Abstract
Angiotensin-converting enzyme functions in the male reproductive system, but the extent of its function in reproduction is not fully understood. The primary objective of this work was to investigate the relationship between the testicular isoform of angiotensin-converting enzyme present in human spermatozoa and semen parameters, human embryo quality, and assisted reproduction success. A total of 81 semen samples and 635 embryos from couples undergoing oocyte donation cycles at the IVI Bilbao Clinic were analyzed. Semen parameters, embryos quality, and blastocyst development were examined according to the World Health Organization standards and the Spanish Association of Reproduction Biology Studies criteria. The percentage of testicular angiotensin-converting enzyme-positive spermatozoa and the number of molecules per spermatozoon were analyzed by flow cytometry. Both parameters were inversely correlated with human sperm motility. Higher percentages of testicular angiotensin-converting enzyme-positive spermatozoa together with fewer enzyme molecules per spermatozoon were positively correlated with better embryo quality and development. Our results suggest that embryos with a higher implantation potential come from semen samples with higher percentages of testicular angiotensin-converting enzyme-positive cells and fewer enzyme molecules per spermatozoon. Based on these findings, we propose that testicular angiotensin-converting enzyme could be used to aid embryologists in selecting better semen samples for obtaining high-quality blastocysts during in vitro fertilization procedures.
Keywords: angiotensin-converting enzyme, ART, embryonic development, human sperm, male fertility
INTRODUCTION
Infertility is recognized as a public health issue by the World Health Organization (WHO)1 and is prevalent around the globe.2 In Western societies, one in six couples of reproductive age is infertile,3 with male factor infertility accounting for 50% of all infertility cases.4,5 Approximately half of the cases of male infertility result from some form of defective sperm production, such as complete blockage of spermatogenesis, low sperm count, or abnormal sperm motility or function.6 A large portion of couples seek medical help to resolve their fertility problems.1 Intracytoplasmic sperm injection (ICSI)7 is currently the primary technique used to achieve pregnancy when male infertility is a factor.8,9 This technique increases the chance of an infertile couple achieving biological parenthood.10 However, defects in the spermatozoa used for ICSI can impede fertilization in several ways.9,11,12,13 Thus, knowledge of molecular markers of different sperm functions could be useful in determining new therapeutic and diagnostic strategies for male infertility.5,9,14
Angiotensin-converting enzyme (ACE; CD143; EC 3.4.15.1) is a zinc-containing dipeptidyl carboxypeptidase broadly distributed in mammalian tissues.15,16 This ectoenzyme catalyzes the cleavage of peptides, including angiotensin I17 and bradykinin,18 and has important functions in the regulation of blood pressure as well as fluid and electrolyte regulation.16 It also plays an important role in the regulation of both male reproduction and female reproduction.19 In human tissues, there are two ACE isozymes: the somatic isoform (sACE) and the testicular or germinal isoform (tACE).16,20 In the male reproductive tract, sACE is a soluble form of the enzyme found in seminal plasma21 and on the surface of epididymal epithelial and Leydig cells,16 while tACE is exclusively expressed in postmeiotic spermatids and spermatozoa.16 Although the activity of sACE in semen is higher than in any other body fluid or tissue,21 the effects of ACE in the male reproductive tract have been attributed to tACE.22
The role of tACE in male fertility has been assessed using several different approaches. Functional studies implicate tACE in sperm functions including motility,23,24,25 capacitation,26,27 the acrosome reaction,27 and sperm-oocyte fusion.28 Unexpectedly, the sperm surface tACE levels change during different phases of fertilization as tACE molecules are released from human spermatozoa during capacitation26,27 and the acrosome reaction,27 suggesting an important role in fertilizing ability.28 Studies in tACE knockout mice29,30 identified normal sperm numbers, morphology, and motility, yet tACE seems to be involved in the transport of spermatozoa through the oviduct and in gamete fusion. In addition, a reduced or lack of expression of human sperm tACE is associated with fertilization failures during in vitro fertilization (IVF) programs.20 Based on these findings, we sought to determine whether tACE can be used as a diagnostic biomarker during ICSI treatments.
PATIENTS AND METHODS
Ethical considerations
This study was approved by the Ethics Committee of the University of the Basque Country, Leioa, Biscay, Spain (CEISH/61/2011). All semen samples were obtained from male partners of couples who underwent oocyte donation cycles at the Clinic IVI Bilbao, Basque Country, Spain. Sperm samples for research were obtained after patients provided written informed consent.
Patients, semen analysis, and preparation
A total of 81 patients were included in this prospective study between September 2014 and July 2015. Normal and pathological semen samples were collected on the day of the oocyte retrieval by masturbation on site after a 2- to 5-day period of sexual abstinence into sterile containers and allowed to liquefy at 37°C and 5% (v/v) CO2 for 10 min before being processing by density-gradient centrifugation. Subsequently, sperm samples were centrifuged at 300 g for 20 min through a discontinuous colloidal silica density gradient of PureSperm (Nidacon, Gothenburg, Sweden). The pellets were then collected and washed (400 g for 5 min) in 2 ml of Global® for fertilization medium (LifeGlobal Group, Brussels, Belgium), and sperm cells were diluted in 0.2–1 ml of medium for ICSI techniques.
Semen volume, concentration, and motility were measured for each sample. All samples were examined in duplicate for sperm concentration and motility in a Makler® Chamber (Sefi Laboratories, Haifa, Israel), counting at least 200 spermatozoa per replicate. The mean from homogenous replicates were considered for the analysis. Motility was evaluated according to the World Health Organization (WHO) standards.31 Briefly, spermatozoa were categorized into three different groups: (1) progressive motility (PR), (2) nonprogressive motility (NP), and (3) immotile spermatozoa (IM).
Surplus spermatozoa remaining after clinical use for ICSI procedures were collected for molecular analysis by flow cytometry. The molecular data obtained were related to basic sperm parameter values (measured in fresh samples as well as after being processed), embryo morphology-quality parameters, and reproductive success.
