Abstract
Background:
Diabetes mellitus type 1 (DM1) is an autoimmune disease characterized by metabolic destruction of pancreatic cells responsible for insulin production, with treatment based on replacing insulin. Long-acting insulin analogs are indicated for patients with DM1 who exhibit important oscillations of their daily glycemia, despite its higher cost. Our study objective was to evaluate the effectiveness and safety of two long-acting insulins, insulin glargine and detemir, in treating patients with DM1.
Methods:
We undertook a systematic review with meta-analysis of observational studies (cohort and registry) available in the databases and the gray literature, and a complementary search in the Diabetes Care journal. Outcomes assessed were: glycated hemoglobin concentration; fasting plasma or capillary glucose; occurrence of episodes of severe hypoglycemia and occurrence of nocturnal hypoglycemia. The assessment of methodological quality was performed using the Newcastle score. The meta-analyses were performed on software Review Manager® 5.2.
Results:
Out of 705 publications, 8 cohort studies were included. The quality of these studies was classified as high. In the meta-analysis, results regarding episodes of severe hypoglycemia (p = 0.02) and fasting glucose (p = 0.01) were in favor of detemir. The glycated hemoglobin (p = 0.49; I2 = 89) showed high heterogeneity and no statistically significant difference between the two. The meta-analysis of total insulin dose favored glargine (p = 0.006; I2 = 75). The rates of nocturnal hypoglycemia (NH) were evaluated only for one study and showed a significant reduction of NH after therapy with detemir, (p < 0.0001).
Conclusion:
Although some outcomes were favorable to detemir insulin analog, it has not been possible to identify important differences of effectiveness and safety between the two analogs. These results can help in the current debate on the inclusion of long-acting analogs on the list of reimbursed medicines in Brazil, especially with the recent introduction of an insulin glargine biosimilar at a considerably lower price.
Keywords: comparative effectiveness, detemir, diabetes mellitus type 1, glargine, systematic review
Introduction
Diabetes mellitus (DM) is a heterogeneous group of metabolic disorders that includes increased levels of blood glucose resulting from defects in insulin action, on insulin secretion, or both. DM is considered a chronic disease with high morbidity and mortality, being one of the leading causes of stroke, myocardial infarction, chronic renal failure, blindness and nontraumatic amputations.1,2 Among the types of DM, DM type 1 (DM1) and DM type 2 (DM2) are the most prevalent.3
According to the International Diabetes Federation (IDF), the number of people with DM in the world increased from 108 million in 1980 to 422 million in 2014, and it is estimated that this number will increase to 642 million by 2040. Approximately 80% of patients with DM live in developing countries due to the growth in populations in these countries, population aging, greater urbanization, the prevalence of obesity and progressive sedentariness, as well as increased survival of patients with DM.4
Among the therapeutic alternatives available on the market for the treatment of DM1, neutral protamine Hagedorn (NPH), which has a profile of intermediate action, is currently considered as standard treatment, and the long-acting insulin analogs, such as insulin glargine (GLA) and insulin detemir (DET), can be combined with fast-acting insulin for better modulation of pharmacotherapy and glycemic control. GLA and DET allow a more stable profile compared with NPH insulin, without a pronounced peak action that does not require homogenization, leading possibly to more flexible administration.5,6
However, a number of meta-analyses and other studies conducted to date do not support the clinical superiority of GLA and DET compared with NPH. In four systematic reviews,6–9 there appeared to be no additional clinical benefit of GLA compared with NPH insulin in terms of effectiveness and side effects. Similar results were observed in a recent cohort study,10 as well as in a recent systematic review comparing the quality of life or patient-reported outcomes in GLA versus NPH insulin.11 Despite these and similar studies of long-acting insulins versus NPH insulin,6–10,12, 13 with concerns echoed by the Brazilian Agency of Health Technology Assessment [Comissão Nacional de Incorporação de Tecnologias no SUS (CONITEC)]14 resulting in long-acting insulins not being recommended for inclusion in the list of official reimbursed medicines, GLA has been incorporated into the list of the Secretary of Health of the State [Secretaria Estadual de Saúde do Estado de Minas Gerais (SES/MG)] in Brazil. This resulted in public spending of approximately US $6 million in 2011 for long-acting insulins, since the difference between the cost of monthly treatment in Brazil was 536% for GLA versus NPH, 377% for DET versus NPH and 34% for GLA versus DET.7,14
Concern about the additional costs of long-acting insulin analogs has resulted in some countries restricting the indications for funding.6 In Brazil, SES/MG attempted to restrict the free supply of GLA to patients with DM1 who demonstrate inadequate glycemic control or episodes of frequent hypoglycemia following NPH insulin; however, there are still requests from patients with DM2 or those patients outside the established criteria.15 Whilst Siebenhofer-Kroitzsch and colleagues also question the clinical relevance of potential minor improvements with insulin analogs versus NPH insulins, they may have a place in selected patients such as those with higher occurrence of nocturnal hypoglycemia.16 It is also worth noting that investment in self-management programs for patients with DM have resulted in sustained clinical gain in terms of glycemic control and a reduced risk of severe hypoglycemia than has been observed with long-acting insulins.14 Nevertheless, long-acting insulin analogs are available in Brazil with restrictions on their use in SES/MG.
