Abstract
Based on our previous docking model, in order to carry out more rational drug design, totally 82 vinyl sulfonyl fluorides, including some 2-(hetero)arylethenesulfonyl fluorides and 1,3-dienylsulfonyl fluorides derivatives as potential human telomerase inhibitors were designed and synthesised. The in vitro anticancer activity assay showed that compound 57 (1E,3E)-4-(4-((E)-2-(fluorosulfonyl)vinyl)phenyl)buta-1,3-diene-1-sulfonyl fluoride exhibited high activity against A375 and MDA-MB-231 cell lines with IC50 1.58 and 3.22 µM, but it manifested obvious un-toxic effect against GES-1 and L-02 with IC50 with IC50 values less than 2.00 mM. By the modified TRAP assay, some compounds including 57 exhibited potent inhibitory activities against telomerase with IC50 values of 0.71–0.97 µM.
Keywords: Ethenesulfonyl fluoride, selective anticancer activity, telomerase, inhibitor
1. Introduction
Telomerase plays an important role in chromosomal integrity. About 80–90% of various cancer cells have detectable telomerase activity, so, it is considered as a potential anticancer target1–4. In the past decade, based on searching for telomerase inhibitors, different approaches have been designed containing G-quadruplex stabilising inhibitors5,6, 2′-O-MeRNA oligonucleotides and peptide nucleic acids targeting telomerase RNA7, ligands targeting telomeric DNA8. But, no compounds that inhibit telomerase activity have reached clinical trials so far9.
Among the whole telomerase, human telomerase reverse transcriptase (TERT) is a key component of telomerase. The expression of TERT is a rate-limiting factor for telomerase activity; also, most human somatic cells do not show detectable telomerase activity due to the lack of TERT. Therefore, TERT is an important target for the drug discovery10–13. Some hTERT inhibitors with good anticancer activity, including BIBR153214 and isothiazolone derivatives have been discovered15–19. However, most of them exhibited potential toxicities against somatic cells, there are no inhibitors targeting hTERT have been approved so far. Therefore, the design of potent hTERT inhibitors with high selectivity against cancer cells and somatic cells is a very important and immediate need.
As we know, chemical environment of the warhead plays a key role in inhibitor design since it enables the tuning of its reactivity and thereby increasing its selectivity and stability20,21. Among them, vinyl sulfone, which is a dominant electrophilic trap containing unsaturated sulfone Michael acceptor, which could inhibit both the proteasome and cysteine proteases and high selectivity through manipulation of the peptidic portion22. Based on the structure of protein TERT using a LigandFit module, the novel docking model was found. The results showed that LYS 189, GLN 308, and ASP 254 were the key residues for telomerase activity. Focusing on these residues and the volume of the active site of hTERT, the fragment of ethenesulfonyl fluoride could be well aligned with the active site. We, therefore, designed some of the drug-like scaffolds which incorporate the moiety of ethenesulfonyl fluoride derivatives and tested their inhibition activity of telomerase (Figure 1).
Figure 1.
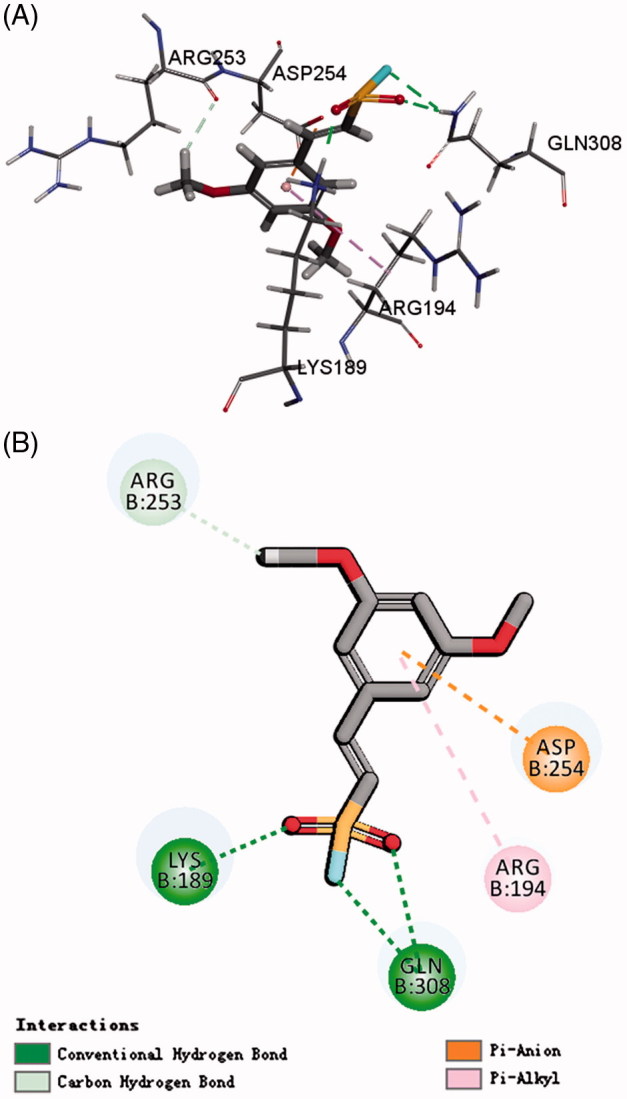
Rational design of target compound. (A) 3D picture of binding was depicted; (B) 2D picture of binding was depicted.