Donors’ and recipients’ stimulation in the assisted reproduction cycles
The use of an oocyte donation model allowed for the analysis of the relationship between tACE and fertilization rates, embryo quality, and the reproductive outcome controlling the potential bias caused by female factor.32 The protocols for donors’ controlled ovarian hyperstimulation, oocyte recruitment and management, and steroid replacement in the receivers have been described previously.32,33
Oocyte insemination techniques
Recovered oocytes were fertilized using ICSI. All oocytes retrieved from a single donor were donated to a single compatible recipient. Oocytes were denuded before sperm injection by enzymatic (in 40 IU ml−1 of hyaluronidase; Hyaluronidase, LifeGlobal Group Guilford, Guilford, CT, USA) and mechanical methods. The oocytes were then placed in 20 μl drops in a preequilibrated culture dish. Subsequently, ICSI was performed when observed in an inverted microscope at ×400 (Nikon Eclipse, Izasa, Barcelona, Spain). Only the mature metaphase II oocytes were selected for sperm injection. Finally, injected oocytes were cultured at 37°C in a 5% (v/v) CO2 controlled atmosphere.
Fertilization rates and embryo morphology and quality
Fertilization was assessed 16–19 h after microinjection by confirmation of two polar bodies (PB) and two pronuclei (PN). A total of 843 oocytes were evaluated in an inverted microscope at ×400.
Embryo quality was assessed both at the cleavage-stage (days 2 and 3), or early embryos, and in the later phase of in vitro development (day 5), at the blastocyst stage. The early embryo quality was evaluated by taking into account the number of blastomeres, type and percentage of fragmentation, the blastomere symmetry (equal, similar, and different), the presence and number of vacuoles (absent, scarce or diameter <5 mm, abundant), the zona pellucida (normal/abnormal), and the presence of multinucleated cells. According to the Spanish Association of Reproduction Biology Studies (ASEBIR) criteria, embryos were classified into four grades (A, B, C, and D) based on their implantation potential and in combination with the various aforementioned morphological parameters34 (Supplementary Figure 1 (200.9KB, tif) and 2 (202.8KB, tif) . Specifically, grade A gave the best and grade D the worst prognosis for implantation.34 The embryo quality was related to sperm tACE in two different ways: (1) grouping the embryos according to their quality (individual concept) and (2) determining the embryo score quality per cohort. To assess the embryo score quality per cohort, each embryo grade was assigned a value (1, 2, 3, and 4); grade A embryos were assigned a value of 1 and those classified in grade D a value of 4. All embryos were included in the determination of the patient's mean embryo score. Moreover, some of the embryo parameters could be calculated similarly as an average of the embryo cohort per patient, including fertilization rate, embryo fragmentation, average number of cells, and symmetry on days 2 and 3.
ASEBIR grading system for embryo quality on day 2 of development.
ASEBIR grading system for embryo quality on day 3 of development.
Human blastocysts were scored on day 5 of embryo development (112–118 h postmicroinjection). Embryos were grouped according to blastocoele expansion degree as either an early (BT), expanding (BC), expanded (BE), hatching or hatched (BHi) blastocyst as previously described.35,36 In addition, the embryos that had a slower development rate and were at the compact morula stage (MC), as well as the embryos that were blocked or degenerated (BD), were also taken into account. To study the relationship between sperm tACE and blastocyst viability, blastocysts were classified as viable (V) or nonviable (NV): viable if they were transferred or frozen and nonviable if they were arrested or were of poor quality.
Embryo transfer, pregnancy, and live-birth outcomes
In each case, one or two embryos were transferred into the uterine cavity on day 3 or day 5 after microinjection. Supernumerary embryos were frozen for eventual future transfers. Clinical pregnancy was determined by observing a gestational sac with fetal heartbeat at 7 weeks of pregnancy. Live-birth outcome was defined by cycles that ended with an infant born alive. In estimating reproductive success, we only included first embryo transfers in our analyses.
Flow cytometry
To measure the tACE levels in sperm samples, we carried out semiquantitative and quantitative flow cytometry assays. To perform quantitative studies, we used the QuantiBRITE™ PE kit (BD Biosciences, San Jose, CA, USA). The same semen samples were simultaneously used for both analyses.
Surplus sperm samples following IVF procedures were fixed in suspension with 4% (w/v) paraformaldehyde (PFA; Sigma-Aldrich, St. Louis, MO, USA), centrifuged at 3500 g for six min, and washed in phosphate-buffered saline (PBS). Samples were incubated in blocking medium (PBS with 10% [w/v] bovine fetal serum [BFS]; Biochrom, Cambridge, United Kingdom) for 30 min and then with a primary antibody. The human ACE monoclonal phycoerythrin (PE)-labeled primary antibody, with a ratio 1:1 (PE: antibody; PE mouse anti-human CD143, 344 204; BioLegend, San Diego, CA, USA) was diluted 1:200 in PBS and incubated overnight at 4°C. Nuclei were stained with 0.5 μg ml−1 Hoechst 33 258 (Molecular Proves, Eugene, OR, USA). Primary antibody specificity was performed using an isotype control antibody (PE mouse IgG1κ isotype control antibody, 400 112; BioLegend) at the same concentration as the primary antibody.
To carry out a quantitative flow cytometry analysis, we plotted a calibration curve with the mean fluorescence intensity (MFI) values obtained from four different populations of PE-conjugated beads with a known number of PE molecules per bead provided for the QuantiBRITE™ PE kit. Briefly, the QuantiBRITE™ PE beads were diluted in 500 μl of 1× PBS with azide plus 0.5% (w/v) bovine serum albumin (BSA, Sigma-Aldrich) and analyzed by flow cytometry. The fluorescence intensity values for semen samples and the different populations of the QuantiBRITE™ PE beads were obtained at the same time and using the same settings for fluorescence and compensation.
