Abstract
Erectile dysfunction is a common side effect of finasteride and dutasteride treatments. The objective of this study was to investigate the structural changes in the penis using a benign prostatic hyperplasia (BPH) rodent model treated with dutasteride or finasteride. Sixty male rats were divided into the following groups: C, untreated control rats; C + D, control rats receiving dutasteride; C + F, control rats receiving finasteride; H, untreated spontaneously hypertensive rats (SHRs); H + D, SHRs treated with dutasteride; and H + F, SHRs treated with finasteride. Treatments were performed for 40 days, and penises were collected immediately thereafter. The organs were analyzed using histomorphometric methods to determine the cross-sectional penile area, as well as the surface density (Sv) of smooth muscle fibers, connective tissue, elastic system fibers, and sinusoidal spaces of the corpus cavernosum. The results were compared using a one-way ANOVA with Bonferroni's posttest. Groups C + D and C + F had a significantly smaller penile cross-sectional area, but more elastic system fiber Sv compared to Group C. Group C + D showed less smooth muscle Sv, and Group H showed more connective tissue but a smaller sinusoidal space Sv in the corpus cavernosum compared to Group C. Groups H + D and H + F had less smooth muscle Sv than Group H. Group H + D also had more connective tissue and elastic system fiber Sv than Group H. Both dutasteride and finasteride promoted penile modifications in the control rat penis, although this affect was greater in Group H animals. In this rodent model, dutasteride was the drug that most affected the corpus cavernosum.
Keywords: animal models, benign prostatic hyperplasia, dutasteride, erectile dysfunction, finasteride, penis
INTRODUCTION
Benign prostatic hyperplasia (BPH) and lower urinary tract symptoms (LUTS) are common conditions seen in the practice of urology. Over half of men, aged 50 years and older, present with BPH and/or LUTS.1 Prostate growth is thought to be stimulated by dihydrotestosterone (DHT), the active metabolite converted from testosterone by the enzyme 5-alpha-reductase (5AR).2 Thus, according to the American Urological Association and European Association of Urology guidelines, the use of finasteride or dutasteride (5AR inhibitors) is a recommended treatment for men with enlarged prostates.3,4
Both finasteride and dutasteride inhibit the conversion of testosterone to DHT, which reduces the prostate volume by 20%–30% after 12 months of treatment.5 However, a common side effect of 5AR inhibitor treatment is erectile dysfunction (ED), which occurs in 5%–16% of men in treatment and may persist even after discontinuing treatment.6,7
Beyond the negative effects on reproduction, ED also has major social and psychological drawbacks. Overall, this condition greatly affects the quality of life of men and their sexual partners.8 ED associated with 5AR inhibitors is thought to be caused by lower DHT concentrations, but the exact pathophysiology is unclear.6 Some studies have shown that the use of 5AR inhibitors alters the morphology and function of the corpus cavernosum.9,10 However, these studies used rats that did not present with BPH or LUTS in the animal models, which may have influenced the results. Thus, the effects of dutasteride or finasteride on the penile morphology of men with BPH remain unknown.
The aim of the present study was to investigate, using histomorphometric methods, the structural changes in the penis of a BPH rodent model that was treated with dutasteride or finasteride.
MATERIALS AND METHODS
Animal model and experimental design
The spontaneously hypertensive rat (SHR) strain was developed by inbreeding Wistar–Kyoto rats.11 These animals are largely used for studying arterial hypertension, its effects on different organs, and treatments.12,13 Recently, the SHR strain has been proposed as a model for studying BPH because SHR animals present prostatic morphological alterations.14 Thus, the SHR strain was used as a model of BPH in the present study, and the Wistar–Kyoto strain was used as a control strain.
Thirty male SHRs and 30 male Wistar–Kyoto rats (3 months old and 243–289 g body weight) were used. All animals were bred in our laboratory. They were kept in a room with a controlled temperature (mean ± standard deviation [s.d.], 25°C ± 1°C) and an artificial light–dark cycle (lights on from 7:00 A.M. to 7:00 P.M.). Rats had free access to standard rat food and water. All experiments were performed according to national and international law for the scientific use of animals. This project was also approved by the Animal Care and Use Committee of the State University of Rio de Janeiro, Rio de Janeiro, RJ, Brazil.
All animals underwent a washout period that lasted 1 month before treatment, during which water was administered daily by gavage. To guarantee that the SHR strain developed the expected hypertension and the Wistar–Kyoto strain was normotensive, systolic blood pressure was assessed weekly using plethysmography during the washout period and through the end of the study.13 The washout period used in this study was important for the animals to adapt to the manipulation procedures (gavage and plethysmography), and it was necessary to avoid acute hypertensive episodes caused by the stress of the experiment.
At 4 months of age, animals were assigned to experimental groups. Wistar–Kyoto rats were randomly assigned to three groups: control Group (C, n = 10), which received distilled water; control plus dutasteride Group (C + D, n = 10), which received 0.5 mg kg−1 dutasteride15 (Dastene 0.5 mg, Aché, Indaiatuba, SP, Brazil); and control plus finasteride Group (C + F, n = 10), which received 5 mg kg−1 finasteride16 (Finasterida 5 mg, Eurofarma, São Paulo, SP, Brazil). SHRs were also randomly divided into three similar groups: BPH Group (H, n = 10), which received distilled water; BPH plus dutasteride Group (H + D, n = 10), which received dutasteride; and BPH plus finasteride Group (H + F, n = 10), which received finasteride. All ten animals in each group were used for the analyses. All drugs were administered orally (diluted to the same final volume) for 40 consecutive days.