2. Experimental
2.1. Cell proliferation assays
The antiproliferative activities evaluation was conducted by using a modified procedure as described in the literature23. Briefly, target tumour cells were grown to log phase in RPMI 1640 medium supplemented with 10% fetal bovine serum. After diluting to 3 × 104 cells mL−1 with the complete medium, 100 µL of the obtained cell suspension was added to each well of 96-well culture plates. The subsequent incubation was performed at 37 °C, 5% CO2 atmosphere for 24 h before subjecting to antiproliferation assessment. The tested samples at pre-set concentrations were added to six wells with Doxorubicin (AMD) co-assayed as a positive reference. After 48 h of exposure period, 25 µL of PBS containing 2.5 mg·mL−1 of MTT was added to each well. After 4 h, the medium was replaced by 150 µL DMSO to dissolve the purple formazan crystals produced. The absorbance at 570 nm of each well was measured using an ELISA plate reader. The data represented the mean of three experiments in triplicate and were expressed as means ± SD using Student’s test. The IC50 value was defined as the concentration at which 50% of the cells could survive.
2.2. Telomerase activity assays
Thirty-six compounds were tested in a search for inhibitors of telomerase activity using the TRAP-PCR-ELISA assay. In detail, the A375 cells were firstly maintained in RPMI 1640 buffer (Hyclone, Miami, FL), supplemented with 10% fetal bovineserum (GIBCO, New York, NY), streptomycin (0.1 mg/mL) and penicillin (100 IU/mL) at 37 °C in a humidified atmosphere containing 5% CO2. After trypsinisation, 5 × 104 cultured cells in logarithmic growth were seeded into T25 flasks (Corning, New York, NY) and cultured to allow to adherence. The cells were then incubated with Staurosporine (Santa Cruz, Santa Cruz,CA) and the drugs with a series of concentration as 60, 20, 6.67, 2.22, 0.75, 0.25 and 0.082 g/mL, respectively. After 24 h treatment, the cells were harvested by cell scraper orderly followed by washing once with PBS. The cells were lysed in 150 µL RIPA cell lysis buffer (Santa Cruz, Santa Cruz, CA), and incubated on ice for 30 min. The cellular supernatants were obtained via centrifugation at 12,000g for 20 min at 4 °C and stored at –80 °C. The TRAP-PCR-ELISA assay was performed using a telomerase detection kit (Roche, Basel, Switzerland) according to the manufacturer’s protocol. In brief, 2 µL of cell extracts were mixed with 48 µL TRAP reaction mixtures. TRAP primers and Taq polymerase are incubated at 25 °C for 30 min. PCR was then initiated at 94 °C, 120 s for predenaturation and performed using 35 cycles each consisting of 94 °C for 30 s, 50 °C for 30 s, 72 °C for 90 s. Then 20 µL of PCR products were hybridised to a digoxigenin (DIG)-labelled telomeric repeat specific detection probe. And the PCR products were immobilised via the biotin-labelled primer to a streptavidin-coated microtiter plate subsequently. The immobilised DNA fragments were detected with a peroxidase-conjugated anti-DIG antibody and visualised following the addition of the stop regent. The microtitre plate was assessed on TECAN Infinite M200 microplate reader (Mannedorf, Switzerland) at a wavelength of 490 nm, and the final value were presented as mean ± SD23.