Fluorescence data from at least 10 000 events were analyzed in a flow cytometer (Gallios™, Becton Dickinson, San Jose, CA, USA). Blue fluorescence (Hoechst 33 258) and red florescence (PE) were collected in the FL9 and FL2 sensors, respectively. To ensure that fluorescence data were from live spermatozoa, we used a discrimination frame around the sperm population on the forward (FSC) and side (SSC) scatter plots and then selected the Hoechst 33 258-positive events. The percentage of PE-positive sperm and the mean fluorescence of sperm samples were determined by subtraction of background fluorescence in each histogram using its control as a reference. The results of PE and Hoechst 33 258 fluorescence were analyzed with Summit software (version 4.3, Beckman Coulter Inc., Los Angeles, CA, USA).
Finally, to perform the semiquantitative flow cytometry analysis, we considered the percentage of cells positive for tACE measured in each semen sample, whereas to carry out the quantitative flow cytometry assay, we determined the average number of tACE molecules per spermatozoon. This average number can be extrapolated from a calibration curve obtained from the populations of PE-conjugated beads, given that the PE:Ab ratio of our primary antibody was 1:1.
Statistical analyses
The number of tACE-positive sperm cells, as well as the average number of molecules of this enzyme per spermatozoon, was compared to basic sperm and embryo quality parameters. The Kolmogorov–Smirnov test was used to determine a normal data distribution. Data were not distributed normally, so nonparametric tests were used. To analyze the correlation between sperm tACE data and basic sperm parameter values and embryo quality parameters, scatter plots and Spearman's rank correlation analysis were used. Statistical significance was considered for nominal P values. Comparisons of tACE-positive sperm cells or tACE molecules per cell for embryos of different quality, viable or nonviable blastocysts, or different reproductive outcomes were analyzed by Kruskal–Wallis and Mann–Whitney U-tests. Statistical significance and high statistical significance were determined by P < 0.05 and < 0.01, respectively. Statistical analyses were performed using the IBM SPSS Statistic 22 software (IBM Corp, Armonk, NY, USA).
RESULTS
Patients and semen samples
The mean age of the men included in this study was 40.25 ± 0.50 years (mean ± standard deviation [s.d.]) and ranged between 32 years and 52 years. The characteristics of the fresh semen samples were (mean ± s.d.): volume, 3.13 ± 1.38 ml; sperm concentration, (76.91 ± 38.61) ×106 sperm per ml; progressive motility, 53.9% ± 17.2%; and immotile spermatozoa, 38.1% ± 14.6%. After sperm preparation for assisted reproduction techniques (ART), characteristics were as follows: volume, 0.76 ± 0.20 ml; sperm concentration, (15.83 ± 12.29) ×106 sperm per ml; progressive motility, 94.1% ± 5.2%; and immotile spermatozoa, 5.1% ± 5.2%.
Flow cytometric analysis showed that the 25.3% ± 1.7% of sperm cells were tACE-positive with an average of 1147.52 ± 91.31 tACE molecules per spermatozoon (Supplementary Figure 3 (460.2KB, tif) ).
Histogram of percentage of positive-tACE sperm cells measured by flow cytometry (n = 3 patients).
Correlation of tACE with basic sperm parameter values
First, we analyzed the association between values of the WHO basic seminal parameters (volume, concentration, and motility)31 and tACE levels, both in fresh and prepared semen samples (Table 1). We found that the average number of tACE molecules per spermatozoon was positively correlated with the volume of fresh semen samples (P < 0.01, Spearman's rank correlation). We did not find any significant association between the volume and concentration of tACE-positive sperm cells in fresh sperm samples (Table 1).
Table 1.
Correlation between sperm testicular angiotensin-converting enzyme and basic sperm parameter values
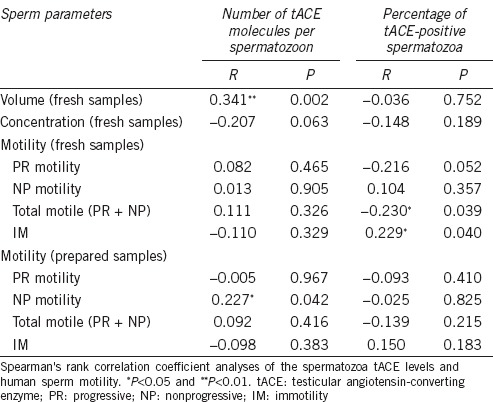
We observed that the number of tACE molecules per spermatozoon was positively associated with the percentage of NP sperm (P < 0.05) in prepared sperm samples. On the other hand, the percentage of tACE-positive cells was negatively correlated with the percentage of total motile spermatozoa (PR + NP) and consequently positively correlated with the percentage of IM spermatozoa in fresh semen samples (P < 0.05, Spearman's rank correlation) (Table 1).
Relationship between tACE, fertilization, and embryo morphology and quality
The total number of recovered oocytes was 991 and the total number of mature oocytes that were donated was 883, with a mean of 10.90 ± 3.24 oocytes received per donor. The overall fertilization rate was 71.9%. After the fertilization assessment, a total of 635 embryos were analyzed, with a mean of 7.86 ± 2.80 embryos per couple.
We first analyzed the relationship between tACE levels and the fertilization rate in the cohort of zygotes obtained per patient. Differences were not statistically significant (r = 0.067, P = 0.554 and r = −0.128, P = 0.256, Spearman's rank correlation).
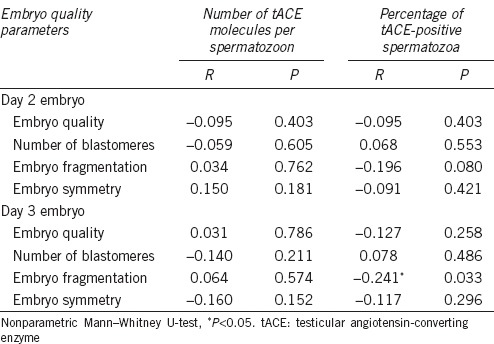
The percentage of good quality embryos (grade A + grade B) on day 2 was 71.9% while on day 3 this percentage was reduced to 48.5%. The mean characteristics of our embryo cohort were as follows (mean ± s.d.): number of blastomeres on day 2, 3.61 ± 0.71; embryo fragmentation on day 2, 2.2% ± 2.4%; number of blastomeres on day 3, 6.47 ± 1.68; and finally embryo fragmentation on day 3, 3.0% ± 3.2%. We did not find any significant difference between the average number of tACE molecules per spermatozoon and the embryo quality grades on days 2 and 3 development (Figure 1a, 1b, Supplementary Table 1 (456.8KB, tif) and 2 (435.1KB, tif) ). However, we observed that embryos with better implantation potential (grades A and B)34 derived from semen samples with higher percentages of tACE-positive spermatozoa on day 2 than lower grade embryos (Figure 1c and Supplementary Table 1 (456.8KB, tif) ). Low percentages of tACE-positive sperm cells were related to the number of grade D embryos on day 3 (Figure 1d and Supplementary Table 2 (435.1KB, tif) ). From analysis of the parameters per early embryo cohort (embryo quality, the number of blastomeres, percentage of embryo fragmentation, and symmetry), we observed a negative correlation between the percentage of tACE-positive spermatozoa and fragmentation on day 3 (Table 2).
