 Cordyceps militaris (C. militaris) is a parasitic fungus that grows on the larvae of Lepidoptera.
Cordyceps militaris (C. militaris) is a parasitic fungus that grows on the larvae of Lepidoptera.
Abstract
Cordyceps militaris (C. militaris) is a parasitic fungus that grows on the larvae of Lepidoptera. It is a well-known fungus with immunomodulatory activity. The study was conducted to clarify the edible safety of C. militaris mycelium for long term use. Eighty Sprague-Dawley (SD) rats were divided into four groups (10 males and 10 females in each group). Rats were orally administrated with reverse osmosis water or 2000, 3000 and 4000 mg per kg BW per day freeze dried C. militaris mycelium powder for 90 consecutive days. Clinical observation was carried out daily. The body weight and feed intake of the rats were recorded weekly. At the end of the study, all rats were sacrificed and the blood and organs were collected for hematology, clinical biochemistry and histopathological examination. All animals survived until the end of the study. During the study period, no abnormality occurred in clinical signs, body weight, feed intake, ophthalmological examination and urinalysis. There were no significant differences upon gross necropsy between the treatment and control group. Hematology, clinical biochemistry parameters and histopathological examination showed no treatment-related change. According to the results, the no-observed-adverse-effect level of C. militaris mycelium is 4000 mg per kg BW per day for male and female SD rats.
Introduction
Cordyceps militaris (C. militaris) (L.) Link is an entomopathogenic fungus belonging to the family Cordycipitaceae and the genus Cordyceps. C. militaris is the type species of Cordyceps, which internally parasitizes larva or pupa of lepidopteran insects and forms fruiting bodies on their insect hosts.1–3 It is one of the most important fungi used in traditional Chinese medicine for the treatment of asthma, and bronchial and lung inflammation.4C. militaris possesses extensive bioactive compounds including polysaccharides, cordycepin, adenosine, amino acids, organic selenium, ergosterol, sterols, cordycepic acid, superoxide dismutase (SOD), and multivitamins with significant pharmacological effects.5–7 C. militaris has been reported to display various biological activities such as anti-cancer,8 immunomodulatory, antioxidant,9 renal-protective,10 antifibrotic,11 antiangiogenetic,12,13 anti-inflammatory,13 and anti-diabetic14 activities.
C. militaris has long been recognized as a desirable alternative to Ophiocordyceps sinensis (O. sinensis) based on the similar compositions and bioactive effects of C. militaris and O. sinensis2,9,15–18 as it has been given the Chinese Licence number Z20030034/35. The demand for O. sinensis is continuously increasing because of its medicinal uses, while the wild resource is decreasing rapidly due to non-sustainable collection.19 Hence, O. sinensis is gradually being replaced by large amounts of cultivated C. militaris manufactured by fermentation technology in the marketplace.
For many years, C. militaris mycelium has beensold as a dietary supplement in many countries, including USA, Canada, Japan, Korea and China. The Taiwan Food and Drug Administration (TFDA) issued a notice of the amendment to “Daily consumption limit and Security Marked Warning of the ingredient ‘Cordyceps militaris fruiting body” draft on Jan 12, 2017. In addition to the C. militaris fruiting body, the manufacturing method and usage amount of C. militaris mycelium are also regulated. Our previous studies demonstrated that no toxic effect was observed in a sub-acute oral toxicity assay,20 3 different test systems of genotoxicity test21 and teratogenicity study.22 The results from these studies suggested that daily treatment with C. militaris mycelium at 3 g per kg BW per day did not induce observable toxicopathologic lesions in male and female rats. However, a 90-day subchronic toxicity study has not yet been conducted for a comprehensive safety profile of this potential mushroom. In the present study, we conducted a 90-day subchronic toxicological assessment of C. militaris mycelium in Sprague-Dawley (SD) rats to confirm the edible safety and evaluate the no-observed-adverse-effect level (NOAEL) of C. militaris mycelium for long term use.
Materials and methods
Preparation of freeze dried C. militaris mycelium powder
For 90-day subchronic toxicological assessment, C. militaris cultured on potato dextrose agar was transferred to a 2.0 L flask containing 1.0 L of PDB. The whole medium was cultivated at 25 °C for 5 days in a rotary shaker (120 rpm) for seed culturing prior to its scale-up production step. The fermented broth (1.0 L) was inoculated into a 500 L fermenter with 60% working volume (2% glucose, 1% yeast extract, 1% soybean powder; pH 6.0), and agitated at 60 rpm with an aeration rate of 0.5 vvm at 25 °C for 3 days. The submerged mycelial culture was heated at 100 °C for 1 h, freeze dried, and ground to powder.
Animals
Eighty 6-week-old SD rats were obtained from BioLASCO Taiwan Co., Ltd (Yilan, Taiwan). After quarantine and accommodation for one week, the rats were randomized based on their body weight, and then entered into the study. The rats had free access to a commercial rodent diet and sterile reverse osmosis water (R.O. water), and were maintained at controlled temperature (22 ± 3 °C), relative humidity (60 ± 10%) and light cycle (12 h light/12 h dark).
Study design
This study was performed based on the safety assessment guideline of Health Food announced by the Ministry of Health and Welfare (Taiwan). The protocol was approved by the Institutional Animal Care and Use Committee (IACUC No. MG103086) before the beginning of the study. The rats were randomly divided into four groups (n = 10 per sex per group) and orally administrated with C. militaris mycelium (2000, 3000 and 4000 mg per kg BW) for 90 days (daily). The body weights of the rats were measured prior to the study and once a week during the study. Measurement of feed intake for all rats was conducted weekly during the study. C. militaris mycelium was prepared freshly by dissolving freeze dried C. militaris mycelium powder into R.O. water to a concentration of 100 mg ml–1, 150 mg ml–1 and 200 mg ml–1, respectively. Clinical observation for all rats was conducted every day by recording abnormal clinical signs or death. At the end of the experiment, the rats were euthanized with CO2 asphyxiation and blood samples were collected.
Urinalysis
One day before scarification, all rats were placed in metabolic cages for 16 h in order to collect urine samples. We used a compact urine analyzer (Urisys 2400; Roche, Germany) to analyze color, glucose, bilirubin, ketone bodies, specific gravity, pH, protein, urobilinogen and occult blood. The sediments of the urine were observed for white blood cells (WBC), red blood cells (RBC), epithelial cells (EP), crystals, microbes etc. by microscopic examination.