2.3. Molecular modelling
The Discovery Studio 2017 was used (Accelrys Software Inc., San Diego, CA). Crystal structure of telomerase TERT (PDB: 3DU6) was used as a template. The active site was defined and sphere of 5 Å was generated around the active site pocket, with the active site pocket of BSAI model using C-DOCKER. The structure of protein, substrate were subjected to energy minimisation using CHARMm forcefield as implemented in DS 2017.
2.4. Statistical analysis
All results are expressed as Mean ± SE. Statistical significance was determined either by the Student’s t-test for comparison between means or one-way analysis of variance with a post-hoc Dunnett’s test.
3. Results and discussion
3.1. Chemistry
In our previous study, 82 structurally diverse vinyl sulfonyl fluorides, including some 2-(hetero)arylethenesulfonyl fluorides and 1,3-dienylsulfonyl fluorides, were synthesised on a decent scale using a general method. Our results of molecular construction revealed that Heck-type reaction had a broad scope for aryl iodides comprising various types of ethenesulfonyl fluoride derivatives. Under mild reaction conditions used simple operations, catalytic amounts of Pd(OAc)2 and stoichiometric amounts of AgTFA affected the transformations of aryl iodides to the corresponding vinyl sulfonyl fluorides with good yields (Figure 2)24.
Figure 2.
Structures of compounds 1–82.
3.2. Anticancer activity
Compounds 1–82 were evaluated for their antiproliferative activities against human melanoma cell A375, human breast cancer cell MDA-MB-231, human hepatoma cell SMMC-7721, human gastric cancer cells SGC-7901 and MGC-803 cell lines. The cells were allowed to proliferate in the presence of tested material for 48 h, and the results were reported with IC50 values. The IC50 values of all tested compounds against five cell lines are summarised in Table 1. From the structure-activity relationships presented, in general, it can be concluded that almost all compounds displayed poor activity against SGC-7901 and MGC-803 cell lines (except for compound 70). Some of the compounds showed moderate activity to MDA-MB-231 cell (such as compounds 1, 5, 6, 11, 12, 18, 22, 35, 69, 70, 71 the IC50s values are between 4.22 and 8.67 µM), among them, compounds 14, 15, 20 and 57 exhibited high activity against MDA-MB-231 with IC50 values at 3.60, 3.64, 3.18 and 3.22 µM, respectively. Compared with MDA-MB-231, the title compound had a certain activity for SMMC-7721 cell, but it is generally weaker than that of MDA-MB-231. One of them, compound 30 is the most potential compound with IC50 value of 2.29 µM, which is comparable with the positive control AMD.