In view of concerns with cost differentials between different long-acting insulins in some countries, improved kidney function in some patients with the long-acting analogs, although still concerns with their overall benefit versus NPH insulins, and potential differences in effectiveness between the long-acting insulins with differences in action between them,6–8,17–19 the objective of this study was to evaluate the effectiveness and safety of GLA in comparison with DET in patients with DM1 through a systematic review and meta-analysis. The results will help inform future decision making in Minas Gerais, as well as wider in Brazil and other countries, especially as more biosimilars of long-acting insulins become available.
Materials and method
This review was conducted in accordance with guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA)20 with registration protocol (available at: http://www.crd.york.ac.uk/PROSPERO/display_record.asp?ID=CRD42017054925).
Database and search strategy
An electronic search was performed in articles published until August to 2017 in databases including MEDLINE (PubMed), Latin American literature and Caribbean Health Sciences (LILACS), EMBASE and the Cochrane Library. Various combinations of terms were used following the peak (population, intervention strategy, comparing, and result): DM1, GLA and DET (Table 1). As a complement to the electronic search, a search was carried out on the references of all included studies, as well as in the electronic journal Diabetes Care from 2003 to August 2017. We also made a search of gray literature studies included in the bank of theses and dissertations of the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) and Digital Library of Theses and Dissertations at the Federal University of Minas Gerais (UFMG), in case we had missed any important studies.
Table 1.
Search strategies.
| Electronic bases | Search strategies | Files retrieved |
|---|---|---|
| MEDLINE (PubMed) | ((((((((((((((((((((((((((‘Diabetes Mellitus, Type 1’ [Mesh]) OR ‘Diabetic Ketoacidosis’ [Mesh]) OR Insulin-Dependent Diabetes Mellitus [Text Word]) OR Diabetes Mellitus, Insulin-Dependent, 1 [Text Word]) OR Diabetes Mellitus Juvenile-Onset [Text Word]) OR Juvenile-Onset Diabetes Mellitus [Text Word]) OR Diabetes Mellitus, Sudden Onset [Text Word])) OR IDDM [Text Word])) OR Juvenile-Onset Diabetes [Text Word])) OR Diabetes Mellitus Brittle [Text Word])) OR Diabetes Mellitus Ketosis-Prone [Text Word])) OR Diabetes, Autoimmune [Text Word])) Or Autoimmune Diabetes [Text Word])) OR Ketoacidoses, Diabetic [Text Word])) OR Acidosis, Diabetic [Text Word])) AND ((((((((((((, Insulin Detemir [MeSH Terms]) OR Basal Insulin Detemir [Text Word])) OR Detemir Basal Insulin, [Text Word])) OR Insulin Detemir, Basal [Text Word])) OR NN304 [Text Word])) OR NN-304 [Text Word])) OR Levemir [Text Word])) AND ((((((((Glargine, Insulin [MeSH Terms]) OR Glargine [Text Word])) OR HOE 901 [Text Word])) OR 901, HOE [Text Word])) OR Lantus [Text Word])) | 117 |
| EMBASE | #1 ‘diabetic ketoacidosis/exp’ OR ‘diabetic acidosis’ OR ‘diabetes’ OR ‘acidosis ketoacidosis diabetes’ OR ‘diabetes’ OR ‘ketosis’ OR ‘diabetic acidosis diabetic ketosis’ OR ‘insulin dependent diabetes mellitus/exp’ OR ‘brittle’ OR ‘brittle diabetes diabetes mellitus’ OR ‘diabetes mellitus type 1’ OR ‘type i diabetes mellitus’ OR ‘diabetes mellitus, insulin-dependent diabetes mellitus’ OR ‘type 1’ OR ‘diabetes, type i diabetes mellitus’ OR ‘brittle’ OR ‘diabetes, insulin dependent diabetes mellitus’ OR ‘juvenile onset’ OR ‘diabetes type 1 diabetes type’ OR ‘i’ OR ‘diabetes, juvenile’ OR ‘dm’ OR ‘1 early onset diabetes