Abstract
Aim
The aim of the study was to detect the level of comprehensive diabetes control among the diabetic patients of Kerala, India.
Methods
Patients (1200) were randomly selected from a diabetes care center. Their blood sugar, biochemical and anthropometric measurements were done and statistically analyzed.
Results
Only 28.3% had their HbA1c at or below 7% and 45% above 9%. One-third of the female and one-fifth of the male patients had coronary artery disease. The prevalence of hypertension was almost equal in both sexes. However, there was a statistically significant higher systolic blood pressure (mean 162.12 mmHg vs 147.49 mmHg, p = 0.01044) among females. The total cholesterol was above 200 mg/dl in 42.1% of males and 45.61% of females. The triglyceride was >150 mg/dl in 38.6% males and 50.88% females. Low high density lipoprotein (HDL) cholesterol levels were found in 20.07% of males and 41.12% of females (p = 0.0445). The mean low density lipoprotein (LDL) was 121.75 (± 32.29)
Conclusion
The mean blood sugar values are found to be high, which will lead to a predictable increase in vascular disease, which in turn will affect the quality of health and productivity of the individual and the economic growth of the society as a whole. Studies suggest that therapeutic interventions to improve glycemic control may reduce the risk of cardiovascular disease and microvascular disease.
This study shows that the level of diabetes control in Kerala is unsatisfactory. We need more medications, better strategies and more emphasis on glycemic management than we are currently able to apply.
Keywords: Diabetes mellitus, Dyslipidemia, Hyperglycemia, Hypertension, Glycemic control, Diabetes control, CAD
1. Introduction
The incidence of diabetes is alarming in both developed and developing countries. In US, the incidence of diabetes in 2010 was 1.7 million new diagnoses/year; in 2012, it increased to 1.9 million.1 This means that we are going to have increasing numbers of cardiovascular events, cerebral vascular events, peripheral vascular and a number of other cardiovascular illnesses.2 For the most part, diabetes has become the leading risk predictor for cardiovascular disease in most clinical cardiology settings. Proper control of hyperglycemia is imperative and significant in preventing both microvascular, and macrovascular complications in diabetes, and reduced control means an even more alarming increase in the complication rates.3 The mean glycated Hb (HbA1c) levels as per the available Indian data are around 9%, which is at least 2% higher than the goal prescribed by international bodies.11 Aim of our study was to identify whether we have achieved a satisfactory level of diabetes control or not in our diabetic population. This study aims to determine the level of diabetic control among a group of diabetic patients visiting a North Malabar diabetic clinic of Kerala to assess the mean glucose burden among the diabetic population as it will help give a direction for the future planning of diabetes management.
2. Materials and methods
Type 2 diabetic patients were recruited from the outpatient clinic of “Diabcare” diabetes care center, Manjeri that is an important secondary care center for diabetes for the whole of Malappuram district, representing a cross section of Malabar. Twelve hundred patients were randomly selected for the study. The age range selected for the study was 18–65 years. Samples for the fasting blood sugar, lipid profile, HbA1c, uric acid, calcium and fasting insulin levels were collected after at least 8 hours of overnight fasting. Samples for post-prandial blood sugars were also collected after 2 hours from the time of starting breakfast, after the patients take their usual medicines/or insulin if he/she is already on any. The study was conducted after getting informed consent. The study was approved by the Institutional Ethical Committee.
