Abstract
Hypertension (HTN) is a complex multi-factorial disease and is considered one of the foremost modifiable risk factors for stroke, heart failure, ischemic heart disease and renal dysfunction. Over the past century, salt and its linkage to HTN and cardiovascular (CV) mortality has been the subject of intense scientific scrutiny. There is now consensus that different individuals have different susceptibilities to blood pressure (BP)-raising effects of salt and this susceptiveness is called as salt sensitivity. Several renal and extra-renal mechanisms are believed to play a role. Blunted activity of the renin–angiotensin–aldosterone system (RAAS), adrenal Rac1-MR-Sgk1-NCC/ENaC pathway, renal SNS-GR-WNK4-NCC pathway, defect of membrane ion transportation, inflammation and abnormalities of Na+/Ca2+ exchange have all been implicated as pathophysiological basis for salt sensitive HTN. While salt restriction is definitely beneficial recent observation suggests that treatment with Azilsartan may improve salt sensitivity by selectively reducing renal proximal tubule Na+/H+ exchange. This encourages the future potential benefits of recognizing and therapeutically addressing the salt sensitive phenotype in humans.
Keywords: Hypertension, Salt sensitivity, Patho-physiological mechanisms, Azilsartan
1. Introduction
Hypertension (HTN) has been attributed as one of the foremost modifiable risk factors for stroke, heart failure, ischemic heart disease and renal dysfunction.1 Data suggests that life expectancy is reduced by approximately 5 years if HTN remains untreated.2 Effective treatment of HTN has been one of the key achievements in last five decades in the field of Medicine. Noteworthy developments in the antihypertensive therapy have resulted in better control of blood pressure (BP) in majority of the patients with HTN. Nevertheless, despite continued research, HTN continues to be a major public health problem. Moreover, the burden of uncontrolled HTN is also increasing dramatically despite the therapeutic advances. One reason for this paradox is that HTN is a multi-factorial disease with various patho-physiologic mechanisms. We undertook this comprehensive review to comprehend salt sensitivity, which is one of the major factors responsible for this growing burden and to better understand the strategies required for addressing this. This review is also expected to elucidate the complex patho-physiological mechanisms of salt sensitivity.
2. Dietary salt
Meticulous physiologic regulation of the sodium levels is of critical importance for optimum efficiency of various physiological functions in the body. Dietary salt i.e. sodium chloride is essential for maintaining extracellular fluid volume and serum osmolality.3 Any changes in the plasma concentration of sodium may be directly detrimental to plasma osmotic pressure, acid-base balance, plasma volume, interstitial fluid volumes, electrical activity of cells and cardiovascular system’s response to circulating endogenous pressor agents.4 Normal human beings can sustain the ill effects of extremely low sodium intake by conserving sodium by way of marked reduction in sodium losses in the urine and sweat. On the other hand, in case of acute or chronic salt challenges, body can quickly excrete very large salt loads without any significant changes in volume homeostasis or BP.3
2.1. Dietary salt and common belief
There is a common belief amongst people that too much of salt in diet will lead to increase in BP. But contrary to this belief, not everyone with high salt diet develops HTN. The effects of dietary sodium on BP vary from person to person because of their differential sensitivity to salt. Thus, those who are salt-sensitive are more likely to develop HTN than those who are resistant to salt.5
2.2. Dietary salt and HTN controversy
Over the past century, salt has been the subject of intense scientific research in relation to HTN and cardiovascular (CV) mortality. Association of dietary salt and HTN has been an area of continuing controversy since many decades. INTERSALT study which was a standardized, worldwide epidemiologic study of large cohort (n = 10,079) revealed no significant relationship between 24-h urinary sodium excretion and BP.6 However, 8 years later, extrapolation of INTERSALT data suggested that reducing salt intake by 1/3rd of the current mean level would reduce BP by an average of 4/2.5 mm Hg in patients with HTN and by 2/1 mm Hg in normotensive people.7 However, this re-analysis being an extrapolation of data has its own limitations. The fact is that different individuals have different susceptibilities to the BP-raising effects of salt.8 While BP in the population as a whole is only modestly affected by the changes in sodium intake, some individuals in response to acute or chronic salt depletion or repletion shows large BP changes and are called as “salt sensitive”.3 Weinberger et al. showed that salt-sensitive subjects had a significantly greater increase in systolic (p < 0.001) and diastolic (p < 0.001) pressure over time than those who were salt resistant.9
3. What is salt sensitivity and how much is the burden?
Salt sensitivity of BP is defined as a physiological trait existing in rodents and other mammals, including humans, by which BP of some members of the population shows changes parallel to changes in salt intake.10 In many individuals, when salt intake increases, the excess amount is excreted by the way of kidney or sweat. However, there are some individuals where this mechanism is faulty and increased salt is retained and manifests as high BP. There is an inter-individual difference in the BP response to changes in dietary sodium chloride intake which could be attributed to salt sensitivity.11, 12 Overall, salt sensitivity appears to be a major public health problem with estimated incidence of 51% in patients with HTN and 26% in normotensive people.12
4. Salt sensitivity and predisposing factors
Excess salt intake along with higher salt sensitivity remains one of the key risk factors for the predisposition to essential HTN. However, the BP response to a change in salt intake is not uniform. Variety of physiological, demographic, genetic and even environmental characteristics differentiate between salt sensitive and resistant population.13 Salt sensitivity appears to be determined by genetic factors, race/ethnicity, age, gender, body mass index and diet. Associated co-morbidities e.g. HTN, diabetes, chronic kidney disease and metabolic syndrome also play a vital role.3, 10 Salt sensitivity is specifically common in older adults, African Americans, and in people with a higher level of BP or other comorbidities.13
Several studies have identified subgroups of the population who are salt sensitive. Black race population manifest a higher BP response to change in salt intake than Whites independent of baseline BP.14 Similarly, data suggests that elderly people and patients with HTN have a greater BP response to a change in salt than young adults and normotensive individuals.9, 15 Furthermore, Weinberger et al observed that BP responses of both normotensive and hypertensive subjects to salt depletion increased significantly as the age advanced with higher response in patients >30 years of age.9 They concluded that salt sensitivity was a predictor of subsequent, age-related BP increase. Another finding of this study was that patients with HTN were more salt sensitive than normotensives, a fact that has been confirmed in numerous subsequent studies.
Other salt sensitive subgroups are females and obese individuals, although evidence for these associations is not too strong.10 Studies have shown that obesity increases sympathetic activity and salt sensitivity. Further, obese Asians may be more prone to develop HTN as compared to obese Caucasians.16 Diet has also been attributed for salt sensitivity. BP response to dietary salt was found to be greater in the setting of a low-potassium intake and in the setting of poor-quality diet compared with the DASH (Dietary Approaches to Stop Hypertension) diet.14, 17, 18
5. Genetics of salt sensitivity
One of the mechanisms for ethnic differences in salt sensitivity could be genetic predisposition for Asian populations. The gene frequency of candidate gene polymorphisms of salt-sensitive HTN such as angiotensinogen gene, alpha-adducin gene, aldosterone synthase gene promoter, β-3 subunit of G-protein (GNB3), etc. was found to be significantly higher in Japanese populations than in Caucasians. This probably accounts for enormous interracial differences in the frequency of salt-sensitive HTN.19
At individual level, though it is crucial to identify the vulnerable people, it has been difficult to discriminate salt-sensitive from salt-resistant individuals with phenotypic studies. Therefore, research is focussed more on identifying the genes that could be involved in salt sensitivity. Several single-gene mutations that directly affect renal sodium reabsorption can cause HTN, but such variants have been observed in only a few individuals.20
There is still an ambiguity in understanding the molecular mechanisms behind the salt sensitivity trait.21 Several evidences strongly suggest the genetic mechanisms determining BP responses to salt intake.22, 23 However, genome wide association studies (GWAS) identified genes that influence only 2% of BP variability and have failed to identify many genes that affect salt sensitivity.24 Similarly, candidate gene association studies revealed only a few genes associated with salt sensitive HTN.24 Recent examples of the discovery of genes involved in salt sensitivity in humans include SLC4A4 and SLC4A5, sodium bicarbonate co-transporters and striatin deficiency.25, 26 Sanada et al proposed that genetic variations in genes involved in the renin–angiotensin–aldosterone system (RAAS) predispose salt sensitivity in carriers.22 Other genes associated with salt sensitivity and HTN are the three endothelial nitric oxide (NOS3) genes, angiotensinogen (AGT) gene, adducin 1 (ADD1), adrenergic receptor beta (ADRB2), AGTR1, CYP11B2, β-3 subunit of G-protein (GNB3).22, 27 Furthermore, genes encoding renal sodium channels and sodium transporters; Na+-K+-Cl−cotransporter-2 (NKCC2), α2-adrenergic receptor, WNK lysine-deficient protein kinase-4, and Na+-Cl−co-transporter (NCC) were linked with salt sensitivity.28, 29
Li et al. carried out genome-wide analyses to identify genomic loci that interact with sodium to influence BP among 1876 Chinese participants of the Genetic Epidemiology Network of Salt-Sensitivity (GenSalt) study. They have identified MKNK1, C2orf80, EPHA6, SCOC-AS1, SCOC, CLGN, MGAT4D, ARHGAP42, CASP4, and LINC01478 that were associated with at least 1 BP phenotype. Thus 8 novel and 1 previously reported BP loci have been recently identified through the examination of single-nucleotide polymorphism and gene-based interactions with sodium.30
6. How to differentiate between salt sensitive and salt resistant
In humans, there is normal distribution of the trait. However, an arbitrary magnitude of the salt-induced change in BP has been used to differentiate between salt sensitive and salt resistant groups.10 Though there is no evidence-based method for the measurement of salt sensitive blood pressure (SSBP) in humans, consensus is reached on sequential low-salt diet and high salt diet protocol to identify a person as salt-sensitive or salt-resistant.10 One of the recommended method is to give four days of low-sodium diet (about 230 mg sodium or 600 mg of table salt per day), followed by a high-sodium diet (about 4.6 g sodium or 12 g of table salt per day) for four days. At the end of the high-sodium period, if BP increases by at least 5% from baseline, the person can be labelled as salt sensitive.31
To define salt sensitivity and resistance, Weinberger et al. have adopted a technique of salt loading with acute intravenous saline challenges after achieving sodium and volume depletion through salt reduction along with diuretic treatment.12 Subjects with a decrease in mean arterial pressure (MAP) ≥10 mm Hg after sodium and volume depletion were considered salt sensitive and those with a decrease ≤5 mm Hg as salt-resistant.12