mellitus’ OR ‘iddm insulin dependent diabetes’ OR ‘juvenile diabetes’ OR ‘juvenile diabetes mellitus’ OR ‘juvenile onset diabetes’ OR ‘juvenile onset diabetes mellitus’ OR ‘ketoacidotic diabetes’ OR ‘labile diabetes mellitus’ OR ‘type 1’ OR ‘type 1 diabetes diabetes mellitus’ OR ‘type i diabetes’ OR ‘type i diabetes mellitus’, #2 ‘/exp’ OR ‘insulin isophane nph insulin glargine’, #3 ‘/exp’ OR ‘abasaglar’ OR ‘abasria’ OR ‘basaglar’ OR ‘insulin glargine’ OR ‘hoe 901’ OR ‘hoe901’ OR ‘insulin glargine recombinant’ OR ‘insulin [a21 glycine b31 b32 arginine arginine]’ OR ‘lantus’ OR ‘lantussolostar’ OR ‘ly’ OR ‘2963016’ OR ‘ly2963016’ OR ‘optisulin optisulin depot’ OR ‘optisulin long’ OR ‘toujeo’, #4 ‘/exp’ OR ‘detemir insulin levemir’ and cohort analysis ‘/exp’ OR ‘controlled clinical trial’ ‘/exp’ OR ‘/exp #1’ AND #2 AND #3 AND #4 | 472 |
| Cochrane Library |
#1 MeSH descriptor: [Diabetes Mellitus, Type 1] explodes all trees #2 MeSH descriptor: [Diabetic Ketoacidosis] explodes all trees #3 Diabetes Mellitus, Insulin-Dependent (Word variations have been searched), Insulin Dependent Diabetes Mellitus #4 (Word variations have been searched) #5 Diabetes Mellitus, Insulin Dependent Juvenile Onset $ #6–#7 Diabetes Mellitus Type 1 Diabetes #8 Diabetes Mellitus, Type I Diabetes, Autoimmune #9 #10 {or #1–#9} #11 MeSH descriptor: [Insulin Glargine] explodes all trees glargine Lantus #13 #12 #14 {#11–#13 or} #15 MeSH descriptor: [Insulin Detemir] explodes all trees #16, Insulin Detemir (Word variations have been searched) Insulin Detemir Basal #17 (Word variations have been searched) #18 Basal Insulin Detemir,: ti, ab, kw (Word variations have been searched) Levemir #19 #20 {or #15–#19} #21 #14 #20 #22 #10 and #21 and #23 MeSH descriptor: [Cohort Studies] explodes all trees $ cohort epidemiologic methods #25 #24 #26 controlled clinical trial #27 {or #23-#26} #28 #22 and #27 |
109 |
| LILACS | ((((((((‘DIABETIC KETOACIDOSIS’) or ‘DIABETES MELLITUS TYPE 1’) or ‘INSULIN-DEPENDENT’ DIABETES MELLITUS) or ‘AUTOIMMUNE DIABETES’) or ‘DIABETES MELLITUS’) or ‘KETOACIDOSIS DIABETICA’) or ‘DIABETES’) or ‘IDDM’) or ‘INSULIN-DEPENDENT DIABETES MELLITUS’ [Words] an d (((‘GLARGINE’) or ‘LANTUS’) or ‘LANTUS SOLOSTAR’) or ‘GLARGINE’ [Words] and ((‘DETEMIR’) or ‘LEVEMIR’) or ‘INSULIN DETEMIR’ [Words] | 7 |
Selection of studies and eligibility criteria
Cohort studies were selected, as well as database records of concurrent and nonconcurrent patients with DM1. Considered studies included those that assessed GLA versus DET, principally in terms of their effectiveness and safety.
We excluded studies that concentrated on dose comparisons, compared other drugs apart from GLA and DET, pregnant patients, clinical protocols, reviews, case reports, studies in animals, in vitro studies, pharmacodynamic studies or studies that combined oral antidiabetic medicines with insulin therapy for DM1, as well as studies that included less than 30 participants or follow-up time was less than 4 weeks, similar to the review by Marra and colleagues.7
Data collection and methodological quality assessment
The studies found in the electronic databases were allocated on a single basis to exclude duplicates using the EndNote software program. Two independent reviewers (TS and PA) evaluated the titles (phase 1), the abstracts (phase 2), and the full text (phase 3). Disagreements were resolved by a third reviewer (VA). The data, including methodological quality, participant information, treatment duration, effectiveness and safety data, were extracted and collected independently with each reviewer on a previously formulated and tested Excel spreadsheet for this purpose.