After the experimental period, animals were euthanized by an overdose of sodium thiopental (Thiopentax 1g, Cristália, Itapira, SP, Brazil) for anesthesia. Following anesthesia, the ventral prostate of each animal was collected and processed to verify the development of the morphological modifications in SHR that are compatible with BPH.
The penises were collected and the skin-denuded middle part of the penile shaft and prostatic fragments were fixed in 4% buffered formaldehyde solution and processed for paraffin embedding. Randomly orientated prostate sections and penile cross-sections (5 mm thick) were obtained and used for histomorphometric evaluations.
Evaluations
Prostate sections were stained with hematoxylin and eosin, and images were captured at ×600 and analyzed to obtain the prostatic epithelium height, as described elsewhere.17,18
The areas of the penis and corpus cavernosum with and without its tunica albuginea were evaluated in hematoxylin and eosin-stained sections. For this purpose, images were captured at ×20 using a digital camera (Axiocam 506 color, Carl Zeiss, Jena, Germany) coupled with a stereomicroscope (Discovery V8, Carl Zeiss). These parameters were measured using the “free hand” tool of the ImageJ software (version 1.45s, National Institutes of Health, Bethesda, MD, USA), and the areas were expressed in square millimeters. The area of the tunica albuginea was estimated by the difference between the area of the corpus cavernosum with and without the tunica albuginea.
To obtain smooth muscle, connective tissue, and sinusoidal space surface densities (Sv), images of Masson's trichrome-stained sections were captured at ×400. To assess the elastic fiber Sv, Weigert's resorcin fuchsin staining was used, and images were captured at ×600 using a digital camera (DP70, Olympus, Tokyo, Japan) that was coupled to a microscope (BX51, Olympus).
The Sv of each structure was measured using the point counting method.19 Briefly, a 100-point grid was superimposed over the images using ImageJ software, and each structure touched by a point was counted as smooth muscle, connective tissue, sinusoidal space, or elastic fiber. The results, expressed as a percentage, were calculated after measuring five different fields from five nonserial sections, for a total of 25 images (or 2500 points) analyzed for each animal.
Picrosirius red-stained sections were observed under polarized light at ×400 to differentiate collagen types III (green) and I (red/orange).20
Statistical analysis
One-way analysis of variance (ANOVA) with Bonferroni's posttest was used to compare mean values. P < 0.05 was used to denote statistical significance. All analyses were performed using GraphPad Prism software (version 5.0, San Diego, CA, USA).
RESULTS
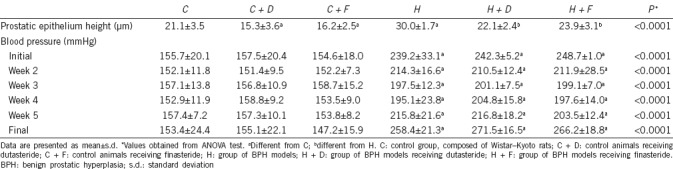
The prostatic epithelium height in Group H was 42.2% higher than that of Group C (P < 0.0001). The use of dutasteride or finasteride restored normal prostatic epithelium height. All SHRs (Groups H, H + D, and H + F) presented with augmented systolic blood pressure throughout the experimental period. These data confirm that our experimental model was that of a hypertensive animal that develops prostatic modifications consistent with BPH and the 5AR inhibitor treatment efficacy (Table 1).
Table 1.
Data of prostatic epithelium and blood pressure at the beginning and in the end of the experiment, confirming the experimental model
Control groups that received dutasteride (C + D) or finasteride (C + F) showed a reduced cross-sectional penile area (39.4% and 40.1%, respectively) compared to Group C. However, this difference was not observed in Groups H + D and H + F (Figure 1). The area of the corpus cavernosum, with and without the tunica albuginea, and the area of the tunica albuginea had similar results, with marked reductions in Groups C + D and C + F (all P < 0.0001) compared to that of Group H. There was no difference among Groups H, H + D, and H + F (all P < 0.05). These data are presented in Table 2.
Figure 1.
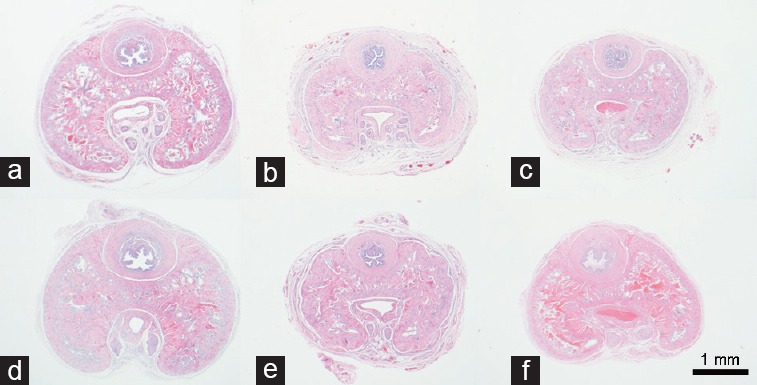
Penile cross-section from control and BPH rats treated with dutasteride or finasteride, and stained using hematoxylin and eosin and captured at ×20. Note that Groups C + D and C + F have reduced cross-sectional penile area when compared to Group C. (a) Control group, composed of Wistar–Kyoto rats (C); (b) control animals receiving dutasteride (C + D); (c) control animals receiving finasteride (C + F); (d) group of BPH models (H); (e) group of BPH models receiving dutasteride (H + D); (f) group of BPH models receiving finasteride (H + F). Scale bars=1 mm. BPH: benign prostatic hyperplasia.