7. Why does salt sensitivity matter?
While not all hypertensives are salt sensitive and not all salt sensitive people are hypertensive, the available evidence suggests that even normotensive salt sensitive individuals are at high cardiovascular (CV) risk and lower survival rate, as BP eventually rises later in life with high salt diet.32 Moreover, salt sensitivity is an independent risk factors for CV disease, beyond the detrimental prognosis conferred by mere HTN. More conclusive evidence of an independent role for salt sensitivity as a CV risk factor was provided by Weinberger et al. in a long-term, 27 year follow-up study. This study revealed that salt sensitivity was associated with an increased mortality risk ratio of 1.73 (95% confidence interval, 1.02–2.94) and normotensive salt sensitive subjects had similar cumulative mortality as that of hypertensive patients. Thus it was established that a unique evidence of a connexion exists between salt sensitivity and mortality independent of elevated BP.32
Weinberger et al in another 10 year follow-up study observed that, there was significantly greater (p < 0.001) increase in BP with age over time in salt sensitive subjects than those who were salt resistant.9 These findings affirm that normotensive subjects who are salt-sensitive may be at an increased risk for developing age-related HTN in later stages of life. This has a definite clinical implication in salt sensitive individuals who are currently normotensive because of risk of future development of HTN. On a positive note, it is therefore conceivable to prevent or delay the subsequent age-related increase in BP, and thus the future development of HTN and thereby reduce the risk of CV events and mortality in salt-sensitive subjects.
Various observations by researchers revealed that “non-dipper” hypertensive patients with blunted nocturnal decline in BP are more likely to exhibit salt sensitivity and disturbances in the circadian rhythm of BP which an independent prognostic- indicator of CV events.33, 34, 35, 36 Studies have also revealed that salt-sensitive patients are prone to not only CV events but also renal events compared with non-salt sensitive hypertensive patients.37 In one study, Morimoto et al. found that total CV events, both fatal and non-fatal, were twice more common in salt-sensitive Japanese hypertensive patients compared with their salt-resistant cohort (4.3 versus 2.0 per 100 patient-years).37 Also, more patients with resistant HTN, defined as BP remaining above target in spite of the use of three or more antihypertensive medications at optimal doses, were found to be salt sensitive.38 Researchers have also suggested a strong relationship between increased salt sensitivity and insulin resistance leading to metabolic syndrome and CV disease. This relationship may be predominantly responsible for epidemic of CV disease in the southern Asian Indian population.39 However, this strong link between salt sensitivity and CV disease and insulin resistance has not received sufficient attention. This may be especially relevant to India where approximately 60% of the world’s cases of CV disease reside and the salt consumption is among the highest of any large population.39 Apart from this, salt sensitivity has also been attributed to end organ damage; increased risk for the development of left ventricular hypertrophy and proteinuria.40, 41 These results corroborate that salt sensitivity is a prognostic factor independent of classic CV risk factors.
Thus, these available evidences provide insight into factors responsible for increased risk of mortality. Apart from the long-recognized risk factors such as age, gender, body mass index, and BP parameters, salt sensitivity has emerged as the crucial silent contributor to the increased mortality in normotensive as well as hypertensive subjects.
8. Mechanism of salt sensitivity
Most genetic aberrations detected to date in salt sensitive hypertensive rodents implicate renal pathology with defective regulation of natriuresis as the main causative mechanism. However, recent evidence utilizing modern techniques of genetic investigation have revealed newer causative mechanisms including cutaneous sodium storage, regulation of regional blood flows, vascular endothelial dysfunction and innate immunity.42, 43 A scientific statement from the American Heart Association highlighted that more than one mechanisms that normally regulate the adaptation of the CV system to a salt load must be impaired in salt sensitivity, indicating multi-factorial causation.10
8.1. Renin-angiotensin-aldosterone system pathway
The RAAS plays a crucial role in the regulation of sodium excretion and equilibrium and is sensitive to changes in sodium intake.44, 45 In salt sensitive individuals, excess sodium is handled less efficiently by the kidney when there are changes in RAAS due to genetic background, age, race, gender and co-morbidities.10
Studies of neuro-hormonal responses to short term dietary salt loading followed by salt deprivation have suggested greater fall in BP with an acute reduction in salt intake in hypertensives compared with normotensives. This was attributable to a less-responsive RAAS in the hypertensive patients. Thus, blunted activity of the RAAS may contribute to salt sensitive HTN in humans.3
Parfrey and co-workers suggested that decrease in BP in salt sensitive subjects might be due to blunted renin response to salt depletion. This observation was well corroborated by the finding that BP was reduced by saralasin in subjects with preserved renin responses but not in those with blunted renin responses to salt depletion.46, 47 In some studies, it was also observed that salt sensitive subjects not only had diminished stimulation of renin consequent to salt depletion, but also exhibited blunted suppression of renin in response to a salt load.48 Other researchers have also confirmed this bidirectional blunting of renin responses to changes in salt intake in salt sensitive individuals, thereby establishing that a blunted RAAS is a phenotypic characteristic of salt sensitivity.49, 50, 51
8.2. Local renal RAS mechanism
It has been postulated that apart from the classical systemic RAAS an independently functioning RAAS within the kidney also play a key role in regulating renal sodium excretion and BP.52, 53 Crowley et al. observed that AT1 receptors in the kidney are principally responsible for mediating angiotensin II (AII)–dependent HTN. Based on this findings, it can be inferred that salt-induced increase in local RAAS formation and the subsequent activation of AT1 receptors in the proximal tubule may contribute to salt sensitive HTN through increased tubular sodium absorption.54
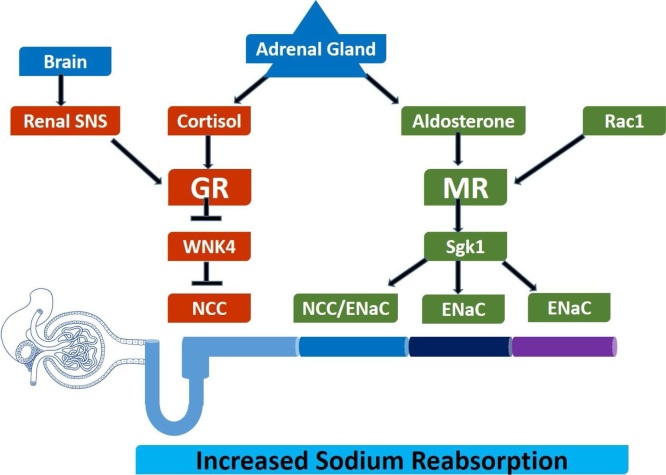
8.2.1. Adrenal RAC1-MR and renal SNS-GR mechanisms
Researchers have proposed that apart from local RAAS pathway at proximal tubule contributing to salt sensitive HTN, defects in sodium handling at other segments of the renal tubules can also lead to increased tubular sodium reabsorption and the consequent salt-sensitive HTN.55 In the distal nephron, aldosterone and cortisol by acting on mineralocorticoid receptor (MR) and the glucocorticoid receptor (GR) respectively, control sodium reabsorption and regulate sodium homeostasis.11 Recently, researchers have identified two novel pathways that are involved in abnormal regulation of renal sodium handling, and consequent salt-sensitive HTN.56, 57
These two pathways; adrenal Rac1-MR-Sgk1-NCC/ENaC pathway (Rac1-mineralocorticoid receptor-glucocorticoid-inducible kinase 1-sodium chloride co-transporter/epithelial sodium channels pathway) and renal SNS-GR-WNK4-NCC pathway (sympathetic nervous system-glucocorticoid receptor-With-no-lysine kinase 4- sodium chloride co-transporter pathway) have been shown to play critical roles in certain rodent models of salt-sensitive HTN11 (Fig. 1). MR and GR are activated by these two pathways resulting into NCC activation in the different distal convoluted tubules segments, thereby leading to abnormal renal excretory function and increased BP.
