Abstract
We summarized our experience in transurethral seminal vesiculoscopy (TSV) for recurrent hemospermia by introducing surgical techniques, intraoperative findings, and treatment outcomes. TSV was performed in 419 patients with an initial diagnosis of persistent hemospermia at Shanghai Changhai Hospital (Shanghai, China) from May 2007 to November 2015. TSV was successfully performed in 381 cases (90.9%). Hemospermia was alleviated or disappeared in 324 (85.0%) patients by 3 months after surgery. Common intraoperative manifestations were bleeding, obstruction or stenosis, mucosal lesions, and calculus. Endoscopic presentation of the ejaculatory duct orifice and the verumontanum was categorized into four types, including 8 (1.9%), 32 (7.6%), 341 (81.4%), and 38 (9.1%) cases in Types A, B, C, and D, respectively. TSV is an effective and safe procedure in the management of seminal tract disorders. This study may help other surgeons to become familiar with and improve this procedure. However, further multicentric clinical trials are warranted to validate these findings.
Keywords: endoscopy, hemospermia, transurethral seminal vesiculoscopy
INTRODUCTION
Disorders of the seminal tract, such as recurrent hemospermia, seminal vesiculitis, and stones, may lead to dysfunction of the reproductive system and cause great psychological burden. However, the traditional management for these diseases is obscure and often invasive. Transrectal ultrasound examination1 and magnetic resonance imaging (MRI)2 are not accurate since the majority of patients do not present obvious imaging changes at an early stage and neither of them would provide information of the possible cause of hemospermia. Other methods of examination, such as vasovesiculography, are invasive and may have negative side effects.3
With advances in endoscopic technology, Yang et al.4 introduced in vivo transurethral seminal vesiculoscopy (TSV) in 2002. Since then, urologists and andrologists have explored the application of TSV as a diagnostic5 and treatment tool for seminal tract stones,6 recurrent hemospermia,5 and ejaculatory duct obstructions.7 However, the safety of this procedure is yet to be validated because the anatomic and histological structure of the ejaculatory duct is poorly understood. In addition, detailed procedures for this surgery have not been clearly illustrated, particularly with regard to gaining access to the ejaculatory duct. More details of this procedure remain unexplored (e.g., the relative location of the ejaculatory duct orifice and the verumontanum, the safety of establishing the surgical path, and the appropriate surgical instruments).
Here, we summarize our experience from 419 TSV cases by illustrating detailed surgical techniques, treatment outcomes, and intraoperative findings, with a discussion on the safety and rationale of this procedure, key steps for this surgery, and the relative location of the ejaculatory duct orifice and verumontanum.
PATIENTS AND METHODS
Study cohort
TSV was performed in 419 patients with an initial diagnosis of persistent hemospermia at Shanghai Changhai Hospital (Shanghai, China). This study was approved by the institutional review board of Shanghai Changhai Hospital. Informed consents were obtained from all patients before administering the treatment.
The indications for each disease have been described previously.5 Briefly, all patients with hemospermia were treated with oral antibiotics for a minimum of 4 weeks before the surgery. All patients were psychologically influenced by persistent or recurrent hemospermia and had a strong wish for surgical intervention. Important preoperative counseling included the duration of hemospermia, the color of hemospermia, pain with ejaculation, hematuria, reproductive history, and PSA level. We also focused on identifying that the blood was in the sperm rather than from the urine or from the bleeding of the urethra. Routine urogenital clinical examinations were performed in all cases, including digital rectal examination (DRE), to rule out the possibility