Table 1.
Antiproliferative activity of compounds 1–82 against A375, MDA-MB-231, SMMC-7721, SGC-7901 and MGC-803 cell lines.a
| Compound | IC50 (μM) |
||||
|---|---|---|---|---|---|
| A375 | MDA-MB-231 | SMMC-7721 | SGC-7901 | MGC-803 | |
| 1 | 5.21 ± 0.39 | 8.67 ± 0.61 | -b | – | – |
| 2 | 22.79 ± 0.71 | 58.56 ± 1.78 | 28.53 ± 1.47 | – | 28.58 ± 1.27 |
| 3 | 27.01 ± 0.82 | 31.83 ± 1.44 | – | – | – |
| 4 | 16.90 ± 1.22 | 24.72 ± 2.15 | 73.79 ± 1.70 | – | – |
| 5 | 5.84 ± 0.33 | 8.39 ± 0.89 | 4.05 ± 0.20 | 32.88 ± 1.85 | – |
| 6 | 5.99 ± 0.49 | 9.20 ± 1.27 | 15.86 ± 0.35 | – | 44.19 ± 1.25 |
| 7 | 9.84 ± 0.78 | 12.26 ± 0.64 | 13.34 ± 0.39 | – | 12.91 ± 0.30 |
| 8 | 9.12 ± 0.82 | 17.47 ± 1.77 | 10.72 ± 0.80 | – | 17.21 ± 0.41 |
| 9 | 24.05 ± 0.99 | 36.52 ± 2.56 | 9.04 ± 0.99 | – | 38.64 ± 1.22 |
| 10 | 5.43 ± 0.40 | 29.78 ± 2.31 | 40.10 ± 1.66 | – | 24.93 ± 0.82 |
| 11 | 10.59 ± 0.90 | 5.66 ± 0.28 | 65.77 ± 1.79 | – | – |
| 12 | 3.58 ± 0.11 | 4.25 ± 0.32 | – | – | – |
| 13 | – | – | – | – | – |
| 14 | 49.97 ± 1.78 | 3.60 ± 0.30 | 26.97 ± 1.01 | – | – |
| 15 | 13.06 ± 0.70 | 3.64 ± 0.22 | – | – | – |
| 16 | 21.14 ± 0.87 | 28.47 ± 1.78 | 45.56 ± 1.29 | – | – |
| 17 | 12.06 ± 1.19 | 10.55 ± 0.91 | – | – | – |
| 18 | 3.82 ± 0.31 | 5.24 ± 0.40 | 24.80 ± 1.58 | – | – |
| 19 | 20.13 ± 0.90 | 12.09 ± 1.52 | – | – | – |
| 20 | 10.43 ± 0.82 | 3.18 ± 0.20 | 20.04 ± 1.74 | – | – |
| 21 | 16.60 ± 0.91 | 23.14 ± 2.78 | 11.77 ± 0.60 | 30.76 ± 2.00 | – |
| 22 | 4.14 ± 0.21 | 6.54 ± 0.74 | 26.12 ± 0.73 | – | – |
| 23 | 25.31 ± 1.33 | 33.75 ± 2.89 | – | – | – |
| 24 | 24.49 ± 1.55 | 41.84 ± 2.97 | 52.75 ± 1.44 | – | – |
| 25 | – | – | – | – | – |
| 26 | – | – | – | – | – |
| 27 | 7.83 ± 0.40 | 39.80 ± 2.55 | 50.66 ± 1.82 | – | – |
| 28 | 10.15 ± 0.75 | 20.55 ± 2.11 | 16.70 ± 0.65 | – | – |
| 29 | 14.34 ± 0.91 | 16.92 ± 0.87 | – | – | – |
| 30 | 27.99 ± 1.31 | 25.09 ± 0.99 | 2.29 ± 0.17 | – | – |
| 31 | 3.49 ± 0.20 | 24.08 ± 1.18 | 33.48 ± 0.88 | – | – |
| 32 | 12.28 ± 0.41 | 16.15 ± 0.41 | 27.02 ± 0.90 | – | – |
| 33 | 4.96 ± 0.51 | 19.04 ± 0.65 | 26.20 ± 0.39 | – | 35.88 ± 0.79 |
| 34 | 5.37 ± 0.60 | 23.39 ± 0.88 | 22.99 ± 0.45 | – | – |
| 35 | 7.41 ± 0.77 | 7.41 ± 0.33 | 36.45 ± 0.70 | – | – |
| 36 | 4.01 ± 0.68 | 37.17 ± 1.40 | 28.98 ± 0.78 | – | – |
| 37 | 9.09 ± 0.81 | 21.48 ± 1.29 | 35.11 ± 0.49 | – | – |
| 38 | 40.81 ± 1.39 | 83.15 ± 2.91 | – | – | – |
| 39 | – | – | – | – | – |
| 40 | 6.73 ± 0.50 | 18.34 ± 0.80 | 35.61 ± 0.55 | 51.30 ± 2.55 | – |
| 41 | 14.55 ± 1.20 | 10.57 ± 0.46 | 28.80 ± 0.75 | – | – |