Figure 1.
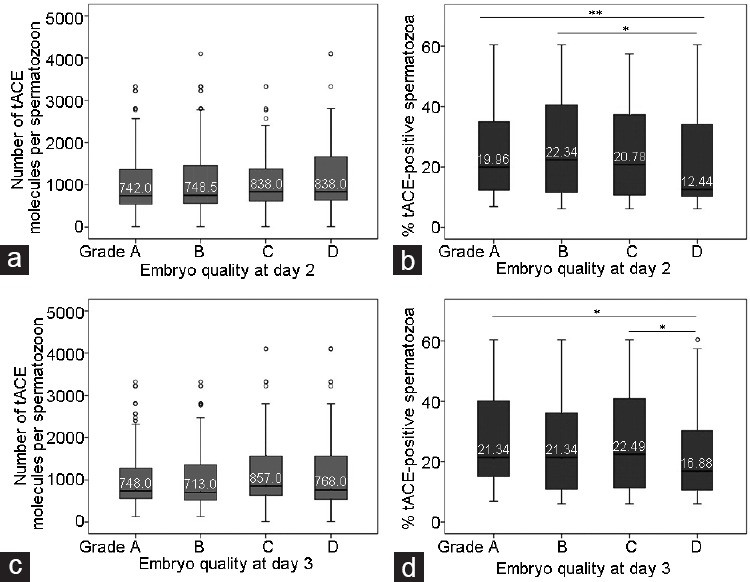
Graphic representation of the scoring of the early embryo quality on days 2 and 3 and the association with sperm tACE. Number of tACE molecules per spermatozoon on (a) day 2, and (b) day 3; and percentage of tACE-positive spermatozoa on (c) day 2, and (d) day 3. Box-plot graph shows the median values as lines across the box. Lower and upper box lines indicate the 25th–75th percentile. Whiskers represent the maximum and minimum values. *P < 0.05 and **P < 0.01. tACE: testicular angiotensin-converting enzyme.
Table 2.
Spearman's rank correlations between sperm testicular angiotensin-converting enzyme and embryo quality score and embryo quality parameters
Early embryo quality at day 2 and their association with percentage of positive-testicular angiotensin-converting enzyme sperm cells and number of testicular angiotensin-converting enzyme molecules per sperm cells
Early embryo quality at day 3 and their association with percentage of positive-testicular angiotensin-converting enzyme sperm cells and number of testicular angiotensin-converting enzyme molecules per sperm cells
Considering that blastocysts have a higher implantation potential than early embryos,37 we also analyzed the association between tACE and the developmental stage of embryos in the later phase of in vitro development. We observed that BD embryos were associated with a higher number of tACE molecules per sperm cell than the most evolved blastocysts (Mann–Whitney U-test), such as expanded (BE; P < 0.01) and hatching/hatched (BHi; P < 0.01) blastocysts. Therefore, early (BT; P < 0.05) and expanded (BE; P < 0.01) blastocysts originated from sperm samples with more tACE molecules per spermatozoon than hatching/hatched blastocysts (BHi, Mann–Whitney U-test) (Figure 2a and Supplementary Table 3 (595.2KB, tif) ). In this case, we did not detect any statistical difference by analyzing the percentages of tACE-positive spermatozoa (Figure 2b and Supplementary Table 3 (595.2KB, tif) ). To evaluate the relationship between tACE and blastocyst viability, we classified embryos in two groups: (1) embryos at the blastocyst stage were considered viable (V) and (2) arrested, degenerated, and blastocysts with lower development were considered nonviable (NV). Approximately 84% of the blastocysts were classified as viable. NV human embryos came from sperm cells with more tACE molecules per spermatozoon on average (P = 0.051, Mann–Whitney U-test; Figure 2c). The percentage of tACE-positive spermatozoa did not show any significant association with blastocyst viability (Figure 2d).
Figure 2.
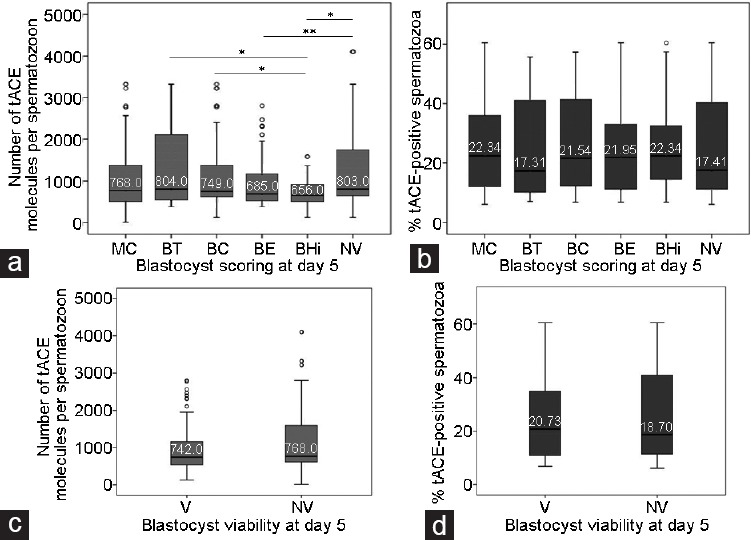
Graphic representation of embryo quality on day 5 and its association with sperm tACE. Embryo development phase at the blastocyst stage and the association with tACE. (a) Number of tACE molecules per spermatozoon and (b) percentage of tACE-positive spermatozoa. Blastocyst viability on day 5 of development and association with tACE: (c) number of tACE molecules per spermatozoon and (d) percentage of tACE-positive spermatozoa. MC: compact morula stage; BT: early blastocyst; BC: expanding blastocyst; BE: expanded blastocyst; BHi: hatching/hatched blastocyst; BD: blocked or degenerated embryos; V: viable blastocyst; NV: non-viable blastocyst. Box-plot graph shows the median values as lines across the box. Lower and upper box lines indicate the 25th–75th percentile. Whiskers represent the maximum and minimum values. *P < 0.05 and **P < 0.01. tACE: testicular angiotensin-converting enzyme.
