 ratio within 6 hours after six transfusions among burn patients with ARDS.15 In this study, we found that ARDS patients were given a significant amount of RBC and FFP compared to non-ARDS patients, but no patient in our study was transfused more than 3 units per day during the treatment period. It is necessary to conduct more research on TRALI for burn patients.
A study by Bhadade and colleagues in 2011 revealed a mortality rate for ARDS of 57%, and up to 88% when ARDS was combined with severe sepsis.11 Recently, Villar et al. reviewed ARDS studies from the year 2000 for all patient categories, and concluded that despite the use of lung protective ventilation, the mortality rate for ARDS is still greater than 40%.16 For burn patients, work by Dancey et al. in 1999 indicated mortality among burn patients with ARDS to be 41.8%.5 Recently, Belenkiy et al. reported that nearly one third of patients with ARDS did not survive. Moderate and severe ARDS increased the odds of death more than fourfold and ninefold, respectively.8A report by Cartotto and colleagues indicated that the mortality rate of severe ARDS according to the Berlin definition was 50% of total cases.9 Like other reports, our study howed that mortality rate among ARDS burn patients in Vietnam was still high (80%) and our results also indicated that ARDS patients with severe oxygenation disorder had a mortality rate of 86.7%, significantly higher than those with moderate hypoxemia (66.7%).
To date, the leading cause of death among patients with ARDS is multiple organ failure (MOF). According to Stapleton et al., 50% of ARDS patients died due to MOF.17 Villar et al. reported that MOF contributed to the highest number of deaths among ARDS patients.16 Bhadade and colleagues concluded that mortality increased with the number of organs that failed.11 Most studies indicated that the main cause of death among burn patients with ARDS was MOF rather than lung disorder.8,9 In this study, MOF contributed to 22 of the 36 patient deaths, accounting for 61.1%. The higher mortality rate in our study may be explained by the severity of the patients’ burns and the high rate of inhalation injury, as well as limitations in the facility’s burn care and therapy, like in other developing countries.
Conclusion
We have shown that ARDS incidence was 28.3% among severe burn patients. Incidence of ARDS increased along with the presence of risk factors, including surface area > 40% TBSA, full thickness burn area > 20% and inhalation injury. Overall mortality was 80% and significantly increased with ARDS severity. MOF was the leading cause of death among patients with ARDS.
References
- 1.Bernard GR, Artigas A, Brigham KL, Carlet J. The American-European Consensus Conference Committee on ARDS. Definitions, mechanisms, relevant outcomes and clinical trial coordination. Am J Respir Crit Care Med. 1994;149:818–824. doi: 10.1164/ajrccm.149.3.7509706. [DOI] [PubMed] [Google Scholar]
- 2.Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND. ARDS Definition Task Force. Acute Respiratory Distress Syndrome The Berlin Definition. JAMA. 2012;307(23):2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 3.Fanelli V, Vlachou A, Ghannadian S, Simonetti U. Acute respiratory distress syndrome: new definition, current and future therapeutic options. J Thorac Dis. 2013;5(3):326–334. doi: 10.3978/j.issn.2072-1439.2013.04.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rubenfeld GD, Caldwell E, Peabody E, Weaver J. Incidence and outcomes of acute lung injury. N Engl J Med. 2005;20(353):1685–1693. doi: 10.1056/NEJMoa050333. [DOI] [PubMed] [Google Scholar]
- 5.Dancey DR, Hayes J, Gomez M, Schouten D. ARDS in patients with thermal injury. Intensive Care Med. 1999;25:1231–1236. doi: 10.1007/pl00003763. [DOI] [PubMed] [Google Scholar]
- 6.The Acute Respiratory Distress Syndrome Network: Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000;342:1301–1308. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 7.NIH NHLBI ARDS Clinical Network. http://www.ardsnet.org/files/ventilator_protocol_2008-07.pdf Mechanical Ventilation Protocol Summary. [Google Scholar]
- 8.Belenkiy SM, Buel AR, Cannon JW. Acute respiratory distress syndrome in wartime military burns: Application of the Berlin criteria. J Trauma Acute Care Surg. 2014;76(3):821–827. doi: 10.1097/TA.0b013e3182aa2d21. [DOI] [PubMed] [Google Scholar]
- 9.Cartotto R, Li Z, Hanna S, Spano S. The Acute Respiratory Distress Syndrome (ARDS) in mechanically ventilated burn patients: An analysis of risk factors, clinical features, and outcomes using the Berlin ARDS definition. Burns. 2016;42(7):1423–1443. doi: 10.1016/j.burns.2016.01.031. [DOI] [PubMed] [Google Scholar]
- 10.Silva L, Garcia L, Oliveira B, Tanita M. Acute respiratory distress syndrome in burn patients: incidence and risk factor analysis. Ann Burns Fire Disasters. 2016;29(3):178–182. [PMC free article] [PubMed] [Google Scholar]
- 11.Bhadade RR, de Souza RA, Harde MJ, Khot A. Clinical characteristics and outcome of patients with acute lung injury and ARDS. J Postgrad Med. 2011;57:286–290. doi: 10.4103/0022-3859.90077. [DOI] [PubMed] [Google Scholar]
- 12.Johnston CJ, Rubenfeld GD, Hudson LD. Effect of age on the development of ARDS in trauma patients. Chest. 2003;124:653–659. doi: 10.1378/chest.124.2.653. [DOI] [PubMed] [Google Scholar]
- 13.Steinvall I, Bak Z, Sjoberg F. Acute respiratory distress syndrome is as important as inhalation injury for the development of respiratory dysfunction in major burns. Burns. 2008;34(4):441–451. doi: 10.1016/j.burns.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 14.Liffner G, Bak Z, Reske A, Sjoberg A. Inhalation injury assessed by score does not contribute to the development of ARDS in burn victims. Burns. 2005;31(3):263–268. doi: 10.1016/j.burns.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 15.Higgins S, Fowler R, Callum J. Transfusion-Related Acute Lung Injury in patients with burns. J Burn Care Res. 2007;28(1):56–64. doi: 10.1097/BCR.0b013E31802C88EC. [DOI] [PubMed] [Google Scholar]
- 16.Villar J, Sulemanji D, Kacmarek RM. The acute respiratory distress syndrome: incidence and mortality, has it changed? Curr Opin Crit Care. 2014;20(1):3–9. doi: 10.1097/MCC.0000000000000057. [DOI] [PubMed] [Google Scholar]
- 17.Stapleton RD, Wang BM, Hudson LD, Rubenfeld GD. Causes and timing of death in patients with ARDS. Chest. 2005;128(2):525–532. doi: 10.1378/chest.128.2.525. [DOI] [PubMed] [Google Scholar]


