Summary
Limited information exists regarding endothelial dysfunction following burn injury. This project aims to evaluate whether thermal injury results in shedding of the endothelial glycocalyx in a manner quantitatively proportional to injury severity, and whether theloss of intact glycocalyx is measurable in end organs. C57BL/6 mice were grouped as uninjured controls, 10% or 25% Total Body Surface Area (TBSA) scald burns. Blood and tissue sampling was performed over a specific time course. Plasma levels of shed syndecan-1, a marker of glycocalyx damage, were quantified by ELISA. Lung and spleen sections were stained with immunofluorescent anti-syndecan-1 antibodies to evaluate intact glycocalyx. Plasma syndecan-1 levels were higher in injured versus uninjured animals. Normalized levels of syndecan-1 in burned mice were significantly increased compared to hour 0 (p<0.05) at hours 4 and 8 post-injury in the 10% TBSA, and at hour 4 in the 25% TBSA group. Levels in the 10% and 25% TBSA groups peaked at hour 4 with fold change of 2.3 and 2.4 respectively. There was less pulmonary syndecan-1 immunostaining in burned animals compared to controls, and the levels inversely correlated with systemic shed syndecan- 1, beginning at hour 4 in the 10% TBSA injury group and at all time points in the 25% TBSA injury group, (0.27±0.06 and 0.14±0.04 respectively for hour 4). Similarly, there was less spleen syndecan-1 immunostaining in burned animals compared to controls at all time points. Burn injury causes shedding of syndecan-1 in a murine model, with levels correlated to injury severity and loss of the glycocalyx in lung and spleen. This work provides further insight into quantification and temporality of glycocalyx damage and systemic response to burn
Keywords: endothelial glycocalyx, Syndecan-1, glycocalyx shedding, endothelial surface layer
Abstract
Les données concernant la dysfonction endothéliale après brûlure sont parcellaires. Les buts de cette étude étaient d’établir une corrélation entre la perte de glycocalyx et la gravité de la brûlure et si cette perte était mesurable au niveau des organes. Des souris C57BL/6 ont été réparties en groupes contrôle, brûlure 10% et brûlure 25% de SCT. Des prélèvements de sang et de tissus ont été réalisés à intervalles prédéterminés. Les taux plasmatiques de syndecan 1 (S1), marqueur de lésion du glycocalyx, ont été mesuré par méthode ELISA. Des échantillons de poumon et de rate ont été mis en présence d’anticorps anti S1, afin d’évaluer le glycocalyx intact. Les taux plasmatiques de S1 étaient plus élevés que ceux du groupe contrôle. Chez les souris brûlées sur 10% de SCT, les taux de S1 à 4h et 8 h étaient supérieurs au taux avant brûlure, ceci n’étant observé qu’à h4 chez les souris brûlées sur 25% de SCT. Le pic de S1 se produisait à h4, avec un rapport de x2,3 (10%) et x2,4 (25%) par rapport à la valeur de base. A partir de h4, on observait une baisse de complexes S1-antiS1 dans les poumons des souris brûlées sur 10% (0,27 +/- 0,06), inversement corrélée aux taux plasmatiques de S1. Cette observation se répétait lors de tous les dosages chez les 25% (0,14 +/- 0,04 à h4). Les mêmes constatations étaient faites sur les échantillons de rate. La brûlure cause des lésions du glycocalyx, parallèles à sa gravité. Ces travaux ouvrent le champ à des recherches futures sur les lésions du glycocalyx et la réponse inflammatoire aux brûlures.