The patients were examined for assessment of height, weight, body mass index (BMI) and waist circumference and waist-hip ratio (WHR). BMI (according to the WHO criteria) <18.5 kg/m2 is underweight, 18.5–24.9 kg/m2 is healthy, 25–29.9 kg/m2 is overweight and 30 kg/m2 and above means obesity. However, the modified Asian criteria defines it differently with <18.5 kg/m2 underweight, 18.5–22.9 kg/m2 is healthy or acceptable risk, 23–24.9 kg/m2 is overweight or high risk and >25 kg/m2 is obese or very high risk. The BMI (body weight in kilograms divided by square of height in meters [kg/m2]) were measured with subjects in light clothing and without chappals. Waist circumference was measured on standing subjects midway between the lowest rib and the iliac crest. Hip circumference was measured at the widest area in the gluteal region and the WHR (according to the WHO criteria, for males normal was < 1 and for females < 0.9 and according to the modified Asian standards normal for men is < 0.90 and for females it was < 0.85) was calculated as a measure of fat distribution (central obesity), whereas BMI was considered a measure of over all adiposity. Two blood pressure readings were obtained from the right arm of the patients in a sitting position after 30 min of rest at 5 min intervals and their mean value was calculated. Systolic blood pressure ≥140 mmHg or diastolic blood pressure ≥90 mmHg (or current use of anti-hypertensive medication) was defined as hypertension.21
Relevant medical history data were collected from the patients including the family history of diabetes and coronary artery disease (CAD) in first-degree relatives. CAD was defined as using nitroglycerine; experiencing typical chest pain or having a history of previous myocardial infarction (MI). The information was validated against ECG changes (Minnesota codes 1.1 −3, 4.1 −4, 5.1 −3) compatible with ischemic heart disease.
Blood glucose was estimated by glucose oxidase-peroxidase enzymatic (GOD-POD), end point colorimetry single reagent chemistry method. Cholesterol estimation was done by enzymatic (Cholesterol Oxidase- peroxidase), end point colorimetry, single reagent chemistry, with lipid clearing factor (LCF). Triglyceride estimation was done by enzymatic (Glycerol 3-Phosphate Oxidase (GPO)/Trinder) end point colorimetry, single reagent chemistry with LCF. HDL cholesterol was estimated using polyethylene glycol-cholesterol oxidase-PAP (Expansion) end point colorimetry, two reagent chemistry with LCF. Auto span semi auto analyzer was used for all the above procedures and calorimetric measurements. HbA1c was measured using Bio-Rad “in2it” HbA1C analyzer using ‘Boronate Affinity Chromatography’ method. Statistical analysis of the data was done with the help of the SPSS v17.
3. Results
When the patients were categorized according to the blood sugar levels fasting and post-prandial, it was found that the majority of patients were poorly controlled. The mean fasting blood sugar was 156.73 (±54.0, p <0.0001), and the postprandial was 232.94 (±6.42, p <0.0001). Among them, 42.1% males and 63% females had a fasting blood sugar in the range of 141–200 mg/dl and 51.72% of males and 54.54% of females had post-prandial blood sugar in the range of 201–300 mg/dl. Only around one-third of the cases had reasonably good (fasting blood sugar <140 mg/dl and post-prandial blood sugar <200 mg/dl) control of blood sugar with only 28.3% of patients having HbA1c at or below 7%, and 45% had HbA1c above 9%, which shows that majority of the study population had poor blood sugar control.
The analysis of the prevalence of CAD (Table 1) showed that one-third of the female and one-fifth of the male patients had CAD. This showed that females had a significantly higher incidence of CAD. However, the prevalence of hypertension was almost equal in both sexes (males- 67.21% and females- 71.19%) (Table 2). There was a statistically significant higher systolic BP (mean 162.12 mmHg vs 147.49 mmHg, p = 0.01044) among females compared to their male counterpart. Regarding family history of diabetes, more than 50% of patients both among males and females had first degree relatives with diabetes (57.38% males vs 52.54% females).
Table 1.
CAD among diabetic patients.
| Male (610) | Female (590) | Total (1200) |
|---|---|---|
| 120 (19.67%) | 180 (30.51%) | 300 (25%) |
Table 2.