Table 2.
Morphometric data of control and benign prostatic hyperplasia rats with dutasteride or finasteride treatment
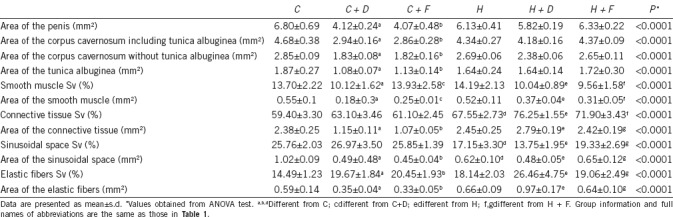
Smooth muscle Sv in the corpus cavernosum of Group C + D was 26.1% lower than that of Group C (P = 0.0016), and no difference was found between Group C + F and Group C (P = 0.8512). In addition, Groups H + D and H + F showed reductions of 29.2% and 32.6%, respectively, in smooth muscle Sv compared to Group H (all P = 0.0002). For connective tissue Sv, Group H had a 13.7% increase compared to Group C (P < 0.0001). In addition, Groups H + D and H + F had an increase in connective tissue Sv of 12.9% and 6.4%, respectively, compared to Group H (all P = 0.0002). Compared to Group C, the sinusoidal space Sv was reduced by 33.4% in Group H (P < 0.0001), while Group H + D showed similar results as Group H (P < 0.05), and Group H + F had increased sinusoidal space Sv compared to Group H + D (P = 0.0003). Figure 2 illustrates the results for smooth muscle, connective tissue, and sinusoidal space Sv.
Figure 2.
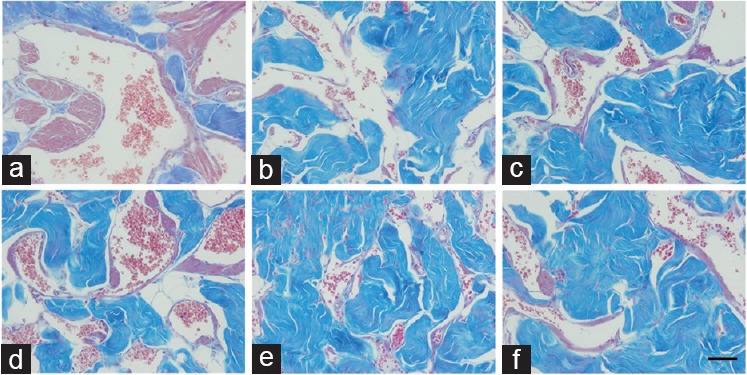
Corpus cavernosum of control and BPH rats treated with dutasteride or finasteride, and stained using Masson's trichrome and captured at ×400. The smooth muscle was reduced in Group C + D (compared to Group C) and in Groups H + D and H + F (compared to Group H). Group H shows more connective tissue (but less sinusoidal space) compared to Group C, and Group H + D has more connective tissue compared to Group H. (a) Control group, composed of Wistar–Kyoto rats (C); (b) control animals receiving dutasteride (C + D); (c) control animals receiving finasteride (C + F); (d) group of BPH models (H); (e) group of BPH models receiving dutasteride (H + D); (f) group of BPH models receiving finasteride (H + F). Scale bars=50 μm. BPH: benign prostatic hyperplasia.
Although there was no difference in elastic fiber Sv between Groups C and H, control animals that received any treatment had an increased elastic fiber Sv. Group C + D had a 35.7% increase and Group C + F had a 41.1% increase in elastic fiber Sv compared to Group C (all P < 0.0001). Group H + D showed an increase of 45.9% in elastic fiber Sv compared to Group H (P = 0.0004). This is illustrated in Figure 3. All histomorphometric data are presented in Table 2.
Figure 3.