Fig. 1.
Role of adrenal glands and central renal SNSs in the development of salt-sensitive hypertension.
(Adopted from Fujita and Nagae et al.)58, 59
NCC/ENaC at distal convoluted tubule (DCT2), ENaC at the connecting tubule (CNT) and cortical collecting duct (CCD) are activated through up-regulated Sgk1 after stimulation of MR by Rac1. Also, NCC activation occurs at DCT1 through WNK4 down regulation because of stimulation of GR by renal SNS over activity. In salt sensitive individuals, upon excessive salt intake, aldosterone secretion is suppressed through inhibition of circulating RAS but Rac 1 is activated in spite of decrease in aldosterone levels. This leads to activation of MR through renal Sgk, (a downstream regulator of MR) which is up regulated due to salt loading resulting into sodium retention and increase in BP.11This paradoxical response of MRs to salt loading in salt-sensitive HTN is because of the abnormal response of Rac1 to salt loading. Thus, Rac1 is an upstream regulator of MRs and serves as a determinant of salt sensitivity. At DCT1, NCC activation via WNK4 down regulation is induced through stimulation of the beta 2 adrenergic receptor (b2-AR) and plays a vital role in the salt induced HTN via increased sodium reabsorption in the DCT segments.11Thus, GR, but not the MR, plays a crucial role in b-AR stimulation-induced WNK4 down regulation and salt-sensitive HTN.57
In salt-sensitive hypertensive rats, renal SNS activity is increased by salt loading. This renal SNS over activity is primarily responsible for salt-induced HTN through impaired excretory function.60 The anti-natriuretic effect of increased renal SNS activity is principally mediated by three major mechanisms: reduced renal blood flow, increased renin secretion and increased tubular reabsorption.61 However, it is not clear how increased renal SNS over activity augments tubular sodium reabsorption and manifests into salt-sensitive HTN.
Thus, the control of renal sodium homeostasis and BP maintenance is primarily regulated by NCC. NCC activation is responsible for salt-sensitive HTN in rodent models through two novel pathways: the Rac1-MR-Sgk1-NCC and beta adrenergic recpetor-GR-WNK4-NCC pathways. Sodium reabsorption is increased by an abnormal Rac1-MR pathway through activation of NCC in the DCT2 segment in addition to activation of epithelial sodium channels (ENaCs) in the DCT2, connecting tubule and cortical collecting duct segments, whereas an aberrant b-AR-GR-WNK4 pathway activates NCCs in the DCT1 segment.11
Moreover, above-mentioned mechanism identified in animal models may be synonymous to those mechanisms found in salt-sensitive humans.62 In future, these two pathways can be potential therapeutic targets for salt-sensitive HTN and salt-mediated cardio-renal injury. However, further exhaustive studies are needed to evaluate the therapeutic value of manipulating these pathways.
8.3. Defective atrial natriuretic peptide (ANP) response
A growing body of evidence has shown that atrial natriuretic peptide (ANP) also plays a crucial role in the regulation of sodium balance and the pathogenesis of salt sensitive HTN. Genetically determined abnormalities in ANP responses to changes in salt balance is the key aberration.10, 63, 64 Animal studies have shown that homozygous deletion of the ANP gene results in HTN with high salt diet and also leads to biventricular hypertrophy and cardio-myocyte enlargement that is independent of BP. Human studies have revealed that in response to high salt diets, secretion of ANP may be blunted in black salt-sensitive hypertensives.65 These observations clearly point to the fact that deficiency in ANP expression is responsible for salt sensitive HTN.3
8.4. Sodium–nervous system interactions
One mechanism that has been hypothesized for variability in salt sensitivity of BP is function of arterial baroreceptor located at the arch of the aorta and carotid sinuses.66 These baro-receptors are stimulated upon increase in BP which results in reduction in sympathetic outflow to resistance vessels and the heart and BP is restored to normal levels. These receptors are involved in the long-term control of BP.67 Furthermore, these baro-receptors appear to be chronically safeguarding the effects of dietary sodium loading on MAP and support the theory that primary baroreceptor dysfunction may play a role in salt-sensitive HTN.3 Various data contribute to a growing consensus from long-term studies that during volume excess and salt-sensitive HTN, neurally induced sodium excretion play a compensatory role in regulation of extracellular fluid volume and arterial pressure.67, 68
8.5. Is salt sensitivity a disorder of the immune system?
Recently, it was observed that salt intake stimulates cutaneous lymphangiogenesis through tissue macrophages and directly alters endothelial cell function. This resulted into increased production of transforming growth factor-β (TGF-β) and nitric oxide (NO). In case of endothelial dysfunction, reduced NO production worsens the vascular effects of TGF-β, thereby promoting decreased arterial compliance and HTN.69
Recent research also suggested that T cells might be involved in the pathogenesis of salt-sensitive HTN. Guzik et al. found that recombinase-activating gene (RAG-1−/−) mice, which lack both T and B cells, showed a blunted increase in BP and reduced vascular oxidative stress in response to desoxycorticosterone acetate (DOCA) salt.70
The potential role of adaptive immunity in vascular pathology associated with salt-sensitive HTN has also been examined using normotensive (Brown Norway), hypertensive (Dahl salt-sensitive) and consomic rats (SSBN2; in which chromosome 2 has been transferred from Brown Norway to Dahl rats).71 Tail-cuff systolic BP were increased in Dahl rats compared with Brown Norway rats and were reduced in SSBN2 rats compared with Dahl rats. Compared with Brown Norway and SSBN2 rats, Dahl rats exhibited increased inflammatory markers in the aorta, including activation of NF-κB and increased infiltration of CD4 + T cells. Also infiltration of cells of the regulatory T cell lineage was found to be decreased in aortic tissue of Dahl rats, relative to the SSBN2 strain. The authors concluded that this genetic rodent model of salt-sensitive HTN exhibited increased vascular inflammatory responses and were reduced by transfer of chromosome 2 from a normotensive strain. This also resulted into enhanced production of immunosuppressive mediators. Although incompletely characterized, the combined findings support a role for adaptive immunity and T cell function in the development of salt-sensitive HTN and end organ damage.71
SH2B3, a genome-wide association studies (GWAS) candidate for HTN and renal disease, encodes an intracellular adaptor protein that functions in many signalling cascades. Rudemiller et al. provided the evidence that the mutation of SH2B3 (LNK) significantly attenuated HTN via immune cell function and renal injury in Dahl salt sensitive hypertensive rats.72
Thus, a role for inflammation in the pathogenesis of HTN is becoming widely accepted and the available data from a variety of experimental models supports this hypothesis. Thus, inflammatory pathways could become therapeutic targets for the treatment of HTN.