For the assessment of methodological quality, we used the Newcastle–Ottawa Scale.21 This scale was originally developed to evaluate the quality of observational studies. On this scale, each study is evaluated in three dimensions. These include the selection of the study groups, comparability of groups and the calculation of exposure or outcome of interest. The total score of nine is considered to be of high quality. In addition, funding sources have been identified and explored in view of concerns with bias identified in the previous systematic review of Marra and colleagues7 and Almeida and colleagues.11 The possibility of publication bias was assessed via analysis using a funnel plot.22 It was considered that there was no conflict of interest in any part of the text if no comment about conflict of interest was found. Conflict of interest refers to sources of funding from pharmaceutical companies or when there was a bond with any of the authors of the study with the pharmaceutical companies. This could include speaker fees or funding for conferences.
Summary of the findings and statistical analysis
The outcomes assessed were the glycated hemoglobin concentration (HbA1c), fasting plasma or capillary glucose, and occurrence of episodes of severe hypoglycemia and occurrence of nocturnal hypoglycemia.
The data from the studies were combined using the random effects model of Review Manager® software version 5.3 Cochrane Community, Haymarket, London, UK. The results were presented by the mean difference (MD) for continuous variables with a 95% confidence interval (CI 95%). Analyses with a heterogeneity (I2) greater than 40%, and a p value chi-square test less than 0.10, were considered as high/significant heterogeneity. Sensitivity analysis was conducted to investigate the causes of the heterogeneity, excluding one study at a time and observed changes in I2 values and p value.22
Results
Included studies
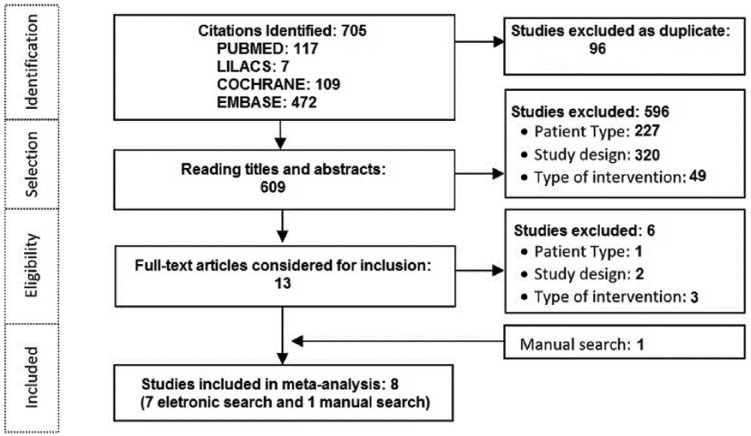
There were 705 publications found in the electronic databases. After deleting duplicates, 609 articles were selected for analysis of the titles and abstracts and 13 for complete reading. After the analysis of the articles using our inclusion criteria, only seven studies were finally selected and the manual search added another publication, totaling eight studies for inclusion in the meta-analysis (Figure 1). Overall, a total of 596 studies were excluded in the first phase after reading titles and abstracts (Figure 1). Following this, as mentioned, 13 studies were progressed to full reading. One study was excluded,23 as the authors had a sample of less than 30 participants. Two studies24,25 were excluded due to the lack of information and a detailed design of the study, and three studies26–28 were excluded due to differences in the type of intervention.
Figure 1.
Study selection chart.
Characteristics of included studies
Of the eight cohort studies retrieved, three were nonconcurrent design29–31 and five, concurrent.32–36 Five studies were multicenter studies30,32,33,35,36 and three were single-center studies.29,31,34 The follow-up time ranged from 3.5 to 54 months (Table 2).
Table 2.
General characteristics of included studies.
