Summary
Enzymatic escharolysis is an innovative, non-surgical treatment method for severe burn patients as it allows very early, nontraumatic removal of necrotic tissue even on patients whose overall clinical conditions would mandate delaying traditional surgical escharectomy. The aim of this work was to examine aspects related to the “quality” of enzymatic debridement, which is inherently different from surgical debridement. To this end, biopsies harvested from partial thickness burn wounds, before and after enzymatic treatment, were histologically assessed. As is well known, surgical escharectomy removes the necrosis as well as some of its neighbouring healthy tissue, sharply and radically, leaving a perfectly clean and viable wound bed. On the other hand, enzymatic escharolysis is more selective, as it completely wipes out the necrotic portion while sparing unharmed and partially damaged tissue. In this study, only mid-deep partial thickness wounds were examined, and it was observed that partially damaged dermis was always spared by the lytic action. This dermis, however, showed some “homogenization” characteristics, had few vital skin annexes in it, and therefore looked very similar to the scaffold of dermal matrices currently available on the market. This scaffold should be safeguarded with a view to possibly achieving a more complete and functional spontaneous tissue regeneration. Conversely, if this dermal portion is mismanaged, it could desiccate, thus leading to the formation of a neo-eschar with unpredictable clinical evolution. Understanding how escharolysis actually works allowed us to extrapolate fruitful usage suggestions to optimize the procedure and fully exploit its potential.
Keywords: NexoBrid, escharolysis, enzymatic
Abstract
La détersion enzymatique est une technique innovante non chirurgicale permettant l’ablation très précoce et non traumatique des tissus nécrosés même chez des patients dont l’état général nécessiterait de repousser une excision chirurgicale. Le but de ce travail était d’évaluer la « qualité » du débridement enzymatique, par essence différent du traitement chirurgical. À cette fin, nous avons examiné histologiquement des biopsies réalisées avant et après détersion. Il est bien connu que la chirurgie emporte totalement et radicalement la nécrose et une partie du tissu environnant, laissant en place un tissu parfaitement propre et viable. Le débridement enzymatique est plus sélectif, emportant tout le tissu nécrosé sans affecter les tissus sains ou viables. Cette étude ne s’est intéressée qu’aux brûlures intermédiaires et nous avons observé que les régions saines étaient toujours préservées. Ce derme restant apparaît toutefois homogénéisé, avec peu d’annexes viables ce qui fait penser aux matrices des dermes artificiels actuellement commercialisés. Il doit être préservé afin de promouvoir une régénération tissulaire complète et fonctionnelle. Ainsi, si ce derme restant n’est pas correctement pris en charge, il peut se dessécher et aboutir à la formation d’un nouvel escarre, d’évolution imprévisible. Le compréhension du mécanisme exact de la lyse de la brûlure permet de développer des protocoles d’optimisation de la technique de lyse enzymatique.
Introduction
It is largely accepted that early escharectomy is the most important procedure in patients with large and deep burn wounds to reduce the effects of SIRS and consequently improve the patient’s prognostic outcome.1-2 The most commonly used technique is surgery (tangential or epifascial escharectomy), but this is particularly invasive and causes extensive bleeding; moreover, it requires availability of an operating room as well as general anesthesia.3,4,5 In severe burn patients, the requirements to perform this type of escharectomy at a relatively early stage (first 24-48 hours) are not always met for several reasons: the patient may be in the most acute phase of shock, possibly with severe organ dysfunctions, or there may be organizational factors (besides clinical ones) to consider, as might happen in the case of mass casualty incidents with a large number of patients that need to be treated simultaneously. The recent introduction of an enzymatic, bromelain-based topical gel (NexoBrid) provides an important alternative to the surgical approach, as this gel is able to remove thermally damaged tissue rapidly and with minimal bleeding.6,7 The treatment can be performed immediately after the trauma, on maximum 15% of TBSA at a time, directly at the patient’s bedside; only one operator and simple analgesia are required, and blood loss is limited. Thanks to these characteristics, all possible drawbacks of the traditional surgical procedure can be easily overcome.8,9,10
The first clinical experiences carried out in several Burn Centres in Italy confirmed the extraordinary ability of this enzymatic mixture to digest necrotic tissue completely. The gel’s main advantage over less selective surgical methods is that “partially damaged” tissues are spared, which in turn leads to easier spontaneous healing, even in areas where skin grafts would have traditionally been used. Further application experience confirmed most of these aspects, but it also raised a few questions concerning, in particular, the quality of the wound bed obtained with enzymatic debridement. Indeed, after a burn wound is enzymatically debrided, one would expect to obtain a perfectly clean wound bed (as with surgical debridement) that can be covered directly with a skin graft. Clinical observation yielded a significantly different picture, though. In the first instance, enzymatic debridement seemed identical to surgical debridement. The subsequent clinical course, however, was sometimes seen to be different. For example, in the case of deep wounds, if skin grafts were applied directly onto a recently debrided area, graft failure would likely occur. On the other hand, partial thickness wounds (which would be expected to heal spontaneously after debridement) could result in the formation of a new, very thin eschar after a few days; in turn, this would either slowly detach and lead to re-epithelialization or become deeper, thus requiring subsequent surgical removal and dermal-epidermal grafting. Questions were raised, therefore, on the reasons behind this difference vis-à-vis surgical debridement. We thought that the best way to shed some light on it was to histologically analyse some bioptic samples taken from burn wounds before and after escharolysis.
