Abstract
Vitamin D has an important role in bone metabolism but recently has been recognized as an immunoregulator, and this has led to investigations on the effect of Vitamin D supplementation in various autoimmune diseases and its anti-inflammatory effects. There is some evidence that Vitamin D can regulate gastrointestinal inflammation. In addition, previous studies have shown that Vitamin D can affect the gut microbiome. The aim of this review is to evaluate the effect of Vitamin D on inflammatory processes, especially its relation to the inflammatory bowel disease (IBD) and gut microbiome. There is some evidence that Vitamin D can regulate gastrointestinal inflammation, with epidemiological studies showing that individuals with higher serum Vitamin D have a lower incidence of IBD, particularly Crohn's disease. Vitamin D changes transcription of cathelicidin and DEFB4 (defensin, beta 4) that can affect the gut microbiome. Several cell types of the immune system express Vitamin D receptor, and hence the use of Vitamin D in immune regulation has some potential. Furthermore, Vitamin D deficiency leads to dysbiosis of gut microbiome and reported to cause severe colitis. Vitamin D supplementation is low cost and available and can be a therapeutic option.
Keywords: Gut microbiome, inflammatory bowel diseases, Vitamin D
INTRODUCTION
Crohn's disease (CD) and ulcerative colitis (UC) are chronic relapsing inflammatory bowel diseases (IBDs) that are increasing in prevalence. In the West, UC affects 120–200/100,000 people and CD affects up to 50–200/100,000 people.[1] IBD also frequently has extraintestinal manifestations; it has a prevalence of 15.4% and leads to a reduced quality of life in these patients.[2] The pathogenesis of IBD is incompletely understood, and it may be due to inappropriate immune responses to the antigens that are present in the gastrointestinal tract.[3] Furthermore, there is a hypothesis that there is an immune dysregulation to normal intestinal microbiome.[4] There is also evidence that inadequate Vitamin D intake is associated with an increased risk for these diseases.[5,6,7]
In the past decade, there has been increasing evidence that Vitamin D is involved in other aspects of human health apart from bone health. Vitamin D has been shown to regulate innate and adaptive immune systems.[8] There appear to be links between Vitamin D status and autoimmune diseases, and Vitamin D insufficiency/deficiency is closely associated with obesity, which is an established risk factor for cardiovascular disease and it is an inflammatory status.[9] Vitamin D deficiency is associated with the onset and activity of IBD and the risk of its malignant transformation.[10,11] Hence, Vitamin D supplements have been suggested as an adjunctive treatment in IBD.[12,13,14] Vitamin D deficiency is also commonly present in IBD, with up to 95% patients with IBD being affected.[15] A large study comprising 3217 patients with CD and UC showed that 32% had Vitamin D deficiency (25(OH)D3 level of <50 nmol/l].[16]
The most common factor accounting for Vitamin D deficiency in IBD patients, as is the case for most other patient groups, has been reported to be inadequate sun exposure, chiefly ultraviolet B radiation.[17] Sun exposure may be affected by outdoor activities, daylight hours, sunscreen use, skin pigmentation, dressing, and latitude. IBD patients often spend less time outdoors because of their disability. Moreover, many use sunscreen because they are often treated with thiopurine drugs that are associated with an increased risk of skin malignancy.[18,19] For these patients, dietary sources of Vitamin D are, therefore, important. The recommended daily allowance of Vitamin D according to Institute of Medicine is 600 IU between ages 1 and 70 years. The optimal intake of Vitamin D varies with individual,[20] and in some circumstances, higher doses are required.[21] Patients with IBD often have clinical features that limit oral absorption of Vitamin D. These include disturbance in bile acids circulation that can lead to malabsorption of fat-soluble vitamins including Vitamin D. Furthermore, disease of the terminal ileum and resection can have an impact on Vitamin D absorption.[22]
The interaction of the gut microbiome, the diet, and other environmental and genetic factors are thought to be important in the development of gastrointestinal disease.[23,24,25,26]
Genetic factors also have an impact on Vitamin D transport proteins and the formation of 25(OH)D3, but these must be investigated in IBD patients.[27] Smoking is a risk factor for CD and is also associated with an increased risk of Vitamin D deficiency.[28,29] Corticosteroid use, long duration of IBD, and body mass index >30 can also lead to Vitamin D deficiency.[30,31]
In this study, Web of Science, PubMed, and Google Scholar have been systematically searched for randomized controlled trials (RCTs), systematic reviews of RCTs, and epidemiologic studies that investigate the concept of Vitamin D, the gut microbiome, and inflammatory bowel diseases.