Ophthalmology
Ophthalmology for all rats was conducted prior to the oral administration and at the end of the study. The external appearance and internal structure of the eyes were evaluated with the naked eye and an ophthalmoscope (Optotechnik K-180, Heine, Germany).
Hematology and serum biochemistry
After overnight fasting, all rats were euthanized with CO2 asphyxiation and blood samples were collected from the abdominal vein. We used an automated hematology analyzer (Sysmex Corporation XT-2000i, Japan) to detect red blood cells (RBC), hemoglobin (HGB), hematocrit (HCT), hemoglobin, mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), mean corpuscular hemoglobin concentration (MCHC), platelets (PLT), mean platelet volume (MPV), white blood cells (WBC), neutrophils (NEUT), lymphocytes (LYMPH), monocytes (MONO), eosinophils (EOS) and basophiles (BASO). Plasma samples were analyzed using an automated coagulated analyzer (Sysmex Corporation CA-1500, Japan) for prothrombin time (PT) and activated partial thromboplastin time (APTT). The following serum biochemistry parameters were analyzed by using an automated biochemistry analyzer (Johnson & Johnson Vitros 5.1 FS, USA): alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), total bilirubin (BIT), γ-glutamyl transpeptidase (GGT), total protein (TP), albumin (ALB), globulin (GLO), amylase (AMY), blood urea nitrogen (BUN), creatinine (CRE), creatine phosphokinase (CPK), glucose (GLU), total cholesterol (TC), triglyceride (TG), sodium (Na), potassium (K), calcium (Cl), calcium (Ca) and inorganic phosphorus (P).
Pathology
Necropsy examination for all rats was conducted at the end of the study. The outer appearance, oral cavity, cranial cavity and all tissues and organs in the thoracic and abdominal cavity were examined visually and recorded. Organ weights including the brain, heart, kidneys, liver, spleen, adrenal, testes or ovaries were measured after the removal of peripheral fat tissue. A histopathological test was performed for the control group and the high dose group to examine the adrenals, aorta, bone, bone marrow, brain, caecum, colon, duodenum, epididymis, esophagus, eyes, Harderian gland, heart, ileum, jejunum, kidneys, liver, lung, lymph nodes, mammary gland, optic nerve, ovaries, oviduct, pancreas, pituitary, prostate gland, rectum, salivary gland, sciatic nerve, seminal vesicle, skeletal muscle, skin, spinal cord, spleen, stomach, testes, thymus, thyroid gland, parathyroid gland, trachea, urinary bladder, uterus and vagina. The collected organs were fixed in 10% neutral formalin buffer. Preserved organs and tissues were dehydrated, clarified, infiltrated with paraffin and embedded after trimming, forming paraffin tissue blocks, and cut into 2 μm thickness of a tissue slice using a paraffin tissue slicing machine (Thermo Shandon Ltd Fitness 325, Cheshire, UK), and stained with Hematoxylin & Eosin (H&E). The histopathological changes were evaluated using an optical microscope (Olympus BX51, Tokyo, Japan). If treatment-related changes occurred in a particular organ or tissue in the high dose group, extended examination for the organ or tissue of medium dose and low dose groups was included.
Statistical analysis
Values are expressed as mean ± standard deviation (SD) and analyzed using one-way analysis of variance (ANOVA) followed by Dunnett's test for comparison of group means. A p value <0.05 is considered statistically significant.
Results
Body weight and feed intake
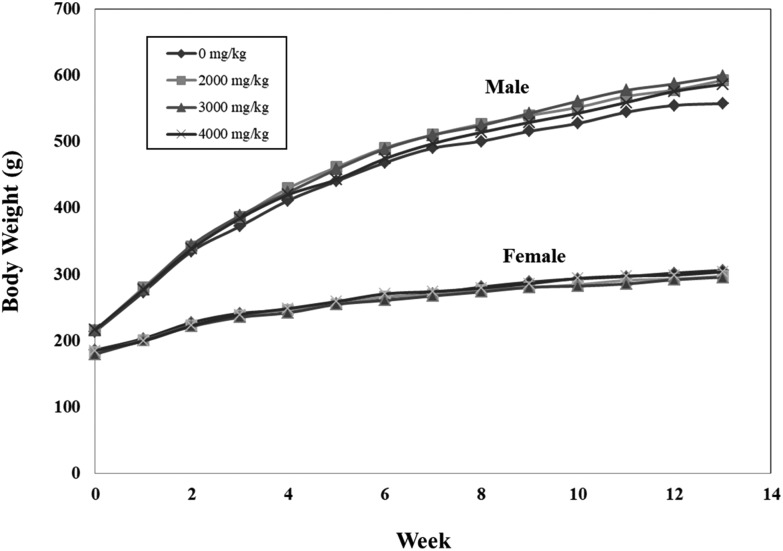
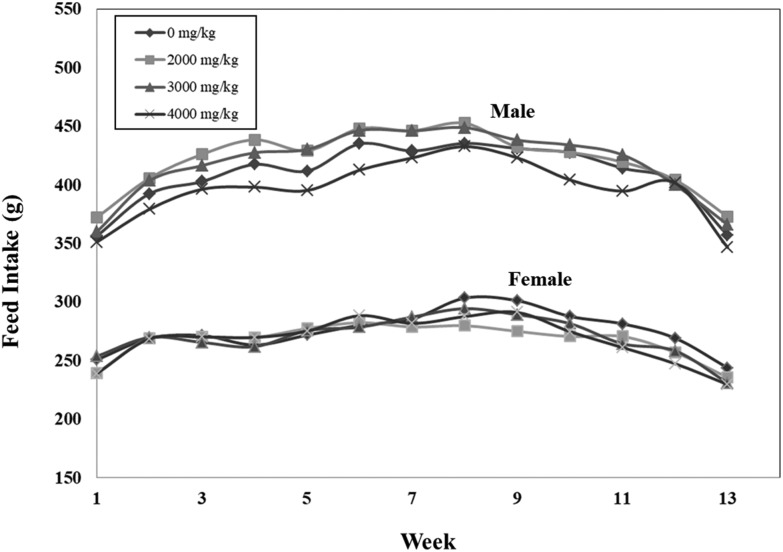
All animals survived during the study (Table 1). No abnormal clinical sign was shown after oral administration of R.O. water and C. militaris mycelium. The body weight of rats receiving C. militaris mycelium was similar to that of the control groups and was not statistically significant (Fig. 1). There is no significant difference in feed intake of both sexes between the treatment and control groups (Fig. 2).