| 42 | – | – | – | – | – |
| 43 | 38.60 ± 0.61 | 43.99 ± 1.20 | 34.48 ± 0.77 | 24.75 ± 0.89 | – |
| 44 | 5.34 ± 0.30 | – | 9.24 ± 0.40 | – | – |
| 45 | 8.61 ± 0.28 | – | 15.59 ± 0.78 | – | – |
| 46 | 1.25 ± 0.07 | – | 5.37 ± 0.11 | – | – |
| 47 | 9.04 ± 0.29 | 10.25 ± 0.44 | 21.15 ± 0.49 | – | – |
| 48 | 27.41 ± 1.50 | 39.70 ± 1.39 | 50.96 ± 1.91 | 49.14 ± 1.39 | – |
| 49 | 32.76 ± 1.44 | 35.23 ± 1.40 | 48.49 ± 1.65 | 49.79 ± 0.47 | – |
| 50 | 20.69 ± 1.39 | 31.56 ± 1.20 | 29.09 ± 1.27 | 26.89 ± 0.56 | – |
| 51 | 42.04 ± 1.60 | 20.87 ± 1.09 | 31.25 ± 1.33 | – | – |
| 52 | 13.29 ± 0.87 | 15.29 ± 0.82 | 24.30 ± 1.19 | – | – |
| 53 | 17.49 ± 0.98 | 28.48 ± 1.14 | 57.32 ± 2.01 | – | – |
| 54 | 17.52 ± 1.52 | 28.51 ± 1.16 | 19.03 ± 0.71 | – | – |
| 55 | 5.87 ± 0.20 | 14.31 ± 1.07 | 34.10 ± 1.45 | – | – |
| 56 | 6.92 ± 0.44 | 12.16 ± 0.92 | 19.31 ± 0.82 | 34.46 ± 1.04 | – |
| 57 | 1.58 ± 0.06 | 3.22 ± 0.18 | – | – | – |
| 58 | – | – | – | – | – |
| 59 | 57.98 ± 1.71 | – | – | – | – |
| 60 | 10.70 ± 0.80 | – | – | – | – |
| 61 | 27.30 ± 1.00 | 26.87 ± 1.20 | 28.20 ± 1.49 | – | 27.06 ± 1.03 |
| 62 | 45.13 ± 1.40 | 86.18 ± 2.44 | – | – | – |
| 63 | – | – | – | – | – |
| 64 | 23.87 ± 1.37 | 38.29 ± 0.90 | – | – | – |
| 65 | 32.68 ± 1.22 | 30.53 ± 1.29 | – | – | – |
| 66 | 8.56 ± 0.49 | 30.29 ± 1.22 | 51.96 ± 1.70 | – | 19.59 ± 1.17 |
| 67 | 8.29 ± 0.42 | 12.07 ± 0.37 | 15.04 ± 0.60 | 50.26 ± 1.17 | 16.13 ± 0.82 |
| 68 | 19.65 ± 1.00 | 21.25 ± 0.80 | – | – | 37.41 ± 1.35 |
| 69 | 8.79 ± 0.61 | 7.95 ± 0.40 | 11.52 ± 0.39 | 36.82 ± 1.25 | 25.09 ± 0.54 |
| 70 | 3.09 ± 0.19 | 4.22 ± 0.20 | 3.00 ± 0.18 | 5.38 ± 0.30 | 3.15 ± 0.27 |
| 71 | 8.65 ± 0.33 | 8.09 ± 0.52 | 23.18 ± 0.78 | – | 20.80 ± 0.79 |
| 72 | 12.98 ± 0.77 | 22.55 ± 1.00 | 16.05 ± 0.91 | – | 44.91 ± 1.13 |
| 73 | 11.16 ± 0.81 | 25.58 ± 0.97 | – | – | 57.70 ± 1.44 |
| 74 | 26.79 ± 1.22 | 34.72 ± 1.20 | – | 31.83 ± 0.82 | 35.31 ± 1.25 |
| 75 | 9.27 ± 0.91 | 21.34 ± 1.04 | 32.07 ± 1.02 | 21.93 ± 0.61 | – |
| 76 | 18.00 ± 1.09 | 36.02 ± 1.20 | – | 50.20 ± 1.79 | – |
| 77 | 13.68 ± 1.29 | 19.44 ± 0.79 | 23.61 ± 1.45 | – | 51.81 ± 0.87 |
| 78 | 26.26 ± 2.35 | 35.22 ± 1.28 | – | – | – |
| 79 | 22.97 ± 1.75 | – | – | – | – |
| 80 | – | – | – | 26.81 ± 1.01 | – |
| 81 | 7.89 ± 0.56 | 27.77 ± 1.32 | 34.92 ± 1.70 | – | – |
| 82 | 24.16 ± 0.60 | 28.67 ± 1.21 | 8.95 ± 0.27 | – | – |
| AMD | 0.52 ± 0.14 | 0.81 ± 0.11 | 1.05 ± 0.09 | 0.92 ± 0.08 | 0.67 ± 0.08 |
The standard deviation (SD) of three time independent tests.
The standard deviation (SD) of three time independent tests.