VITAMIN D AND IMMUNITY
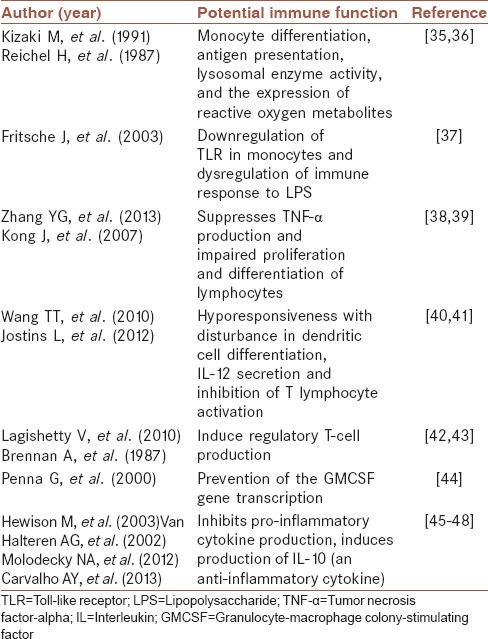
A potential link between vitamin D and the immune system was suggested when it was observed that Vitamin D affected leukemia cell differentiation.[32] It has now been confirmed that peripheral blood mononuclear cells have Vitamin D receptors (VDRs);[33,34] VDR is also found on other hematopoietic cells.[35] Furthermore, macrophage and dendritic cells (DCs) have been shown to elaborate active Vitamin D (1,25[OH]2D).[36,37] We have listed potential immune functions of Vitamin D in Table 1.
Table 1.
Potential immune functions of Vitamin D
VITAMIN D AND INTESTINAL IMMUNITY
While the role of Vitamin D deficiency in the etiology of IBD is unclear, it has been shown to have some potentially beneficial effects in vitro and in vivo.
Vitamin D reduces the permeability of intestinal cells in animal models of colitis.[38,39] In VDR knock-out mice, dextran sodium sulfate induces colitis that is associated with decreased immunostaining of zonula occludens-1 and occluding proteins on epithelial cells of the colon and associated with decreased transepithelial resistance and increased permeability. The nucleotide-binding oligomerization domain-containing protein 2 (NOD2) is an intracellular pattern recognition receptor. It activates NF-kB and increases Vitamin D-mediated transcription of cathelicidin and DEFB4 (defensin, beta 4).[40] If the innate immune system cannot control the microbiome, it could be lead to tissue inflammation.
NOD2 is thought to be important in CD susceptibility.[41] It is possible that Vitamin D modulates NOD2 and protects against CD with antibacterial responses.
Angiogenin-4 is another antimicrobial protein. It has bactericidal activity against intestinal microbiome. In mice with Vitamin D deficiency, several genes have been reported consistently to be upregulated or downregulated when compared with Vitamin D-sufficient mice.[42] Angiogenin-4 was one of these genes. This change was associated with increasing number of colonic bacteria up to 50 fold. Therefore, Vitamin D deficiency can lead to dysbiosis and it may, therefore, be a factor in the pathogenesis of CD.
DCs are antigen-presenting cells that express the VDR and can produce 1,25(OH)2D3.[43] Treatment of these cells with 25(OH)D3 and 1,25(OH)2D3 lead to a weakened capacity for antigen presentation. Therefore, the response of the DC to antigens become weaker and they cannot activate adaptive immune system effectively (tolerogenesis).[44,45] Furthermore, 1,25(OH)2D3-treated DCs express more interleukin (IL)-10, which is an anti-inflammatory and immunosuppressive cytokine.[46]
VITAMIN D AND INFLAMMATORY BOWEL DISEASE
The incidence of IBD has increased in recent years and has been estimated to affect 1/200 people in the developed countries.[47] IBD is a more severe disease in children and adolescents.