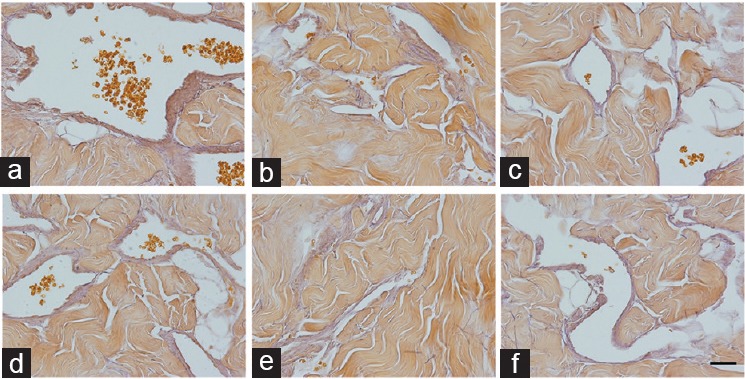
Corpus cavernosum from control and BPH rats treated with dutasteride or finasteride, and stained using Weigert's resorcin fuchsin and captured at ×600. Groups C + D and C + F show increased amounts of elastic fibers (compared to Group C), while Group H + D shows increased amounts compared to Group H. (a) Control group, composed of Wistar–Kyoto rats (C); (b) control animals receiving dutasteride (C + D); (c) control animals receiving finasteride (C + F); (d) group of BPH models (H); (e) group of BPH models receiving dutasteride (H + D); (f) group of BPH models receiving finasteride (H + F). Scale bars=25 μm. BPH: benign prostatic hyperplasia.
When analyzing the collagen type that was predominant in the corpus cavernosum, all animals showed a predominance of Type I collagen (reddish appearance), with no difference among the groups (Figure 4).
Figure 4.
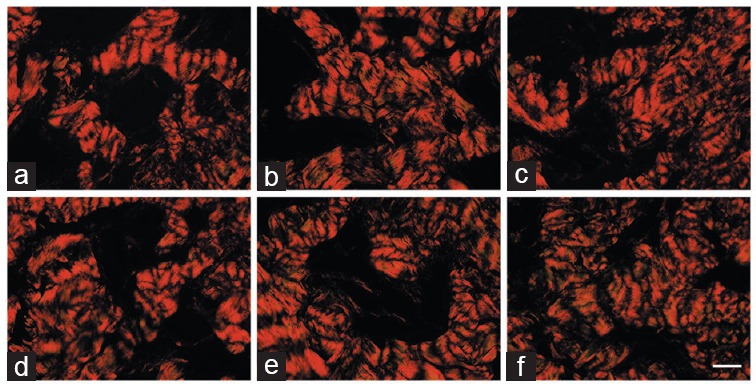
Corpus cavernosum of control and BPH rats treated with dutasteride or finasteride, and stained using picrosirius red. Samples were observed under polarized light, and images captured at ×400. These images show that all groups had a predominance of type I collagen. (a) Control group, composed of Wistar–Kyoto rats (C); (b) control animals receiving dutasteride (C + D); (c) control animals receiving finasteride (C + F); (d) group of BPH models (H); (e) group of BPH models receiving dutasteride (H + D); (f) group of BPH models receiving finasteride (H + F). Scale bars=50 μm. BPH: benign prostatic hyperplasia.
DISCUSSION
Dutasteride and finasteride are drugs that are routinely prescribed for BPH treatment. However, adverse effects of dutasteride and finasteride, such as reduced libido, ejaculatory disorders, infertility, and ED, have been associated with these treatments.21,22,23,24,25,26,27 Some patients treated with 5AR inhibitors showed persistent adverse effects, even after discontinuing treatment, which is of major concern.28
Penile morphology (mainly the corpus cavernosum structure) is related to erectile function. Costa et al.29 showed that men with ED had altered proportions of the extracellular matrix components in their corpus cavernosum. Here, we observed penile morphological alterations in some parameters of animals treated with 5AR inhibitors. This was more clearly observed in the BPH Groups (H, H + D, and H + F). Thus, it is reasonable to assume that these alterations, induced by finasteride and dutasteride, may be related to ED.
Penile morphology can be altered in several situations.30,31,32 For example, testosterone depletion promotes drastic morphological alterations; however, after a period of hormonal replacement therapy, the corpus cavernosum almost completely recovers its normal morphology.33
Smooth muscle tissue is the major contractile component in the corpus cavernosum, and as these muscle cells relax, blood fills the sinusoidal space to start the penile erection.34,35 The reduction in the amount of smooth muscle in the corpus cavernosum is commonly associated with ED in rodent models. This was observed by our group under multiple experimental conditions such as chronic stress,19 anabolic steroid abuse,36 and testosterone depletion.33 This latter model (testosterone depletion) may be comparable in some ways to the animals used in this study. In Groups C + D, C + F, H + D, and H + F, in which the conversion of the testosterone to its active metabolite was inhibited, the results for smooth muscle density were similar to those observed by Miranda et al.33 Thus, it is possible that testosterone (and DHT) may play an important role in maintaining normal amounts of cavernosal smooth muscle.
The reduction in sinusoidal space density found in SHR animals was previously described by Felix-Patricio et al.13 and it may be a consequence of decreased intracavernosal pressure. This decreased intracavernosal pressure was demonstrated in SHR animals by other authors.34 In addition, the reduction in sinusoids space may cause the inability to compress the deep circumflex veins against the tunica albuginea, and this may result in venous leak and low intracavernosal pressure.33 All groups treated with 5AR inhibitors showed similar values of sinusoidal space density compared to its strain control (i.e., Groups C + D and C + F were similar to Group C). This indicates that the drugs do not interfere significantly in the sinusoidal space, and there was a slight increase in the sinusoidal space density in Group H + F. Although Group H + F did not have statistically significant differences in sinusoidal space density compared to Group H, a difference was observed when it was compared to Group H + D, indicating that finasteride may have some benefit over dutasteride regarding sinusoidal space density.