Introduction
The endothelial glycocalyx (EGL) is a complex and highly dynamic layer of carbohydrate and protein components coating the endothelial surface of blood vessels, which contributes to the maintenance of vascular integrity. The glycocalyx is bound to endothelial cells through a backbone of core proteins of each molecule.1,2 The glycoproteins are comprised of adhesion molecules, as well as molecules important in coagulation, fibrinolysis and hemostasis. The proteoglycans, such as syndecan-1, contain negatively charged glycosaminoglycan (GAG) moieties, which include heparan sulfate, chondroitin sulfate and hyaluronan chains.1-4 The complex sulfate patterns of these GAG side chains allow for ligand specificity and dynamic plasma protein interactions, generating continuous structural changes in the EGL.1-3 The EGL is variable in structure and has diverse functionality in endothelial physiology. It regulates cell adhesion and migration, signaling pathways, vascular tone and endothelial permeability, and inhibits intravascular thrombosis.1-3,5-7 As a result of shear forces and numerous biological stressors, the surface layer equilibrium is easily disrupted and may cause components of the EGL to be cleaved by various proteases or mechanical shearing in a process termed shedding.1-8 This process occurs to a certain degree in normal endothelial cell turnover, and at much higher rates in pathologic states. Factors contributing to shedding include alterations in pH, mechanical stimuli, loss of anionic charges and direct endothelial injury.5 Disruptions in EGL integrity can involve loss of the backbone molecules including GAG side chains entirely, or limited damage to individually cleaved side chains.9 Subsequently, shed components circulate in the bloodstream and mediate physiologic changes at distant sites resulting in altered vascular tone, endothelial integrity and permeability, inflammation and coagulopathy. 2,5,7,10-14 Syndecan-1 (SDC-1), a major endothelial cell-bound proteoglycan, is an important biomarker of glycocalyx damage when measured in plasma.5,15,16 EGL shedding has been examined in various pathologic states including diabetes, ischemia/reperfusion, atherosclerosis, pulmonary fibrosis, sepsis, and surgical and traumatic injury. 4-6,9,12-19 Several studies have shown increased shedding of syndecan-1 following hemorrhagic shock, with high levels associated with excessive inflammation, aberrancies in coagulation, and increased mortality in both animal models and human studies.11,14,15,17,19 Rahbar et al. demonstrated that increased syndecan-1 shedding in trauma patients was associated with low plasma oncotic pressure, increased endothelial cell permeability and higher injury severity.6 There is evidence that the shed GAG side chains exhibit anticoagulant activity in a phenomenon termed endogenous heparinization, which may play a role in trauma-induced coagulopathy.9
Systemic impacts of burn injury remain poorly understood. Massive tissue destruction, edema, intravascular volume depletion, cytokine activation and increased systemic vascular resistance all contribute to the development of burn shock, and mitigating these factors while managing fluid resuscitation is complicated. To date, only one scientific group has examined the effect of burn injury on the EGL by looking at the relationship between shed syndecan-1 and pseudomonas infectivity. This work additionally outlines a potential role for syndecan- 1 in the innate immune system.8 However, no prior work has documented the effect of burn injury severity on EGL degradation. To explore this relationship and the systemic impact of EGL degradation, a controlled murine scald burn model was implemented, hypothesizing that thermal injury results in shedding of syndecan-1 in a burn-size-dependent manner, and that the loss of glycocalyx can be quantified by measuring intact syndecan-1 in an end organ.
Materials and methods
Animals
The MedStar Health Research Institute Institutional Animal Care and Use Committee (IACUC) reviewed and approved all procedures and animal work described. Male C57BL/6 mice, 8-10 weeks old, weighing approximately 30g, were obtained from Jackson Laboratory and used in all experiments. Animals were maintained and housed per facility standard operating procedures.
Injury
Animals were fully anesthetized with isoflurane gas induction followed by an intraperitoneal injection of Ketamine/Xylazine (100 mg/kg and 12.5 mg/kg) prior to all procedures, and were subsequently monitored for signs of pain and distress. The mice then had their dorsum, flank and ventral areas clipped of hair, followed by application of Veet hair remover (Reckitt Benckiser, Parsippany, NJ) to ensure complete alopecia. Mice were then randomly assigned to one of three groups: uninjured control, 10% TBSA burn, and 25% TBSA burn. Using a previously described model,20-22 animals were then secured within a protected plastic template with a window exposing only the predetermined skin surface area, as calculated by the Meeh equation.23 Thermal injury was induced by submerging the exposed skin area in a 100°C-water bath. A 2 cm x 4.5 cm window with 7-second exposure was used to create a single burn on animals assigned to the 10% TBSA injury. A 2 cm x 5 cm window was implemented to approximate a 25% TBSA group, creating both a dorsal (7-second exposure) and a ventral injury (2-second exposure). Injury depth was confirmed by histological examination of biopsied sections of the wounds. Immediately post-injury, mice were administered intraperitoneal buprenorphine (0.05 mg/kg) for analgesia, and 1 or 1.5 mL of Lactated Ringer’s solution respectively for the 10% vs. 25% injury groups. Sham animals received analgesia and resuscitation equivalent to the 10% TBSA group and were used as controls at equivalent time points.23,24 Groups of animals were then euthanized post-injury at hours 0, 2, 4 or 8. The “Hour 0” group was euthanized approximately 10 minutes after injury, following LR resuscitation. All others were maintained on a heating pad until recovery. All time points and injury severity groups included n=3 animals. Euthanasia and blood collection were performed via cardiac stick. Lungs and spleens were harvested and stored in 10% neutral buffered formalin.