of advanced prostate cancer. Routine urine and blood tests were performed. None of these patients were with concurrent hematuria or bladder cancer (ruled out during the operation by examination of the bladder). PSA test was done for all patients. If the PSA level is higher than 4 ng ml−1, another PSA test was prescribed for the patients and a prostate biopsy is considered. No prostate cancer patient was detected. All patients received at least one kind of imaging examination, including transrectal ultrasound, pelvic computed tomography, and MRI, before and after the surgery. We performed these examinations to have better information about the anatomy and lesions of the seminal vesicle or the ejaculatory duct, such as the dilation of the ejaculatory duct, stones and blood clot in the seminal tract, and to rule out the possibility of prostate cancer and malignancy of the seminal vesicle. Among these examinations, MRI is the most informative one and previous studies have illustrated imaging findings of seminal tract disorders.8
Surgical procedures
All procedures were performed with the patients in a lithotomy position with laryngeal mask general anesthesia or spinal anesthesia. The endoscopic procedure was performed through the normal anatomic path of the distal seminal tracts with a 7-F rigid vesiculoscope in the initial 154 cases, and a 5-F or 6-F rigid vesiculoscope in later cases (Supplementary Figure 1 (110.4KB, tif) ). The TSV procedure is illustrated in Figure 1. First, the vesiculoscope was inserted into the urethra and a careful search for the ejaculatory duct orifice was performed on the outside wall of the verumontanum. Second, the vesiculoscope was inserted to access the ejaculatory duct. There are two ways to enter the ejaculatory duct depending on the endoscopic presentation (Figure 2). In some patients, the orifice of the ejaculatory duct was observed from the urethra, and then the vesiculoscope was inserted directly into the ejaculatory duct (Type A), whereas in other patients, when the ejaculatory duct orifice could not be identified on the surface of the verumontanum (Types B, C, and D), a surgical path was established. In Type B patients, the ejaculatory duct and the verumontanum were only separated by a thin layer of white membrane-like tissue, and a “Zebra guidewire” (Straight Tip, black/white PTFE-coated Jacket guidewire, ST-32150, Urovision, Oberbayern, Germany) could be used to establish the surgical path from the verumontanum to the ejaculatory duct. However, in Type C patients, the membrane-like tissue was not identifiable at the first sight; tentative punctures using the guidewire on the suspected spot on the inner wall of the verumontanum were applied. The course of the ejaculatory duct is just beneath the inner wall of verumontanum, approximately 45° from the central line on the sagittal plane. However, the surgical path could not be established in some cases, leading to the failure of the procedure (Type D). Third, the vesiculoscope was inserted into the seminal vesicle. Close observation and treatments were performed according to endoscopic findings. A typical manifestation of the seminal vesicle was honeycomb-like tissues, and the surgeon needed to go through every small cavity in the seminal vesicles. In cases of stenosis of the ejaculatory duct orifice, holmium (Ho:YAG) or thulium fiber laser was applied to expand the surgical path. Typically, blood mixed with seminal plasma fluid could be identified in hemospermia patients. Continuous saline irrigation with a 50-ml syringe was applied to clear out the seminal plasma fluid, blood, and small calculi. Grasping forceps were applied for larger calculi. In cases with hemorrhagic spots, a low-power holmium fiber laser was applied for hemostasis. The catheter was placed after surgery and removed on the 2nd day.
Figure 1.
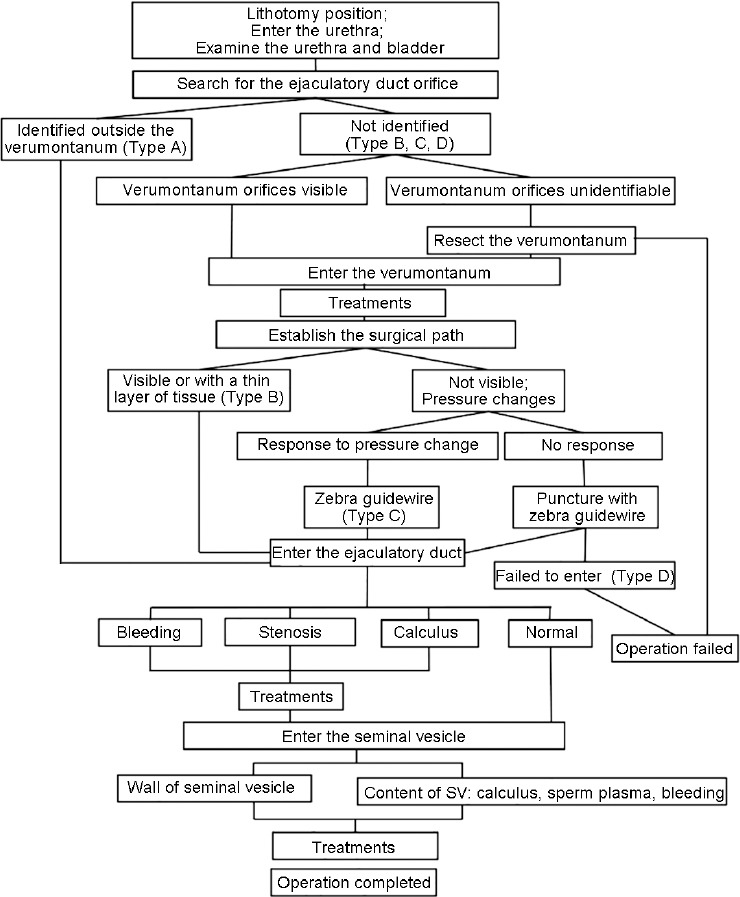
Flowchart of the surgery. For each key step in the surgery, “positive or negative” findings together with further guidance of the next move are listed in the flowchart.