Group H showed an increase in the amount of connective tissue, which is in agreement with previously reported findings.13 This may reflect penile fibrosis, which is commonly related to aging, diabetes mellitus, cavernous nerve damage, chronic stress, and/or androgen deprivation.19,37 The SHR animals that received 5AR inhibitors showed even higher amounts of cavernous connective tissue. This may be a direct effect of DHT suppression and it warrants further investigation.
The role of elastic fibers in the erection process is not clear. Costa et al.29 observed a reduction in the amount of these fibers in the corpus cavernosum in men with severe ED. This reduction was also observed in some experimental models of ED. For example, a reduction in the amount of elastic fibers was observed in the penis of diabetic rats.38 However, paradoxically, some experimental studies showed an increased amount of elastic fibers in rats with (supposed) ED.39 Few studies have investigated elastic fibers in corpus cavernosum, and there is not much information about the relationship between elastic fibers and erection. In our study, the use of 5AR inhibitors altered the normal amount of elastic fibers (both in controls and in Group H animals). We believe that any alteration of the normal corpus cavernosum morphology may affect normal penile physiology, and we see these corpus cavernosum morphological alterations as a negative aspect that should be investigated further.
Modifications in the proportion of the corpus cavernosum components were observed in the present study, which suggests that time after treatment is completed is necessary for a return to a normal penile morphology. Thus, the altered penile morphology observed in the groups treated with 5AR inhibitors can be returned to normal after a washout period without dutasteride or finasteride exposure. Taking into account that normal penile morphology is necessary for a normal erection, the results of the present study may help explain the persistence of adverse effects in erectile function after discontinuing treatment with 5AR inhibitors that is observed in some men.
How the corpus cavernosum reacts after discontinuing treatment with dutasteride or finasteride is unknown. It is also not clear if a completely normal penile architecture can be achieved after the washout period. In addition, it is not known how long it takes to restore normal morphology. Thus, future studies are warranted to evaluate the corpus cavernosum after discontinuing treatment with 5AR inhibitors.
The morphological alterations observed in this study were present in either the SHRs (models of BPH) or in control animals. While the SHRs were used to simulate patients undergoing treatment for BPH, control groups are similar to patients without BPH but who are using dutasteride or finasteride, such as patients undergoing androgenic alopecia treatment. In these patients, ED is also observed and it is related to 5AR inhibitor treatment. The present study showed that, in the rats that correspond to this group of patients (C + F and C + D), there were changes in penile morphology that explained clinically observed ED.
The animal model used in these experiments is considered to be the most suitable for BPH studies.40,41 We confirmed the previously described prostatic alterations in our SHRs. However, this strain of rats presents systemic arterial hypertension, and penile morphological alterations and predisposition toward ED were previously described in this model13,34 and confirmed in this study. Thus, it is not possible to confirm that the alterations observed in Group H were the result of prostatic alterations, or a previous described characteristic of the SHR strain. However, this does not discount the main purpose of the study, which was to examine penile alterations related to 5AR inhibitor treatments. Thus, both 5AR inhibitors negatively affected the corpus cavernosum morphology in the BPH model.
The probable mechanism involved in the observed morphological modifications is DHT depletion in penile tissues. The penis is an androgen-dependent organ, and a decrease in masculine hormones leads to morphological and functional penile prejudice.25,42 Further, DHT is a potent activator of the enzyme nitric oxide synthase and this enzyme is involved in one of the main mechanisms of smooth muscle relaxation, which ultimately results in penile erection. Thus, the reduction of DHT, caused by the 5AR inhibitor treatment, decreases the nitric oxide relaxation response, impairing normal penile erections.9 Because testosterone levels are increased in individuals taking 5AR inhibitors,43 and testosterone is not being converted to DHT, the alterations observed in penile morphology are justified.
Among the 5AR inhibitors used in this study, dutasteride had a higher potency, because it inhibited both isoforms of the enzyme 5AR, while finasteride only inhibited the type II isoform.21 This may explain why dutasteride promoted more prominent alterations in penile morphology compared to finasteride-treated animals. Further, this characteristic reinforces the postulated mechanism that the observed response is a result of low DHT levels in penile tissues.
Thus, we suggest that finasteride should be preferred instead of dutasteride when prescribing 5AR inhibitors for patients in whom ED is an important issue. However, other classes of drugs approved for BPH treatment (such as alpha-1-adrenergic blockers) may be selected instead of 5AR inhibitors. Future studies are necessary to investigate if alpha-1-adrenergic blockers may also interfere with the normal penile morphology.
The possibility of recovering normal penile morphology after discontinuing these drugs and whether either of the drugs is associated with a faster or more complete return to a normal penile morphology remains unknown. Further, the dose of these drugs varies, especially for patients taking androgenic alopecia treatment; thus, future studies comparing different doses will be important.
The present study used the rat as an animal model, which is a limitation because the results may not reflect what occurs in other species. This model is widely used because it has been shown in different clinical situations that rat penile morphology is altered in a similar manner as in humans.9,13,33,44 However, the results obtained in this model should not be directly translated to men.