Embryo developmental phase at blastocyst stage at day 5 and their association with percentage of positive-testicular angiotensin-converting enzyme sperm cells and number of testicular angiotensin-converting enzyme molecules per sperm cells
Relationship between tACE and reproductive outcomes
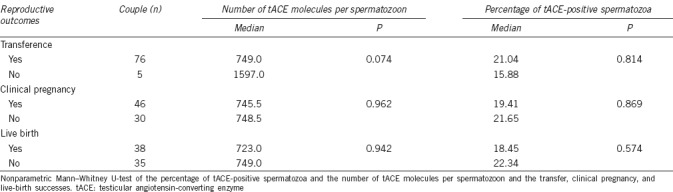
Finally, we sought to determine the association between sperm tACE and ART success. The mean characteristics of the reproductive outcomes were as follows (mean ± s.d.): the number of embryos transferred per patient was 1.47 ± 0.59, the implantation rate was 50.0% ± 44.72%, the mean frozen embryos per patient was 2.59 ± 2.51, and the number of viable embryos (transferred and cryopreserved) per patient was 4.12 ± 2.67.
To conclude, we evaluated the role of tACE in embryo transfer, clinical pregnancy, and live-birth success. We found 93.8%, 58.9%, and 52.1% of the analyzed cycles resulted in embryo transfer, clinical pregnancy, and live birth, respectively. However, when we analyzed the relationship between these outcomes with both the percentage of tACE-positive spermatozoa and the average number of tACE molecules per spermatozoon, we did not find any statistically significant relationships (Table 3).
Table 3.
Relationship between sperm testicular angiotensin-converting enzyme and reproductive outcome
DISCUSSION
The diagnosis of male infertility depends on a morphofunctional descriptive evaluation of the semen, taking into account the concentration and motility of spermatozoa in the ejaculate.5 However, a normal seminogram does not necessarily correlate with sperm fertilization ability,9 suggesting that other sperm dysfunctions not observed in a conventional semen analysis can be responsible for male infertility.5 Several studies have been conducted to identify the molecular biomarkers related to male fertility potential.38,39 Among these, tACE has been identified as a marker of male fertility.
tACE is expressed in human spermatozoa at the gene and protein level16,20,40 and is found at the acrosomal region, the midpiece, and the flagellum of ejaculated human spermatozoa.41 However, the levels of tACE on the surface of spermatozoa change during the different phases of fertilization, since the tACE molecules are released from human spermatozoa during capacitation26,27 and acrosome reaction.27 This is consistent with our analysis in which we detected lower percentages of tACE-positive sperm cells in prepared cells. Although sperm tACE seems to be involved in different steps of fertilization such as motility, capacitation, or sperm-oocyte interactions,23,24,25,26,27,28 the physiological role of tACE in male reproduction is not fully understood.
Given that tACE plays an important role in sperm physiology,42 our first aim was to evaluate the association of tACE levels with basic sperm parameter values. From this, the average number of tACE molecules per spermatozoon was positively associated with the semen volume of the fresh samples. We also observed an association between tACE and sperm motility. Specifically, sperm motility was negatively correlated with both the percentage of tACE-positive spermatozoa and the number of tACE molecules per spermatozoon. This inverse relationship between enzyme activity and the motility of human spermatozoa has been described in previous studies,23,24,43 and is also consistent with the ACE inactivation of bradykinin,44 which is known to be a stimulator of sperm motility.45
Some recent reports demonstrate that sperm molecular characteristics may contribute to oocyte fertilization and embryo development.11,12 We also evaluated the relationship between sperm tACE levels and embryo development in humans using an oocyte donation model. The use of donated oocytes allowed us to avoid the bias related to oocyte quality.
Functional and knockout studies implicate tACE as a key factor in the processes of fertilization.28,30,43,46 In addition, absent or aberrant expression of human tACE protein on spermatozoa causes low fertilization rates and fertilization failure.20 Nevertheless, after analyzing our results, we did not observe any relationship between the tACE levels and fertilization rate. Even so, this could be because all cycles included in this study were inseminated by ICSI, in which the previous steps that take place in natural conception (such as reaching the fertilization site, recognizing and fusing with the oocyte) have been bypassed by the embryologist, and only those factors involved in embryo development are needed.11,38
Ang II, the resulting peptide of ACE action on angiotensinogen (AGT), plays a role in development as a growth factor.47 Similarly, the embryo is sensitive to Ang II that is present in the early gestational environment. However, because AGT, as well as renin and ACE, does not appear to be expressed by the preimplantation embryos, Ang II may be a maternal product.48 Nevertheless, it could be that the tACE molecules present in the spermatozoon that fertilized the oocyte contribute to the production of Ang II. Moreover, since a defective sperm contribution may extend beyond fertilization, highlighting the fact that early and late paternal effects may be determinants of normal embryo development,11 we evaluated the relationship between tACE and embryo quality. We observed that semen samples with lower percentages of positive-tACE spermatozoa were related to worse early embryo quality. In fact, it has been previously reported that lacking or aberrant expression of human sperm tACE protein causes low fertilization rates and fertilization failure.20 Our results, therefore, suggest that the presence of tACE in sperm cells influences the quality of embryos reinforcing the role of sperm proteins in embryonic development. However, not only the presence of tACE sperm protein, but also the level of this protein on the surface membrane, is related to embryonic development. Semen samples with fewer tACE molecules present on the surface membranes were related to blastocysts with higher implantation potential, such as fully expanded and hatching blastocysts.49 This is consistent with the fact that a reduction of the sperm surface tACE levels plays an important role in their fertilizing ability,28 suggesting that not only the presence or absence of this sperm enzyme but also the number of molecules per spermatozoon is involved in human embryo development.