Table 1. Mortality and incidence of abnormal clinical signs of the rats after 90 days of C. militaris mycelium administration.
| Control |
Low dose |
Medium dose |
High dose |
|||||
| 0 mg kg–1 |
2000 mg kg–1 |
3000 mg kg–1 |
4000 mg kg–1 |
|||||
| Mortality of the rats | ||||||||
| Sex | M | F | M | F | M | F | M | F |
| Number of rats in each group | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 |
| Number of rats died during the study | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Incidence of abnormal clinical sign | ||||||||
| Sex | M | F | M | F | M | F | M | F |
| Number of rats in each group | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 |
| Number of rats exhibiting abnormal clinical sign | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
Fig. 1. Effects of C. militaris mycelium on body weight in male and female SD rats during the 90-day safety assessment. Data expressed as mean ± S.D., n = 10. Error bars have been omitted for simple presentation.
Fig. 2. Effects of C. militaris mycelium on feed intake in male and female SD rats during the 90-day safety assessment. Data expressed as mean ± S.D., n = 5 (2 rats in one cage). Error bars have been omitted for simple presentation.
Urinalysis
There was no significant difference in the examination of urine sediments and the routine test of the urinalysis between the treatment and control groups of both sexes (Tables 2 and 3).
Table 2. Microscopic examination of urinary sediments of rats after 90 days of C. militaris mycelium administration.
| Items | Dosage (mg per kg BW) |
|||||||||
| Male |
Female |
|||||||||
| 0 | 2000 | 3000 | 4000 | 0 | 2000 | 3000 | 4000 | |||
| Cell number (HPF) | EP | 0–1 | 10/10 | 10/10 | 10/10 | 10/10 | 10/10 | 10/10 | 10/10 | 9/10 |
| 0–2 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | ||
| 1–2 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | ||
| 1–3 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | ||
| 2–3 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 1/10 | ||
| WBC | 0–1 | 0/10 | 0/10 | 0/10 | 0/10 | 10/10 | 10/10 | 10/10 | 10/10 | |
| 0–2 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | ||
| 0–3 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | ||
| 1–2 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | ||
| 1–3 | 10/10 | 9/10 | 9/10 | 10/10 | 0/10 | 0/10 | 0/10 | 0/10 | ||
| 2–3 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | ||
| 2–4 | 0/10 | 0/10 | 1/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | ||
| RBC | 0–1 | 10/10 | 9/10 | 9/10 | 10/10 | 10/10 | 10/10 | 10/10 | 10/10 | |
| 0–2 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | ||
| 0–3 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | ||
| 0–4 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | ||
| 1–2 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | ||
| 1–3 | 0/10 | 0/10 | 1/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | ||
| Crystals | None | 0/10 | 0/10 | 0/10 | 0/10 | |||||
| Triple phosphate | 10/10 | 10/10 | 10/10 | 10/10 | ||||||
Table 3. Urinalysis of rats after 90 days of C. militaris mycelium administration.
| Items |
Dosage (mg per kg b.w.) |
||||||||
| Male |
Female |
||||||||
| 0 | 2000 | 3000 | 4000 | 0 | 2000 | 3000 | 4000 | ||
| Appearance | Yellow | 0/10 | 0/10 | 0/10 | 0/10 | 1/10 | 3/10 | 0/10 | 4/10 |
| Brown | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | |
| Amber | 10/10 | 10/10 | 10/10 | 10/10 | 9/10 | 7/10 | 10/10 | 6/10 | |
| Glucose | N | 10/10 | 10/10 | 10/10 | 10/10 | 10/10 | 10/10 | 10/10 | 10/10 |
| Bilirubin | N | 8/10 | 9/10 | 9/10 | 8/10 | 10/10 | 9/10 | 9/10 | 9/10 |
| P | 2/10 | 1/10 | 1/10 | 2/10 | 0/10 | 1/10 | 1/10 | 1/10 | |
| Ketone bodies | N | 0/10 | 0/10 | 0/10 | 0/10 | 2/10 | 2/10 | 1/10 | 1/10 |
| 1+ | 0/10 | 0/10 | 1/10 | 1/10 | 5/10 | 3/10 | 2/10 | 7/10 | |
| 2+ | 9/10 | 10/10 | 9/10 | 9/10 | 3/10 | 5/10 | 7/10 | 2/10 | |
| 3+ | 1/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | |
| Specific gravity | ≦1.030 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 |
| 1.031–1.040 | 3/10 | 0/10 | 3/10 | 2/10 | 5/10 | 4/10 | 0/10 | 7/10 | |
| 1.041–1.050 | 7/10 | 10/10 | 7/10 | 8/10 | 5/10 | 6/10 | 10/10 | 3/10 | |
| >1.050 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | |
| pH | ≦6.5 | 1/10 | 0/10 | 2/10 | 2/10 | 7/10 | 7/10 | 4/10 | 8/10 |
| 7.0 | 9/10 | 10/10 | 5/10 | 8/10 | 3/10 | 3/10 | 5/10 | 2/10 | |
| 7.5 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | |
| 8.0 | 0/10 | 0/10 | 3/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | |
| 8.5 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | |
| ≧9.0 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 1/10 | 0/10 | |
| Protein | N | 8/10 | 10/10 | 10/10 | 10/10 | 8/10 | 10/10 | 10/10 | 10/10 |
| 1+ | 2/10 | 0/10 | 0/10 | 0/10 | 2/10 | 0/10 | 0/10 | 0/10 | |
| Urobilinogen | Normal | 0/10 | 1/10 | 3/10 | 1/10 | 2/10 | 3/10 | 0/10 | 2/10 |
| 1+ | 8/10 | 9/10 | 7/10 | 9/10 | 8/10 | 7/10 | 9/10 | 8/10 | |
| 2+ | 2/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 1/10 | 0/10 | |
| Occult blood | N | 9/10 | 9/10 | 9/10 | 10/10 | 10/10 | 10/10 | 10/10 | 9/10 |
| ± | 1/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | |
| 1+ | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | |
| 2+ | 0/10 | 0/10 | 1/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | |
| 3+ | 0/10 | 1/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 1/10 | |
Ophthalmology
The results revealed that no abnormal findings were observed in ophthalmological examination with the naked eye and by ophthalmoscopic diagnosis for the treated and control groups of both sexes prior to the oral administration and at the end of the study (Table 4).