In conclusion, we have shown that both 5AR inhibitors (dutasteride and finasteride) promote penile morphological modifications in control and BPH rat models although these modifications were more prominent in the BPH animal model than in controls. Dutasteride was the drug that most affected the corpus cavernosum in this rodent model.
AUTHOR CONTRIBUTIONS
MHADS, WSC, FJBS, and DBDS contributed to the research design. MHADS performed the experiments and data acquisition. MHADS and DBDS performed the analysis and interpretation of the data and drafted the manuscript. WSC, FJBS and DBDS revised the manuscript. All authors read and approved the final version of the manuscript.
COMPETING INTERESTS
All authors declared no competing interests.
ACKNOWLEDGMENTS
This study was supported by grants from the Foundation for Research Support of Rio de Janeiro (FAPERJ), the Coordination for the Improvement of Postgraduate Students (CAPES), and the National Council for Scientific and Technological Development (CNPq). These foundations had no involvement in the study design, data collection, analysis, and interpretation, drafting the manuscript and in the decision to submit for publication.
REFERENCES
- 1.Egan KB. The epidemiology of benign prostatic hyperplasia associated with lower urinary tract symptoms: prevalence and incident rates. Urol Clin North Am. 2016;43:289–97. doi: 10.1016/j.ucl.2016.04.001. [DOI] [PubMed] [Google Scholar]
- 2.Jarvis TR, Chughtai B, Kaplan SA. Testosterone and benign prostatic hyperplasia. Asian J Androl. 2015;17:212–6. doi: 10.4103/1008-682X.140966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Madersbacher S, Alivizatos G, Nordling J, Sanz CR, Emberton M, et al. EAU 2004 guidelines on assessment, therapy and follow-up of men with lower urinary tract symptoms suggestive of benign prostatic obstruction (BPH guidelines) Eur Urol. 2004;46:547–54. doi: 10.1016/j.eururo.2004.07.016. [DOI] [PubMed] [Google Scholar]
- 4.McVary KT, Roehrborn CG, Avins AL, Barry MJ, Bruskewitz RC, et al. Update on AUA guideline on the management of benign prostatic hyperplasia. J Urol. 2011;185:1793–803. doi: 10.1016/j.juro.2011.01.074. [DOI] [PubMed] [Google Scholar]
- 5.Nickel JC, Gilling P, Tammela TL, Morrill B, Wilson TH, et al. Comparison of dutasteride and finasteride for treating benign prostatic hyperplasia: the Enlarged Prostate International Comparator Study (EPICS) BJU Int. 2011;108:388–94. doi: 10.1111/j.1464-410X.2011.10195.x. [DOI] [PubMed] [Google Scholar]
- 6.Erdemir F, Harbin A, Hellstrom WJ. 5-alpha reductase inhibitors and erectile dysfunction: the connection. J Sex Med. 2008;5:2917–24. doi: 10.1111/j.1743-6109.2008.01001.x. [DOI] [PubMed] [Google Scholar]
- 7.Traish AM, Hassani J, Guay AT, Zitzmann M, Hansen ML. Adverse side effects of 5alpha-reductase inhibitors therapy: persistent diminished libido and erectile dysfunction and depression in a subset of patients. J Sex Med. 2011;8:872–84. doi: 10.1111/j.1743-6109.2010.02157.x. [DOI] [PubMed] [Google Scholar]
- 8.Li H, Gao T, Wang R. The role of the sexual partner in managing erectile dysfunction. Nat Rev Urol. 2016;13:168–77. doi: 10.1038/nrurol.2015.315. [DOI] [PubMed] [Google Scholar]
- 9.Pinsky MR, Gur S, Tracey AJ, Harbin A, Hellstrom WJ. The effects of chronic 5-alpha-reductase inhibitor (dutasteride) treatment on rat erectile function. J Sex Med. 2011;8:3066–74. doi: 10.1111/j.1743-6109.2011.02425.x. [DOI] [PubMed] [Google Scholar]
- 10.Zhang MG, Wu W, Zhang CM, Wang XJ, Gao PJ, et al. Effects of oral finasteride on erectile function in a rat model. J Sex Med. 2012;9:1328–36. doi: 10.1111/j.1743-6109.2012.02661.x. [DOI] [PubMed] [Google Scholar]
- 11.Okamoto K, Aoki K. Development of a strain of spontaneously hypertensive rats. Jpn Circ J. 1963;27:282–93. doi: 10.1253/jcj.27.282. [DOI] [PubMed] [Google Scholar]
- 12.Bechara GR, de Souza DB, Simoes M, Felix-Patricio B, Medeiros JL, Jr, et al. Testicular morphology and spermatozoid parameters in spontaneously hypertensive rats treated with enalapril. J Urol. 2015;194:1498–503. doi: 10.1016/j.juro.2015.06.073. [DOI] [PubMed] [Google Scholar]