8.6. Aberrant adiponectin response or its polymorphism
Preliminary evidence shows that salt could modulate adiponectin level in normal individuals. Fuqiang et al. hypothesized that abnormalities of adiponectin and inflammation may be the potential mechanism of salt sensitivity. They observed that the usual increase in adiponectin in response to high salt diet in normotensive salt sensitive subjects may be blunted. Thus, during high salt diet, the disturbance of adiponectin exists in normotensive salt sensitive subjects, which may be a novel underlying mechanism of salt sensitivity. Adiponectin gene variation might be mechanistically involved in the salt sensitivity.73
8.7. Ion transportation
Currently, several studies have revealed that salt sensitive HTN may also be attributable to the ion transportation, and the high-salt intake could lead to the defect of membrane ion transportation and abnormalities of Na+/Ca2+ exchange.74, 75, 76 The data suggests that phenomenon of intracellular calcium overload may be prevalent in salt-sensitive population. This suggests that pressure boosting response of salt sensitive HTN is positively linked with the intracellular Na+ and Ca2+ levels.77, 78 Studies by Guo et al. and Jiang et al. revealed that activities of Na+-Ca2+ pump in the vascular smooth muscle cells of spontaneously hypertensive rat were decreased and antihypertensive treatment restored them.79, 80 Thus, it could be speculated that the reduced activities of Na+ and Ca2+ pump leading to the vasoconstriction and increase in BP might be one of the pathogeneses of salt sensitive HTN. Also, reversal of depressed Na+-K+-ATPase and Ca2+-ATPase activities by RAAS inhibitors could be one of the mechanisms that RAAS inhibitors reduced the BP of salt-sensitive hypertensive rats.80
8.8. Hyperinsulinemia
It was observed that normotensive and hypertensive salt sensitive subjects are more insulin resistant than their salt resistant subjects independent of BP.49 However, it is not very clear whether the stimulatory effect of insulin on tubular sodium reabsorption, sympathetic activity, or vascular remodelling is responsible for the development of salt sensitive HTN.10
8.9. Salt sensitivity and vascular endothelial dysfunction
Vascular endothelium has been a source of intense research as it was observed that vascular endothelium is more than a simple barrier and plays a crucial role in mediating vascular relaxation through endothelial-derived vasodilator NO.81 Endothelial dysfunction, especially the NO system has been implicated in both experimental and clinical HTN. Clinical data suggests that in response to salt intake, salt-sensitive patients may be unable to up-regulate the production of nitric oxide.82 It has also been documented in some experimental studies and human clinical trials that endothelial function is impaired by high salt intake independent of BP by causing oxidative stress and increased endothelial cell stiffness. Also transforming growth factor beta promotes increased arterial stiffness in the presence of endothelial dysfunction.83
In addition, contrary to the traditional view that salt sensitivity is solely a consequence of renal dysfunction, recent studies suggest that non-osmotic salt accumulation in the skin interstitium and the endothelial dysfunction caused by the deterioration of vascular endothelial glycocalyx layer and the epithelial Na+ channel on the endothelial luminal surface (EnNaC) also play crucial role in storage of salt. These new concepts highlight that sodium homeostasis and salt sensitivity seem to be related not only to the renal dysfunction but also to the endothelial dysfunction.84 In a study by Matsumoto et al., it was observed that Azilsartan restored endothelial function by reducing vascular inflammation and by increasing the phosphorylation ratio Ser1177/Thr497 of endothelial nitric oxide synthase (eNOS) in diabetic mice.85 Azilsartan has also been shown to restore vascular dysfunction by reducing tumour necrosis factor-α and IL-1β levels, and up-regulating the vascular endothelial growth factor.86
8.10. Salt preference, prehypertension and sympathovagal balance
Prehypertension has been linked to the damage to the coronary vasculature and adverse CV events. In a study by Pal et al. to assess the correlation of salt preference to development of prehypertension in Indian adults, it was observed that almost 50% of the study population had salt-preference. However, the percentage of prehypertensives was significantly higher (32.48%) among salt-preference subjects than the no-salt-preference subjects (7.47%).87
In addition, among the prehypertensives, 80.9% subjects were salt-preference subjects and19.1% subjects were no-salt-preference prehypertensives. These findings highlighted the higher prevalence of prehypertension in salt-preferring young Indian subjects. Furthermore, spectral analysis of heart rate variability showed that sympathovagal imbalance was more intense in salt-preferring prehypertensives than in salt-preferring normotensives. Thus salt-preference has been linked to sympathovagal imbalance caused by sympathetic overactivity and vagal withdrawal. Therefore, salt-preferring subjects should be encouraged to restrict salt intake to maintain their sympathovagal balance and BP homeostasis.87
9. Salt sensitivity: what to do about it?
At this moment, definitive management strategy for salt sensitivity per se or the treatment of HTN in salt sensitive patients is unclear. Documenting the existence of salt sensitivity undoubtedly will be of critical importance to ultimate treatment plan. A greater understanding of the risk factors such as salt sensitivity that account for an increase in HTN burden or uncontrolled HTN could potentially contribute to its future prevention by addressing its root cause. It has been hypothesized that a low-salt educational intervention customized to cultural practices and needs of population along with standard HTN medication management would contribute in a big way towards greater BP reduction than standard care alone. Recent observation that treatment with the specific angiotensin receptor blocker (ARB), Azilsartan may improve salt sensitivity in mice by selectively reducing renal proximal tubule Na+/H+ exchange encourages the future potential of recognising and effectively treating the salt sensitive phenotype in humans.88 At the moment, an integrated approach to BP reduction offers the best option for HTN management in patients with salt sensitivity.
9.1. Possible role of dietary modifications
Reduction in salt intake at a population level to reduce BP would appear to be the most cost-effective method to lower the burden of HTN and thereby CV disease. The Chennai Urban Rural Epidemiology Study (CURES), a population based study in India has observed that mean dietary salt intake (8.5 g per day) in this population was higher than that recommended by the World Health Organization (<5 g per day).89 In UK and Europe, based on the relationship of salt intake with BP and CV disease, salt intake of less than 6 g per day (∼100 mmol Na per day) is recommended.90 On the other hand, a NICE guideline has recommended even greater restrictions to below 60 mmol Na per day. Nonetheless, a recent systematic review suggested an adverse effect of salt intakes as low as 3.5 g per day.91 A greater BP lowering effect of salt restriction in hypertensive compared with normotensive subject has been observed in meta-analyses of salt restriction studies. When salt intake is reduced to 100 mmol per day, average reduction in BP by 7/4 mm Hg can be achieved.92 Moreover, INTERSALT study established the positive benefits of reducing dietary salt intake on BP reduction in normotensive people.7 Results of the DASH trial provide additional confirmation that dietary salt reduction significantly reduces BP in both hypertensive and normotensive populations.18 The available evidence has shown that a universal reduction in dietary intake of sodium by 50 mmol per day (equivalent to 1.2 g of salt per day) would not only lead to a 50% reduction in the number of people requiring anti-hypertensive therapy but also to 22% reduction in number of deaths due to stroke and a 16% reduction in number of deaths from coronary artery disease.93
In a supplementary study to DASH-Sodium trial, Akita et al. investigated the effects of DASH diet on the pressure-natriuresis relationship.94 It was observed that BP of subjects on DASH diet was much less sodium-sensitive than those who were on control diet. It has been postulated that the beneficial effects of DASH diet in reducing the BP in salt sensitive subjects is mainly attributable to ability of DASH diet to make them sodium-insensitive through its diuretic action. The authors inferred that the increased slope of the pressure-natriuresis curve ascertains a natriuretic action of the DASH diet and possibly linked to its high potassium and calcium content.94 Increasing potassium consumption in the form of fruits like banana and vegetable has a beneficial effect of reducing health consequences in salt sensitive patients.
Basu et al. in their study on a mathematical model of dietary salt intake, BP and subsequent myocardial infarction (MI) and stroke events, found that nearly 400,000 cases and about 81,000 deaths from MI and stroke could be reduced in India by reducing the dietary salt by 3 g over 30 years.95 This approximates the overall reduction in the mortality due to MI and stroke by 6.7% and 8.8% respectively among different subpopulations of the country. Thus, moderate reductions in dietary salt intake could considerably reduce CV morbidity and mortality in Indian population.95
However, despite of potential benefits of reducing dietary salt intake on controlling BP and reducing CV morbidity and mortality, adherence to dietary advice is frequently poor, resulting in smaller changes (<1 mm Hg) in BP. This demands a relook at other strategies including antihypertensive agents which can be an alternative to salt restriction.
9.2. Antihypertensive agent with proven benefits in salt sensitivity
In the last 6 decades, since the introduction of the thiazide diuretics, many classes of antihypertensive drugs have been approved for use in HTN. Diuretics, beta-receptor blockers, angiotensin-converting–enzyme (ACE) inhibitors, calcium-channel blockers, and ARBs are the primary treatment options. Among them, ARBs have been recommended by various guidelines as one of the first choice due to their favourable effects in reducing CV morbidity and mortality. Furthermore, ARBs alone or in combination are considered among the best available therapeutic options for the treatment of HTN even in patients with compelling indications, such as heart failure, diabetes and previous MI.96 In the light of escalating burden of salt sensitivity, its impact on control of BP and CV mortality coupled with failure of patient population to comply with dietary recommendations, it has become imperative to research an antihypertensive agent which can better handle this complex entity.