Similarly, we observed that viable embryos were related to semen samples with a lower number of molecules of tACE per spermatozoon. Previous studies have already reported that the ACE/AngII/AT1R/AT2R axis appears to be involved in the advanced stages of embryo development, since the use of blockers to Ang II receptors induces an increase in the number of hatched embryos.49 Our results suggest that the presence of fewer tACE molecules in sperm cells seem to be beneficial to late embryo development, as has been described for other proteins.32
Finally, we also evaluated the role of tACE in reproductive success. Although we did not find any relationship between embryo transfer rate, clinical pregnancy, or live birth and tACE, we cannot deny a possible effect of this enzyme on the studied parameters because we only analyzed the first embryo transfer, where only the high-quality embryos were transferred. In consequence, this positive selection could be a factor of bias in our analysis, as described previously.32
Conclusion
Our results suggest that the presence of tACE sperm protein as well as the level of the enzyme on sperm surface membranes could play a role during embryo development, even before the activation of the translational machinery of the embryo.11,32,38 Specifically, semen samples with higher percentages of positive-tACE sperm cells together with fewer tACE molecules per spermatozoa on its surface membrane are associated with better embryo quality and viability. Our results suggest that not only the presence or absence of sperm proteins, but also the number of molecules per spermatozoa, could provide very valuable information regarding embryo development, quality, and viability. As such, tACE could be useful as a biomarker to aid in informing embryologists in the selection of a sperm population with a high potential to produce high-quality embryos during ICSI.
AUTHOR CONTRIBUTIONS
MG designed the experiments, evaluated semen quality parameters, performed the statistical analysis, and wrote the manuscript; IUA and IMH conducted flow cytometry experiments; ZL carried out the IVF procedures and evaluated human embryo quality; NG and LC participated in critical discussion and helped draft the manuscript; JI conceived of the study, participated in critical discussion, and helped draft the manuscript; NS conceived of the study, participated in its design and coordination, and helped draft the manuscript. All authors read and approved the final manuscript.
COMPETING INTERESTS
All authors declared no competing interests.
ACKNOWLEDGMENTS
The authors thank Alejandro Díez, Ph.D., technician of the Analytical and High-Resolution Microscopy and Cytometry Laboratory in Biomedicine Service of the University of Basque Country (UPV/EHU), for his technical assistance with samples processing by flow cytometry. This study was supported by grants from the Basque Government (GIC12/173; to MG and IMH) and University of the Basque Country (UPV/EHU) (EHUA14/17; to MG and IUA).
Supplementary information is linked to the online version of the paper on the Asian Journal of Andrology website.
REFERENCES
- 1.Boivin J, Bunting L, Collins JA, Nygren KG. International estimates of infertility prevalence and treatment-seeking: potential need and demand for infertility medical care. Hum Reprod. 2007;22:1506–12. doi: 10.1093/humrep/dem046. [DOI] [PubMed] [Google Scholar]
- 2.Inhorn MC, Patrizio P. Infertility around the globe: new thinking on gender, reproductive technologies and global movements in the 21st century. Hum Reprod Update. 2015;21:411–26. doi: 10.1093/humupd/dmv016. [DOI] [PubMed] [Google Scholar]
- 3.McLernon DJ, Maheshwari A, Lee AJ, Bhattacharya S. Cumulative live birth rates after one or more complete cycles of IVF: a population-based study of linked cycle data from 178,898 women. Hum Reprod. 2016;31:572–81. doi: 10.1093/humrep/dev336. [DOI] [PubMed] [Google Scholar]
- 4.Nallella KP, Sharma RK, Aziz N, Agarwal A. Significance of sperm characteristics in the evaluation of male infertility. Fertil Steril. 2006;85:629–34. doi: 10.1016/j.fertnstert.2005.08.024. [DOI] [PubMed] [Google Scholar]
- 5.Samplaski MK, Agarwal A, Sharma R, Sabanegh E. New generation of diagnostic tests for infertility: review of specialized semen tests. Int J Urol. 2010;17:839–47. doi: 10.1111/j.1442-2042.2010.02619.x. [DOI] [PubMed] [Google Scholar]
- 6.Jamsai D, O’Bryan MK. Mouse models in male fertility research. Asian J Androl. 2011;13:139–51. doi: 10.1038/aja.2010.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Palermo G, Joris H, Devroey P, Van Steirteghem AC. Pregnancies after intracytoplasmic injection of single spermatozoon into an oocyte. Lancet. 1992;340:17–8. doi: 10.1016/0140-6736(92)92425-f. [DOI] [PubMed] [Google Scholar]
- 8.Khorram O, Patrizio P, Wang C, Swerdloff R. Reproductive technologies for male infertility. J Clin Endocrinol Metab. 2001;86:2373–9. doi: 10.1210/jcem.86.6.7571. [DOI] [PubMed] [Google Scholar]
- 9.Muratori M, Marchiani S, Tamburrino L, Forti G, Luconi M, et al. Markers of human sperm functions in the ICSI era. Front Biosci (Landmark Ed) 2011;16:1344–63. doi: 10.2741/3793. [DOI] [PubMed] [Google Scholar]