Based on MDA-MB-231, the further structure–activity relationship was summarised as follows. First, about 2-phenylethenesulfonyl fluoride series, compounds with non-substituted or para-substituted of the benzene ring showed better activity (compounds 1, 5, 6, 11, 12, 14 and 15); For the meta-substituted compounds, strong electron withdrawing group, such as F, CF3, NO2 reflected great contribution to the activity (compounds 18, 20 and 22). Two substitutions containing Cl were beneficial to the activity (compounds 69 and 71). For the R-ethenesulfonyl fluoride, when R is heterocyclic or benzo heterocyclic, it was not beneficial to the activity (compounds 44, 45, 46, 58 and 59). Second, for the series of diene-1-sulfonyl fluoride, most of these compounds had certain activity (compounds 51–56), among them, compound 2-(fluorosulfonyl)vinyl)phenyl)buta-1,3-diene-1-sulfonyl fluoride showed the best activity with IC50 value of 3.22 µM.
It was obvious from the data that most compounds showed good activity against A375 cell (compounds 12, 18, 31, 46, 57 and 70, their IC50s value is around 3.0 µM), among them, compound 46 possessed the highest activity with the IC50 value of 1.25 µM, which is comparable with the positive control AMD. For the phenylethenesulfonyl fluoride series, most of the para-substituted derivatives showed good activity except for –CN and –COOEt substitutions. But, in addition to F substitution, most of the meta-substituted derivatives displayed weak activity. We then investigated the SAR profiles of the multi-substituted group of ethenesulfonyl fluoride, compared to phenylethenesulfonyl fluoride moiety, the kind of diene-1-sulfonyl fluoride moiety could not significantly improve the activity.
3.3. Inhibition assay of human normal cell
In order to determine the selective cancer cell toxicity of some title compounds. We subsequently conducted a proliferative inhibition assay with human normal liver cell (L-02) and gastric mucosa cell (GES-1). As shown in Table 2, all compounds manifested obvious un-toxic effect on GES-1 and L-02 with IC50 from 1.33 to 2.80 mM. The data indicated that the title compounds have good selectivity against tumour cells over somatic cells (Tables 1 and 2).
Table 2.
Selected compounds against normal cells L-02 and GES-1proliferation.a
| Compound | L-02 (IC50, mM) | GES-1 (IC50, mM) |
|---|---|---|
| 1 | 1.97 ± 0.20 | 1.45 ± 0.17 |
| 5 | 1.33 ± 0.15 | 2.00 ± 0.25 |
| 15 | 2.11 ± 0.19 | 2.50 ± 0.22 |
| 25 | 2.45 ± 0.25 | 2.80 ± 0.31 |
| 31 | 1.41 ± 0.10 | 1.65 ± 0.18 |
| 46 | 2.06 ± 0.18 | 1.82 ± 0.11 |
| 57 | 2.01 ± 0.16 | 2.19 ± 0.18 |
| 75 | 2.44 ± 0.22 | 1.89 ± 0.17 |
MTT assays were used for evaluation, and values were expressed as mean IC50 of the triplicate experiment.
3.4. Telomerase activity
Screening results of cell activity showed that most compounds had good activity against A375 cell, due to high expression of telomerase in melanoma cells A375, so, to confirm if the title compounds performed anticancer activity via telomerase inhibition, some selected compounds were evaluated using an extraction from A375 cells. The results are summarised in Table 3. Among them, compounds 5, 31, 36, 57, 46 and 70 showed potent inhibitory activities against telomerase with IC50 values less than 1.5 µM, better than positive control staurosporine with IC50 value 8.67 µM. One of them, compound 5 showed the most potent activity against telomerase with IC50 value of 0.71 µM, which is 12 times as much as the positive control staurosporine.
Table 3.
Some compounds inhibitory activity against telomerase.a
| Compound | IC50 (µM) | Compound | IC50 (µM) |
|---|---|---|---|
| 1 | 2.41 ± 0.51 | 46 | 1.12 ± 0.27 |
| 5 | 0.71 ± 0.28 | 47 | 2.13 ± 0.60 |
| 6 | 2.98 ± 0.59 | 48 | 40.15 ± 1.30 |
| 7 | 3.02 ± 0.44 | 55 | 47.22 ± 1.52 |
| 8 | 4.33 ± 0.77 | 56 | 3.20 ± 0.55 |
| 57 | 0.97 ± 0.12 | ||
| 12 | 2.77 ± 0.58 | 58 | 39.88 ± 1.29 |
| 13 | 38.99 ± 1.29 | 59 | 44.21 ± 2.26 |
| 15 | 1.87 ± 0.41 | 62 | 39.89 ± 1.40 |
| 17 | 10.08 ± 1.13 | 63 | 45.33 ± 1.87 |
| 18 | 1.09 ± 0.11 | 67 | 1.87 ± 0.47 |
| 20 | 4.17 ± 0.39 | 69 | 4.22 ± 0.71 |
| 22 | 2.25 ± 0.30 | 70 | 1.01 ± 0.13 |
| 25 | no | 71 | 6.08 ± 1.30 |
| 31 | 0.81 ± 0.07 | 75 | 4.40 ± 2.00 |
| 36 | 1.44 ± 0.22 | 80 | 49.27 ± 2.55 |
| 39 | 48.76 ± 2.22 | 81 | 2.55 ± 0.40 |
| 42 | no | 82 | 37.88 ± 2.19 |
| 44 | 15.22 ± 1.56 | ||
| Staurosporineb | 8.67 ± 0.18 | Staurosporineb | 8.67 ± 0.18 |
aTelomerase supercoiling activity.