Figure 2.
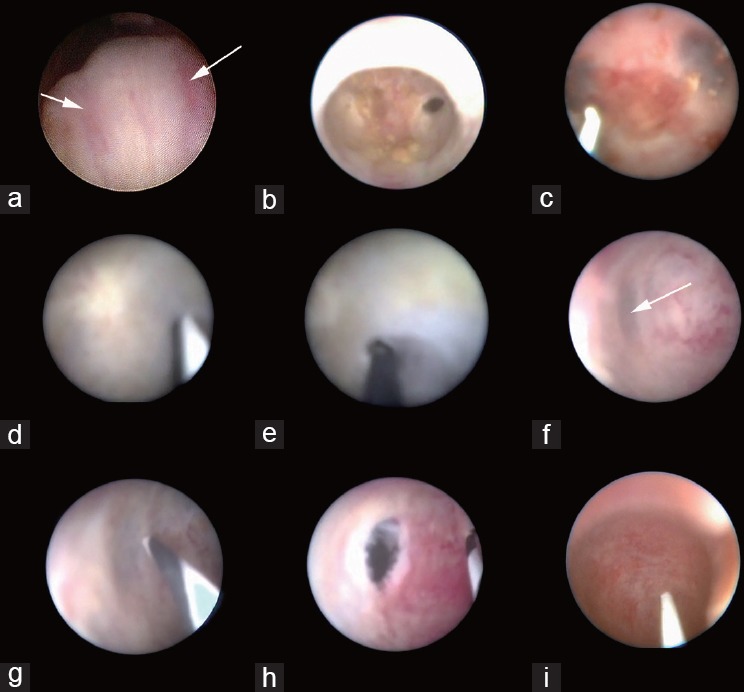
Endoscopic patterns of the orifice of the ejaculatory duct and the surgical path in Type A patients where the orifice of the ejaculatory duct could be observed from the urethra (a); in Type B patients, the ejaculatory duct and the verumontanum were only separated by a thin layer of white membrane-like tissue on the left side (b) or on both sides (c); the membrane-like tissue was not identified at first sight in Type C patients (d), and tentative puncture at the membrane-like tissue were performed in the same patient (e); pressure changes indicated the suspected location for tentative puncture (f) and tentative puncture performed at the suspected location in Type C patients (g); the surgical pathway was established after the tentative puncture in Type C patients (h); the surgical path could not be established in Type D patients (i).
A rigid vesiculoscope applied in the surgery.
Data collection and statistical methods
We prospectively recorded demographic and operative data, including age, baseline symptoms, and other clinical parameters. Therapeutic effects were recorded. Patients were asked to abstain from ejaculation for 3 weeks after surgery. Follow-up was performed monthly during the first 6 months after surgery and then bimonthly during the subsequent 6 months. Mean, range, and statistical significance were used to report continuous and categorical data. All the analyses were conducted using Stata 13.0 (Stata Corp., College Station, TX, USA). Statistical significance was determined based on a two-sided significance level of 0.05.
RESULTS
Preoperative data and treatment outcomes
The mean age of all patients was 49 years (standard deviation [s.d.]: 7.2). The median time of follow-up was 7.3 months and the mean length of surgery was 21 min. The procedure was completed in 381 patients (90.9%). Hemospermia was alleviated or resolved in 324 (85.0%) patients by 3 months after surgery and 24 (6.3%) patients experienced recurrence by 12 months after surgery. The effect of TVS was not immediate. Hemospermia was usually alleviated or resolved after three to five ejaculations. As the patients were abstaining from ejaculation for 3 weeks after the surgery, most patients experienced alleviated hemospermia by 2–3 months after the surgery. The treatment for patients who failed to have alleviated symptoms is mostly antibiotics. However, the effectiveness of such treatment is not good.