Enzyme-linked immunosorbent assays (ELISA)
Whole blood was collected into BD Microtainer tubes with dipotassium EDTA (BD Biosciences, San Jose, CA) and centrifuged per manufacturer guidelines to obtain plasma. Plasma samples were stored at -80°C until use. To assess endothelial degradation, syndecan-1 was quantified in plasma using a commercial ELISA per the manufacturer’s protocol (mybiosource.com, MBS745791, San Diego, CA). Monoclonal anti-SDC-1 antibody and a SDC-1-HRP conjugate were utilized in a competitive ELISA. Samples were run in duplicate at a 1:4 dilution in phosphate-buffered saline (PBS). Absorbance was measured at 450 nm and corrected by comparison to wells containing PBS alone. ELISA standard curves were fitted using a four-parameter polynomial function.
Syndecan-1 immunofluorescence
Lung or spleen tissue was fixed, sectioned and stained with rabbit anti-mouse SDC-1 antibody (Biorbyt, Cambridge, UK) and fluorescent goat anti-rabbit IgG Cy5 secondary antibody (Abcam, Cambridge, MA), following deparaffinization and antigen retrieval with citrated buffer.11,25 Sections were counterstained with DAPI to visualize nuclei. Images of 5 different high powered-fields were taken from each lung or spleen section at 40X using a Zeiss Axio Imager microscope and fluorescence was quantified using Zen software (Zeiss Microscopy, Oberkochen, Germany). Fluorescence measurements were normalized to DAPI to account for differences in cellularity. In the case of the spleen tissue, which showed extreme variability in the Hour 0 levels of syndecan-1, the hour 2, 4, and 8 time points were normalized to Hour 0 within each injury group. Secondary controls incubated without primary antibody were performed and factored into the analysis.
Statistical analysis
All statistical analyses were conducted using commercially available software (GraphPad Prism 6.0, La Jolla, CA). Both ELISA and immunofluorescence data were grouped by time point and injury severity and differences between experimental groups and sham controls were assessed using a student’s ttest. Data is expressed as means plus standard deviation, with n=3 animals per group. Two-way ANOVA with Tukey’s test to correct for multiple comparisons was used to evaluate ELISA data expressed as concentration and fold change. Significance was achieved when p<0.05.