Hypertension among diabetic patients.
| Male (610) | Female (590) | Total (1200) |
|---|---|---|
| 410 (67.21%) | 420 (71.19%) | 830 (69.17%) |
Regarding lipid abnormalities (Table 3), the mean total cholesterol was 201.20 (± 38.52) and was above 200 mg/dl in 42.1% of males and 45.61% of females, whereas the average triglyceride was 151.255 (± 81.14). The triglyceride level was high (>150 mg/dl) in 38.6% males and 50.88% females. Low HDL cholesterol levels were found in 20.07% of males <40 mg/dl and 41.12% of females (<50 mg/dl) (mean 50.62 ± 13.78). This difference was found to be statistically significant (p = 0.0445). Among the various lipid fractions analyzed, high LDL cholesterol (>100 mg/dl) was the most prominent abnormality (71.93% of males and 82.46% of females) found among the study population. The mean LDL was 121.74 (± 32.29). Lower HDL and higher LDL cholesterol were found more among female diabetics compared to males.
Table 3.
Distribution of different Lipid components among diabetics.
| Sex (M = 570,F = 570) | TC (n ≤ 200) | TGL (n ≤ 150) | HDL (n-M ≥ 40mg/dl, n-F ≥ 50mg/dl) | LDL (N ≤ 100mg%) |
|---|---|---|---|---|
| Male Normal | 330 (57.89%) | 350 (61.40%) | 410 (71.93%) | 160 (28.07%) |
| Male Abnormal | 240 (42.11%) | 220 (38.6%) | 160 (20.07%) | 410 (71.93%) |
| Female Normal | 310 (54.39%) | 280 (49.12%) | 290 (50.88%) | 100 (17.54%) |
| Female Abnormal | 260 (45.61%) | 290 (50.88%) | 280 (49.12%) | 470 (82.46%) |
TC = Total Cholesterol, TGL = Triglycerides, HDL = HDL Cholesterol, LDL = LDL Cholesterol, IR = Insulin resistance.
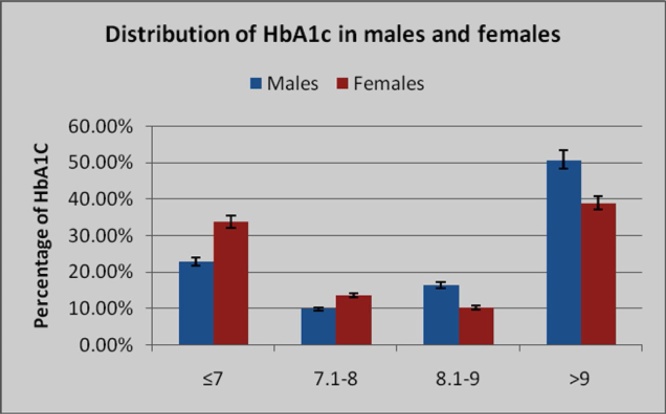
A total of 1200 patients (610 males and 590 females) were categorized according to their HbA1c value. Among males, 50.8% and among females 38.9% were having an HbA1c value above 9% indicating uncontrolled diabetes. Of 1200 patients, 13.3% had a value between 7.1% and 8% (reasonable/fair control), and 11.7% of patients had values between 8.1% and 9% (poor control). This shows an overall poor control of diabetes among the majority of the study population. Among them, only an aggregate of 28.3% of patients showed a good control of their diabetes with an HbA1c value of ≤7% (Fig. 1). From the results, among either sex, females show a better control of their diabetes than males. (Table 4 and Fig. 2). The mean values of calcium were slightly lower in both sexes, and was 8.13 mg/dL among males and 8.14 mg/dL among females, (p value = 0.71872-NS). Similarly, the mean uric acid values were 6.114 mg/dL in males and 5.558 mg/dL in females (p value = 0.71872-NS).
Fig. 1.
Distribution of diabetic patients according to HbA1c value. It is found that the HbA1c value is high in the majority of the diabetic patients, in both sexes (females faired better than males).
Table 4.