Endoscopic pattern of ejaculatory duct orifice in relation to the verumontanum
The endoscopic presentation of the ejaculatory duct orifice and the verumontanum was categorized into four types, including 8 (1.9%), 32 (7.6%), 341 (81.4%), and 38 (9.1%) cases in Types A, B, C, and D, respectively.
Intraoperative findings
In patients who successfully underwent the surgery, bleeding in the seminal vesicle occurred in 362 (95.0%) patients, whereas ejaculatory duct stenosis or obstructions, mucosal lesions in the seminal vesicle, and calculus in the seminal vesicle or in the verumontanum were presented in 312 cases (81.9%), 209 cases (54.9%), and 19 cases (5.0%), respectively (Table 1 and Figure 3). Cystadenoma and hemangioma of the seminal vesicles were observed in only one case each. Irrigation was applied to flush out bleeding and small calculi in the verumontanum, ejaculatory duct, or seminal vesicle.
Table 1.
Main intraoperative manifestations (in successful cases)
![]()
Figure 3.
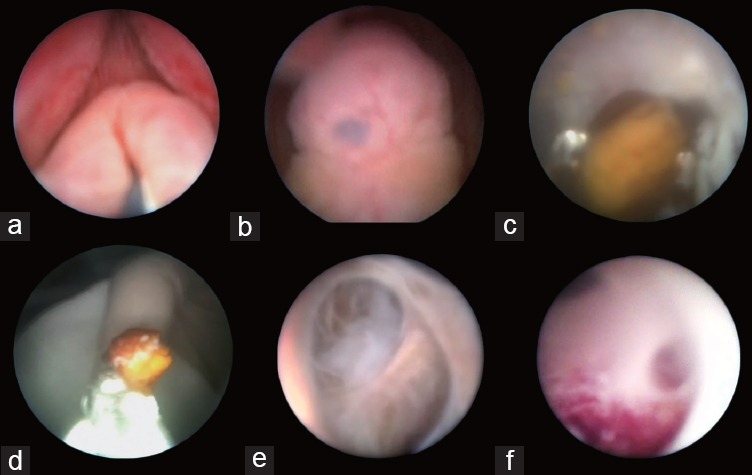
Endoscopic view of the seminal tract. (a) Opening of the verumontanum; (b) orifice of the verumontanum; (c) calculus in the ejaculatory duct; (d) grasping forceps applied for removal of calculus in the ejaculatory duct; (e) normal seminal vesicle; (f) inflammatory lesions in the seminal vesicle.
Complications
Complications were as follows: retrograde ejaculation in one patient, who received transurethral resection of the verumontanum. In our initial cases, we applied transurethral resection of the verumontanum in five cases. Among these patients, one patient developed retrograde ejaculation. We considered that retrograde ejaculation might be associated with this procedure. Thus, we suggested that we should be especially careful and cautious about applying this technique and try not to apply the resection unless we fully acknowledged its risk. No seminal vesicle perforation, erectile dysfunction, urinary reflux into the ejaculatory duct, rectourethral fistula, or other severe complications was observed. Two cases with epididymitis were observed, and symptoms disappeared after 3–7 days of intravenous antibiotics.
DISCUSSION
Our report represents the largest series of TSV cases, demonstrating satisfactory short-term symptomatic improvements. Since its application in 2002, andrologists and urologists including us have proposed and expanded the indication of this procedure. Asian andrologists and urologists have acknowledged this procedure very fast and performed this procedure in several centers in China, Korea, and other countries. However, we found that the development of this procedure was confined to some extent. In our view, there are a couple of issues preventing the wide application of TVS. First, the procedures for TVS are currently not standardized, making it hard to be learned by other surgeons. Second, failure to identify the ejaculatory duct orifice and failure to establish the surgical path are the major causes of failure.5 Under these conditions, there is no well-recognized instruction that we could follow to identify the proper surgical path. With accumulated surgical experience and knowledge of applied anatomy of the seminal tract, we made some improvements in this procedure and drafted a flowchart for standardized procedure.