Results
Plasma syndecan-1 quantification
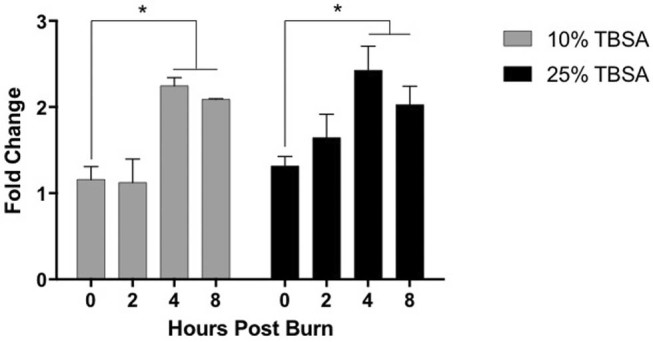
Mice were thermally injured with either a 10% TBSA (9.88 ± 0.37) or a 25% TBSA (25.81 ± 0.57) scald burn. Higher levels of syndecan-1 were measured in the plasma of burned mice compared to sham animals at all time points, with statistical significance noted at hour 4 and 8 (Fig. 1). The level of syndecan-1 in sham animals did not show significant change over time. Data for injured animals were normalized to uninjured sham animals at equivalent time points and are presented as fold changes (Fig. 2). In the 10% TBSA injury group, the amount of shed SDC-1 peaked at hour 4, exceeding twice the amount shed in sham animals. The normalized levels at hours 4 and 8 were significantly increased from hour 0 (p=0.0034 and p=0.0169 respectively). In the 25% TBSA injury group, a nearly 2.5-fold increase in shed syndecan-1 was observed compared to uninjured sham animals by hour 4, which is a significant increase when compared to hour 0 (p=0.0209). Examining the two experimental groups side by side demonstrates a clear peak in shed syndecan-1 at hour 4, the significance of which decreases over subsequent time points. There was a trend towards earlier and higher level of shedding in the 25% injury group compared to the 10% injury group.
Fig. 1. Syndecan-1 quantified in plasma. Shed syndecan-1 quantified using ELISA in mice injured with either a 10% TBSA or 25% TBSA scald injury compared to uninjured sham animals over time. Data is shown as mean ±SD, n=3 per group. p<0.05.
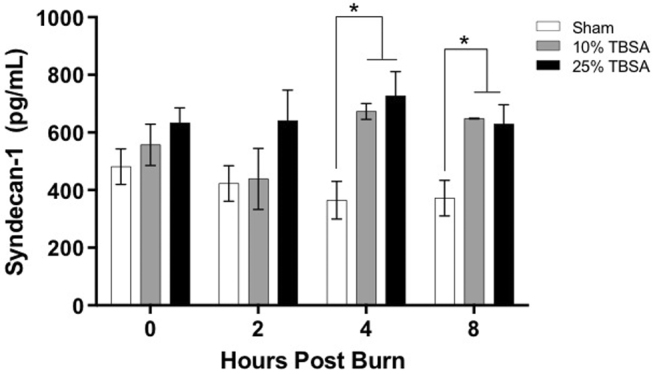
Fig. 2. Shedding of syndecan-1 is dynamic. Shed Syndecan-1 in plasma of burn-injured animals was normalized to levels in uninjured sham animals at similar time points. Data is shown as fold-change from sham levels with standard deviation, n=3 per, p<0.05.
Pulmonary and spleen syndecan-1 immunofluorescence
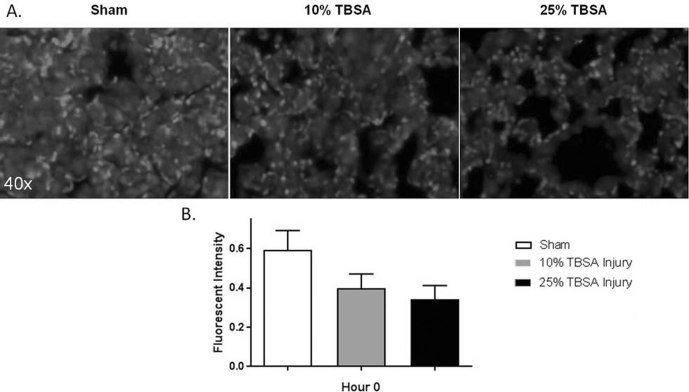
The inverse correlation between end-organ levels of syndecan and shed plasma syndecan has been identified in prior studies examining non-burn injuries.25,26 In our study design, immunostaining targeted intact syndecan-1 present in pulmonary and spleen tissue, with less intense staining presumably resulting from higher proportions of syndecan-1 shedding. Pulmonary syndecan-1 immunostaining fluorescent intensity was lower in injured animals compared to sham animals immediately post-injury for both injury groups (Fig. 3A). At hour 0, fluorescent intensities of 0.60±0.10, 0.40±0.07 and 0.30±0.07 were quantified in sham, 10% TBSA and 25% TBSA groups respectively (Fig. 3B), demonstrating less intact pulmonary syndecan-1 in response to injury.