| Study | Year of publication |
Type of study |
Number of participants* |
Male (%) | Mean age |
Patients | Location of study |
Scope | Conflict of interest | Financing | Classification of the duration of the study**** | Total score in Newcastle–Ottawa Scale |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Abali et al. 29 | 2014 | Cohort no (concurrent) | 117 (85/32) |
55 | 12 ± 3.4 | Pediatric | Turkey | SC | NR | NR | Long | 7 |
| Haukka et al. 30 | 2013 | Cohort no (concurrent) | 7110 (3359/7110) | NR | NR | Pediatric and adult | Finland | MC | Yes | Novo Nordisk | Long | 6 |
| Kabadi et al. 31 | 2008 | Cohort no (concurrent) | 45 (24/21) |
56 | NR | Adult | USA | SC | No | No | Intermediate | 7 |
| Jinno et al. 32 | 2012 | Cohort no concurrent | 90 (29/90) |
36 | 11.9 ± 3.8 | Pediatric | Japan | MC | NR | Japan Diabetes Foundation | Long | 6 |
| Hermansen et al. 33 | 2009 | Cohort no (concurrent) | 643 (643/643) |
39 | 40.3 ±15.7 | Adult | 6 countries ** | MC | Yes | Novo Nordisk | Intermediate | 7 |
| Preumont et al. 34 | 2009 | Cohort no (concurrent) | 181 (181/181) |
NR | NR | Adult | Belgium | SC | Yes | Novo Nordisk | Intermediate | 6 |
| Yenigun et al. 35 | 2009 | Cohort no (concurrent) |
508 (482/26) |
48 | NR | Adult | 11 countries *** | MC | Yes | Novo Nordisk | Long | 7 |
| Dornhorst et al. 36 | 2007 | Cohort no (concurrent) | 3330 (1665/1665) | NR | NR | Adult | Austria, Czech Republic and Denmark | MC | Yes | Novo Nordisk | Short | 5 |
The value in brackets indicates (ni/nc) where, ni = the number of participants in the intervention group (glargine), and nc = the number of participants in the control group (detemir).
Czech Republic, United Kingdom, Republic of Ireland, Netherlands, Sweden and Finland.
Austria, Czech Republic, Denmark, Finland, Germany, Ireland, Israel, Netherlands, Sweden, Turkey and United Kingdom.
Studies with follow-up time up to 3 months were classified as short, 3–6 months as intermediate and >6 months as long.
NR, not reported; Cohort no, study with declaration of no conflict of interest; SC, single center; MC, multicenter.
Five studies30,33,34–36 declared conflicts of interest, one31 stated the absence of conflicts of interest and two32,37 didn’t mention this. Only two studies32,37 did not report funding, five studies30,33–36 were funded by the pharmaceutical industry and a single study31 had its own financing. Two studies evaluated only pediatric patients, five studies, only adult patients, and one study, both adults and children. The eight studies included a total of 9375 patients (Table 2).
With respect to the characteristics of patients, the average age ranged between 12 and 49 years, 56% were men, and the average disease duration ranged from 4 to 21 years.
Methodological quality
No studies obtained the maximum score of nine stars on the Newcastle–Ottawa Scale (Table 2). Four studies scored seven, three scored six, and one scored five. The quality of the included studies was ranked as high. There was asymmetry in the funnel plot (Figure 2) for the HbA1c outcome, suggesting an influence of publication bias.
Figure 2.
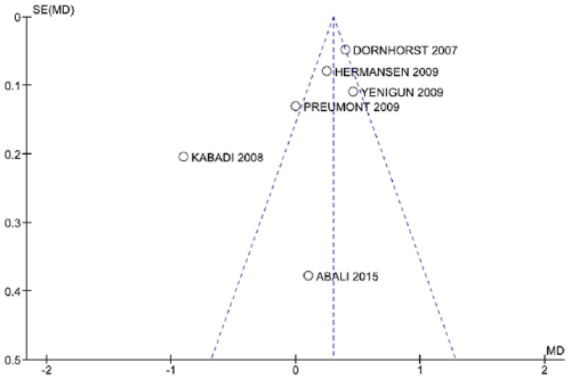
Funnel plot of mean difference in glycated hemoglobin.
MD mean difference, SE standard error.
Summary of the findings
To assess the effectiveness and safety of the different long-acting insulins in meta-analysis, the following outcomes were included: HbA1c, severe hypoglycemia, total dose of insulin and fasting glucose. As for the outcome of events of NH, we described only the results presented in each study, since they did not provide data in pairs that could be combined in a meta-analysis.
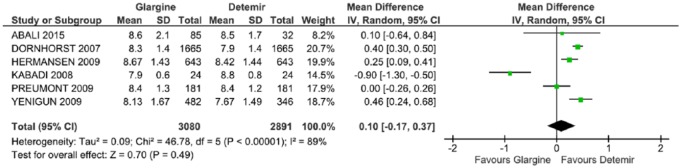
HbA1c analysis was included six studies.29,31,33–36 The results did not favor any of the two long-acting insulins (p = 0.49), with an average difference of 0.10 (CI: −0.17, 0.37, p < 0.00001; I2 = 89%), and significant heterogeneity (Figure 3).
Figure 3.
Meta-analysis of glycated hemoglobin (%).