- 10.Chemes HE, Alvarez Sedo C. Tales of the tail and sperm headaches: changing concepts on the prognostic significance of sperm pathologies affecting the head, neck and tail. Asian J Androl. 2012;14:14–23. doi: 10.1038/aja.2011.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barroso G, Valdespin C, Vega E, Kershenovich R, Avila R, et al. Developmental sperm contributions: fertilization and beyond. Fertil Steril. 2009;92:835–48. doi: 10.1016/j.fertnstert.2009.06.030. [DOI] [PubMed] [Google Scholar]
- 12.Govindaraju A, Dogan S, Rodriguez-Osorio N, Grant K, Kaya A, et al. Delivering value from sperm proteomics for fertility. Cell Tissue Res. 2012;349:783–93. doi: 10.1007/s00441-012-1452-2. [DOI] [PubMed] [Google Scholar]
- 13.Said TM, Land JA. Effects of advanced selection methods on sperm quality and ART outcome: a systematic review. Hum Reprod Update. 2011;17:719–33. doi: 10.1093/humupd/dmr032. [DOI] [PubMed] [Google Scholar]
- 14.Muratori M, Luconi M, Marchiani S, Forti G, Baldi E. Molecular markers of human sperm functions. Int J Androl. 2009;32:25–45. doi: 10.1111/j.1365-2605.2008.00875.x. [DOI] [PubMed] [Google Scholar]
- 15.Costerousse O, Jaspard E, Wei L, Corvol P, Alhenc-Gelas F. The angiotensin I-converting enzyme (kininase II): molecular organization and regulation of its expression in humans. J Cardiovasc Pharmacol. 1992;20(Suppl 9):S10–5. doi: 10.1097/00005344-199200209-00004. [DOI] [PubMed] [Google Scholar]
- 16.Pauls K, Metzger R, Steger K, Klonisch T, Danilov S, et al. Isoforms of angiotensin I-converting enzyme in the development and differentiation of human testis and epididymis. Andrologia. 2003;35:32–43. doi: 10.1046/j.1439-0272.2003.00535.x. [DOI] [PubMed] [Google Scholar]
- 17.Skeggs LT, Jr, Kahn JR, Shumway NP. The preparation and function of the hypertensin-converting enzyme. J Exp Med. 1956;103:295–9. doi: 10.1084/jem.103.3.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang HY, Erdos EG, Levin Y. A dipeptidyl carboxypeptidase that converts angiotensin I and inactivates bradykinin. Biochim Biophys Acta. 1970;214:374–6. doi: 10.1016/0005-2795(70)90017-6. [DOI] [PubMed] [Google Scholar]
- 19.Paul M, Poyan Mehr A, Kreutz R. Physiology of local renin-angiotensin systems. Physiol Rev. 2006;86:747–803. doi: 10.1152/physrev.00036.2005. [DOI] [PubMed] [Google Scholar]
- 20.Li LJ, Zhang FB, Liu SY, Tian YH, Le F, et al. Human sperm devoid of germinal angiotensin-converting enzyme is responsible for total fertilization failure and lower fertilization rates by conventional in vitro fertilization. Biol Reprod. 2014;90:125. doi: 10.1095/biolreprod.113.114827. [DOI] [PubMed] [Google Scholar]
- 21.Cushman DW, Cheung HS. Concentrations of angiotensin-converting enzyme in tissues of the rat. Biochim Biophys Acta. 1971;250:261–5. doi: 10.1016/0005-2744(71)90142-2. [DOI] [PubMed] [Google Scholar]
- 22.Mukhopadhyay AK, Cobilanschi J, Schulze W, Brunswig-Spickenheier B, Leidenberger FA. Human seminal fluid contains significant quantities of prorenin: its correlation with the sperm density. Mol Cell Endocrinol. 1995;9:219–24. doi: 10.1016/0303-7207(95)03505-2. [DOI] [PubMed] [Google Scholar]
- 23.Siems WE, Heder G, Hilse H, Baeger I, Engel S, et al. Angiotensin-converting enzyme and other peptidolytic enzymes in human semen and relations to its spermatologic parameters. Andrologia. 1991;23:185–9. doi: 10.1111/j.1439-0272.1991.tb02533.x. [DOI] [PubMed] [Google Scholar]
- 24.Shibahara H, Kamata M, Hu J, Nakagawa H, Obara H, et al. Activity of testis angiotensin converting enzyme (ACE) in ejaculated human spermatozoa. Int J Androl. 2001;24:295–9. doi: 10.1046/j.1365-2605.2001.00301.x. [DOI] [PubMed] [Google Scholar]
- 25.Yamaguchi R, Yamagata K, Ikawa M, Moss SB, Okabe M. Aberrant distribution of ADAM3 in sperm from both angiotensin-converting enzyme (Ace)- and calmegin (Clgn)-deficient mice. Biol Reprod. 2006;75:760–6. doi: 10.1095/biolreprod.106.052977. [DOI] [PubMed] [Google Scholar]
- 26.Foresta C, Indino M, Manoni F, Scandellari C. Angiotensin-converting enzyme content of human spermatozoa and its release during capacitation. Fertil Steril. 1987;47:1000–3. doi: 10.1016/s0015-0282(16)59236-x. [DOI] [PubMed] [Google Scholar]
- 27.Kohn FM, Miska W, Schill WB. Release of angiotensin-converting enzyme (ACE) from human spermatozoa during capacitation and acrosome reaction. J Androl. 1995;16:259–65. [PubMed] [Google Scholar]
- 28.Foresta C, Mioni R, Rossato M, Varotto A, Zorzi M. Evidence for the involvement of sperm angiotensin converting enzyme in fertilization. Int J Androl. 1991;14:333–9. doi: 10.1111/j.1365-2605.1991.tb01101.x. [DOI] [PubMed] [Google Scholar]
- 29.Krege JH, John SW, Langenbach LL, Hodgin JB, Hagaman JR, et al. Male-female differences in fertility and blood pressure in ACE-deficient mice. Nature. 1995;375:146–8. doi: 10.1038/375146a0. [DOI] [PubMed] [Google Scholar]
- 30.Hagaman JR, Moyer JS, Bachman ES, Sibony M, Magyar PL, et al. Angiotensin-converting enzyme and male fertility. Proc Natl Acad Sci U S A. 1998;95:2552–7. doi: 10.1073/pnas.95.5.2552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.World Health Organization. Geneva: World Health Organization; 2010. WHO laboratory manual for the examination and processing of human semen. [Google Scholar]
- 32.Meseguer M, de los Santos MJ, Simon C, Pellicer A, Remohi J, et al. Effect of sperm glutathione peroxidases 1 and 4 on embryo asymmetry and blastocyst quality in oocyte donation cycles. Fertil Steril. 2006;86:1376–85. doi: 10.1016/j.fertnstert.2006.03.053. [DOI] [PubMed] [Google Scholar]