Distribution of HbA1c in males and females
| HbA1c | Males (n = 610) | Females (n = 590) | Total (n = 1200) |
|---|---|---|---|
| ≤7 | 140 (22.95%) | 200 (33.9%) | 340 (28.3%) |
| 7.1–8 | 60 (9.84%) | 80 (13.56%) | 140 (11.7%) |
| 8.1–9 | 100 (16.4%) | 60 (10.17%) | 160 (13.3%) |
| >9 | 310 (50.8%) | 230 (38.98%) | 540 (45%) |
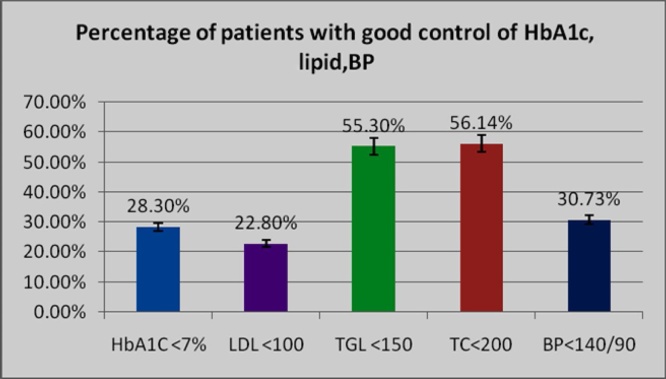
Fig. 2.
Percentage of patients with good control of HbA1c, lipids and BP control within the goals prescribed by world bodies. HbA1c, LDL Cholesterol and BP are well below a third of the goal set by them.
4. Discussion
The mean blood sugar values are found to be high in our study group. Mean fasting blood sugar value was 156.73 mg/dL, and the average post-prandial blood sugar was 232.95 mg/dl. This is quite high, indicating poor control of blood sugar in the diabetic population. Sixty-three percent females and 42.1% males had fasting blood sugar values between 141 and 200 mg/dl. Among the males, 51.72% and among females 54.54% had post-prandial blood sugar between 201 mg/dl and 300 mg/dl. Among males 50.8% and among females 38.9% were having an HbA1c value above 9% indicating uncontrolled diabetes. 13.3% patients had a value between 7.1% and 8% (reasonable/fair control), and 11.7% of patients had values between 8.1% and 9%(poor control). This shows an overall poor control of diabetes among the majority of the study population. Among them, only an aggregate of 28.3% of patients showed a good control of their diabetes with an HbA1c value of ≤7%. A similar trend was seen in the ICMR-INDIAB study where there was only 31.1% of patients with HbA1c <7% from the three Indian states of Tamilnadu, Maharashtra, and Jharkhand, and one Union Territory-Chandigarh.12 Some of the other studies show lesser levels of blood sugar in their diabetic study population. Most of them had fasting blood sugar values between 130 and 140 mg/dl.4, 5, 6 These data indicate that the majority of Indian diabetic population is poorly controlled.
Studies show that elevated blood glucose levels and the development of atherosclerosis are linked, suggesting that therapeutic interventions to improve glycemic control may reduce the risk of cardiovascular disease (CVD).19 Studies have also shown that post prandial hyperglycemia is a risk factor for atherosclerosis and CVD.7 This is caused by direct endothelial damage,8 through similar mechanisms as with insulin resistance and central obesity, like increased oxidative stress, reduced nitric oxide synthesis, and bioavailability.9, 10, 11 In addition, hyperglycemia may cause LDL glycosylation and oxidation, activate coagulation pathways, increase circulating levels of adhesion molecules involved in early atherogenesis, and increase levels of some of the inflammatory markers.11 Several large epidemiologic studies have shown that postprandial hyperglycemia is associated with increased incidence of cardiovascular disease.10, 13, 14, 15, 16, 17 In our study, total 25% (19.67% males and 30.51% females) of the patients have ECG evidence of CAD, which is slightly higher than that recorded in 2001 in the Chennai Urban Population Study (CUPS), a population based study in Chennai in South India, which showed a prevalence of 21.4% among their diabetic patients.18 This substantiates the growing epidemiological evidence for the association of (post-prandial) hyperglycemia and macrovascular complications in diabetic individuals.13, 14, 22, 23, 24