CI, confidence interval; df., degrees of freedom; I2, Inconsistency; SD, standard deviation.
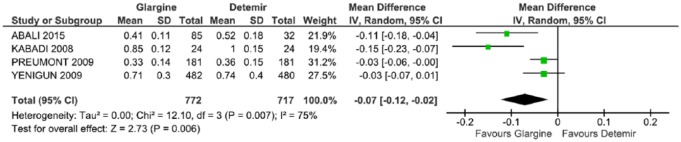
In the meta-analysis that assessed the total dose of insulin administered, four studies were included.29,31,34,35 There was a statistically significant difference favoring GLA (p = 0.006) in −0.07 (CI: −0.12, 0.02, p = 0.007; I2 = 75%) and with a significant heterogeneity (Figure 4).
Figure 4.
Meta-analysis of full insulin dose (U/kg/day).
CI, confidence interval; df., degrees of freedom; I2, Inconsistency; SD, standard deviation.
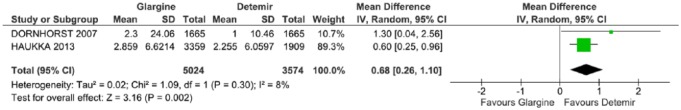
In the meta-analysis that assessed the occurrence of severe hypoglycemia, only two studies were included.30,36 The data showed a statically significant difference favoring DET (p = 0.002), with an average difference of 0.68 (CI: 0.26, 1.10, p = 0.30; I2 = 8%) (Figure 5).
Figure 5.
Meta-analysis of severe hypoglycemia (episodes/person-year).
CI, confidence interval; df., degrees of freedom; I2, Inconsistency; SD, standard deviation.
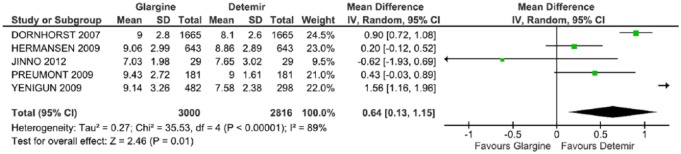
Five studies were included in the evaluation of fasting glucose levels.32–36 The result was statistically significant, favoring DET (p = 0.01), with an average difference of 0.64 (CI: 0.13, 1.15, p < 0.00001; I2 = 89%) with a high heterogeneity (Figure 6).
Figure 6.
Meta-analysis of fasting glucose (mmol/l).
CI, confidence interval; df., degrees of freedom; I2, Inconsistency; SD, standard deviation.
Nocturnal hypoglycemia events were assessed in the meta-analysis because the studies did not present data that could be combined. In the study of Yenigun and colleagues,35 nocturnal hypoglycemia events per patient-year were reduced to 10.01 with GLA once a day and to 3.77 with DET once a day (p < 0.0001). Dornhost and colleagues36 noted a decrease of 10.1 nocturnal hypoglycemia events per patient-year with GLA versus DET (p < 0.0001).
In the study of Haukka and colleagues,30 DET presented a lower risk of 13.1% (1.0%), 29.6−23.6% (−47.8%) and 17.9–5.1% (3.6–30.1%) for the occurrence of the first recurring hypoglycemia, as well as hypoglycemia and coma hypoglycemia (p = 0.034, p = 0.021, p = 0.016), respectively, versus GLA.
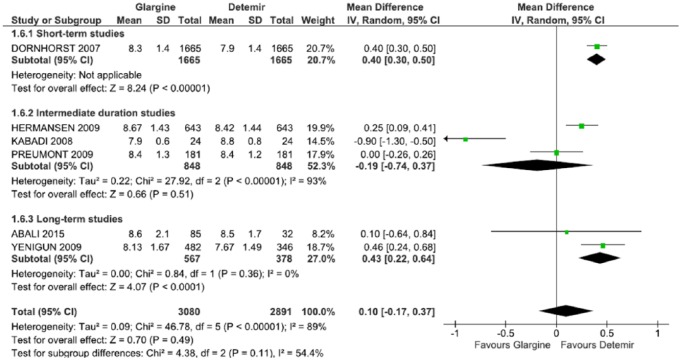
Analysis of subgroups
The outcome of HbA1c was also evaluated in two subgroups: the time of follow up and the presence of conflict of interest.