Although patients with CD have a significantly lower serum level of 25(OH)D as compared with control group, it is unknown whether this was cause or effect of IBD activity.[48] Furthermore, seasonal changes in serum 25(OH)D level are associated with CD activity.[49]
Genetic predisposition appears to be an important factor in the development of IBD, and 163 distinct genetic variants have been reported to be associated with IBD risk. Host–microbiome interactions have been implicated in risk of IBD and even progression of disease.[41]
However, few studies have investigated the association between Vitamin D deficiency and risk of IBD. The Nurses’ Health Study has shown that participants in the highest quartile of Vitamin D had a lower risk of developing CD, but this was nonsignificant for UC (odds ratio [OR] for CD: 0.54; 95% confidence interval [CI]: 0.30–0.99; P = 0.02). The insignificant result for UC may have been because of smaller number of UC patients compared with CD patients.[10]
Cohort studies have found an association between CD activity and low serum 25(OH)D3 levels.[17,50,51] A cross-sectional study has reported that higher levels of fecal calprotectin (as a marker for intestinal inflammation) was associated with lower serum 25(OH)D3 level and it indicated that Vitamin D may be of potential value in treating intestinal inflammation.[52] However, some studies have not shown any significant association between serum 25(OH)D3 level and CD and UC activity;[53,54] these studies may be limited by the low number of participants.
A large multicentral IBD cohort has shown that serum 25(OH)D3 level <50 nmol/l has increased risk of surgery (OR: 1.76; 95% CI: 1.24–2.51) or hospitalization (OR: 2.07; 95% CI: 1.59–2.68).[16] This result suggests that Vitamin D deficiency may have an adverse effect on the course of IBD.
VITAMIN D AND THE GUT MICROBIOME
The gut microbiome has effects on the host immune system and has the potential to change disease development.
Guo et al. have shown that Vitamin D induces the expression of cathelicidin antimicrobial peptide (CAMP) gene [Figure 1]. CAMP expresses by immune and epithelial cells and enhances barrier function.[55] These results suggest that Vitamin D has antibacterial effects.
Figure 1.
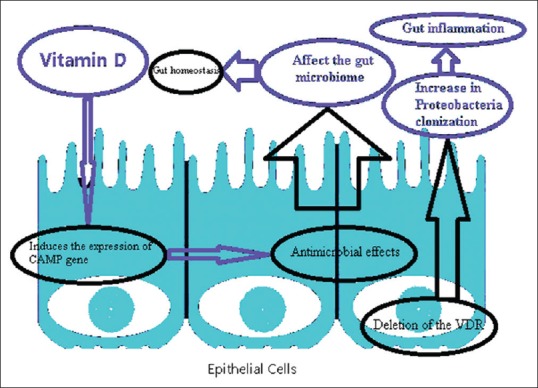
Interaction between Vitamin D and gut microbiome can affect gut homeostasis or gut inflammation. VDR: Vitamin D Receptor, CAMP: Cathelicidin antimicrobial peptide
The intestinal microbiome is involved in the synthesis of secondary bile acids. Lithocholic acid (LCA) is a secondary bile acid that is hepatotoxic and toxic for intestinal cells and may also induce enteric cancer. Interestingly, LCA can bind to the VDR. When Vitamin D activates the VDR, it induces the expression of cytochrome CYP3A, which is involved in the detoxification of LCA in intestine and liver.[56,57] Some probiotics increase serum Vitamin D level. In a multicenter study, oral supplementation with Lactobacillus reuteri (NCIMB30242), a probiotic, increased serum 25(OH)D concentration.[58]
Some bacteria, for example, Chlamydia trachomatis, reduces VDR activity.[59] The probable mechanism for this is through bacterial capnine that inhibits ligand-binding pocket in the nuclear VDR.[60] The VDR response is inhibited by such bacteria and leads to upregulation of the miRNA, hsa-mir-21 by monocytes, thereby potentially blocking the immune responses that are stimulated by Vitamin D.[61]
A reduction in the production of 1,25(OH)2D3 or expression of VDR may lead to gut inflammation and an increase in Proteobacteria colonization. This leads to a shift in the balance of the microbiome composition [Figure 1].
Deletion of the VDR in intestinal epithelial cells results to a defective autophagy in colitis. Defective autophagy has seen in IBD. There is an essential correlation between autophagy, VDR, and gut microbiome that is important for intestinal homeostasis, these have a role in pathophysiology of IBD.[62] These data suggest that Vitamin D is an important factor in determining the composition of the gut microbiome and inflammatory processes in IBD.