bStaurosporine is reported as a control.
From the structure–activity relationships presented in Table 3, it can be concluded that most compounds had high telomerase activity. Overall, the compounds with low activity against cancer cell expressed the same weak telomerase activity (compounds 25 and 42). There was also a good correlation between the antiproliferative activity and the IC50s of telomerase inhibition activity (compounds 31, 47, 57 and 70).
In order to further analyse the SAR, the 82 compounds were divided into two types, seriers one is R-ethenesulfonyl fluoride skeleton and series two is R1-diene-1-sulfonyl fluoride skeleton. In general, the activity of series one is generally higher than the activity of series two (compounds 5, 15, 18, 31, 36, 46, 47, 58, 67 with 48 and 55). Further SAR analysis was focused on different substitutes of R. For the series one skeleton, when R was a different substitute phenyl、thick ring and heterocyclic ring showed significant effect on the activity against telomerase (compounds 5, 31, 46, 47 and 58). For series two skeleton, the nucleus of different rings had little effect on the activity of telomerase (compounds 48 and 55). The result of enzyme inhibition test showed that title compounds had moderate activity against telomerase. For this reason, it is not certain that TERT is the only protein target, responsible for the biological activity.
4. Conclusions
Inspired by the dual warhead approach, based on our previous TERT model of rational drug design, in this study, 82 sulfonyl fluoride derivatives were designed, synthesised and biologically evaluated as potential telomerase inhibitors. Total 82 compounds were evaluated for their antiproliferative activities against A375, MDA-MB-231, SMMC-7721, SGC-7901 and MGC-803 cell lines. The structure–activity relationships were discussed in depth. The results showed most compounds had good activity against A375 cell with lower toxicity against normal cells in vitro. Moreover, compounds 5, 31 and 57 displayed high inhibitory activity against telomerase with IC50 = 0.71, 0.81 and 0.97 µM, respectively. These results are of help in rational design of more efficient telomerase TERT inhibitors in the future.
Funding Statement
The authors would like to thank the National Natural Science Foundation of China [No. 21272008, 21572003].
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- 1.Stewart SA, Bertuch AA. The role of telomeres and telomerase in cancer research. Cancer Res 2010;70:7365–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shay JW, Wright WE. Telomerase: a target for cancer therapeutics. Cancer Cell 2002;2:257–65. [DOI] [PubMed] [Google Scholar]
- 3.Shay JW, Wright WE. Telomerase therapeutics for cancer: challenges and new directions. Nat Rev Drug Discov 2006;5:577–84. [DOI] [PubMed] [Google Scholar]
- 4.Corey DR. Telomeres and telomerase: from discovery to clinical trials. Chem Biol 2009;16:1219–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bhattacharya S, Chaudhuri P, Jain AK, et al. . Symmetrical bisbenzimidazoles with benzenediyl spacer: the role of the shape of the ligand on the stabilization and structural alterations in telomeric G-quadruplex DNA and telomerase inhibition. Bioconjug Chem 2010;21:1148–59. [DOI] [PubMed] [Google Scholar]
- 6.Paul A, Maji B, Misra SK, et al. . Stabilization and structural alteration of the G-quadruplex DNA made from the human telomeric repeat mediated by Troger's base based novel benzimidazole derivatives. J Med Chem 2012;55:7460–741. [DOI] [PubMed] [Google Scholar]