Studies classified as intermediate follow up31,33,34 (Table 2) had nonstatistically significant findings (p = 0.51) (MD = −0.19; CI: −0.74, 0.37, p < 0.0001; I2 = 93%). In longer duration studies,29,35 the difference of the average was estimated at 0.43 (CI: 0.22, 0.64, p = 0.36; I2 = 0%) favoring DET. When consolidated, an estimate of the difference of the average was 0.10 (CI: –0.17, 0.37, p < 0.00001; I2 = 89%) with high heterogeneity and did not favor either of the two long-acting insulins. Sensitivity analyses excluding one study31 affected the outcome favoring DET (p = 0.0005) and decreasing the heterogeneity for I2 = 61% (Table 3, Figure 7).
Table 3.
Result of meta-analysis.
| Outcome | Number of studies | Participants | Estimated effect (CI 95%) | I2 (%) | p value* |
|---|---|---|---|---|---|
| (1) HbA1c (%) | 6 | 5971 | 0.10 (−0.17, 0.37) | 89 | 0.49 |
| (1.1) Studies of intermediate duration | 3 | 1696 | −0.20 (−0.77, 0.38) | 91 | 0.51 |
| Long-term studies | 2 | 945 | 0.43 (0.64, 0.22) | 0 | <0.0001 |
| Studies of short duration | 1 | 3330 | 0.40 (0.30, 0.50) | – | <0.00001 |
| (1.2) With conflict of interest | 4 | 5806 | 0.30 (0.12, 0.47) | 0 | <0.00001 |
| Without conflict of interest | 2 | 165 | −0.45 (−1.43, 0.52) | 86 | 0.0007 |
| (2) Severe hypoglycemia (episodes/person-year) | 2 | 8598 | 0.68 (0.26, 1.10) | 8 | 0.002 |
| (3) Total dose of insulin (U/kg/day) | 4 | 1489 | −0.07 (−0.12, −0.02) | 75 | 0.006 |
| (4) Fasting glucose (mmol/l) | 5 | 5816 | 0.64 (0.13, 1.15) | 89 | 0.01 |
p value of the test for general effect.
CI, confidence interval; HbA1c, glycated hemoglobin.
Figure 7.
Meta-analysis of glycated hemoglobin (%) in a subgroup of the study duration.
CI, confidence interval; df., degrees of freedom; I2, Inconsistency; SD, standard deviation.
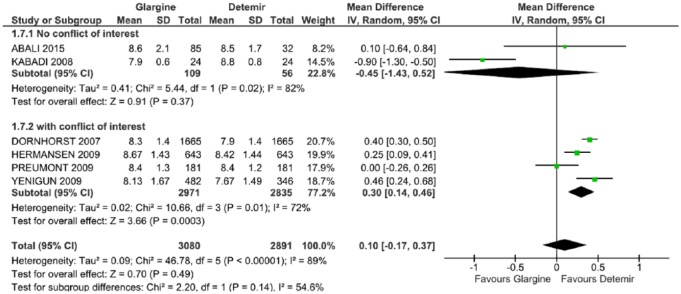
In the subgroup of studies without conflicts of interest,29,31 there were no statistically significant differences in HbA1c (DM = −0.45, CI = −1.43, 0.52, p = 0.02; I2 = 82%). In the subgroup of studies with conflicts of interest,33–36 there was an estimated average difference of 0.30 (CI: 0.14, 0.46, p = 0.01; I2 = 72%) favoring DET. All the results showed no statistically significant difference between the two long-acting insulins with a mean difference of 0.10 (CI: –0.17, 0.37, p < 0.00001; I2 = 89%), with high heterogeneity (Table 3, Figure 8). The exclusion of any studies in the sensitivity analyses affected the direction of the result.
Figure 8.
Meta-analysis of glycated hemoglobin (%) in the conflict-of-interest subgroup.
CI, confidence interval; df., degrees of freedom; I2, Inconsistency; SD, standard deviation.
Discussion
Faced with a chronic disease such as DM1, which requires that patients take care of themselves over a long period of time, it is necessary to outline a plan of action which can be modified when new clinical findings or laboratory results justify such modification. Intensive therapy, bringing together multiple daily injections and self monitoring, aiming to achieve improved glycemic control, are considered the optimal treatment for the DM1 to reduce the risk of complications. Strict control of DM1 can delay the progression of chronic microvascular complications in approximately 50% of cases, which makes the treatment of DM1 cost effective.38
The availability of long-acting insulins adds to the armamentarium where there are concerns about HbA1c control and hypoglycemia with current approaches. The findings from our meta-analysis of Hb1Ac found no differences between the two long-acting insulins (GLA and DET) in terms of glycemic control. Similar results were also described in randomized clinical trials (RCTs) and systematic reviews that compared GLA, DET and NPH insulin.9–12,33,34,39 Swinnen and colleagues, in their earlier systematic review of RCTs comparing GLA versus DET, also showed that glycemic control, as measured by the Hb1Ac, did not differ statistically significantly between the different long-acting insulins,37 supporting our findings.