VITAMIN D AS A TREATMENT FOR IBD PATIENTS
It has been proposed that the benefits of supplementation with Vitamin D in IBD because it can regulate homeostasis in the gut. Vitamin D modifies epithelial cells’ integrity, immune responses especially for innate immune system, and the diversity and the composition of the human gut microbiome.[63] In Vitamin D/IL-10 deficient mice, administration of 1,25(OH)2D was found to reduce the severity of colitis.[64]
In man, there have been few studies to evaluate the therapeutic effects of Vitamin D in IBD patients. In one clinical trial in CD patients, receiving 1200 IU/day of Vitamin D for 1 year, reduced the relapse rate from 29% to 13% when compared with placebo group.[14] However, this reduction was nonsignificant (P = 0.06) and was of insufficient power due to the small sample size. Miheller et al. have compared the effects of 1,25 dihydroxyvitamin D and 25 hydroxyvitamin D on disease activity of CD patients. Their results have shown that, after 6-week, Crohn's Disease Activity Index reduced significantly with 1,25 dihydroxyvitamin D.[65]
In CD patients, supplementation with 1000 IU/day of Vitamin D3 until the serum level of 25(OH)D reached to 100 nmol/l was associated with a significant reduction in Crohn's Disease Activity Index scores. However, inflammatory markers did not change significantly.[13]
In addition to the effects of Vitamin D for treatment of IBD patients, it has effects on other medical treatments in these patients. Zator et al. evaluated the effect of the serum level of 25 hydroxyvitamin D and duration of responses to antitumor necrosis factor-α as a treatment in IBD patients. Interestingly, there was a significant association between Vitamin D insufficiency and early failure of treatment with antitumor necrosis factor-a (hazard ratio: 2.13; 95% CI: 1.34–4.39; P = 0.04).[12] Furthermore, in patients that did not receive Vitamin D supplements, this failure was stronger. This effect is due to the effects of suppression of the colon tumor necrosis factor-a genes by Vitamin D.
The studies support Vitamin D supplementation in IBD patients. However, this is important to investigate the Vitamin D doses according to some other factors such as the human gut microbiome or genetic factors.
CONCLUSION
Several cell types of the immune system express VDR, and hence the use of Vitamin D in immune regulation has some potential. Recent studies confirm an association between Vitamin D status and the development of IBD, particularly CD. There have been few clinical trials that have evaluated the effects of Vitamin D supplementation in the treatment of IBD. Vitamin D has been reported to have effects on the severity (increasing hospitalization and surgery) and pathogenesis of IBD. In animal studies, Vitamin D deficiency has been reported to cause severe colitis, but in man, it has not been proven that low serum level of Vitamin D is a consequence of disease or it is the cause of IBD. Therefore, further research is necessary in this field and for using Vitamin D as an adjunct therapy for IBD patients.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Cosnes J, Gower-Rousseau C, Seksik P, Cortot A. Epidemiology and natural history of inflammatory bowel diseases. Gastroenterology. 2011;140:1785–94. doi: 10.1053/j.gastro.2011.01.055. [DOI] [PubMed] [Google Scholar]
- 2.Fatemi A, Jazi HH, Emami MH, Kazemizadeh A, Tavakkoli H, Smiley A, et al. Relationship between articular and nonarticular manifestations in inflammatory bowel diseases. J Res Med Sci. 2016;21:48. doi: 10.4103/1735-1995.183989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Imanzadeh F, Nasri P, Sadeghi S, Sayyari A, Dara N, Abdollah K, et al. Food allergy among iranian children with inflammatory bowel disease: A preliminary report. J Res Med Sci. 2015;20:855–9. doi: 10.4103/1735-1995.170605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wallace KL, Zheng LB, Kanazawa Y, Shih DQ. Immunopathology of inflammatory bowel disease. World J Gastroenterol. 