Table 4. Ophthalmological examination before and at the end of the study.
| Group |
||||
| Control |
High dose |
|||
| Male | Female | Male | Female | |
| Before the study | ||||
| Number of rats in each group | 10 | 10 | 10 | 10 |
| Number of rats exhibiting ophthalmic abnormality | 0 | 0 | 0 | 0 |
| At the end of the study | ||||
| Number of rats in each group | 10 | 10 | 10 | 10 |
| Number of rats exhibiting ophthalmic abnormality | 0 | 0 | 0 | 0 |
Hematology
Five hematological parameters in male rats were noted to be statistically significant (Table 5). In male rats, MPV of the low dose group, WBC of the low dose and high dose groups and LYMPH of the low dose and medium dose groups were significantly lower than the control group (p < 0.05). NEUT in the low dose and medium dose groups of C. militaris-treated males and EOS in the high dose group of C. militaris-treated males were significantly higher than in the control (p < 0.05). Four hematological parameters in female rats were noted to be statistically significant (Table 5). In female rats, MCHC of the low dose and medium dose groups and NEUT of the medium dose and high dose groups were significantly higher than in the control (p < 0.05). MCV in the medium dose group of C. militaris-treated females and LYMPH in the medium dose and high dose groups of C. militaris-treated females were significantly lower than in the control (p < 0.05).
Table 5. Hematology of rats after 90 days of C. militaris mycelium administration.
| Items | Hematology
a
|
|||
| Control | Low dose | Medium dose | High dose | |
| 0 mg kg–1 | 2000 mg kg–1 | 3000 mg kg–1 | 4000 mg kg–1 | |
| Male | ||||
| RBC (106 μl–1) | 9.143 ± 0.473 | 9.158 ± 0.419 | 8.969 ± 0.37 | 8.966 ± 0.303 |
| HGB (g dL–1) | 15.86 ± 0.57 | 15.94 ± 0.57 | 15.82 ± 0.61 | 15.71 ± 0.53 |
| HCT (%) | 50.11 ± 2.21 | 49.77 ± 1.88 | 49.65 ± 2.24 | 50.13 ± 1.96 |
| MCV (fl) | 54.83 ± 1.82 | 54.39 ± 1.38 | 55.46 ± 3.69 | 55.94 ± 2.21 |
| MCH (pg) | 17.37 ± 0.47 | 17.42 ± 0.33 | 17.67 ± 0.94 | 17.54 ± 0.73 |
| MCHC (%) | 31.67 ± 0.51 | 32.04 ± 0.30 | 31.86 ± 0.59 | 31.36 ± 0.76 |
| PLT (103 μl–1) | 1097.0 ± 90.6 | 1213.4 ± 124.0 | 1100.8 ± 119.8 | 1008.5 ± 312.5 |
| MPV (fl) | 8.05 ± 0.34 | 7.63 ± 0.22* | 7.77 ± 0.30 | 8.04 ± 0.50 |
| WBC (μl) | 12 120.0 ± 2802.8 | 9341.0 ± 1670.9* | 11 411.0 ± 2436.7 | 9512.0 ± 1129.0* |
| NEUT (%) | 14.81 ± 2.66 | 24.86 ± 5.30* | 21.84 ± 9.27* | 18.46 ± 5.89 |
| LYMPH (%) | 78.35 ± 2.60 | 67.00 ± 6.34* | 69.67 ± 9.83* | 73.51 ± 7.66 |
| MONO (%) | 5.89 ± 1.28 | 6.82 ± 1.95 | 7.24 ± 2.41 | 6.49 ± 2.32 |
| EOS (%) | 0.81 ± 0.32 | 1.21 ± 0.36 | 1.11 ± 0.36 | 1.43 ± 0.58* |
| BASO (%) | 0.14 ± 0.05 | 0.11 ± 0.06 | 0.14 ± 0.07 | 0.11 ± 0.07 |
| PT (s) | 11.42 ± 1.13 | 12.13 ± 0.89 | 11.88 ± 1.11 | 12.01 ± 0.80 |
| APTT (s) | 23.37 ± 3.77 | 22.80 ± 1.28 | 25.75 ± 3.99 | 25.70 ± 2.53 |
| Female | ||||
| RBC (106 μl–1) | 8.215 ± 0.243 | 8.212 ± 0.389 | 8.214 ± 0.50 | 8.197 ± 0.442 |
| HGB (g dL–1) | 15.26 ± 0.36 | 15.14 ± 0.63 | 15.25 ± 0.93 | 15.11 ± 0.74 |
| HCT (%) | 50.65 ± 1.57 | 48.25 ± 2.30 | 47.94 ± 2.98 | 49.15 ± 2.30 |
| MCV (fl) | 61.69 ± 2.54 | 58.82 ± 3.21 | 58.39 ± 1.91* | 60.04 ± 2.88 |
| MCH (pg) | 18.59 ± 0.60 | 18.47 ± 0.77 | 18.58 ± 0.51 | 18.45 ± 0.58 |
| MCHC (%) | 30.14 ± 0.64 | 31.41 ± 0.63* | 31.80 ± 0.36* | 30.74 ± 0.69 |
| PLT (103 μl–1) | 941.2 ± 136.8 | 991.4 ± 178.8 | 1008.5 ± 88.2 | 953.9 ± 72.8 |
| MPV (fl) | 7.71 ± 0.25 | 7.63 ± 0.20 | 7.56 ± 0.26 | 7.58 ± 0.28 |
| WBC (μl) | 7896.0 ± 2049.5 | 7810.0 ± 1941.0 | 7464.0 ± 1649.9 | 6599.0 ± 1358.6 |
| NEUT (%) | 12.22 ± 2.70 | 15.45 ± 2.32 | 16.46 ± 4.64* | 16.14 ± 2.97* |
| LYMPH (%) | 80.90 ± 4.42 | 77.36 ± 3.34 | 76.30 ± 4.66* | 76.19 ± 3.62* |
| MONO (%) | 5.67 ± 2.49 | 5.83 ± 1.94 | 5.93 ± 1.64 | 6.05 ± 1.94 |
| EOS (%) | 1.08 ± 0.40 | 1.22 ± 0.45 | 1.18 ± 0.36 | 1.49 ± 0.54 |
| BASO (%) | 0.13 ± 0.08 | 0.14 ± 0.05 | 0.13 ± 0.05 | 0.13 ± 0.08 |
| PT (s) | 9.42 ± 0.85 | 9.16 ± 0.24 | 9.22 ± 0.36 | 9.16 ± 0.28 |
| APTT (s) | 18.78 ± 3.86 | 19.62 ± 3.60 | 19.35 ± 2.94 | 20.79 ± 2.78 |
aData expressed as mean ± S.D., n = 10.