Fig. 3. Pulmonary syndecan-1 is decreased after burn injury. (A) Representative images of lung tissue immunofluorescently stained for intact syndecan- 1 immediately post injury at hour 0. (B). Syndecan-1 immunostaining in lung tissue was decreased when compared to sham animals immediately post-injury in both injury groups.
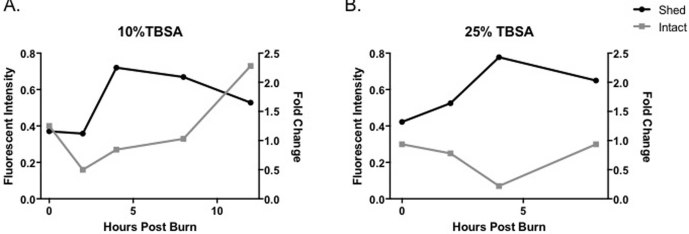
Quantified pulmonary syndecan-1 continued to decrease at hour 4 with 0.27±0.06 in the 10% TBSA injury and 0.14±0.04 in the 25% TBSA injury. The smallest amounts of SDC-1 in the lung tissue were quantified at hour 2 in the 10% TBSA injured group and at hour 4 in the 25% injured group, corresponding to the peak in shed syndecan-1 quantified in the plasma at the same time point. When compared, immunostaining of intact syndecan-1 showed an inverse relationship with systemically shed syndecan-1 detected in plasma beginning at hour 4 in the 10% TBSA injury (Fig. 4A), and at all time points in the 25% TBSA injury (Fig. 4B). Intact syndecan-1 levels in the lung approximated baseline levels at hour 8. These findings may be a result of protein upregulation following disruption, potentially demonstrating an early restorative response, which may provide an interesting target for future research.
Fig. 4. Pulmonary syndecan-1 inversely correlates with systemic shed syndecan-1 in plasma. (A) Comparison of intact pulmonary (measured in fluorescent intensity, left Y-axis) and shed plasma syndecan-1 (measured in fold change, right Y-axis) after a 10% TBSA and a 25% TBSA burn injury over time. Immunostaining of intact syndecan-1 inversely correlated with systemically shed syndecan-1 detected in plasma beginning at hour 4 in the 10% TBSA injury and (B) at all time points in the 25% TBSA injury.
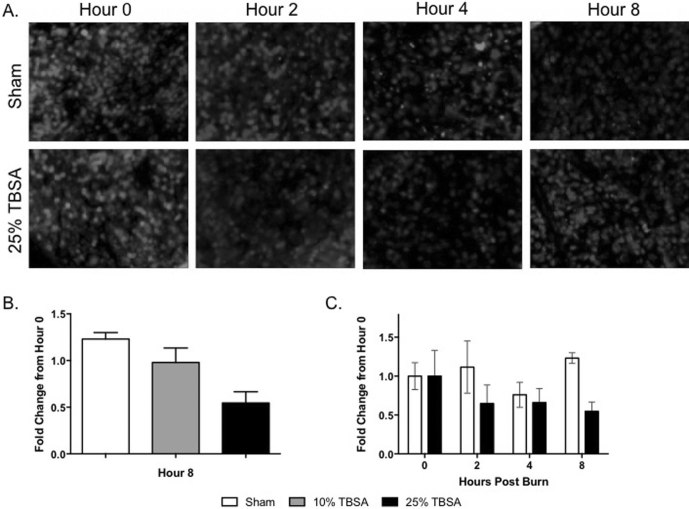
Intact syndecan-1 was also identified in spleen tissue in the burn and sham groups. At hour 0, the sham and 25% TBSA groups had high levels of intact syndecan (Fig. 5A). In the sham group, this intact syndecan-1 did not change strikingly over time, while the 25% TBSA group showed less intact staining after burn injury. Similar to the hour 0 pulmonary staining discussed above, at hour 8, spleen syndecan-1 immunostaining intensity was lower in 10% and 25% injured animals in a manner proportional to injury severity, being 1.23±0.11 vs. 0.98±0.26 vs. 0.55±0.17 for sham, 10% TBSA, and 25% TBSA respectively (Fig. 5B). At hours 2 (1.12±0.58 vs. 0.65±0.33), 4 (0.92±0.22 vs. 0.67±0.31) and 8 (1.23±0.11 vs. 0.54±0.17), there were higher levels of syndecan-1 in the sham group than in the 25% TBSA injured group (Fig. 5C).