2014;20:6–21. doi: 10.3748/wjg.v20.i1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Spedding S, Vanlint S, Morris H, Scragg R. Does Vitamin D sufficiency equate to a single serum 25-hydroxyvitamin D level or are different levels required for non-skeletal diseases? Nutrients. 2013;5:5127–39. doi: 10.3390/nu5125127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bolland MJ, Grey A, Gamble GD, Reid IR. The effect of Vitamin D supplementation on skeletal, vascular, or cancer outcomes: A trial sequential meta-analysis. Lancet Diabetes Endocrinol. 2014;2:307–20. doi: 10.1016/S2213-8587(13)70212-2. [DOI] [PubMed] [Google Scholar]
- 7.Grant WB. 25-hydroxyvitamin D and breast cancer, colorectal cancer, and colorectal adenomas: Case-control versus nested case-control studies. Anticancer Res. 2015;35:1153–60. [PubMed] [Google Scholar]
- 8.Hewison M. An update on Vitamin D and human immunity. Clin Endocrinol (Oxf) 2012;76:315–25. doi: 10.1111/j.1365-2265.2011.04261.x. [DOI] [PubMed] [Google Scholar]
- 9.Tabatabaeizadeh SA, Avan A, Bahrami A, Khodashenas E, Esmaeili H, Ferns GA, et al. High dose supplementation of Vitamin D affects measures of systemic inflammation: Reductions in high sensitivity C-reactive protein level and neutrophil to lymphocyte ratio (NLR) distribution. J Cell Biochem. 2017;118:4317–22. doi: 10.1002/jcb.26084. [DOI] [PubMed] [Google Scholar]
- 10.Ananthakrishnan AN, Khalili H, Higuchi LM, Bao Y, Korzenik JR, Giovannucci EL, et al. Higher predicted Vitamin D status is associated with reduced risk of Crohn's disease. Gastroenterology. 2012;142:482–9. doi: 10.1053/j.gastro.2011.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ananthakrishnan AN, Cheng SC, Cai T, Cagan A, Gainer VS, Szolovits P, et al. Association between reduced plasma 25-hydroxy Vitamin D and increased risk of cancer in patients with inflammatory bowel diseases. Clin Gastroenterol Hepatol. 2014;12:821–7. doi: 10.1016/j.cgh.2013.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zator ZA, Cantu SM, Konijeti GG, Nguyen DD, Sauk J, Yajnik V, et al. Pretreatment 25-hydroxyvitamin D levels and durability of anti-tumor necrosis factor-α therapy in inflammatory bowel diseases. JPEN J Parenter Enteral Nutr. 2014;38:385–91. doi: 10.1177/0148607113504002. [DOI] [PubMed] [Google Scholar]
- 13.Yang L, Weaver V, Smith JP, Bingaman S, Hartman TJ, Cantorna MT, et al. Therapeutic effect of Vitamin D supplementation in a pilot study of Crohn's patients. Clin Transl Gastroenterol. 2013;4:e33. doi: 10.1038/ctg.2013.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jørgensen SP, Agnholt J, Glerup H, Lyhne S, Villadsen GE, Hvas CL, et al. Clinical trial: Vitamin D3 treatment in Crohn's disease – A randomized double-blind placebo-controlled study. Aliment Pharmacol Ther. 2010;32:377–83. doi: 10.1111/j.1365-2036.2010.04355.x. [DOI] [PubMed] [Google Scholar]
- 15.Mouli VP, Ananthakrishnan AN. Review article: Vitamin D and inflammatory bowel diseases. Aliment Pharmacol Ther. 2014;39:125–36. doi: 10.1111/apt.12553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ananthakrishnan AN, Cagan A, Gainer VS, Cai T, Cheng SC, Savova G, et al. Normalization of plasma 25-hydroxy Vitamin D is associated with reduced risk of surgery in Crohn's disease. Inflamm Bowel Dis. 2013;19:1921–7. doi: 10.1097/MIB.0b013e3182902ad9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Joseph AJ, George B, Pulimood AB, Seshadri MS, Chacko A. 25 (OH) Vitamin D level in Crohn's disease: Association with sun exposure & disease activity. Indian J Med Res. 2009;130:133–7. [PubMed] [Google Scholar]
- 18.Ariyaratnam J, Subramanian V. Association between thiopurine use and nonmelanoma skin cancers in patients with inflammatory bowel disease: A meta-analysis. Am J Gastroenterol. 2014;109:163–9. doi: 10.1038/ajg.2013.451. [DOI] [PubMed] [Google Scholar]