9.2.1. Azilsartan and salt sensitivity
In past, RAAS blockers had been considered unfavourable for the treatment of salt-sensitive HTN as the preliminary findings with earlier RAAS blockers were not encouraging. Initial observations by Herlitz et al., Fang et al. and Xu et al. had shown that the antihypertensive effects of chronic enalapril, captopril and losartan treatment were apparently nullified with high salt loading in hypertensive patients and animal models.97, 98, 99 In fact, it has been reported that treatment with RAAS blockers (captopril and olmesartan) enhanced salt sensitivity.100, 101 However, recent data suggests that a newly approved ARB; Azilsartan improved salt sensitivity. In a study by Hatanaka et al. in salt sensitive hypertensive mice, urinary fractional excretion of sodium with Azilsartan was significantly higher than the candesartan and vehicle groups (Azilsartan: 21.37 ± 3.69%; candesartan: 14.17 ± 1.42%; vehicle: 13.85 ± 5.30%; P < 0.05 Azilsartan vs. candesartan or vehicle).88 Also, pressure-natriuresis curve confirmed that Azilsartan treatment restored salt sensitivity. To unravel the mechanism of improved natriuresis by Azilsartan, authors assessed expression of the major tubular sodium transporter in proximal tubules (Na+-H+ exchanger-3, NHE3), NKCC2 in the loop of Henle, NCC in distal tubules, and ENaC in the collecting ducts in mice on a high-salt diet. It was also observed that expression of NHE3 was decreased via ubiquitin-proteasomal degradation by Azilsartan.88
This observation of reduction in salt sensitivity with Azilsartan, by selectively reducing renal proximal tubule Na+/H+ exchange is well supported by the findings that NHE3 expression is decreased and natriuresis is increased in proximal tubule-specific angiotensin II type-1a receptor (AT1aR) knockout mice.102 The decreased NHE3 expression could contribute to natriuresis.
Crosstalk exists between AT1R and AT2R, and they have counter-regulatory functions in several systems, especially the CV system.103 Growing evidence suggests that AT1R mediates the classical actions of AT II (e.g. vasoconstriction and CV hypertrophy), whereas AT2R is directly involved in vasodilation and anti-growth effects.104 However, in a study by Hatanaka et al, the effect of Azilsartan on NHE3 protein expression was not affected by the AT2R antagonist PD123319.88 This suggested that Azilsartan augmented NHE3 ubiquitination via AT1R blockade and not by counter-stimulation of the AT2R.88
10. Summary
Despite of continued research, HTN continues to be a major public health problem and the global burden of HTN is mounting day by day. Over the past century, salt has been the subject of intense scientific research linked to HTN and CV mortality. By way of neuro-hormonal and hemodynamic responses, human body has unparalleled capability to adjust to the extremes of salt intake. There is now consensus that different individuals have different susceptibilities to the BP-raising effects of salt. While BP in the population as a whole is only modestly affected by the changes in salt intake, some individuals in response to acute or chronic salt depletion or repletion show large BP changes and are called “salt sensitive”. Salt sensitivity appears to be a multifactorial entity with strong determinants being genetic factors, race/ethnicity, age, gender, body mass index, associated co-morbidities and diet. Salt sensitivity is an independent risk factor for CV disease and mortality above and beyond that conferred by mere HTN. Various mechanisms; blunted activity of the RAAS, adrenal Rac1-MR-Sgk1-NCC/ENaC pathway, renal SNS-GR-WNK4-NCC pathway, deficiency in ANP expression, primary baroreceptor dysfunction, inflammation, aberrant adiponectin response or its polymorphism, defect of membrane ion transportation and abnormalities of Na+/Ca2+ exchange have been implicated as patho-physiological basis for salt sensitive HTN. A low-salt educational intervention customised to individual cultures, practices and needs of population along with standard HTN medication management has been postulated to result in greater BP reduction than standard care alone. Recent observation that treatment with the specific ARB, Azilsartan may improve salt sensitivity by selectively reducing renal proximal tubule Na+/H+ exchange encourages the future potential benefits of recognising and therapeutically addressing the salt sensitive phenotype in humans. An integrated approach, involving multiple successful approaches to BP reduction offers the best option for HTN management in patients with salt sensitivity.
Conflicts of interest
None.
References
- 1.Pickering G. 2nd ed. J&A Churchill; London, UK: 1968. High blood pressure; p. 229. [Google Scholar]
- 2.Franco O.H., Peeters A., Bonneux L., de Laet C. Blood pressure in adulthood and life expectancy with cardiovascular disease in men and women. Hypertension. 2005;46:280–286. doi: 10.1161/01.HYP.0000173433.67426.9b. [DOI] [PubMed] [Google Scholar]
- 3.Franco V., Oparil S. Salt sensitivity, a determinant of blood pressure, cardiovascular disease and survival. J Am Coll Nutr. 2006;25(Suppl. 3):247S–255S. doi: 10.1080/07315724.2006.10719574. [DOI] [PubMed] [Google Scholar]
- 4.Grollman A. The role of salt in health and disease. Am J Cardiol. 1961;8:593–601. [PubMed] [Google Scholar]
- 5.Angeloni E. Azilsartan medoxomil in the management of hypertension: an evidence-based review of its place in therapy. Core Evid. 2016;11:1–10. doi: 10.2147/CE.S81776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.INTERSALT Cooperative Research Group Intersalt: an international study of electrolyte excretion and blood pressure: results for 24-hour urinary sodium and potassium excretion. BMJ. 1998;297(6644):319–328. doi: 10.1136/bmj.297.6644.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Elliott P., Stamler J., Nichols R. Intersalt revisited: further analyses of 24-hour sodium excretion and blood pressure within and across populations. BMJ. 1996;312:1249–1253. doi: 10.1136/bmj.312.7041.1249. INTERSALT Cooperative Research Group. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Luft F.C., Weinberger M.H. Heterogenous responses to changes in dietary salt intake: the salt-sensitivity paradigm. Am J Clin Nutr. 1997;65(Suppl. 2):612S–617S. doi: 10.1093/ajcn/65.2.612S. [DOI] [PubMed] [Google Scholar]
- 9.Weinberger M.H., Fineberg N.S. Sodium and volume sensitivity of blood pressure: age and pressure change over time. Hypertension. 1991;18:67–71. doi: 10.1161/01.hyp.18.1.67. [DOI] [PubMed] [Google Scholar]
- 10.Elijovich F., Weinberger M.H., Anderson C.A.M. Salt sensitivity of blood pressure a scientific statement from the American Heart Association. Hypertension. 2016;68:e7–e46. doi: 10.1161/HYP.0000000000000047. [DOI] [PubMed] [Google Scholar]
- 11.Fujita Toshiro. Mechanism of salt-sensitive hypertension: focus on adrenal and sympathetic nervous systems. J Am Soc Nephrol. 2014;25:1148–1155. doi: 10.1681/ASN.2013121258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weinberger M.H., Miller J.Z., Luft F.C., Grim C.E., Fineberg N.S. Definitions and characteristics of sodium sensitivity and blood pressure resistance. Hypertension. 1986;8(6 pt 2):II127–II134. doi: 10.1161/01.hyp.8.6_pt_2.ii127. [DOI] [PubMed] [Google Scholar]
- 13.Weinberger M.H. Salt sensitivity of blood pressure in humans. Hypertension. 1996;27(pt 2):481–490. doi: 10.1161/01.hyp.27.3.481. [DOI] [PubMed] [Google Scholar]