Methods
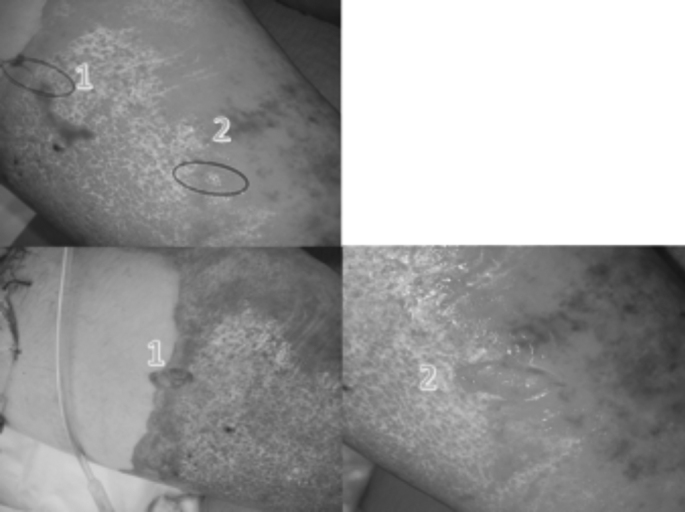
Between December 2015 and May 2016, eight patients with mid-deep and deep burn wounds (average TBSA approximately 30%) were studied. Preferably, wounds located on the front surface of lower limbs were examined. Patient average age was 49 years, with prevalence of males (5 cases). In all cases, the enzymatic treatment was performed within the first 24 hours after the trauma. After carefully removing all epidermal debris and thoroughly rinsing the wound with saline, a biopsy was harvested with a biopsy punch ø 4 mm (Fig. 1) from areas with apparently mid-deep burn wounds. Then, the enzymatic gel was applied. Shortly after the enzymatic debridement, another biopsy was taken from an adjoining area. In one case, two skin lozenges were harvested too, 24 hours after the debridement. These were approximately 3 cm long and included areas of morphologically different tissue (unevenly off-white zones and ecchymotic ones) (Fig. 2). In all, 18 samples were taken: 8 before and 10 after debridement. All samples were immediately fixed in 10% formalin and sent to the Pathological Anatomy Unit of the University Hospital of Pisa. Histology slides were prepared using hematoxylin and eosin staining, then they were photographed under different magnification.
Fig. 1. Post-debridement harvest with Biopsy Punch 4 mm.
Fig. 2. Post-debridement harvest areas (lozenges of skin, 3 cm).
Results
The histology reports provided very similar information in all cases under examination. In biopsies taken before enzymatic debridement, patterns compatible with mid-deep burn wounds were obviously described: completely de-epithelialized areas with foci of coagulative necrosis unevenly widespread throughout the dermis. Inflammatory cells were almost constantly found (predominantly PMN neutrophils) with interstitial or perivascular distribution. Skin annexes looked either partly preserved or with cytologic alterations (pyknotic nuclei with a perinuclear halo) attributable to thermal damage.
In the biopsies taken after escharolytic treatment, the most significant characteristic was observed in the upper area of dermal remnants, which looked “homogenized” (Fig. 3). The few annex structures were described as poorly viable. In some areas (namely those that looked particularly ecchymotic to the naked eye) significant vascular congestion was observed with frequent blood extravasation due to capillary disruption. In posttreatment samples as well, many inflammatory cells were observed. On the other hand, the deep dermis seemed to retain its morphological features and looked normal, although with greater presence of inflammatory infiltrates.