Fig. 5. Spleen syndecan-1 is decreased after burn injury. (A) Representative images of spleen tissue immunofluorescently stained for intact syndecan- 1 immediately post injury at hour 0 and at hours 2, 4, and 8. (B) Syndecan-1 immunostaining in spleen tissue was decreased when compared to sham animals at hour 8 in both injury groups. (C) At each time point examined, levels of syndecan-1 in the spleen were lower in the 25% TBSA injury group compared to the sham group when fluorescent intensity was quantified. Syndecan-1 (Pink) DAPI (Blue).
Discussion
Our study demonstrates syndecan-1 shedding as a result of burn injury in a rodent scald model, and explores the variations in shedding in relation to injury severity and time, an entity previously undocumented in the literature. We report reproducible trends that point to a dose-dependency between burn severity and EGL degradation, with valuable dynamics in the temporality of syndecan shedding. Our findings are further substantiated by a predictable reduction in intact syndecan-1 in end organ measurements.
The relationship between injury severity and glycocalyx degradation is testament to the massive capillary leak and other systemic implications seen in burn shock patients. Previous research has shown mitigation of glycocalyx damage with manipulation of resuscitation in hemorrhagic shock, specifically with the administration of fresh frozen plasma (FFP).17,26,27 It is hypothesized that FFP mobilizes existing intracellular syndecan- 1 to restore the endothelial surface layer, and inhibits protease activity to halt continued shedding.17 Administration of FFP after burn injury results in decreased volume requirements, intra-abdominal pressures and edema.28,29 The beneficia effects of FFP administration likely are, at least in part, secondary to decreased syndecan-1 shedding and improved resolution of EGL damage. Based on these data, it is possible that FFP and other colloids could improve outcomes in both the experimental and clinical setting, although our laboratory has not yet explored this prospect.
We recognize there are several limitations to this study. Future work will require inclusion of additional animals and larger injury groups to empower our findings. As evidenced in the literature, syndecan-1 is found in both endothelial and epithelial cell surfaces, and can be shed from a variety of tissue types, making the origin of shed syndecan-1 in plasma uncertain. Acknowledging the prior work in a hemorrhagic shock model by Peng et al., which demonstrated a similar inverse correlation between plasma and intact pulmonary syndecan-1, we agree that although SDC-1 is likely shed from multiple anatomic locations as a systemic response to injury, the lung and its vasculature are reliable and measurable sources of intact SDC-1. Existing literature analyzing this relationship, as well as prior researcher experience, were the primary reasons for selecting SDC-1 as a target marker and lung as a focus organ in our initial model. It is possible that the lung is of particular vulnerability to endothelial damage in response to burn injury, similarly to the known models in hemorrhagic shock.11,25 To expand upon the findings obtained with the lung tissue, spleen tissue was also stained for syndencan-1 and was able to confirm our findings. There seems to be a slightly different time dependency in the shedding of syndecan-1 from lung compared to spleen. This relationship should be further explored. In our experimental protocol, additional organs, including skin, were harvested and preserved for the possibility of future analysis not performed for the purposes of this small pilot project.
Conclusion
Ongoing research is directed at analyzing the impact of burn-induced glycocalyx degradation both at the organ-specific and at the systemic levels. Delineating the effects of thermal injury on endotheliopathy, including vascular permeability, inflammation and coagulation, will guide future therapeutic interventions to maintain vascular integrity through regulation of glycocalyx shedding. Improved insight into burn-induced glycocalyx damage and its downstream effects is essential to understanding shock and refining resuscitation regimens. This work expands the current acumen by exploring quantification and temporality of damage to the glycocalyx following burn injury, underscoring the impact of endotheliopathy on treatment paradigms and patient outcomes.