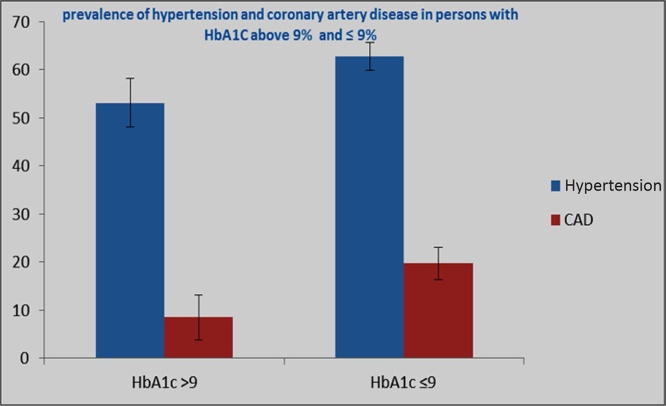
In the present study, the prevalence of hypertension was 69.17% (males- 67.21% and females- 71.19%) (Table 2). This was higher than the data published by Mohan et al (2007) in their CURES-38 study, which reported a prevalence of hypertension of 38.2% among their diabetes population.20 On further analysis, it was found that only 53.1% patients with HbA1c > 9% have hypertension, whereas 62.7% have hypertension in the HbA1c ≤9% group (Fig. 3, Table 5). In the case of CAD, only 8.5% of patients with HbA1c >9% were having CAD, whereas 19.7% of patients with HbA1c ≤ 9% had CAD. So unlike post-prandial hyperglycemia, no significant causal relationship of elevated glycated hemoglobin level with CAD or hypertension was identified in the present study.
Fig. 3.
Among the 1200 persons studied, only 53.1% patients with HbA1C > 9% have Hypertension, whereas 62.7% have hypertension in the HbA1C ≤9% group. In case of coronary artery disease, only 8.5% of patients with HbA1c >9% are having CAD, whereas 19.7% of patients with HbA1c ≤ 9% have CAD.
Table 5.
Distribution of CAD and Hypertension in patients with HbA1c > 9 and below ≤ 9
| Hypertension% | S E (Hypertension) | CAD% | S E (CAD) | |
|---|---|---|---|---|
| HbA1c > 9 | 53.1 | 5.11 | 8.5 | 4.65 |
| HbA1c ≤ 9 | 62.7 | 2.85 | 19.7 | 3.3 |
| Quality control values | ||||
|---|---|---|---|---|
| Test Constituent | Value (mg/dl) | Range (mg/dl) | CV | SDI |
| Glucose | 130 | 109.5–145.1 | 8.7 | 0.24 |
| Cholesterol | 115 | 85.1–115.1 | 8.4 | 1.77 |
| Triglyceride | 124 | 92.7–152.1 | 11.5 | 0.15 |
| Uric Acid | 7.4 | 5.2–7.2 | 11.9 | 1.63 |
Although targeting associated risk factors is much more likely to be cardioprotective than controlling the glucose level alone, good glycemic control is warranted to reduce the risks of nephropathy, retinopathy and neuropathy.25 The importance of tighter glycemic control is underscored by the American Diabetes Association decision to change the definition of “impaired fasting glucose” by lowering the glucose threshold to 100 mg/dl from 110 mg/dl.26
Glycemic control in diabetics, around the world, seems to be worsening despite the newer additions to the medical armamentarium to treat diabetes. In contrast, hypertension control and cholesterol control have got better, not worse, in the same interval. So, there is an absolute absence of progress, in fact, a reversal in the area of glucose control.
5. Conclusion
The above observations, it is found that the majority of the study patients have poor glycemic control. We can confidently conclude that poor glycemic control indicates the increased chance of developing macrovascular complications as well as microvascular disease in the near future, which will not only affect the productivity and the quality of life of the individuals but also thrust a significant financial burden on their family and the government.