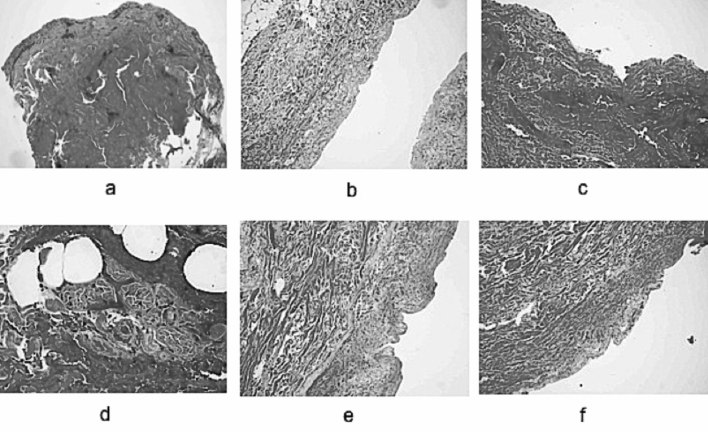
Fig. 3. Biopsy sections before and after debridement with NexoBrid® on partial thickness burn wounds. a) biopsy before NexoBrid. De-epithelialized dermis with areas of coagulative necrosis; b) biopsy after NexoBrid. The dermis is de-epithelialized and the most superficial area looks homogenized; c) post-debridement biopsy on ecchymotic area. The superficial dermis has become denser and infiltrated with erythrocytes; d) at 40x magnification, capillary disruption with blood extravasation is observed. Inflammatory infiltrates and partially damaged annex structures; e) homogenized superficial dermis; deep dermis with normal structure; f) the most superficial dermis shows the presence of areas with residual thermal necrosis and partially viable skin annexes.
Discussion
In our view, histology results made it possible to better understand what happens to tissues that undergo enzymatic treatment. First and foremost, it should be borne in mind that three different zones can be seen in any thermally damaged tissue: the zone of necrosis (at the centre), the zone of stasis (surrounding the former) and the zone of hyperemia (outermost). 11,12 The enzymatic mixture acts fast and quickly only on the zone of necrosis. On the surrounding areas (zone of stasisand hyperemia) this effect is milder: the dermis that is partially damaged by the thermal trauma is only partly affected by enzymes and becomes “homogenized”, thus showing few viable epithelial elements.13 In the areas corresponding to the “zone of hematic stasis”, diffuse hemorrhagic infiltration was observed, which was due to capillary disruption and transfer of erythrocytes to the interstitial space. After debridement, these zones looked like ecchymotic areas surrounding the more severely damaged ones to the naked eye. The natural characteristics of deeper reticular dermis always seemed to be preserved. What does that “homogenized” dermis mean? The answer to the initial clinical question on the difference between enzymatic and surgical escharectomy lies exactly in this layer of not fully viable tissue, whose evolution can be different according to the circumstances. Indeed, if it is protected from desiccation, this dermis might provide a scaffold just like synthetic dermal substitutes. Progressive recolonization of connective, endothelial and epithelial cellular elements would take place, slowly resulting in reepithelialization. On the contrary, if this dermal portion is mismanaged it might desiccate, thus causing the formation of the above mentioned neo-eschar. The possibility of spontaneous wound healing in this latter case is linked to the quantity of viable dermis underneath the neo-eschar: if there is enough of it (i.e. the wounds are not too deep or located in body regions with thicker dermis) the neoeschar will progressively unroof due to the underlying reepithelialization process, but if the dermis is insufficient (i.e. the wounds are deep or located in body regions with thin dermis) the neo-eschar will inevitably affect almost all of it. Consequently, the wound will require surgical cleansing and coverage with skin autografts.
In full thickness wound areas, the complete removal of necrotic tissue exposes a partially viable subcutaneous layer (as with the partial thickness wounds described above). This should be taken into account when considering coverage with a skin graft.