- 13.Felix-Patricio B, Medeiros JL, Jr, De Souza DB, Costa WS, Sampaio FJ. Penile histomorphometrical evaluation in hypertensive rats treated with sildenafil or enalapril alone or in combination: a comparison with normotensive and untreated hypertensive rats. J Sex Med. 2015;12:39–47. doi: 10.1111/jsm.12750. [DOI] [PubMed] [Google Scholar]
- 14.Lujan M, Ferruelo A, Paez A, Moreno A, Berenguer A. Prostate apoptosis after doxazosin treatment in the spontaneous hypertensive rat model. BJU Int. 2004;93:410–4. doi: 10.1111/j.1464-410x.2003.04627.x. [DOI] [PubMed] [Google Scholar]
- 15.Wang D, Zha X, Nagase K, Akino H, Muramatsu I, et al. Effects of the 5α-reductase inhibitor dutasteride on rat prostate α1A-adrenergic receptor and its mediated contractility. Urology. 2015;85:704. e9–14. doi: 10.1016/j.urology.2014.12.002. [DOI] [PubMed] [Google Scholar]
- 16.Garcia PV, Barbieri MF, Perobelli JE, Consonni SR, Mesquita Sde F, et al. Morphometric-stereological and functional epididymal alterations and a decrease in fertility in rats treated with finasteride and after a 30-day post-treatment recovery period. Fertil Steril. 2012;97:1444–51. doi: 10.1016/j.fertnstert.2012.03.025. [DOI] [PubMed] [Google Scholar]
- 17.Furriel A, Campos-Silva P, Silva PC, Costa WS, Sampaio FJ, et al. Diets rich in saturated and polyunsaturated fatty acids induce morphological alterations in the rat ventral prostate. PLoS One. 2014;9:e102876. doi: 10.1371/journal.pone.0102876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vargas RA, Oliveira LP, Frankenfeld S, Souza DB, Costa WS, et al. The prostate after administration of anabolic androgenic steroids: a morphometrical study in rats. Int Braz J Urol. 2013;39:675–82. doi: 10.1590/S1677-5538.IBJU.2013.05.10. [DOI] [PubMed] [Google Scholar]
- 19.de Souza DB, Silva D, Cortez CM, Costa WS, Sampaio FJ. Effects of chronic stress on penile corpus cavernosum of rats. J Androl. 2012;33:735–9. doi: 10.2164/jandrol.111.014225. [DOI] [PubMed] [Google Scholar]
- 20.Montes GS. Structural biology of the fibres of the collagenous and elastic systems. Cell Biol Int. 1996;20:15–27. doi: 10.1006/cbir.1996.0004. [DOI] [PubMed] [Google Scholar]
- 21.Alcantara-Montero A, Brenes-Bermudez FJ. Finasteride or dutasteride for the pharmacological treatment for male lower urinary tract symptoms due to benign prostatic hyperplasia? Actas Urol Esp. 2016;40:268–9. doi: 10.1016/j.acuro.2015.12.006. [DOI] [PubMed] [Google Scholar]
- 22.Boyapati A, Sinclair R. Combination therapy with finasteride and low-dose dutasteride in the treatment of androgenetic alopecia. Australas J Dermatol. 2013;54:49–51. doi: 10.1111/j.1440-0960.2012.00909.x. [DOI] [PubMed] [Google Scholar]
- 23.Favilla V, Russo GI, Privitera S, Castelli T, Giardina R, et al. Impact of combination therapy 5-alpha reductase inhibitors (5-ARI) plus alpha-blockers (AB) on erectile dysfunction and decrease of libido in patients with LUTS/BPH: a systematic review with meta-analysis. Aging Male. 2016;19:175–81. doi: 10.1080/13685538.2016.1195361. [DOI] [PubMed] [Google Scholar]
- 24.Hirshburg JM, Kelsey PA, Therrien CA, Gavino AC, Reichenberg JS. Adverse effects and safety of 5-alpha reductase inhibitors (finasteride, dutasteride): a systematic review. J Clin Aesthet Dermatol. 2016;9:56–62. [PMC free article] [PubMed] [Google Scholar]
- 25.Liu L, Zhao S, Li F, Li E, Kang R, et al. Effect of 5alpha-reductase inhibitors on sexual function: a meta-analysis and systematic review of randomized controlled trials. J Sex Med. 2016;13:1297–310. doi: 10.1016/j.jsxm.2016.07.006. [DOI] [PubMed] [Google Scholar]
- 26.Shanshanwal SJ, Adwani G, Dandale AL, Dhurat RS. Case of unilateral temporal triangular alopecia. Indian Dermatol Online J. 2017;8:161. doi: 10.4103/2229-5178.202276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tsunemi Y, Irisawa R, Yoshiie H, Brotherton B, Ito H, et al. Long-term safety and efficacy of dutasteride in the treatment of male patients with androgenetic alopecia. J Dermatol. 2016;43:1051–8. doi: 10.1111/1346-8138.13310. [DOI] [PubMed] [Google Scholar]
- 28.Hagberg KW, Divan HA, Persson R, Nickel JC, Jick SS. Risk of erectile dysfunction associated with use of 5-alpha reductase inhibitors for benign prostatic hyperplasia or alopecia: population based studies using the Clinical Practice Research Datalink. BMJ. 2016;354:i4823. doi: 10.1136/bmj.i4823. [DOI] [PubMed] [Google Scholar]