Serum biochemistry
Serum biochemistry analysis revealed a lower (p < 0.05) AST, CPK and P of three treatment groups. Besides, TC in the medium dose and high dose groups of C. militaris-treated males were significantly lower than in the control group (p < 0.05). In female rats, GGT of the medium dose and high dose groups, TG of the low dose group and P of the high dose group were significantly lower than the control group (p < 0.05). Cl in the low dose, medium dose and high dose groups of C. militaris-treated females and K in the medium dose group of C. militaris-treated females were significantly higher than in the control group (p < 0.05, Table 6).
Table 6. Clinical chemistry of rats after 90 days of C. militaris mycelium administration.
| Items | Clinical chemistry
a
|
|||
| Control | Low dose | Medium dose | High dose | |
| 0 mg kg–1 | 2000 mg kg–1 | 3000 mg kg–1 | 4000 mg kg–1 | |
| Male | ||||
| ALT (U L–1) | 34.7 ± 5.3 | 35.3 ± 5.2 | 34.6 ± 4.8 | 34.3 ± 2.5 |
| AST (U L–1) | 114.2 ± 16.6 | 97.3 ± 13.7* | 90.5 ± 9.2* | 95.1 ± 9.8* |
| ALP (U L–1) | 92.4 ± 16.7 | 91.2 ± 13.3 | 92.6 ± 16.8 | 89.2 ± 8.4 |
| TBIL (mg dL–1) | 0.014 ± 0.007 | 0.015 ± 0.007 | 0.013 ± 0.005 | 0.011 ± 0.0 |
| GGT (U L–1) | 1.7 ± 0.5 | 1.3 ± 0.5 | 1.5 ± 0.7 | 1.4 ± 0.5 |
| Total protein (g dL–1) | 7.1 ± 0.4 | 7.1 ± 0.4 | 7.0 ± 0.2 | 6.8 ± 0.3 |
| Albumin (g dL–1) | 4.4 ± 0.2 | 4.4 ± 0.3 | 4.4 ± 0.1 | 4.3 ± 0.2 |
| Globulin (g dL–1) | 2.7 ± 0.2 | 2.7 ± 0.2 | 2.7 ± 0.1 | 2.5 ± 0.2 |
| Amylase (U L–1) | 1979.4 ± 254.2 | 1858.4 ± 247 | 1761.6 ± 200.6 | 1801.4 ± 215.5 |
| BUN (mg dL–1) | 16.3 ± 1.1 | 15.4 ± 1 | 16.2 ± 1.8 | 15.9 ± 2.2 |
| Creatinine (mg dL–1) | 0.65 ± 0.05 | 0.60 ± 0.05 | 0.61 ± 0.0 | 0.64 ± 0.1 |
| CPK (U L–1) | 200.9 ± 69.1 | 103.5 ± 24.2* | 92.0 ± 38.4* | 122.8 ± 42.3* |
| Glucose (mg dL–1) | 178.6 ± 21.7 | 200.7 ± 33.7 | 202.6 ± 23.4 | 175.7 ± 28.4 |
| TC (mg dL–1) | 84.9 ± 16.7 | 70.5 ± 10.1 | 67.6 ± 17.7* | 62.6 ± 14.1* |
| Triglyceride (mg dL–1) | 68.2 ± 25.1 | 51.3 ± 17 | 54.6 ± 25.6 | 59.6 ± 16.3 |
| Sodium (mmol L–1) | 147.1 ± 1.7 | 147.7 ± 1.5 | 147.8 ± 1.4 | 147.5 ± 1.4 |
| Potassium (mmol L–1) | 7.1 ± 1.2 | 6.8 ± 0.8 | 6.7 ± 0.9 | 6.5 ± 0.9 |
| Chloride (mmol L–1) | 97.7 ± 2.0 | 99.3 ± 1.6 | 99.7 ± 1.9 | 99.0 ± 0.9 |
| Calcium (mg dL–1) | 12.5 ± 1 | 12.3 ± 0.4 | 13.0 ± 0.7 | 13.0 ± 0.8 |
| Phosphorus (mg dL–1) | 11.6 ± 1.1 | 10.3 ± 0.5* | 10.2 ± 0.6* | 10.5 ± 0.7* |
| Female | ||||
| ALT (U L–1) | 35.1 ± 11.0 | 35.6 ± 8.0 | 34.8 ± 9.4 | 35.5 ± 17.4 |
| AST (U L–1) | 107.4 ± 14.7 | 96.5 ± 15.8 | 90.3 ± 12.1 | 97.6 ± 17.5 |
| ALP (U L–1) | 40.9 ± 8.9 | 47.9 ± 7.5 | 43.7 ± 19.0 | 47.5 ± 10.4 |
| TBIL (mg dL–1) | 0.043 ± 0.032 | 0.020 ± 0.012 | 0.024 ± 0.022 | 0.020 ± 0.0 |
| GGT (U L–1) | 1.8 ± 0.6 | 1.3 ± 0.5 | 1.1 ± 0.3* | 1.2 ± 0.4* |
| Total protein (g dL–1) | 7.9 ± 0.6 | 7.8 ± 0.5 | 7.9 ± 0.6 | 7.6 ± 0.4 |
| Albumin (g dL–1) | 5.2 ± 0.5 | 4.9 ± 0.2 | 5.0 ± 0.4 | 4.9 ± 0.4 |
| Globulin (g dL–1) | 2.8 ± 0.2 | 2.8 ± 0.2 | 2.9 ± 0.2 | 2.7 ± 0.2 |
| Amylase (U L–1) | 1443 ± 380.7 | 1382.3 ± 242.1 | 1266.4 ± 342.4 | 1350.9 ± 328.0 |
| BUN (mg dL–1) | 18.2 ± 2.7 | 17.5 ± 1.8 | 19.3 ± 4.7 | 19.2 ± 3.0 |
| Creatinine (mg dL–1) | 0.71 ± 0.06 | 0.68 ± 0.04 | 0.67 ± 0.1 | 0.72 ± 0.1 |
| CPK (U L–1) | 141.2 ± 39.1 | 112.0 ± 42.2 | 95.3 ± 22.7 | 122.7 ± 57.4 |
| Glucose (mg dL–1) | 167.4 ± 28.6 | 160.9 ± 22.6 | 150.2 ± 16.5 | 158.6 ± 25.1 |
| TC (mg dL–1) | 100.7 ± 25.4 | 95.4 ± 28.0 | 96.9 ± 23.2 | 87.9 ± 10.3 |
| Triglyceride (mg dL–1) | 62.2 ± 19.0 | 43.5 ± 9.7* | 47.6 ± 10.9 | 51.7 ± 12.1 |
| Sodium (mmol L–1) | 145.4 ± 0.7 | 144.9 ± 1.3 | 144.4 ± 0.8 | 145.2 ± 1.2 |
| Potassium (mmol L–1) | 6.2 ± 0.7 | 6.6 ± 0.7 | 7.3 ± 0.8* | 6.3 ± 0.5 |
| Chloride (mmol L–1) | 97.6 ± 1.7 | 99.6 ± 2.0* | 99.5 ± 1.2* | 99.9 ± 0.7* |
| Calcium (mg dL–1) | 12.2 ± 0.6 | 12.0 ± 0.3 | 12.5 ± 0.5 | 12.0 ± 0.3 |
| Phosphorus (mg dL–1) | 8.9 ± 0.8 | 8.4 ± 0.7 | 8.8 ± 0.8 | 7.4 ± 0.7* |
aData expressed as mean ± S.D., n = 10.