Histological observations and better understanding of the actual debridement action of the enzymatic mixture allowed us to outline a few clinical indications aimed at improving the enzymatic escharolysis procedure. First of all, the appropriateness of the first dressing post debridement and the use of skin grafts were examined. As for the first dressing, the use of proteolytic enzymes (collagenase) is justified as they complete the degradation of necrotic residue and contribute to preventing desiccation of the dermal scaffold. The wet-to-dry technique is also justified because it can mechanically remove part of the necrotic residue that is still found on the wound bed. The use of skin substitutes in this phase should be carefully considered against the need to prevent desiccation of the dermal scaffold, because this would foster the formation of the neo-eschar. Preference should be given to devices that are able to maintain some degree of local moisture (e.g. hydrogels, hydrocolloids) over other synthetic materials. As regards skin grafts, early use of homografts seems to be beneficial, as they can better protect the dermal remnants and facilitate subsequent regenerative processes. Before applying a skin graft, however, it is advisable to scrub the wounds vigorously to perfect the wound bed cleansing and reactivate circulation by reopening occluded capillaries. This procedure is all the more necessary if the decision is taken to use skin autografts right away to repair obvious subdermal wounds. In such a case, it is strongly recommended that the debridement process is completed surgically (with dermatome, dermabrader or Versajet) so as not to jeopardize the chances of graft take.
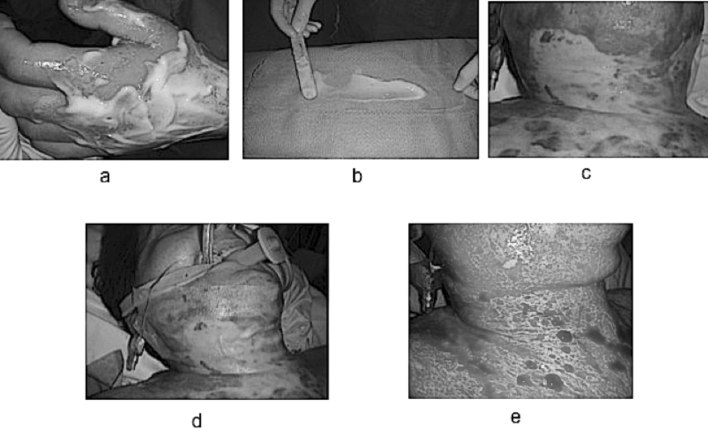
Another - merely technical - aspect that was examined dealt with how to apply the enzymatic gel to burn wounds. Direct observation of what happens immediately after applying the gel, particularly in the case of mid-deep wounds, showed that wounds start bleeding after a very short time. This light blood flow, combined with gravity acting on sloping surfaces, causes the gel to slide off: consequently, its action on those areas may not be complete (could this be the cause for the partial debridement of partially viable zones?) (Fig. 4a). To minimize this, it was deemed appropriate to “block” the gel into position by spreading it on a non-adhesive dressing (Fig. 4b) before applying it directly onto the wound (Figs. 4c and 4d). This technique reduced gel loss and increased the effectiveness of the lytic action (Fig. 4e).
Fig. 4. a) sliding of the enzymatic gel due to bleeding and gravity; b) “blocking” the gel into position by spreading it on a non-adhesive dressing; c) deep burn wound on front neck and thorax; d) applying it directly onto the wound; e) increased the effectiveness of the lytic action.
Finally, this study helped to identify patients who, in our view, are potentially eligible for enzymatic treatment: all patients with mid-deep or deep burn wounds, regardless of burn size, who cannot undergo surgery for various reasons, obviously fall into this category. In those cases in which early surgery is an option, the choice whether to complement it with enzymatic escharolysis should take into account some factors, namely wound location and size. We believe that small, deep wounds located in functionally irrelevant areas should preferably be treated with surgical escharectomy followed by immediate repair with autografts. This approach might be reconsidered and escharolysis might be preferred if functionally sensitive areas are involved, or if surgical debridement proves particularly complex (for instance on hands, neck, perineum and feet). Enzymatic escharolysis plays a significant role with larger wounds (TBSA > 20-30%): indeed, in these cases it should be used in conjunction with surgery to achieve complete removal of the necrosis faster, less invasively and with reduced blood loss. Because of its selectiveness, then, escharolysis might encourage spontaneous healing in “borderline” areas with mid-deep wounds (which are difficult to spare with the surgical approach), thus curtailing the need to use skin autografts, whose availability may be limited
Conclusion