- 29.Costa WS, Carrerete FB, Horta WG, Sampaio FJ. Comparative analysis of the penis corpora cavernosa in controls and patients with erectile dysfunction. BJU Int. 2006;97:567–9. doi: 10.1111/j.1464-410X.2005.05917.x. [DOI] [PubMed] [Google Scholar]
- 30.Pereira VA, Abidu-Figueiredo M, Pereira-Sampaio MA, Chagas MA, Costa WS, et al. Sinusoidal constriction and vascular hypertrophy in the diabetes-induced rabbit penis. Int Braz J Urol. 2013;39:424–31. doi: 10.1590/S1677-5538.IBJU.2013.03.17. [DOI] [PubMed] [Google Scholar]
- 31.Abidu-Figueiredo M, Ribeiro IC, Chagas MA, Cardoso LE, Costa WS, et al. The penis in diabetes: structural analysis of connective tissue and smooth muscle alterations in a rabbit model. BJU Int. 2011;108:400–4. doi: 10.1111/j.1464-410X.2010.09944.x. [DOI] [PubMed] [Google Scholar]
- 32.Okabe H, Hale TM, Kumon H, Heaton JP, Adams MA. The penis is not protected – In hypertension there are vascular changes in the penis which are similar to those in other vascular beds. Int J Impot Res. 1999;11:133–40. doi: 10.1038/sj.ijir.3900394. [DOI] [PubMed] [Google Scholar]
- 33.Miranda AF, Gallo CB, De Souza DB, Costa WS, Sampaio FJ. Effects of castration and late hormonal replacement in the structure of rat corpora cavernosa. J Androl. 2012;33:1224–32. doi: 10.2164/jandrol.112.017012. [DOI] [PubMed] [Google Scholar]
- 34.Behr-Roussel D, Chamiot-Clerc P, Bernabe J, Mevel K, Alexandre L, et al. Erectile dysfunction in spontaneously hypertensive rats: pathophysiological mechanisms. Am J Physiol Regul Integr Comp Physiol. 2003;284:R682–8. doi: 10.1152/ajpregu.00349.2002. [DOI] [PubMed] [Google Scholar]
- 35.Gratzke C, Angulo J, Chitaley K, Dai YT, Kim NN, et al. Anatomy, physiology, and pathophysiology of erectile dysfunction. J Sex Med. 2010;7:445–75. doi: 10.1111/j.1743-6109.2009.01624.x. [DOI] [PubMed] [Google Scholar]
- 36.Sena Ade S, Vargas RA, Souza DB, Costa WS, Sampaio FJ. Morphometric study of the corpus cavernosum after anabolic androgenic steroid administration in pubertal and adult rats. Acta Cir Bras. 2015;30:478–83. doi: 10.1590/S0102-865020150070000005. [DOI] [PubMed] [Google Scholar]
- 37.El-Sakka AI, Yassin AA. Amelioration of penile fibrosis: myth or reality. J Androl. 2010;31:324–35. doi: 10.2164/jandrol.109.008730. [DOI] [PubMed] [Google Scholar]
- 38.Mostafa ME, Senbel AM, Mostafa T. Effect of chronic low-dose tadalafil on penile cavernous tissues in diabetic rats. Urology. 2013;81:1253–9. doi: 10.1016/j.urology.2012.12.068. [DOI] [PubMed] [Google Scholar]
- 39.Medeiros JL, Jr, Costa WS, Felix-Patricio B, Sampaio FJ, Cardoso LE. Protective effects of nutritional supplementation with arginine and glutamine on the penis of rats submitted to pelvic radiation. Andrology. 2014;2:943–50. doi: 10.1111/andr.134. [DOI] [PubMed] [Google Scholar]
- 40.Shimizu S, Shimizu T, Tsounapi P, Higashi Y, Martin DT, et al. Effect of silodosin, an alpha1a-adrenoceptor antagonist, on ventral prostatic hyperplasia in the spontaneously hypertensive rat. PLoS One. 2015;10:e0133798. doi: 10.1371/journal.pone.0133798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Saito M, Shimizu S, Ohmasa F, Oikawa R, Tsounapi P, et al. Characterization of silodosin and naftopidil in the treatment of bladder dysfunction in the spontaneously hypertensive rat. Neurourol Urodyn. 2013;32:393–8. doi: 10.1002/nau.22297. [DOI] [PubMed] [Google Scholar]
- 42.Hwang I, Lee HS, Yu HS, Kim ME, Lee JS, et al. Testosterone modulates endothelial progenitor cells in rat corpus cavernosum. BJU Int. 2016;117:976–81. doi: 10.1111/bju.13438. [DOI] [PubMed] [Google Scholar]
- 43.Uygur MC, Arik AI, Altug U, Erol D. Effects of the 5 alpha-reductase inhibitor finasteride on serum levels of gonadal, adrenal, and hypophyseal hormones and its clinical significance: a prospective clinical study. Steroids. 1998;63:208–13. doi: 10.1016/s0039-128x(98)00005-1. [DOI] [PubMed] [Google Scholar]
- 44.Hannan JL, Blaser MC, Pang JJ, Adams SM, Pang SC, et al. Impact of hypertension, aging, and antihypertensive treatment on the morphology of the pudendal artery. J Sex Med. 2011;8:1027–38. doi: 10.1111/j.1743-6109.2010.02191.x. [DOI] [PubMed] [Google Scholar]