Pathology
No gross lesions were observed at necropsy (Table 7). In male rats, except for the adrenals and adrenals-to-brain weight ratio of the low dose and medium dose groups and kidneys of the medium dose group, no significant difference in organ weights was observed between the treatment and control groups. In female rats, there were no significant differences in organ weight between the treatment and control groups (Table 8).
Table 7. Incidence of gross lesion after 90 days of C. militaris mycelium administration.
| Control | Low dose | Medium dose | High dose | |
| 0 mg kg–1 | 2000 mg kg–1 | 3000 mg kg–1 | 4000 mg kg–1 | |
| Male | ||||
| Number of rats in each group | 10 | 10 | 10 | 10 |
| Number of rats exhibiting gross lesion | 0 | 0 | 0 | 0 |
| Female | ||||
| Number of rats in each group | 10 | 10 | 10 | 10 |
| Number of rats exhibiting gross lesion | 0 | 0 | 0 | 0 |
Table 8. Organ weight of rats after 90 days of C. militaris mycelium administration.
| Absolute organ weight
a
|
|||||
| Control | Low dose | Medium dose | High dose | ||
| 0 mg kg–1 | 2000 mg kg–1 | 3000 mg kg–1 | 4000 mg kg–1 | ||
| Male | |||||
| Organ | Weight | ||||
| Brain | Weight (g) | 2.164 ± 0.081 | 2.188 ± 0.086 | 2.204 ± 0.103 | 2.193 ± 0.089* |
| Adrenals | Weight (g) | 0.0423 ± 0.0065 | 0.0532 ± 0.0094* | 0.0583 ± 0.0075* | 0.0479 ± 0.0102 |
| Ratio (%) | 1.9589 ± 0.3192 | 2.4287 ± 0.4018* | 2.6459 ± 0.3296* | 2.1793 ± 0.4339 | |
| Heart | Weight (g) | 1.578 ± 0.132 | 1.661 ± 0.139 | 1.677 ± 0.141 | 1.655 ± 0.151 |
| Ratio | 0.730 ± 0.066 | 0.758 ± 0.070 | 0.760 ± 0.052 | 0.754 ± 0.055 | |
| Kidneys | Weight (g) | 3.724 ± 0.272 | 4.136 ± 0.519 | 4.349 ± 0.525* | 4.098 ± 0.288 |
| Ratio | 1.724 ± 0.154 | 1.892 ± 0.243 | 1.974 ± 0.224 | 1.873 ± 0.151 | |
| Liver | Weight (g) | 15.627 ± 1.842 | 16.110 ± 2.485 | 16.473 ± 1.684 | 16.046 ± 1.008 |
| Ratio | 7.230 ± 0.887 | 7.382 ± 1.260 | 7.486 ± 0.802 | 7.327 ± 0.547 | |
| Spleen | Weight (g) | 0.781 ± 0.094 | 0.755 ± 0.077 | 0.777 ± 0.153 | 0.803 ± 0.121 |
| Ratio | 0.359 ± 0.041 | 0.343 ± 0.035 | 0.352 ± 0.059 | 0.367 ± 0.050 | |
| Testes | Weight (g) | 3.800 ± 1.196 | 3.490 ± 0.345 | 3.673 ± 0.221 | 3.492 ± 0.353 |
| Ratio | 1.752 ± 0.510 | 1.596 ± 0.140 | 1.669 ± 0.122 | 1.593 ± 0.142 | |
| Thymus | Weight (g) | 0.306 ± 0.062 | 0.281 ± 0.061 | 0.328 ± 0.066 | 0.340 ± 0.080 |
| Ratio | 0.142 ± 0.028 | 0.128 ± 0.030 | 0.149 ± 0.030 | 0.156 ± 0.041 | |
| Female | |||||
| Organ | Weight | ||||
| Brain | Weight (g) | 1.943 ± 0.097 | 1.971 ± 0.082 | 1.991 ± 0.058 | 1.979 ± 0.077 |
| Adrenals | Weight (g) | 0.0554 ± 0.0094 | 0.0615 ± 0.0095 | 0.0681 ± 0.0161 | 0.0592 ± 0.0070 |
| Ratio (%) | 2.8459 ± 0.4254 | 3.1299 ± 0.5245 | 3.4153 ± 0.7895 | 3.0008 ± 0.4290 | |
| Heart | Weight (g) | 0.941 ± 0.096 | 0.911 ± 0.080 | 0.923 ± 0.093 | 0.959 ± 0.100 |
| Ratio | 0.486 ± 0.059 | 0.463 ± 0.051 | 0.464 ± 0.049 | 0.485 ± 0.051 | |
| Kidneys | Weight (g) | 1.994 ± 0.180 | 2.039 ± 0.160 | 2.124 ± 0.265 | 2.063 ± 0.167 |
| Ratio | 1.025 ± 0.065 | 1.038 ± 0.102 | 1.068 ± 0.133 | 1.044 ± 0.105 | |
| Liver | Weight (g) | 8.541 ± 0.710 | 7.953 ± 0.982 | 8.239 ± 1.247 | 8.202 ± 0.725 |
| Ratio | 4.403 ± 0.379 | 4.048 ± 0.581 | 4.136 ± 0.610 | 4.151 ± 0.385 | |
| Spleen | Weight (g) | 0.413 ± 0.069 | 0.472 ± 0.165 | 0.455 ± 0.045 | 0.435 ± 0.052 |
| Ratio | 0.215 ± 0.040 | 0.240 ± 0.085 | 0.228 ± 0.025 | 0.220 ± 0.026 | |
| Testes | Weight (g) | 0.0670 ± 0.0176 | 0.0686 ± 0.0100 | 0.0767 ± 0.0162 | 0.0774 ± 0.0281 |
| Ratio | 0.0342 ± 0.0080 | 0.0348 ± 0.0054 | 0.0386 ± 0.0071 | 0.0392 ± 0.0140 | |
| Thymus | Weight (g) | 0.254 ± 0.060 | 0.233 ± 0.067 | 0.202 ± 0.040 | 0.247 ± 0.059 |
| Ratio | 0.131 ± 0.032 | 0.117 ± 0.035 | 0.101 ± 0.021 | 0.124 ± 0.031 | |
aData expressed as mean ± S.D., n = 10.