- 14.Weinberger M.H., Luft F.C., Bloch R. The blood pressure-raising effects of high dietary sodium intake: racial differences and the role of potassium. J Am Coll Nutr. 1982;1:139–148. doi: 10.1080/07315724.1982.10718981. [DOI] [PubMed] [Google Scholar]
- 15.He J., Gu D., Chen J. Gender difference in blood pressure responses to dietary sodium intervention in the GenSalt study. J Hypertens. 2009;27:48–54. doi: 10.1097/hjh.0b013e328316bb87. GenSalt Collaborative Research Group. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kario K. Evidence and perspectives on the 24-hour management of hypertension: hemodynamic biomarker-initiated ‘anticipation medicine’ for zero cardiovascular event. Prog Cardiovasc Dis. 2016;59(3):262–281. doi: 10.1016/j.pcad.2016.04.001. [DOI] [PubMed] [Google Scholar]
- 17.Morris R.C., Jr, Sebastian A., Forman A., Tanaka M., Schmidlin O. Normotensive salt sensitivity: effects of race and dietary potassium. Hypertension. 1999;33:18–23. doi: 10.1161/01.hyp.33.1.18. [DOI] [PubMed] [Google Scholar]
- 18.Sacks F.M., Svetkey L.P., Vollmer W.M. Effects on blood pressure of reduced dietary sodium and the dietary approaches to stop hypertension (DASH) diet. N Engl J Med. 2001;344(1):3–10. doi: 10.1056/NEJM200101043440101. DASH-sodium collaborative research group. [DOI] [PubMed] [Google Scholar]
- 19.Katsuya T., Ishikawa K., Sugimoto K., Rakugi H., Ogihara T. Salt sensitivity of Japanese from the viewpoint of gene polymorphism. Hypertens Res. 2003;26:521–525. doi: 10.1291/hypres.26.521. [DOI] [PubMed] [Google Scholar]
- 20.Lifton R.P., Gharavi A.G., Geller D.S. Molecular mechanisms of human hypertension. Cell. 2001;104:545–556. doi: 10.1016/s0092-8674(01)00241-0. [DOI] [PubMed] [Google Scholar]
- 21.Weinberger M.H. Pathogenesis of salt sensitivity of blood pressure. Curr Hypertens Rep. 2006;8:166–170. doi: 10.1007/s11906-006-0014-y. [DOI] [PubMed] [Google Scholar]
- 22.Sanada H., Jones J.E., Jose P.A. Genetics of salt sensitive hypertension. Curr Hypertens Rep. 2011;13:55–66. doi: 10.1007/s11906-010-0167-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miller J.Z., Weinberger M.H., Christian J.C., Daugherty S.A. Familial resemblance in blood pressure response to sodium restriction. Am J Epidemiol. 1987;126:822–830. doi: 10.1093/oxfordjournals.aje.a114719. [DOI] [PubMed] [Google Scholar]
- 24.Wain L.V., Verwoert G.C., O’Reilly P.F. Genome-wide association study identifies six new loci influencing pulse pressure and mean arterial pressure. Nat Genet. 2011;43:1005–1011. doi: 10.1038/ng.922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kelly T.N., He J. Genomic epidemiology of blood pressure salt sensitivity. J Hypertens. 2012;30:861–873. doi: 10.1097/HJH.0b013e3283524949. [DOI] [PubMed] [Google Scholar]
- 26.Carey R.M., Schoeffel C.D., Gildea J.J. Salt sensitivity of blood pressure is associated with polymorphisms in the sodium bicarbonate co-transporter. Hypertension. 2012;60:1359–1366. doi: 10.1161/HYPERTENSIONAHA.112.196071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pereira T.V., Rudnicki M., Cheung B.M. Three endothelial nitric oxide (NOS3) gene polymorphisms in hypertensive and normotensive individuals: meta-analysis of 53 studies reveals evidence of publication bias. J Hypertens. 2007;25:1763–1774. doi: 10.1097/HJH.0b013e3281de740d. [DOI] [PubMed] [Google Scholar]
- 28.Carmosino M., Rizzo F., Ferrari P. NKCC2 is activated in Milan hypertensive rats contributing to the maintenance of salt-sensitive hypertension. Pflugers Arch. 2011;462(2):281–291. doi: 10.1007/s00424-011-0967-9. [DOI] [PubMed] [Google Scholar]
- 29.Mu S., Shimosawa T., Ogura S. Epigenetic modulation of the renal beta-adrenergic-WNK4 pathway in salt-sensitive hypertension. Nat Med. 2011;17(5):573–580. doi: 10.1038/nm.2337. [DOI] [PubMed] [Google Scholar]
- 30.Li C., He J., Chen J. Genome-wide gene–sodium interaction analyses on blood pressure. Hypertension. 2016;68:348–355. doi: 10.1161/HYPERTENSIONAHA.115.06765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sullivan J.M. Salt sensitivity. Definition, conception, methodology, and long-term issues. Hypertension. 1991;17(Suppl. 1):I61–I68. doi: 10.1161/01.hyp.17.1_suppl.i61. [DOI] [PubMed] [Google Scholar]
- 32.Weinberger Myron H., Fineberg Naomi S., Edwin Fineberg S., Weinberger Morris. Salt sensitivity, pulse pressure, and death in normal and hypertensive humans. Hypertension. 2001;37(part 2):429–432. doi: 10.1161/01.hyp.37.2.429. [DOI] [PubMed] [Google Scholar]
- 33.Uzu T., Kazembe F.S., Ishikawa K., Nakamura S., Inenaga T., Kimura G. High sodium sensitivity implicates nocturnal hypertension in essential hypertension. Hypertension. 1996;28(1):139–142. doi: 10.1161/01.hyp.28.1.139. [DOI] [PubMed] [Google Scholar]
- 34.Uzu T., Ishikawa K., Fujii T., Nakamura S., Inenaga T., Kimura G. Sodium restriction shifts circadian rhythm of blood pressure from non-dipper to dipper in essential hypertension. Circulation. 1997;96(6):1859–1862. doi: 10.1161/01.cir.96.6.1859. [DOI] [PubMed] [Google Scholar]
- 35.Kario K. Morning surge in blood pressure and cardiovascular risk: evidence and perspectives. Hypertension. 2010;56(5):765–773. doi: 10.1161/HYPERTENSIONAHA.110.157149. [DOI] [PubMed] [Google Scholar]
- 36.Ohkubo T., Hozawa A., Yamaguchi J. Prognostic significance of the nocturnal decline in blood pressure in individuals with and without high 24-h blood pressure: the Ohasama study. J Hypertens. 2002;20(11):2183–2189. doi: 10.1097/00004872-200211000-00017. [DOI] [PubMed] [Google Scholar]
- 37.Morimoto A., Uzu T., Fujii T. Sodium sensitivity and cardiovascular events in patients with essential hypertension. Lancet. 1997;350(9093):1734–1737. doi: 10.1016/S0140-6736(97)05189-1. [DOI] [PubMed] [Google Scholar]
- 38.Pimenta E., Gaddam K.K., Oparil S. Effects of dietary sodium reduction on blood pressure in subjects with resistant hypertension: results from a randomized trial. Hypertension. 2009;54:475–481. doi: 10.1161/HYPERTENSIONAHA.109.131235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ganda O.P., Fonseca V.A. Salt sensitivity, insulin resistance, and public health in India. Endocr Pract. 2010;16(6):940–944. doi: 10.4158/EP10103.OR. [DOI] [PubMed] [Google Scholar]
- 40.Bihorac A., Tezcan H., Ozener C., Oktay A., Akoglu E. Association between salt sensitivity and target organ damage in essential hypertension. Am J Hypertens. 2000;13:864–872. doi: 10.1016/s0895-7061(00)00253-3. [DOI] [PubMed] [Google Scholar]
- 41.Bigazzi R., Bianchi S., Baldari D., Sgherri G., Baldari G., Campese V.M. Microalbuminuria in salt-sensitive patients: a marker for renal and cardiovascular risk factors. Hypertension. 1994;23:195–199. doi: 10.1161/01.hyp.23.2.195. [DOI] [PubMed] [Google Scholar]
- 42.Kopp C., Linz P., Dahlmann A. 23Na magnetic resonance imaging-determined tissue sodium in healthy subjects and hypertensive patients. Hypertension. 2013;61:635–640. doi: 10.1161/HYPERTENSIONAHA.111.00566. [DOI] [PubMed] [Google Scholar]
- 43.Wiig H., Schroder A., Neuhofer W. Immune cells control skin lymphatic electrolyte homeostasis and blood pressure. J Clin Invest. 2013;123:2803–2815. doi: 10.1172/JCI60113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Graudal N.A., Galloe A.M., Garred P. Effects of sodium restriction on blood pressure, renin, aldosterone, catecholamines, cholesterols, and triglycerides. JAMA. 1998;279:1383–1391. doi: 10.1001/jama.279.17.1383. [DOI] [PubMed] [Google Scholar]