Acknowledgments
Funding.Financial support and sponsorship: DC Firefighters Burn Foundation, MedStar Washington Hospital Center Graduate Medical Education Resident Research Grant
Conflict of interestNone
References
- 1.Reitsma X, Slaaf DW, Vink H. The endothelial glycocalyx: composition, functions, and visualization. Pflugers Archiv - European Journal of Physiology, 2007;454(3):345–359. doi: 10.1007/s00424-007-0212-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alphonsus CS, Rodseth RN. The endothelial glycocalyx: a review of the vascular barrier. Anaesthesia. 2014;69(7):777–784. doi: 10.1111/anae.12661. [DOI] [PubMed] [Google Scholar]
- 3.Tarbell JM, Pahakis MY. Mechanotransduction and the glycocalyx. J Intern Med. 2006;259(4):339–350. doi: 10.1111/j.1365-2796.2006.01620.x. [DOI] [PubMed] [Google Scholar]
- 4.Chappell D, Brettner F, Doerfler N. Protection of glycocalyx decreases platelet adhesion after ischaemia/reperfusion: an animal study. Eur J Anaesthesiol. 2014;31(9):474–481. doi: 10.1097/EJA.0000000000000085. [DOI] [PubMed] [Google Scholar]
- 5.Chelazzi C, Villa G, Mancinelli P. Glycocalyx and sepsis-induced alterations in vascular permeability. Critical Care (London, England) 2015;19:26. doi: 10.1186/s13054-015-0741-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rahbar E, Cardenas JC, Baimukanova G. Endothelial glycocalyx shedding and vascular permeability in severely injured trauma patients. J Transl Med. 2015;13:117. doi: 10.1186/s12967-015-0481-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Becker BF, Chappell D, Bruegger D. Therapeutic strategies targeting the endothelial glycocalyx: acute deficits, but great potentiaL. Cardiovasc Res. 2010;87(2):300–310. doi: 10.1093/cvr/cvq137. [DOI] [PubMed] [Google Scholar]
- 8.Haynes A 3rd, Ruda F, Oliver J. Syndecan 1 shedding contributes to Pseudomonas aeruginosa sepsis. Infection & Immunity. 2005;73(12):7914–7321. doi: 10.1128/IAI.73.12.7914-7921.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ostrowski SR, Johansson PI. Endothelial glycocalyx degradation induces endogenous heparinization in patients with severe injury and early traumatic coagulopathy. [Article]. J Trauma Acute Care. 2012;73(1):60–66. doi: 10.1097/TA.0b013e31825b5c10. [DOI] [PubMed] [Google Scholar]
- 10.Fitzgerald ML, Wang Z, Park PW. Shedding of syndecan-1 and - 4 ectodomains is regulated by multiple signaling pathways and mediated by a TIMP-3-sensitive metalloproteinase. J Cell Biol. 2000;148(4):811–824. doi: 10.1083/jcb.148.4.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Peng Z, Pati S, Potter D. Fresh frozen plasma lessens pulmonary endothelial inflammation and hyperpermeability after hemorrhagic shock and is associated with loss of syndecan 1. [Miscellaneous Article] Shock. 2013;40(3):195–202. doi: 10.1097/SHK.0b013e31829f91fc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hayashida K, Parks WC. Syndecan-1 shedding facilitates the resolution of neutrophilic inflammation by removing sequestered CXC chemokines. Blood. 2009;114(14):3033–3043. doi: 10.1182/blood-2009-02-204966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Torres LN, Sondeen JL, Dubick MA. Systemic and microvascular effects of resuscitation with blood products after severe hemorrhage in rats. J Trauma Acute Care Surg. 2014;77(5):716–723. doi: 10.1097/TA.0000000000000448. [DOI] [PubMed] [Google Scholar]
- 14.Kozar RA, Peng Z, Zhang R. Plasma restoration of endothelial glycocalyx in a rodent model of hemorrhagic shock. [Report]. Anesthesia & Analgesia. 2011;112(6):1289–1295. doi: 10.1213/ANE.0b013e318210385c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johansson PI, Stensballe J, Rasmussen LS. high admission syndecan-1 level, a marker of endothelial glycocalyx degradation, is associated with inflammation, protein C depletion, fibrinolysis and increased mortality in trauma patients. [Article]. Annals of Surgery. 2011;254(2):194–200. doi: 10.1097/SLA.0b013e318226113d. [DOI] [PubMed] [Google Scholar]