- 7.Herbert B, Pitts AE, Baker SI, et al. . Inhibition of human telomerase in immortal human cells lead to progressive telomere shortening and cell death. Proc Natl Acad Sci USA 1999;96:14276–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sun D, Thompson B, Cathers BE, et al. . Inhibition human telomerase G-quadruplex-interactive compound. J Med Chem 1997;40:2113–2116. [DOI] [PubMed] [Google Scholar]
- 9.Autexier C, Lue NF. The structure and function of telomerase reverse transcriptase. Annu Rev Biochem 2006;75:493–517. [DOI] [PubMed] [Google Scholar]
- 10.Hahn W, Stewart SA, Brooks MW, et al. . Inhibition for telomerase limits the growth of human cancel cells. Nat Med 1999;5:1164–70. [DOI] [PubMed] [Google Scholar]
- 11.Li Y, Pan G, Chen Y, et al. . Inhibitor of the human telomerase reverse trancriptase (hTERT) gene promoter induces cell apoptosis via a mitochondrial-dependent pathway. Eur J Med Chem 2018;145:370–8. [DOI] [PubMed] [Google Scholar]
- 12.Wang Y, Cheng FX, Yuan XL, et al. . Dihydropyrazole derivatives as telomerase inhibitors: structure-based design, synthesis, SAR and anticancer evaluation in vitro and in vivo. Eur J Med Chem 2016;112:231–51. [DOI] [PubMed] [Google Scholar]
- 13.Chen YY, Wu XQ, Tang WJ, et al. . Novel dihydropyrazole-chromen: design and modulates hTERT inhibition proliferation of MGC-803. Eur J Med Chem 2016;110:65–75. [DOI] [PubMed] [Google Scholar]
- 14.Barma DK, Elayadi A, Falck JR, et al. . Inhibition of telomerase by BIBR 1532 and related analogues. Bioorg Med Chem Lett 2003;13:1333–6. [DOI] [PubMed] [Google Scholar]
- 15.Makhlouf Brahmi M, Portmann C, D'Ambrosio D, et al. . Telomerase inhibitors from cyanobacteria: isolation and synthesis of sulfoquinovosyl diacylglycerols from Microcystis aeruguinosa PCC 7806. Chem Eur J 2013;19:4596–601. [DOI] [PubMed] [Google Scholar]
- 16.Sekaran V, Soares J, Jarstfer MB. Telomere maintenance as a target for drug discovery. J Med Chem 2014;57:521–38. [DOI] [PubMed] [Google Scholar]
- 17.Ruden M, Puri N. Novel anticancer therapeutics targeting telomerase. Cancer Treat Rev 2013;39:444–56. [DOI] [PubMed] [Google Scholar]
- 18.Gillis AJ, Schuller AP, Skordalakes E. Structure of the Tribolium castaneum telomerase catalytic subunit TERT. Nature 2008;455:633–7. [DOI] [PubMed] [Google Scholar]
- 19.Zheng QZ, Zhang XM, Xu Y, et al. . Synthesis, biological evaluation, and molecular docking studies of 2-chloropyridine derivatives possessing 1,3,4-oxadiazole moiety as potential antitumor agents. Bioorg Med Chem 2010;18:7836–41. [DOI] [PubMed] [Google Scholar]
- 20.Brouwer AJ, Álvarez NH, Ciaffoni A, et al. . Proteasome inhibition by new dual warhead containing peptide vinyl sulfonyl fluorides. Bioorg Med Chem 2016;24:3429–35. [DOI] [PubMed] [Google Scholar]
- 21.Alvarez NH, Langemheen HVD, Brouwer AJ, et al. . Potential peptidic proteasome inhibitors by incorporation of an electrophilic trap based on amino acid derived a-substituted sulfonyl fluorides. Bioorg Med Chem 2017;25:5055–63. [DOI] [PubMed] [Google Scholar]
- 22.Kisselev AF, Van der Linden WA, Overkleeft HS. Proteasome inhibitors: an expanding army attacking a unique target. Chem Biol 2012;19:99–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang JQ, Yang MD, Wang Y, et al. . Discovery of new chromen-4-one derivative as telomerase inhibitor through regulating expression of dyskerin. J Enzym Inhib Med Chem 2018; in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zha GF, Zheng QH, Leng J, et al. . Palladium-catalyzed fluorosulfonylvinylation of organic iodides. Angew Chem Int Ed 2017;56:4849–52. [DOI] [PMC free article] [PubMed] [Google Scholar]