- 45.Rasmussen M.S., Simonsen J.A., Sandgaard N.C.F., Hoilund-Carlsen P.F., Bie P. Mechanisms of acute natriuresis in normal humans on low sodium diet. J Physiol. 2003;546:591–603. doi: 10.1113/jphysiol.2002.027425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Parfrey P.S., Markandu N.D., Roulston J.E., Jones B.E., Jones J.C., MacGregor G.A. Relation between arterial pressure, dietary sodium intake, and renin system in essential hypertension. Br Med J (Clin Res Ed) 1981;283:94–97. doi: 10.1136/bmj.283.6284.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cappuccio F.P., Markandu N.D., Sagnella G.A., MacGregor G.A. Sodium restriction lowers high blood pressure through a decreased response of the renin system: direct evidence using saralasin. J Hypertens. 1985;3:243–247. doi: 10.1097/00004872-198506000-00008. [DOI] [PubMed] [Google Scholar]
- 48.Yatabe M.S., Yatabe J., Yoneda M. Salt sensitivity is associated with insulin resistance, sympathetic over activity, and decreased suppression of circulating renin activity in lean patients with essential hypertension [published correction appears in Am J ClinNutr, 2010;92:1002] Am J Clin Nutr. 2010;92:77–82. doi: 10.3945/ajcn.2009.29028. [DOI] [PubMed] [Google Scholar]
- 49.Laffer C.L., Elijovich F. Differential predictors of insulin resistance in non-diabetic salt-resistant and salt-sensitive subjects. Hypertension. 2013;61:707–715. doi: 10.1161/HYPERTENSIONAHA.111.00423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Laffer C.L., Laniado-Schwartzman M., Wang M.H., Nasjletti A., Elijovich F. 20-HETE and furosemide-induced natriuresis in salt-sensitive essential hypertension. Hypertension. 2003;41(pt 2):703–708. doi: 10.1161/01.HYP.0000051888.91497.47. [DOI] [PubMed] [Google Scholar]
- 51.Elijovich F., Laffer C.L., Schiffrin E.L., Gavras H., Amador E. Endothelin aldosterone interaction and proteinuria in low-renin hypertension. J Hypertens. 2004;22:573–582. doi: 10.1097/00004872-200403000-00021. [DOI] [PubMed] [Google Scholar]
- 52.Gonzalez-Villalobos R.A., Billet S., Kim C. Intrarenal angiotensin-converting enzyme induces hypertension in response to angiotensin I infusion. J Am Soc Nephrol. 2011;22:449–459. doi: 10.1681/ASN.2010060624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gonzalez-Villalobos R.A., Janjoulia T., Fletcher N.K. The absence of intrarenal ACE protects against hypertension. J Clin Invest. 2013;123:2011–2023. doi: 10.1172/JCI65460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Crowley S.D., Gurley S.B., Oliverio M.I. Distinct roles for the kidney and systemic tissues in blood pressure regulation by the renin angiotensin system. J Clin Invest. 2005;115:1092–1099. doi: 10.1172/JCI200523378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Keszei A.P., Tislér A., Backx P.H., Andrulis I.L., Bull S.B., Logan A.G. Molecular variants of the thiazide-sensitive Na+-Cl− co-transporter in hypertensive families. J Hypertens. 2007;25:2074–2081. doi: 10.1097/HJH.0b013e3282a9be1b. [DOI] [PubMed] [Google Scholar]
- 56.Shibata S., Mu S., Kawarazaki H. Rac1 GTPase in rodent kidneys is essential for salt-sensitive hypertension via a mineralocorticoid receptor-dependent pathway. J Clin Invest. 2011;121:3233–3243. doi: 10.1172/JCI43124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mu S.Y., Shimosawa T., Ogura S. Epigenetic modulation of the renal b-adrenergic-WNK4 pathway in salt-sensitive hypertension. Nat Med. 2011;17:573–580. doi: 10.1038/nm.2337. [DOI] [PubMed] [Google Scholar]
- 58.Fujita T. Mineralocorticoid receptors, salt-sensitive hypertension and metabolic syndrome. Hypertension. 2010;55:813–818. doi: 10.1161/HYPERTENSIONAHA.109.149062. [DOI] [PubMed] [Google Scholar]
- 59.Nagae A., Fujita M., Kawarazaki H., Matsui H., Ando K., Fujita T. Sympatho-excitation by oxidative stress in the brain mediates arterial pressure elevation in obesity-induced hypertension. Circulation. 2009;119:978–986. doi: 10.1161/CIRCULATIONAHA.108.824730. [DOI] [PubMed] [Google Scholar]
- 60.Jacob F., Clark L.A., Guzman P.A., Osborn J.W. Role of renal nerves in development of hypertension in DOCA-salt model in rats: a telemetric approach. Am J Physiol Heart Circ Physiol. 2005;289:H1519–H1529. doi: 10.1152/ajpheart.00206.2005. [DOI] [PubMed] [Google Scholar]
- 61.DiBona G.F. Physiology in perspective: the wisdom of the body. Neural control of the kidney. Am J Physiol Regul Integr Comp Physiol. 2005;289:R633–R641. doi: 10.1152/ajpregu.00258.2005. [DOI] [PubMed] [Google Scholar]
- 62.Snyder E.M., Turner S.T., Joyner M.J., Eisenach J.H., Johnson B.D. The Arg16Gly polymorphism of the b2-adrenergic receptor and the natriuretic response to rapid saline infusion in humans. J Physiol. 2006;574:947–954. doi: 10.1113/jphysiol.2006.107672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Feng J.A., Perry G., Mori T., Harashi T., Oparil S., Chen Y.F. Pressure independent enhancement of cardiac hypertrophy in atrial natriuretic peptide-deficient mice. Clin Exp Pharmacol Physiol. 2003;30:343–349. doi: 10.1046/j.1440-1681.2003.03836.x. [DOI] [PubMed] [Google Scholar]
- 64.John S.W.M., Krege J.H., Oliver P.M. Genetic decreases in atrial natriuretic peptide and salt-sensitive hypertension. Science. 1995;267:679–681. doi: 10.1126/science.7839143. [DOI] [PubMed] [Google Scholar]
- 65.Rutledge D.R., Sun Y., Ross E.A. Polymorphisms within the atrial natriuretic peptide gene in essential hypertension. J Hypertens. 1995;13:953–955. doi: 10.1097/00004872-199509000-00003. [DOI] [PubMed] [Google Scholar]
- 66.Heymans C., Neil E. Reflexogenic areas of the cardiovascular system. Postgrad Med J. 1959;35(401):160–161. [Google Scholar]
- 67.Thrasher T.N. Unloading arterial baroreceptors causes neurogenic hypertension. Am J Physiol Regul Integr Comp Physiol. 2002;282(4):R1044–53. doi: 10.1152/ajpregu.00431.2001. [DOI] [PubMed] [Google Scholar]
- 68.Lohmeier T.E., Hildebrandt D.A. Renal nerves promote sodium excretion in angiotensin-induced hypertension. Hypertension. 1998;31:429–434. doi: 10.1161/01.hyp.31.1.429. [DOI] [PubMed] [Google Scholar]
- 69.Kanbay M., Chen Y., Solak Y., Sanders P.W. Mechanisms and consequences of salt sensitivity and dietary salt intake. Curr Opin Nephrol Hypertens. 2011;20(1):37–43. doi: 10.1097/MNH.0b013e32834122f1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Guzik T.J., Hoch N.E., Brown K.A. Role of the T cell in the genesis of angiotensin II induced hypertension and vascular dysfunction. J Exp Med. 2007;204(10):2449–2460. doi: 10.1084/jem.20070657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Viel E.C., Lemarié C.A., Benkirane K., Paradis P., Schiffrin E.L. Immune regulation and vascular inflammation in genetic hypertension. Am J Physiol Heart Circ Physiol. 2010;298(3):H938–H944. doi: 10.1152/ajpheart.00707.2009. [DOI] [PubMed] [Google Scholar]
- 72.Rudemiller N.P., Lund H., Priestley J.R.C. Mutation of SH2B3 (LNK), a GWAS candidate for hypertension, attenuates Dahl SS hypertension via inflammatory modulation. Hypertension. 2015;65(5):1111–1117. doi: 10.1161/HYPERTENSIONAHA.114.04736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Fuqiang L., Chu C., Mu J. The association of adiponectin or its polymorphism with salt sensitivity: a human dietary intervention study. J Am Coll Cardiol. 2016;68(Suppl. 16) [Google Scholar]
- 74.Iwamoto T. Salt-sensitive hypertension and Na+/Ca2+ exchange: old and new mechanisms for linking high salt intake to vascular tone. Nihon Yakurigaku Zasshi. 2006;127:387–392. doi: 10.1254/fpj.127.387. [DOI] [PubMed] [Google Scholar]