Lack of patient awareness and more conservative attitude of treating physicians towards diabetes management may be the reasons for poor control of diabetes among the patients. We need more affordable and effective medications, better strategies, and more emphasis on glycemic management than we are currently able to apply.
References
- 1.Data from the National Diabetes Statistics Report, 2014, (released June 10, 2014) –accessed at http://www.diabetes.org/diabetes-basics/statistics/#sthash.CQKBBu1E.dpuf.
- 2.Lars Rydén, Grant Peter J., Stefan Anker D. ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD The Task Force on diabetes, pre-diabetes, and cardiovascular diseases of the European Society of Cardiology (ESC) and developed in collaboration with the European Association for the Study of Diabetes (EASD) Eur Heart J. 2013:eht108. doi: 10.1093/eurheartj/eht108. [DOI] [PubMed] [Google Scholar]
- 3.David Nathan David M. The diabetes control and complications trial/epidemiology of diabetes interventions and complications study at 30 years: overview. Diabetes Care. 2014;37(1):9–16. doi: 10.2337/dc13-2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hayashi Tomoshige, Edward Boyko J., Sato Kyoko Kogawa. Patterns of insulin concentration during the OGTT predict the risk of type 2 diabetes in Japanese Americans. Diabetes Care. 2013;36(5):1229–1235. doi: 10.2337/dc12-0246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jl Vijay, Eric S.K., Paul E.J., Hn David A., Stephen L.A. Biological variation of homeostasis model AssessmentDerived insulin resistance in type 2 diabetes. Diabetes Care. 2002;25:2022–2025. doi: 10.2337/diacare.25.11.2022. [DOI] [PubMed] [Google Scholar]
- 6.Sangeeta R.K., Linda J.R., Jennifer L. Insulin resistance is associated with impaired nitric oxide synthase activity in skeletal muscle of type 2 diabetic subjects. J Clin Endocrinol Metabol. 2005;90:1100–1105. doi: 10.1210/jc.2004-0745. [DOI] [PubMed] [Google Scholar]
- 7.Haffner S.M., Lehto S., Ronnemaa T., Pyorala K., Laakso M. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med. 1998;339:229–234. doi: 10.1056/NEJM199807233390404. [DOI] [PubMed] [Google Scholar]
- 8.Lisa Kitasato, Tojo Taiki, Hatakeyama Yuko, Kameda Ryo, Hashikata Takehiro, Yamaoka-Tojo Minako. Postprandial hyperglycemia and endothelial function in type 2 diabetes: focus on mitiglinide. Cardiovasc Diabetol. 2012;11(1):79. doi: 10.1186/1475-2840-11-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ceriello A., Quagliaro L., Catone B. Role of hyperglycemia in nitrotyrosine postprandial generation. Diabetes Care. 2002;25:1439–1443. doi: 10.2337/diacare.25.8.1439. [DOI] [PubMed] [Google Scholar]
- 10.Ceriello A., Hanefeld M., Leiter L. Postprandial glucose regulation and diabetic complications. Arch Intern Med. 2004;164:2090–2095. doi: 10.1001/archinte.164.19.2090. for the International Prandial Glucose Regulation (PGR) Study Group. [DOI] [PubMed] [Google Scholar]
- 11.Joshi S.R., Das A.K., Vijay V.J. Challenges in Diabetes care in India: sheer numbers, lack of awareness and inadequate control. J Assoc Phys India. 2008;56(June):443–450. [PubMed] [Google Scholar]