No significant treatment-related changes for all rats were observed in the adrenals, brain, kidney, liver, spleen, testis (male) and ovary (female) of the treatment and control groups (Table 9). In male rats, focal, slight to moderate severe cortical lipidosis was found in the adrenal gland of the control and high dose groups (incidence rate of 3/10 and 2/10). Only one male rat in the control group exhibited focal and slight mononuclear cell infiltration in Harderian gland. Focal, minimal to slight mononuclear cell infiltration was found in the heart of the control and high dose male rats (incidence rate of 1/10 and 2/10) and control female rats (incidence rate of 1/10). Focal, minimal to slight interstitial fibrosis and focal, minimal to slight tubular cast were observed in the kidney of the control and high dose male rats (incidence rate of 1/10 and 1/10). Focal, minimal to slight tubular mineralization was also observed in the kidney of the control and high dose female rats (incidence rate of 4/10 and 5/10). There was one male rat in the control group that exhibited focal and slight sperm degeneration and necrosis in the epididymis.
Table 9. Histopathological examination of rats after 90 days of C. militaris mycelium administration.
| Organ | Lesions | Group |
|||
| Control |
High dose |
||||
| Male | Female | Male | Female | ||
| Adrenal gland | |||||
| Lipidosis, cortex, focal, slight to moderate severe | 3/10 | — | 2/10 | — | |
| Aorta | — | — | — | — | |
| Bain | |||||
| Fore | — | — | — | — | |
| Middle | — | — | — | — | |
| Cerebellum | — | — | — | — | |
| Bone | — | — | — | — | |
| Bone marrow | — | — | — | — | |
| Epididymis | N | N | |||
| Degeneration/necrosis, sperm, focal, slight | 1/10 | — | |||
| Esophagus | — | — | — | — | |
| Eyes | — | — | — | — | |
| Harderian gland | |||||
| Infiltration, mononuclear cell, focal, slight | 1/10 | — | — | — | |
| Heart | |||||
| Infiltration, mononuclear cell, focal, minimal to slight | 1/10 | 1/10 | 2/10 | — | |
| Intestine, small duodenum | — | — | — | — | |
| Jejunum | — | — | — | — | |
| Ileum | — | — | — | — | |
| Intestine, large | |||||
| Caecum | — | — | — | — | |
| Colon | — | — | — | — | |
| Rectum | — | — | — | — | |
| Kidney | |||||
| Fibrosis, interstitial, focal, minimal to slight | 1/10 | — | 1/10 | — | |
| Cast, tubular, focal, minimal to slight | 1/10 | — | 1/10 | — | |
| Mineralization, tubular, focal, minimal to slight | — | 4/10 | — | 5/10 | |
| Liver | — | — | — | — | |
| Lung | — | — | — | — | |
| Lymph node | |||||
| Cervical | — | — | — | — | |
| Mesenteric | — | — | — | — | |
Discussion
Because of long-term excessive collection of wild C. militaris, supply of wild C. militaris is failing to meet the market demands. Artificially cultivated C. militaris is a better alternative for healthcare product development. Liu et al. (2015) yielded mass production of C. militaris mycelium using a variety of carbon sources, nitrogen sources and inorganic salts combined with a liquid fermentation technique in a 50 ton fermenter, offering the obvious advantages of less pollution and a short production cycle.21 Furthermore, the functional component contents and physiological activity of the produced C. militaris mycelium were not inferior to those of wild C. militaris mycelium. Previous studies reported the proximate composition of natural and cultivated Cordyceps mycelium. Cordyceps contains a high amount of polysaccharide with a wide range.23,24 Protein levels in Cordyceps mycelium have been reported to be approximately 5.6–31.6 g per 100 g.24–26 Fat contents reported previously were above 4.5 g per 100 g.24,27–29 We have performed an analysis of bioactive compounds and found that the adenosine, cordycepin, N6-(2-hydroxyethyl)-adenosine and ergosterol contents in C. militaris mycelium were 1.7, 0.3, 0.1 and 2.2 mg g–1, respectively.21 The nutritional values of C. militaris detected indicate its potential use in well-balanced diets and as a source of bioactive compounds.
During the study period, no abnormality occurred in clinical signs, body weight, feed intake and ophthalmological examination. There were no significant differences in urinalysis between the treatment and control group. A few parameters in hematology and clinical biochemistry analysis showed significant differences between the treatment and control group, including MPV, WBC, NEUT, LYMPH, EOS, AST, CPK, P, and TC in male rats, and MCHC, MCV, NEUT, LYMPH, GGT, TG, Cl, K and P in female rats. The above findings are changes within the range observed in normal SD rats,30 and the changes were not found to be dose-dependent.
Necropsy and histopathological examination found no treatment-related change. Although the organ weights and organ-to-brain weight ratio of the adrenal gland of the low and medium dose groups and the organ weights of the kidneys of the medium dose group in male rats were significantly higher than the control group, no differences (p > 0.05) were found between treatments regarding the serum biochemical parameters and histopathological effects of these organs. Non-specific histopathological changes were observed, including focal cortical lipidosis in the adrenal gland, focal mononuclear cell infiltration in the Harderian gland and heart, focal interstitial fibrosis, focal tubular cast and focal tubular mineralization in the kidney, and focal sperm degeneration and necrosis in the epididymis. According to the results, the incidence of histopathological change was not directly correlated with the oral administration of C. militaris mycelium. These histopathological changes were non-specific, and not induced by oral administration of C. militaris mycelium.