The histological assessment of wounds before and after the escharolytic treatment was extremely useful for understanding how the enzymatic mixture actually works. Nexo- Brid®’s cleansing action is excellent on full thickness wounds and offers all the benefits of early removal of the necrosis and minimal blood loss on the patient’s metabolic homeostasis as well as on severity and evolution of SIRS and the incidence of possible infectious complications. It was deemed advisable to shed light on some clinical aspects concerning the spontaneous healing potential of partial thickness wounds, which seemed to vary from case to case with sometimes unexpected or unforeseeable outcomes. The histological findings of post-debridement biopsies showed structural alterations affecting the dermal remnants after removal of the necrosis. As a matter of fact, only the deeper layers of this dermis are normal. In its most superficial ones, a sort of “homogenization” is observed, with partial viability and residual thermal damage. With adequate management, though, this dermal portion can recover. This tissue may indeed be compared to a scaffold that is subsequently and progressively revitalized from the bottom, thus slowly leading to spontaneous healing. Recovery of this dermal layer seems to be linked to better morphological results after the treatment. On the contrary, if the scaffold is not adequately managed (inappropriate dressings, infections, etc.) a neo-eschar may develop, and its evolution may be either favourable, with spontaneous detachment and healing, or unfavourable, thus requiring a surgical reconstruction treatment. Microscopic evaluation of the quality of tissue remnants after escharolysis and macroscopic observation of usual events provided useful indications on how to optimize the whole procedure involving enzymatic gel – from its application onto wounds to the management of the post-debridement phase and the methods and timings for the use of autologous and homologous skin grafts. Finally, potentially eligible patients for enzymatic treatment were defined, and it was highlighted that this method should be adopted in conjunction with surgery on severe burn patients in order to mitigate invasiveness of surgical procedures and hopefully reduce the need for skin autografts thanks to its greater selectivity.
References
- 1.Janzekovic J. new concept of early excision and immediate grafting of burns. J Trauma. 1970;10:1103–1108. [PubMed] [Google Scholar]
- 2.Herndon DN. A comparison of conservative versus early excision therapies in severely burned patients. Ann Surg. 1989;209:547–553. doi: 10.1097/00000658-198905000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mzezewa S. A prospective double blind randomized study comparing the need for blood transfusion with terlipressin or a placebo during early excision and grafting of burns. Burns. 2004;30(3):236–240. doi: 10.1016/j.burns.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 4.Mosier MJ, Gibran NS. Surgical excision of the burn wound. Clin Plast Surg. 2009;36:617–625. doi: 10.1016/j.cps.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 5.Rosenberg L. Selectivity of a bromelain-based enzymatic debridement agent: A porcine study. Burns. 2012;30:466–474. doi: 10.1016/j.burns.2012.02.011. [DOI] [PubMed] [Google Scholar]
- 6.Rosenberg L. A novel rapid and selective enzymatic debridement agent for burn wound management: A multi-center RCT. Burns. 2009;40:466–474. doi: 10.1016/j.burns.2013.08.013. [DOI] [PubMed] [Google Scholar]
- 7.Hauben DJ, Mahler D. Histological investigation of burn eschar and underlying recipient area in tangential early excision of burns. Burns. 1979;5:160. [Google Scholar]
- 8.Desai MH. Early burn wound excision significantly reduces blood loss. Ann Surg. 1990;211(6):753–759. doi: 10.1097/00000658-199006000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Luo G. Blood loss during extensive escharectomy and auto-microskin grafting in adult male major burn patients. Burns. 2011;37(5):790–793. doi: 10.1016/j.burns.2011.01.021. [DOI] [PubMed] [Google Scholar]
- 10.Adam J, Singer MD. Rapid and selective enzymatic debridement of porcine comb burns with bromelain-derived debrase: acute-phase preservation of noninjured tissue and zone of stasis. J Burn Care Res. 2010;31(2):304–309. doi: 10.1097/BCR.0b013e3181d0f4d4. [DOI] [PubMed] [Google Scholar]
- 11.Kagan RJ. American Burn Association White Paper. Surgical management of the burn wound and use of skin substitutes. American Burn Association. 2009 doi: 10.1097/BCR.0b013e31827039a6. [DOI] [PubMed] [Google Scholar]
- 12.Adam J, Singer MD. The effects of rapid enzymatic debridement of deep partial-thickness burns with Debrase® on wound reepithelialization in swine. J Burn Care Res. 2010;31(5):795–802. doi: 10.1097/BCR.0b013e3181eed48e. [DOI] [PubMed] [Google Scholar]
- 13.Rosenberg L. Safety and efficacy of a proteolytic enzyme for enzymatic burn debridement: a preliminary report. Burns. 2004;30:843–850. doi: 10.1016/j.burns.2004.04.010. [DOI] [PubMed] [Google Scholar]