- 19.Attard NR, Karran P. UVA photosensitization of thiopurines and skin cancer in organ transplant recipients. Photochem Photobiol Sci. 2012;11:62–8. doi: 10.1039/c1pp05194f. [DOI] [PubMed] [Google Scholar]
- 20.Carlberg C. Genome-wide (over) view on the actions of Vitamin D. Front Physiol. 2014;5:167. doi: 10.3389/fphys.2014.00167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ross AC, Manson JE, Abrams SA, Aloia JF, Brannon PM, Clinton SK, et al. The 2011 report on dietary reference intakes for calcium and Vitamin D from the institute of medicine: What clinicians need to know. J Clin Endocrinol Metab. 2011;96:53–8. doi: 10.1210/jc.2010-2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tajika M, Matsuura A, Nakamura T, Suzuki T, Sawaki A, Kato T, et al. Risk factors for Vitamin D deficiency in patients with Crohn's disease. J Gastroenterol. 2004;39:527–33. doi: 10.1007/s00535-003-1338-x. [DOI] [PubMed] [Google Scholar]
- 23.Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI, et al. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444:1027–31. doi: 10.1038/nature05414. [DOI] [PubMed] [Google Scholar]
- 24.Gentschew L, Ferguson LR. Role of nutrition and microbiota in susceptibility to inflammatory bowel diseases. Mol Nutr Food Res. 2012;56:524–35. doi: 10.1002/mnfr.201100630. [DOI] [PubMed] [Google Scholar]
- 25.Ferguson LR. Nutrigenetics, nutrigenomics and inflammatory bowel diseases. Expert Rev Clin Immunol. 2013;9:717–26. doi: 10.1586/1744666X.2013.824245. [DOI] [PubMed] [Google Scholar]
- 26.Goldsmith JR, Sartor RB. The role of diet on intestinal microbiota metabolism: Downstream impacts on host immune function and health, and therapeutic implications. J Gastroenterol. 2014;49:785–98. doi: 10.1007/s00535-014-0953-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Powe CE, Evans MK, Wenger J, Zonderman AB, Berg AH, Nalls M, et al. Vitamin D-binding protein and Vitamin D status of black Americans and white Americans. N Engl J Med. 2013;369:1991–2000. doi: 10.1056/NEJMoa1306357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Suibhne TN, Cox G, Healy M, O’Morain C, O’Sullivan M. Vitamin D deficiency in Crohn's disease: Prevalence, risk factors and supplement use in an outpatient setting. J Crohns Colitis. 2012;6:182–8. doi: 10.1016/j.crohns.2011.08.002. [DOI] [PubMed] [Google Scholar]
- 29.Lawrance IC, Murray K, Batman B, Gearry RB, Grafton R, Krishnaprasad K, et al. Crohn's disease and smoking: Is it ever too late to quit? J Crohns Colitis. 2013;7:e665–71. doi: 10.1016/j.crohns.2013.05.007. [DOI] [PubMed] [Google Scholar]
- 30.Liel Y, Ulmer E, Shary J, Hollis BW, Bell NH. Low circulating Vitamin D in obesity. Calcif Tissue Int. 1988;43:199–201. doi: 10.1007/BF02555135. [DOI] [PubMed] [Google Scholar]
- 31.Zhou C, Assem M, Tay JC, Watkins PB, Blumberg B, Schuetz EG, et al. Steroid and xenobiotic receptor and Vitamin D receptor crosstalk mediates CYP24 expression and drug-induced osteomalacia. J Clin Invest. 2006;116:1703–12. doi: 10.1172/JCI27793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Abe E, Miyaura C, Sakagami H, Takeda M, Konno K, Yamazaki T, et al. Differentiation of mouse myeloid leukemia cells induced by 1 alpha, 25-dihydroxyvitamin D3. Proc Natl Acad Sci U S A. 1981;78:4990–4. doi: 10.1073/pnas.78.8.4990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Provvedini DM, Tsoukas CD, Deftos LJ, Manolagas SC. 1,25-dihydroxyvitamin D3 receptors in human leukocytes. Science. 1983;221:1181–3. doi: 10.1126/science.6310748. [DOI] [PubMed] [Google Scholar]
- 34.Bhalla AK, Amento EP, Clemens TL, Holick MF, Krane SM. Specific high-affinity receptors for 1,25-dihydroxyvitamin D3 in human peripheral blood mononuclear cells: Presence in monocytes and induction in T lymphocytes following activation. J Clin Endocrinol Metab. 1983;57:1308–10. doi: 10.1210/jcem-57-6-1308. [DOI] [PubMed] [Google Scholar]
- 35.Kizaki M, Norman AW, Bishop JE, Lin CW, Karmakar A, Koeffler HP, et al. 1,25-dihydroxyvitamin D3 receptor RNA: Expression in hematopoietic cells. Blood. 1991;77:1238–47. [PubMed] [Google Scholar]