Rational of effectiveness and safety considerations
The diagnosis of seminal tract disorders mainly relies on typical clinical manifestations. In most cases, hemospermia is a sign of benign disease, but it can cause psychological distress. In this study, at least oral antibiotics were administered for at least 4 weeks before the surgery. Patients were diagnosed with persistent or refractory hemospermia if their symptoms persisted. A high proportion of these patients were willing to undergo surgery.
The diagnosis of seminal tract disorders mainly relies on typical clinical manifestations, which means that the initial diagnosis only focuses on a part of the lesions. However, we suggest that these disorders are usually correlated and may influence each other. In the absence of malignancy and obvious congenital malformations, recurrent or persistent hemospermia may be caused by obstruction or stricture at the seminal tract or the presence of calculi. Calculi and blood clots further block the drainage of seminal vesicle fluid and cause inflation and the formation of calculi and aggravate the stricture, making stricture, calculus formation, and inflammation a vicious circle. As both a diagnostic and a therapeutic approach, TSV may be effective for these patients, especially for those without a clear diagnosis but with persistent and recurrent symptoms.5 However, it is vital for surgeons to fully evaluate the duration of hemospermia and its psychological burden on patients to select only proper candidates for this procedure.
As illustrated by Nguyen et al.,9 the wall of the ejaculatory duct consists of three layers: an inner epithelial layer, a middle collagenous layer, and a very thin outer muscular coat. To confirm their findings, we have investigated eight pairs of autopsy specimens from healthy men aged 20–43 years. The histology slides illustrated that the tissue between the verumontanum lumen and the ejaculatory duct was quite thin, without nerves or vessels (Figure 4).
Figure 4.
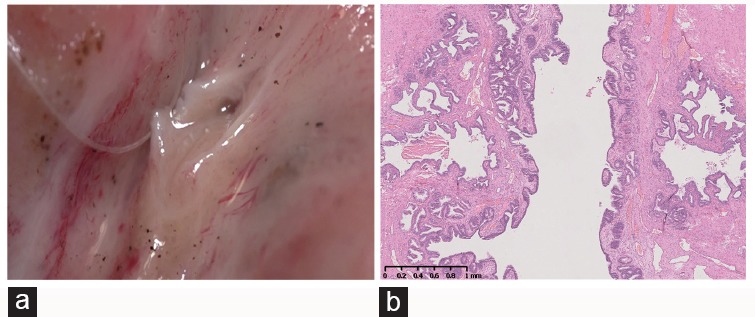
Pathological review: (a) the verumontanum was split in the middle of the sagittal plane. (b) The histological structure of the ejaculatory duct, the verumontanum, and the membrane between them.
Key step in TSV: identify the ejaculatory duct orifice and establish the proper surgical path
Finding the orifice in the urethra from the outside of verumontanum or establishing the surgical path to the ejaculatory duct is the key step in this procedure. Knowledge of the relative location of the ejaculatory duct and the verumontanum is crucial for surgeons to establish the surgical path. However, these data may vary because experienced surgeons may find more Type C cases and fewer Type D cases compared with inexperienced surgeons. We suggest that changes in irrigation pressure may provide further information about the proper site for tentative puncture to establish the surgical path. The membraniform wall typically protrudes slightly when irrigation pressure is lower and becomes depressed when the pressure increases.
Limitations and future prospects
The major limitation of this procedure is the uncertainty of its long-term safety. For instance, although we have gained experience in manipulating the TSV, there is still a chance that we may have failed to identify the proper surgical path. It would be easier for the surgeon to identify the proper site to perform puncture if antegrade vaso-vesiculography with dye or contrast agents were performed during the operation. However, such examinations may cause complications. Furthermore, flexible vesiculoscopy would greatly improve manipulation during surgery, improve the view of the surgeon, and make it easier to detect minor lesions in smaller cavities in seminal vesicle and even in the vas deferens.10 In addition, techniques that improve the safety of this surgery should be studied, especially in monitoring the pressure of irrigation, allowing stable irrigation in the seminal tract. Finally, it should be emphasized that hemospermia in most patients is caused by benign and self-limited diseases. This technique should only be performed in patients with persistent, recurrent symptom and intensive anxiety. In summary, TSV is an effective and safe procedure in the management of seminal tract disorders. Improvements in surgical techniques, applied anatomic structure, and novel vesiculoscopy may contribute to more successful outcomes for this surgery. Further multicentric clinical trials are needed to validate the effectiveness of TSV.