- 33.Munoz E, Fernandez I, Martinez M, Tocino A, Portela S, et al. Oocyte donation outcome after oncological treatment in cancer survivors. Fertil Steril. 2015;103:205–13. doi: 10.1016/j.fertnstert.2014.09.027. [DOI] [PubMed] [Google Scholar]
- 34.ASEBIR. Madrid: Góbalo; 2008. Criteria for morphological assessment of oocytes, embryos early and human blastocysts. Clinical embryologist notebooks. [Google Scholar]
- 35.Elgindy E, Elsedeek MS. Day 5 expanded blastocysts transferred on same day have comparable outcome to those left for more extended culture and transferred on day 6. J Assist Reprod Genet. 2012;29:1111–5. doi: 10.1007/s10815-012-9837-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Alpha Scientists in Reproductive Medicine and ESHRE Special Interest Group of Embryology. The Istanbul consensus workshop on embryo assessment: proceedings of an expert meeting. Hum Reprod. 2011;26:1270–83. doi: 10.1093/humrep/der037. [DOI] [PubMed] [Google Scholar]
- 37.Papanikolaou EG, Kolibianakis EM, Tournaye H, Venetis CA, Fatemi H, et al. Live birth rates after transfer of equal number of blastocysts or cleavage-stage embryos in IVF. A systematic review and meta-analysis. Hum Reprod. 2008;23:91–9. doi: 10.1093/humrep/dem339. [DOI] [PubMed] [Google Scholar]
- 38.Garrido N, Remohi J, Martinez-Conejero JA, Garcia-Herrero S, Pellicer A, et al. Contribution of sperm molecular features to embryo quality and assisted reproduction success. Reprod Biomed Online. 2008;17:855–65. doi: 10.1016/s1472-6483(10)60415-4. [DOI] [PubMed] [Google Scholar]
- 39.Sakkas D, Ramalingam M, Garrido N, Barratt CL. Sperm selection in natural conception: what can we learn from mother nature to improve assisted reproduction outcomes? Hum Reprod Update. 2015;21:711–26. doi: 10.1093/humupd/dmv042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lattion AL, Soubrier F, Allegrini J, Hubert C, Corvol P, et al. The testicular transcript of the angiotensin I-converting enzyme encodes for the ancestral, non-duplicated form of the enzyme. FEBS Lett. 1989;252:99–104. doi: 10.1016/0014-5793(89)80897-x. [DOI] [PubMed] [Google Scholar]
- 41.Kohn FM, Dammshauser I, Neukamm C, Renneberg H, Siems WE, et al. Ultrastructural localization of angiotensin-converting enzyme in ejaculated human spermatozoa. Hum Reprod. 1998;13:604–10. doi: 10.1093/humrep/13.3.604. [DOI] [PubMed] [Google Scholar]
- 42.Nikolaeva MA, Balyasnikova IV, Alexinskaya MA, Metzger R, Franke FE, et al. Testicular isoform of angiotensin I-converting enzyme (ACE, CD143) on the surface of human spermatozoa: revelation and quantification using monoclonal antibodies. Am J Reprod Immunol. 2006;55:54–68. doi: 10.1111/j.1600-0897.2005.00326.x. [DOI] [PubMed] [Google Scholar]
- 43.Kohn FM, Muller C, Drescher D, Neukamm C, el Mulla KF, et al. Effect of angiotensin converting enzyme (ACE) and angiotensins on human sperm functions. Andrologia. 1998;30:207–15. doi: 10.1111/j.1439-0272.1998.tb01162.x. [DOI] [PubMed] [Google Scholar]
- 44.Ehlers MR, Riordan JF. Angiotensin-converting enzyme: new concepts concerning its biological role. Biochemistry. 1989;28:5311–8. doi: 10.1021/bi00439a001. [DOI] [PubMed] [Google Scholar]
- 45.Miska W, Schill WB. Enhancement of sperm motility by bradykinin and kinin analogs. Arch Androl. 1990;25:63–7. doi: 10.3109/01485019008987595. [DOI] [PubMed] [Google Scholar]
- 46.Fuchs S, Frenzel K, Hubert C, Lyng R, Muller L, et al. Male fertility is dependent on dipeptidase activity of testis ACE. Nat Med. 2005;11:1140.2. doi: 10.1038/nm1105-1140. author reply 1142-3. [DOI] [PubMed] [Google Scholar]
- 47.Tebbs C, Pratten MK, Broughton Pipkin F. Angiotensin II is a growth factor in the peri-implantation rat embryo. J Anat. 1999;195(Pt 1):75–86. doi: 10.1046/j.1469-7580.1999.19510075.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pijacka W, Hunter MG, Broughton Pipkin F, Luck MR. Expression of renin-angiotensin system components in the early bovine embryo. Endocr Connect. 2012;1:22–30. doi: 10.1530/EC-12-0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Machtinger R, Racowsky C. Morphological systems of human embryo assessment and clinical evidence. Reprod Biomed Online. 2013;26:210–21. doi: 10.1016/j.rbmo.2012.10.021. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
ASEBIR grading system for embryo quality on day 2 of development.
ASEBIR grading system for embryo quality on day 3 of development.
Histogram of percentage of positive-tACE sperm cells measured by flow cytometry (n = 3 patients).
Early embryo quality at day 2 and their association with percentage of positive-testicular angiotensin-converting enzyme sperm cells and number of testicular angiotensin-converting enzyme molecules per sperm cells
Early embryo quality at day 3 and their association with percentage of positive-testicular angiotensin-converting enzyme sperm cells and number of testicular angiotensin-converting enzyme molecules per sperm cells
Embryo developmental phase at blastocyst stage at day 5 and their association with percentage of positive-testicular angiotensin-converting enzyme sperm cells and number of testicular angiotensin-converting enzyme molecules per sperm cells