- 36.Reichel H, Koeffler HP, Norman AW. Synthesis in vitro of 1,25-dihydroxyvitamin D3 and 24,25-dihydroxyvitamin D3 by interferon-gamma-stimulated normal human bone marrow and alveolar macrophages. J Biol Chem. 1987;262:10931–7. [PubMed] [Google Scholar]
- 37.Fritsche J, Mondal K, Ehrnsperger A, Andreesen R, Kreutz M. Regulation of 25-hydroxyvitamin D3-1 alpha-hydroxylase and production of 1 alpha, 25-dihydroxyvitamin D3 by human dendritic cells. Blood. 2003;102:3314–6. doi: 10.1182/blood-2002-11-3521. [DOI] [PubMed] [Google Scholar]
- 38.Zhang YG, Wu S, Sun J. Vitamin D, Vitamin D receptor, and tissue barriers. Tissue Barriers. 2013;1 doi: 10.4161/tisb.23118. pii: e23118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kong J, Zhang Z, Musch MW, Ning G, Sun J, Hart J, et al. Novel role of the Vitamin D receptor in maintaining the integrity of the intestinal mucosal barrier. Am J Physiol Gastrointest Liver Physiol. 2008;294:G208–16. doi: 10.1152/ajpgi.00398.2007. [DOI] [PubMed] [Google Scholar]
- 40.Wang TT, Dabbas B, Laperriere D, Bitton AJ, Soualhine H, Tavera-Mendoza LE, et al. Direct and indirect induction by 1,25-dihydroxyvitamin D3 of the NOD2/CARD15-defensin beta2 innate immune pathway defective in Crohn disease. J Biol Chem. 2010;285:2227–31. doi: 10.1074/jbc.C109.071225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jostins L, Ripke S, Weersma RK, Duerr RH, McGovern DP, Hui KY, et al. Host-microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Nature. 2012;491:119–24. doi: 10.1038/nature11582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lagishetty V, Misharin AV, Liu NQ, Lisse TS, Chun RF, Ouyang Y, et al. Vitamin D deficiency in mice impairs colonic antibacterial activity and predisposes to colitis. Endocrinology. 2010;151:2423–32. doi: 10.1210/en.2010-0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brennan A, Katz DR, Nunn JD, Barker S, Hewison M, Fraher LJ, et al. Dendritic cells from human tissues express receptors for the immunoregulatory Vitamin D3 metabolite, dihydroxycholecalciferol. Immunology. 1987;61:457–61. [PMC free article] [PubMed] [Google Scholar]
- 44.Penna G, Adorini L. 1 alpha, 25-dihydroxyvitamin D3 inhibits differentiation, maturation, activation, and survival of dendritic cells leading to impaired alloreactive T cell activation. J Immunol. 2000;164:2405–11. doi: 10.4049/jimmunol.164.5.2405. [DOI] [PubMed] [Google Scholar]
- 45.Hewison M, Freeman L, Hughes SV, Evans KN, Bland R, Eliopoulos AG, et al. Differential regulation of Vitamin D receptor and its ligand in human monocyte-derived dendritic cells. J Immunol. 2003;170:5382–90. doi: 10.4049/jimmunol.170.11.5382. [DOI] [PubMed] [Google Scholar]
- 46.van Halteren AG, van Etten E, de Jong EC, Bouillon R, Roep BO, Mathieu C, et al. Redirection of human autoreactive T-cells upon interaction with dendritic cells modulated by TX527, an analog of 1,25 dihydroxyvitamin D(3) Diabetes. 2002;51:2119–25. doi: 10.2337/diabetes.51.7.2119. [DOI] [PubMed] [Google Scholar]
- 47.Molodecky NA, Soon IS, Rabi DM, Ghali WA, Ferris M, Chernoff G, et al. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology. 2012;142:46–5.e42. doi: 10.1053/j.gastro.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 48.Carvalho AY, Bishop KS, Han DY, Ellett S, Jesuthasan A, Lam WJ, et al. The role of Vitamin D level and related single nucleotide polymorphisms in Crohn's disease. Nutrients. 2013;5:3898–909. doi: 10.3390/nu5103898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kini GP, Young B, Herbison P, Schultz M. Does seasonal level of serum 25-OH Vitamin D correlate with the activity of Crohn's disease? N Z Med J. 2014;127:51–9. [PubMed] [Google Scholar]
- 50.Jørgensen SP, Hvas CL, Agnholt J, Christensen LA, Heickendorff L, Dahlerup JF, et al. Active Crohn's disease is associated with low Vitamin D levels. J Crohns Colitis. 2013;7:e407–13. doi: 10.1016/j.crohns.2013.01.012. [DOI] [PubMed] [Google Scholar]