AUTHOR CONTRIBUTIONS
YHS and ZYL conceived of the study, designed and carried out the surgeries. RC and LW drafted the manuscript, and carried out the analysis of the data. RC and LW, XS, HL, TZ, GC, CZ, DK, GX, YL, RC, XS, XC, HL, YL, YL, TZ, GC, CZ, DK, GX, XL, LW, XWN and ZYJ participated in the performing of the surgeries, the design of this study and the editing of the manuscript. All authors read and approved the final manuscript.
COMPETING INTERESTS
The authors declare no competing interests.
ACKNOWLEDGMENTS
This study was funded by “Key Project of Shanghai Commission of Science and Technology”: Mechanism and clinical implication of dysfunction of the gonadal axis in climacteric man (09DJ1400400) and the “1255 Project” of the Second Military Medical University: Applied Anatomy of Seminal Tract (125540300).
Supplementary information is linked to the online version of the paper on the Asian Journal of Andrology website.
REFERENCES
- 1.Lotti F, Maggi M. Ultrasound of the male genital tract in relation to male reproductive health. Hum Reprod Update. 2015;21:56–83. doi: 10.1093/humupd/dmu042. [DOI] [PubMed] [Google Scholar]
- 2.Ammar T, Sidhu PS, Wilkins CJ. Male infertility: the role of imaging in diagnosis and management. Br J Radiol. 2012;85:S59–68. doi: 10.1259/bjr/31818161. Special Issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fourcade R, Jardin A. Vaso-vesiculography: assessment in andrology and urology. Arch Androl. 1981;6:273–80. doi: 10.3109/01485018108987538. [DOI] [PubMed] [Google Scholar]
- 4.Yang SC, Rha KH, Byon SK, Kim JH. Transutricular seminal vesiculoscopy. J Endourol. 2002;16:343–5. doi: 10.1089/089277902760261347. [DOI] [PubMed] [Google Scholar]
- 5.Xing C, Zhou X, Xin L, Hu H, Li L, et al. Prospective trial comparing transrectal ultrasonography and transurethral seminal vesiculoscopy for persistent hematospermia. Int J Urol. 2012;19:437–42. doi: 10.1111/j.1442-2042.2011.02952.x. [DOI] [PubMed] [Google Scholar]
- 6.Song T, Zhang X, Zhang L, Zhang F, Fu WJ. Transurethral seminal vesiculoscopy in the diagnosis and treatment of seminal vesicle stones. Chin Med J (Engl) 2012;125:1475–8. [PubMed] [Google Scholar]
- 7.Wang H, Ye H, Xu C, Liu Z, Gao X, et al. Transurethral seminal vesiculoscopy using a 6F vesiculoscope for ejaculatory duct obstruction: initial experience. J Androl. 2012;33:637–43. doi: 10.2164/jandrol.111.013912. [DOI] [PubMed] [Google Scholar]
- 8.Li BJ, Zhang C, Li K, Zhang J, Zhang Y, et al. Clinical analysis of the characterization of magnetic resonance imaging in 102 cases of refractory haematospermia. Andrology. 2013;1:948–56. doi: 10.1111/j.2047-2927.2013.00132.x. [DOI] [PubMed] [Google Scholar]
- 9.Nguyen HT, Etzell J, Turek PJ. Normal human ejaculatory duct anatomy: a study of cadaveric and surgical specimens. J Urol. 1996;155:1639–42. doi: 10.1016/s0022-5347(01)66150-0. [DOI] [PubMed] [Google Scholar]
- 10.Trottmann MB, Becker A, Liedl B, Stief C, Graw M, et al. Endoscopy of the vas deference – A new diagnostic and therapeutic tool in andrology. Eur Urol Suppl. 2013;12:e844. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A rigid vesiculoscope applied in the surgery.