- 75.Linde C.I., Antos L.K., Golovina V.A., Blaustein M.P. Nanomolar ouabain increases NCX1 expression and enhances Ca2+ signaling in human arterial myocytes: a mechanism that links salt to increased vascular resistance. Am J Physiol Heart Circ Physiol. 2012;303:H784–H794. doi: 10.1152/ajpheart.00399.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Popov S., Silveira A., Wågsäter D. Salt-inducible kinase 1 influences Na(+), K(+)-ATPase activity in vascular smooth muscle cells and associates with variations in blood pressure. J Hypertens. 2011;29:2395–2403. doi: 10.1097/HJH.0b013e32834d3d55. [DOI] [PubMed] [Google Scholar]
- 77.Iwamoto T. Na+/Ca2+ exchanger (NCX1) and salt-sensitive hypertension. Nihon Rinsho. 2006;64:167–176. [PubMed] [Google Scholar]
- 78.Hauck C., Frishman W.H. Systemic hypertension: the roles of salt, vascular Na+/K+ ATPase and the endogenous glycosides, ouabain and marinobufagenin. Cardiol Rev. 2012;20:130–138. doi: 10.1097/CRD.0b013e31823c835c. [DOI] [PubMed] [Google Scholar]
- 79.Guo Y., Shang Q., Wu Q., Jiang Q., Zhang G. Effects of lisinopril on the activities and mRNA expression of ion pumps in aortic smooth muscle cells from spontaneously hypertensive rats. Chin J Arterioscler. 2010;18:441–444. [Google Scholar]
- 80.Jiang Q., Yin Y. Impacts of telmisartan and ramipril on Na+-K+-ATPase and Ca2+-ATPase in thoracic aortic smooth muscle cells of salt-sensitive hypertensive rat. Int J Clin Exp Med. 2016;9(6):11009–11015. [Google Scholar]
- 81.Furchgott R.F., Zawadzki J.V. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980;288:373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- 82.Bragulat E., Sierra A. Salt intake, endothelial dysfunction, and salt-sensitive hypertension. J Clin Hypertens. 2002;4:41–46. doi: 10.1111/j.1524-6175.2002.00503.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Edwards D.G., Farquhar W.B. Vascular effects of dietary salt. Curr Opin Nephrol Hypertens. 2015;24:8–13. doi: 10.1097/MNH.0000000000000089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Choi H.Y., Park H.C., Ha S.K. Salt sensitivity and hypertension a paradigm shift from kidney malfunction to vascular endothelial dysfunction. Electrolyte Blood Press. 2015;13:7–16. doi: 10.5049/EBP.2015.13.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Matsumoto S., Shimabukuro M., Fukuda D. Azilsartan, an angiotensin II type 1 receptor blocker, restores endothelial function by reducing vascular inflammation and by increasing the phosphorylation ratio Ser1177/Thr497 of endothelial nitric oxide synthase in diabetic mice. Cardiovasc Diabetol. 2014;13:30. doi: 10.1186/1475-2840-13-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Araújo A.A., Varela H., Medeiros C.A. Azilsartan reduced TNF-α and IL-1β levels, increased IL-10 levels and upregulated VEGF, FGF, KGF, and TGF-α in an oral mucositis model. PLoS One. 2015;10(2):e0116799. doi: 10.1371/journal.pone.0116799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Pal G.K., Adithan C., Dutta T.K. Preference for salt contributes to sympathovagal imbalance in the genesis of prehypertension. Eur J Clin Nutr. 2013;67:586–591. doi: 10.1038/ejcn.2013.64. [DOI] [PubMed] [Google Scholar]
- 88.Hatanaka M., Kaimori J.Y., Yamamoto S. Azilsartan improves salt sensitivity by modulating the proximal tubular Na+-H+ exchanger-3 in mice. PLoS One. 2016;11(1):e0147786. doi: 10.1371/journal.pone.0147786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Radhika G., Sathya R.M., Sudha V., Ganesan A., Mohan V. Dietary salt intake and hypertension in an urban south Indian population – [CURES 53] J Assoc Physicians India. 2007;55:405–411. [PubMed] [Google Scholar]
- 90.FS Agency . Medical Research Council. Human Nutrition Research; 2005. Why 6g? A summary of the scientific evidence for the salt intake target.http://www.food.gov.uk/ Available from: [Google Scholar]
- 91.Taylor R.S., Ashton K.E., Moxham T., Hooper L., Ebrahim S. Reduced dietary salt for the prevention of cardiovascular disease. Cochrane Database Syst Rev. 2011;7:CD009217. doi: 10.1002/14651858.CD009217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.He F.J., MacGregor G.A. Effect of modest salt reduction on blood pressure: a meta-analysis of randomized trials. Implications for public health. J Hum Hypertens. 2002;16:761–770. doi: 10.1038/sj.jhh.1001459. [DOI] [PubMed] [Google Scholar]
- 93.Reddy K.S., Katan B.M. Diet, nutrition and the prevention of hypertension and cardiovascular diseases. Public Health Nutr. 2004;7:167–186. doi: 10.1079/phn2003587. [DOI] [PubMed] [Google Scholar]
- 94.Akita S., Sacks F.M., Svetkey L.P., Conlin P.R., Kimura G. Effects of the Dietary Approaches to Stop Hypertension (DASH) diet on the pressure-natriuresis relationship. Hypertension. 2003;42:8–12. doi: 10.1161/01.HYP.0000074668.08704.6E. DASH-Sodium Trial Collaborative Research Group. [DOI] [PubMed] [Google Scholar]
- 95.Basu S., Stuckler D., Vellakkal S., Ebrahim S. Dietary salt reduction and cardiovascular disease rates in India: a mathematical model. PLoS One. 2012;7(9):e44037. doi: 10.1371/journal.pone.0044037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.National Institutes of Health, National Heart, Lung, and Blood Institute . US Department of Health and Human Services; Bethesda, MD: 2004. The seventh report of the Joint National Committee on Prevention, Detection, Evaluation and Treatment of High Blood Pressure.http://www.nhlbi.nih.gov/guidelines/hypertension/jnc7full.pdf Available from: [PubMed] [Google Scholar]
- 97.Herlitz H., Dahlof B., Jonsson O., Friberg P. Relationship between salt and blood pressure in hypertensive patients on chronic ACE-inhibition. Blood Press. 1998;7(1):47–52. doi: 10.1080/080370598437565. [DOI] [PubMed] [Google Scholar]
- 98.Fang Z., Sripairojthikoon W., Calhoun D.A., Zhu S., Berecek K.H., Wyss J.M. Interaction between lifetime captopril treatment and NaCI-sensitive hypertension in spontaneously hypertensive rats and Wistar-Kyoto rats. J Hypertens. 1999;17(7):983–991. doi: 10.1097/00004872-199917070-00015. [DOI] [PubMed] [Google Scholar]
- 99.Xu L., Brooks V.L. Sodium intake, angiotensin II receptor blockade: and baroreflex function in conscious rats. Hypertension. 1997;29(1 Pt 2):450–457. doi: 10.1161/01.hyp.29.1.450. [DOI] [PubMed] [Google Scholar]
- 100.Endo S., Mori T., Yoneki Y. Blockade of angiotensin II type-1 receptor increases salt sensitivity in Sprague-Dawley rats. Hypertens Res. 2009;32(6):513–519. doi: 10.1038/hr.2009.40. [DOI] [PubMed] [Google Scholar]
- 101.Kimura G., Deguchi F., Kojima S. Antihypertensive drugs and sodium restriction. Analysis of their interaction based on pressure-natriuresis relationship. Am J Hypertens. 1988;1(4 Pt 1):372–379. doi: 10.1093/ajh/1.4.372. [DOI] [PubMed] [Google Scholar]
- 102.Gurley S.B., Riquier-Brison A.D., Schnermann J. AT1A angiotensin receptors in the renal proximal tubule regulate blood pressure. Cell Metab. 2011;13(4):469–475. doi: 10.1016/j.cmet.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hernandez Schulman I., Zhou M.S., Raij L. Cross-talk between angiotensin II receptor types 1 and 2: potential role in vascular remodelling in humans. Hypertension. 2007;49(2):270–271. doi: 10.1161/01.HYP.0000253966.21795.d3. [DOI] [PubMed] [Google Scholar]
- 104.Widdop R.E., Vinh A., Henrion D., Jones E.S. Vascular angiotensin AT2 receptors in hypertension and ageing. Clin Exp Pharmacol Physiol. 2008;35(4):386–390. doi: 10.1111/j.1440-1681.2008.04883.x. [DOI] [PubMed] [Google Scholar]