- 51.Ulitsky A, Ananthakrishnan AN, Naik A, Skaros S, Zadvornova Y, Binion DG, et al. Vitamin D deficiency in patients with inflammatory bowel disease: Association with disease activity and quality of life. JPEN J Parenter Enteral Nutr. 2011;35:308–16. doi: 10.1177/0148607110381267. [DOI] [PubMed] [Google Scholar]
- 52.Garg M, Rosella O, Lubel JS, Gibson PR. Association of circulating Vitamin D concentrations with intestinal but not systemic inflammation in inflammatory bowel disease. Inflamm Bowel Dis. 2013;19:2634–43. doi: 10.1097/01.MIB.0000436957.77533.b2. [DOI] [PubMed] [Google Scholar]
- 53.Levin AD, Wadhera V, Leach ST, Woodhead HJ, Lemberg DA, Mendoza-Cruz AC, et al. Vitamin D deficiency in children with inflammatory bowel disease. Dig Dis Sci. 2011;56:830–6. doi: 10.1007/s10620-010-1544-3. [DOI] [PubMed] [Google Scholar]
- 54.Blanck S, Aberra F. Vitamin d deficiency is associated with ulcerative colitis disease activity. Dig Dis Sci. 2013;58:1698–702. doi: 10.1007/s10620-012-2531-7. [DOI] [PubMed] [Google Scholar]
- 55.Guo C, Sinnott B, Niu B, Lowry MB, Fantacone ML, Gombart AF, et al. Synergistic induction of human cathelicidin antimicrobial peptide gene expression by Vitamin D and stilbenoids. Mol Nutr Food Res. 2014;58:528–36. doi: 10.1002/mnfr.201300266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Makishima M, Lu TT, Xie W, Whitfield GK, Domoto H, Evans RM, et al. Vitamin D receptor as an intestinal bile acid sensor. Science. 2002;296:1313–6. doi: 10.1126/science.1070477. [DOI] [PubMed] [Google Scholar]
- 57.Cheng J, Fang ZZ, Kim JH, Krausz KW, Tanaka N, Chiang JY, et al. Intestinal CYP3A4 protects against lithocholic acid-induced hepatotoxicity in intestine-specific VDR-deficient mice. J Lipid Res. 2014;55:455–65. doi: 10.1194/jlr.M044420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jones ML, Martoni CJ, Prakash S. Oral supplementation with probiotic L. reuteri NCIMB 30242 increases mean circulating 25-hydroxyvitamin D: A post hoc analysis of a randomized controlled trial. J Clin Endocrinol Metab. 2013;98:2944–51. doi: 10.1210/jc.2012-4262. [DOI] [PubMed] [Google Scholar]
- 59.He Q, Ananaba GA, Patrickson J, Pitts S, Yi Y, Yan F, et al. Chlamydial infection in Vitamin D receptor knockout mice is more intense and prolonged than in wild-type mice. J Steroid Biochem Mol Biol. 2013;135:7–14. doi: 10.1016/j.jsbmb.2012.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Marshall TG. Bacterial Capnine Blocks Transcription of Human Antimicrobial Peptides. Abstract presentation, Metagenomics, San Diego. 2007 doi:10.1038/npre.2007.164.1. [Google Scholar]
- 61.Liu PT, Wheelwright M, Teles R, Komisopoulou E, Edfeldt K, Ferguson B, et al. MicroRNA-21 targets the Vitamin D-dependent antimicrobial pathway in leprosy. Nat Med. 2012;18:267–73. doi: 10.1038/nm.2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wu S, Zhang YG, Lu R, Xia Y, Zhou D, Petrof EO, et al. Intestinal epithelial Vitamin D receptor deletion leads to defective autophagy in colitis. Gut. 2015;64:1082–94. doi: 10.1136/gutjnl-2014-307436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cantorna MT, McDaniel K, Bora S, Chen J, James J. Vitamin D, immune regulation, the microbiota, and inflammatory bowel disease. Exp Biol Med (Maywood) 2014;239:1524–30. doi: 10.1177/1535370214523890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cantorna MT, Munsick C, Bemiss C, Mahon BD. 1,25-dihydroxycholecalciferol prevents and ameliorates symptoms of experimental murine inflammatory bowel disease. J Nutr. 2000;130:2648–52. doi: 10.1093/jn/130.11.2648. [DOI] [PubMed] [Google Scholar]
- 65.Miheller P, Muzes G, Hritz I, Lakatos G, Pregun I, Lakatos PL, et al. Comparison of the effects of 1,25 dihydroxyvitamin D and 25 hydroxyvitamin D on bone pathology and disease activity in Crohn's disease patients. Inflamm Bowel Dis. 2009;15:1656–62. doi: 10.1002/ibd.20947. [DOI] [PubMed] [Google Scholar]