- 16.Jepsen CH, deMoya MA, Perner A. Effect of valproic acid and injury on lesion size and endothelial glycocalyx shedding in a rodent model of isolated traumatic brain injury. [Article]. Journal of Trauma and Acute Care Surgery. 2014;77(2):292–297. doi: 10.1097/TA.0000000000000333. [DOI] [PubMed] [Google Scholar]
- 17.Haywood-Watson RJ, Holcomb JB, Gonzalez EA. Modulation of syndecan-1 shedding after hemorrhagic shock and resuscitation. PLoS ONE [Electronic Resource. 2011;6(8):e23530. doi: 10.1371/journal.pone.0023530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sillesen M, Rasmussen LS, Jin G. Assessment of coagulopathy, endothelial injury, and inflammation after traumatic brain injury and hemorrhage in a porcine model. [Article]. J Trauma Acute Care Surg. 2014;76(1):12–20. doi: 10.1097/TA.0b013e3182aaa675. [DOI] [PubMed] [Google Scholar]
- 19.Rehm M, Bruegger D, Christ F. Shedding of the endothelial glycocalyx in patients undergoing major vascular surgery with global and regional ischemia. Circulation. 2007;116(17):1896–1906. doi: 10.1161/CIRCULATIONAHA.106.684852. [DOI] [PubMed] [Google Scholar]
- 20.Walker HL, Mason AD. A standard animal burn. J Trauma. 1968;8(6):1049–1051. doi: 10.1097/00005373-196811000-00006. [DOI] [PubMed] [Google Scholar]
- 21.Song J, Saeman MR, De Libero J. Skeletal muscle loss is associated with TNF mediated insufficient skeletal myogenic activation after burn. Shock. 2015;44(5):479–486. doi: 10.1097/SHK.0000000000000444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Plackett TP, Colantoni A, Heinrich SA. The early acute phase response after burn injury in mice. J Burn Care Res. 2007;28(1):167–172. doi: 10.1097/BCR.0b013E31802CB84F. [DOI] [PubMed] [Google Scholar]
- 23.Karas RE, Fish MJ, Brown PJ, Danneman AZ. American College of Laboratory Animal Medicine Series A2 - Anesthesia and Analgesia in Laboratory Animals (Second Edition), Academic Press, San Diego. 2008 [Google Scholar]
- 24.Song J, Saeman MR, De Libero J. Skeletal muscle loss is associated with TNF mediated insufficient skeletal myogenic activation after burn. [Miscellaneous Article]. Shock. 2015;44(5):479–486. doi: 10.1097/SHK.0000000000000444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kliment CR, Englert JM, Gochuico BR. Oxidative stress alters syndecan-1 distribution in lungs with pulmonary fibrosis. J Biol Chem. 2009;284(6):3537–3545. doi: 10.1074/jbc.M807001200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Peng Z, Pati S, Potter D. Fresh frozen plasma lessens pulmonary endothelial inflammation and hyperpermeability after hemorrhagic shock and is associated with loss of syndecan 1. Shock. 2013;40(3):195–202. doi: 10.1097/SHK.0b013e31829f91fc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kozar RA, Peng Z, Zhang R. Plasma restoration of endothelial glycocalyx in a rodent model of hemorrhagic shock. Anesthes Analg. 2011;112(6):1289–1295. doi: 10.1213/ANE.0b013e318210385c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.O’Mara MS, Slater H, Goldfarb IW. A prospective, randomized evaluation of intra-abdominal pressures with crystalloid and colloid resuscitation in burn patients. J Trauma. 2005;58(5):1011–1018. doi: 10.1097/01.ta.0000162732.39083.15. [DOI] [PubMed] [Google Scholar]
- 29.Du GB, Slater H, Goldfarb IW. Influences of different resuscitation regimens on acute early weight gain in extensively burned patients. Burns. 1991;17(2):147–150. doi: 10.1016/0305-4179(91)90139-8. [DOI] [PubMed] [Google Scholar]