- 12.Unnikrishnan R., Anjana R.M., Deepa M. Glycemic control among individuals with self reported diabetes in India-The ICMR-INDIAB Study. Diabetes Technol Ther. 2014;16(September(a)):596–603. doi: 10.1089/dia.2014.0018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hanefeld M., Fischer, Julius U. DIS Group. Risk factors for myocardial infarction and death in newly detected NIDDM: the Diabetes Intervention Study, 11-year follow-up. Diabetologia. 1996;39:1577–1583. doi: 10.1007/s001250050617. [DOI] [PubMed] [Google Scholar]
- 14.Barrett-Connor E., Ferrara A. Isolated postchallengehyperglycemia and the risk of fatal cardiovascular disease in older women and men: the Rancho Bernardo Study. Diabetes Care. 1998;21:1236–1239. doi: 10.2337/diacare.21.8.1236. [DOI] [PubMed] [Google Scholar]
- 15.Hanefeld M., Koehler C., Schaper F. Postprandial plasma glucose is an independent risk factor for increased carotid intima-media thickness in non-diabetic individuals. Atherosclerosis. 1999;144:229–235. doi: 10.1016/s0021-9150(99)00059-3. [DOI] [PubMed] [Google Scholar]
- 16.The DECODE Study Group Glucose tolerance and mortality: comparison of WHO and American Diabetic Association diagnostic criteria. Lancet. 1999;354:617–621. [PubMed] [Google Scholar]
- 17.Tominaga M., Eguchi H., Manaka H. Impaired glucose tolerance is a risk factor for cardiovascular disease but not impaired fasting glucose. The Funagata Diabetes Study. Diabetes Care. 1999;22:920–924. doi: 10.2337/diacare.22.6.920. [DOI] [PubMed] [Google Scholar]
- 18.Mohan Viswanathan, Deepa Raj, Shanthi Rani Subramanian, Premalatha Gopal. Prevalence of coronary artery disease and its relationship to Lipids in a selected population in South India. The Chennai urban Population Study (CUPS No. 5) J Am Coll Cardiol. 2001;38(3):682–687. doi: 10.1016/s0735-1097(01)01415-2. [DOI] [PubMed] [Google Scholar]
- 19.Gregg E.W., Li Y., Wang J. Changes in diabetes-related complications in the United States. N Engl J Med. 2014;370:1514–1523. doi: 10.1056/NEJMoa1310799. [DOI] [PubMed] [Google Scholar]
- 20.Mohan V., Sandeep S., Deepa M., Gokulakrishnan K., Datta M., Deepa R. A diabetes risk score helps identify metabolic syndrome and cardiovascular risk in Indians-the Chennai Urban Rural Epidemiology Study (CURES-38) Diabetes ObesMetab. 2007;9:337–343. doi: 10.1111/j.1463-1326.2006.00612.x. [DOI] [PubMed] [Google Scholar]
- 21.Aram Chobanian V. The seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure. JAMA. 2004:11–12. doi: 10.1161/01.HYP.0000107251.49515.c2. NIH Publication No. 04–5230. [DOI] [PubMed] [Google Scholar]
- 22.Kawamori R. Asymptomatic hyperglycemia and early atherosclerotic changes. Diabetes Res Clin Pract. 1998;40:35–42. doi: 10.1016/s0168-8227(98)00041-2. Suppl. [DOI] [PubMed] [Google Scholar]
- 23.Temelkova-Kurktschiev T.S., Koehler C., Henkel E. Postchallengeplasma glucose and glycemic spikes are more strongly associated with atherosclerosis than fasting glucose or HbA1c level. Diabetes Care. 2000;23:1830–1834. doi: 10.2337/diacare.23.12.1830. [DOI] [PubMed] [Google Scholar]
- 24.The DECODE Study Group Glucose tolerance and cardiovascular mortality. Comparison of fasting and 2-hour diagnostic criteria. Arch Intern Med. 2001;161:397–404. doi: 10.1001/archinte.161.3.397. [DOI] [PubMed] [Google Scholar]
- 25.Caren G., Solomon M.D. Reducing cardiovascular risk in type 2 diabetes. Editorial New England J Med. 2003;348(5):457–459. doi: 10.1056/NEJMe020172. [DOI] [PubMed] [Google Scholar]
- 26.American Diabetes Association Diagnosis and classification of diabetes mellitus. Diabetes Care. 2014;37(Supplement 1):S81–S90. doi: 10.2337/dc14-S081. [DOI] [PubMed] [Google Scholar]