Conclusion
The present study demonstrates that the 90-day subchronic toxicological assessment showed no systemic toxicity attributable to dietary exposure to the powder of C. militaris submerged mycelial culture, and no significant toxicity occurred even at the highest dose of 4000 mg per kg BW per day in SD rats. The results from the 90-day subchronic toxicity study of C. militaris mycelium do not raise concern with respect to its possible use as a functional food ingredient. According to the results, the no-observed-adverse-effect level (NOAEL) of C. militaris mycelium was 4000 mg per kg BW per day in SD rats. This information provides evidence supporting the use of the C. militaris fermentation product as a safe agent for functional foods.
Conflicts of interest
There are no conflicts of interest to declare.
References
- Kuo Y. C., Tsai W. J., Wang J. Y., Chang S. C., Lin C. Y., Shiao M. S. Life Sci. 2001;68(9):1067–1082. doi: 10.1016/s0024-3205(00)01011-0. [DOI] [PubMed] [Google Scholar]
- Meng Q. F., Lin X. Y., Lin W., Feng Y., Teng L. R. Amino Acids Biotic Resour. 2005;27:33–34. [Google Scholar]
- Duh P. D. J. Agric. Food Chem. 2007;55(17):7215–7216. [Google Scholar]
- Lin X. X., Xie Q. M., Shen W. H., Chen Y. China J. Chin. Mater. Med. 2001;26(9):622–625. [PubMed] [Google Scholar]
- Buenz E. J., Bauer B. A., Osmundson T. W., Motley T. J. J. Ethnopharmacol. 2005;96(1):19–29. doi: 10.1016/j.jep.2004.09.029. [DOI] [PubMed] [Google Scholar]
- Wen T. C., Lei B. X., Kang J. C., Li G. R., He J. Food Ferment. Ind. 2009;35(8):49–53. [Google Scholar]
- Li H. C., Sun P., Feng C. Q. J. Jinggangshan Univ. (Natural Science) 2010;31(2):93–96. [Google Scholar]
- C. Park, S. H. Hong, J. Y. Lee, G. Y. Kim, B. T. Choi, Y. T. Lee, D. I. Park, Y. M. Park, Y. K. Jeong and Y. H. Choi, Growth inhibition of U937 leukemia cells by aqueous extract of Cordyceps militaris through induction of apoptosis, Oncol. Rep., 2005, 13(6), 1211–1216
- Ng T. B., Wang H. X. J. Pharm. Pharmacol. 2005;57(12):1509–1519. doi: 10.1211/jpp.57.12.0001. [DOI] [PubMed] [Google Scholar]
- Zhao-Long W., Xiao-Xia W., Wei-Ying C. Cell Biochem. Funct. 2000;18(2):93–97. doi: 10.1002/(SICI)1099-0844(200006)18:2<93::AID-CBF854>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Nan J. X., Park E. J., Yang B. K., Song C. H., Ko G., Sohn D. H. Arch. Pharmacal Res. 2001;24(4):327–332. doi: 10.1007/BF02975101. [DOI] [PubMed] [Google Scholar]
- Yoo H. S., Shin J. W., Cho J. H., Son C. G., Lee Y. W., Park S. Y., Cho C. K. Acta Pharmacol. Sin. 2004;25(5):657–665. [PubMed] [Google Scholar]
- Won S. Y., Park E. H. J. Ethnopharmacol. 2005;96(3):555–561. doi: 10.1016/j.jep.2004.10.009. [DOI] [PubMed] [Google Scholar]
- Choi S. B., Park C. H., Choi M. K., Jun D. W., Park S. Biosci., Biotechnol., Biochem. 2004;68(11):2257–2264. doi: 10.1271/bbb.68.2257. [DOI] [PubMed] [Google Scholar]
- Xu J. T., Medicinal Fungus in China, Beijing Medical University, Peking Union Medical College Joint Press, Beijing, China, 1997. (Chinese). [Google Scholar]
- Kim H. O., Yun J. W. J. Appl. Microbiol. 2005;99(4):728–738. doi: 10.1111/j.1365-2672.2005.02682.x. [DOI] [PubMed] [Google Scholar]
- Yu H. M., Wang B. S., Huang S. C., Duh P. D. J. Agric. Food Chem. 2006;54(8):3132–3138. doi: 10.1021/jf053111w. [DOI] [PubMed] [Google Scholar]
- Gu Y. X., Song Y. W., Fan L. Q., Yuan Q. S. ChinaChina J. Chin. Mater. Med.J. Chin. Mater. Med. 2007;32(11):1028–1031. [PubMed] [Google Scholar]
- P. E. Mortimer, S. C. Karunarathna, Q. Li, H. Gui, X. Yang, X. Yang, J. He, L. Ye, J. Guo, H. Li, P. Sysouphanthong, D. Zhou, J. Xu and K. D. Hyde, Sysouphanthong, Prized edible Asian mushrooms: ecology, conservation and sustainability, Fungal Divers., 2012, 56(1), 31–47
- Liu H. H., Yeh S. H., Chen C. H., Chen C. C. J. Biomed. Lab. Sci. 2016;28(3):104–114. [Google Scholar]
- Liu H. H., Xie Y. X., Chen J. R., Shih C. H., Peng Y. T., Chen C. C. J. Test. Qual. Assur. 2015;4:23–33. [Google Scholar]
- Jhou B. Y., Liu H. H., Yeh S. H., Chen C. C. J. Test. Qual. Assur. 2016;5:58–65. [Google Scholar]
- Yan J. K., Wang W. Q., Wu J. Y. J. Funct. Foods. 2014;6:33–47. doi: 10.1016/j.jff.2013.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X., Huang K., Zhou J. J. Food Nutr. Res. 2014;2:74–79. [Google Scholar]
- Dong C. H., Yao Y. J. LWT – Food Sci. Technol. 2008;41:669–677. doi: 10.1016/j.lwt.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z. Y., Lei Z. F., Lu Y., Lu Z. Z., Chen Y. J. Biosci. Bioeng. 2008;106:188–193. doi: 10.1263/jbb.106.188. [DOI] [PubMed] [Google Scholar]
- Hsu T. H., Shiao L. H., Hsieh C. H., Chang D. M. Food Chem. 2002;78:463–469. [Google Scholar]
- Oh S. W., Kim S. H., Song H. N., Han D. Korean J. Food Sci. Technol. 2003;35:15–22. [Google Scholar]
- Li C., Li Z., Fan M., Cheng W., Long Y., Ding T., Ming L. J. Food Compos. Anal. 2006;19:800–805. [Google Scholar]
- Medgaea Life Sciences Institute, Clinical chemistry and haematology historical data in control Sprague-Dawley rats from pre-clinical toxicity studies, 2014. [DOI] [PubMed] [Google Scholar]