When evaluating the results of HbA1c, the studies-without-conflict-of-interest subgroup did not show a statistically significant difference between GLA and DET. In the subgroup with conflicts of interest, the results favored DET, but the reference values for HbA1c control, recommended by the American Diabetes Association as below 7.0%, were not achieved.38 Bekelman and colleagues claim that financial relations between industry, researchers and academic institutions, can lead to favorable results for the sponsor, which can compromise subsequent patient welfare.40 Similar results were found in a previous meta-analysis.10
Two studies were included in the meta-analysis of doses used, with the results favorable to GLA. A daily dose (possibly two) is a basal scheme, with lispro/asparte/glulisine before each meal or, in the case of unpredictability of food intake (common in children), immediately after the meal. Despite GLA and DET having very similar absorption curves, there are differences between the two insulins, as a side-chain fatty acid promotes the formation of hexamers in the injection site, decreasing the absorption of DET and prolonging even further its action, indicating that the doses of DET should be about 30% higher than the doses of NPH used previously.41 On the other hand, there seems to be less intra-individual variation with the use of DET compared with GLA and NPH.42
The results of the meta-analysis of severe hypoglycemia involving 8598 patients showed statistical significance, favoring DET. Singh and colleagues8 showed that the DET reduced the risk of occurrence of episodes of severe and nocturnal hypoglycemia in relation to NPH, an advantage not seen with GLA insulin. Pieber and colleagues43 showed that the use of DET is equally effective in glycemic control versus GLA in patients with DM1, but with less daytime or severe hypoglycemia. However, in relation to the control of hypoglycemia episodes (any episode of hypoglycemia), the meta-analysis by Monami and colleagues9 showed that the incidence of any event of hypoglycemia was equal among the long-acting insulin analogs and NPH insulin.
In this context, self management is integral to the control of DM1, as it allows patients to assess their individual response to therapy with insulin, as well as monitor whether blood glucose targets are being effectively achieved, and may be useful in preventing hypoglycemia or hyperglycemia symptoms and therapeutic adjustment.44
The results of the meta-analysis of fasting glucose also favored DET, with lower values in patients treated with DET when compared with GLA. However, recent studies have questioned this parameter to monitor the glycemic control of patients, because it reflects a one-time nonrecurring measure, at the time of blood collection.37
Although some results favored the DET, in most cases, the therapeutic goal for glycemic control was not achieved in the groups of patients monitored. This can be due to barriers the disease imposes, such as the occurrence and fear of hypoglycemic events, the complexity of daily treatment, the need for self monitoring and frequent adjustments of insulin doses, and because in routine clinical care, the results from long-acting insulin analogs may not duplicate those observed in RCTs.4–8 Consequently, the choice of long-acting insulin analogs should be based on the individual characteristics of the patient, the effectiveness of existing therapies and any cost differential between the different insulins.
Currently, the annual cost of treating people with DM represents approximately 12% of total health expenditure in the world.4 Whilst not the subject of this review, the cost differential between GLA, DET and NPH insulins must be considered, especially in healthcare systems striving for, or currently attaining, universal access within finite resources. A study conducted by the Canadian Agency for Drugs and Technologies in Health (CADTH) compared the cost effectiveness of GLA and DET with NPH in patients with DM1 and DM2, and noted that the long-action analogs are not cost effective and that the substitution of NPH by DET and GLA in patients with DM1 would be costly to the Canadian health system.13 Evaluations carried out in the United Kingdom estimated savings of up to US $836 million over a decade with greater use of NPH versus long-acting analogs.45 The savings would have been higher if you take into account the Brazilian perspective, since in the United Kingdom, the cost differential between long-acting analogs and NPH insulin is lower than the 536% differential that currently exists in Brazil.
However, in 2017, the National Agency of Sanitary Vigilance [Agência Nacional de Vigilância Sanitaria (ANVISA)] of Brazil registered a GLA biosimilar (Abasagar), with its price determined by the Regulation of the Marketing of Medicines [Câmara de Regulação do Mercado de Medicamentos (CMED)] at 70% lower than originator GLA and 45% lower than DET.46,47 This systematic review and meta-analysis, along with other studies and economic analyses, can help health authorities, and those responsible for the coordination of health programs and services within finite resources in Brazil and other countries with universal healthcare, re-evaluate the possible incorporation of different long-acting analogs into the list of publicly funded of medicines, as well as potentially help with price negotiations. In Germany, after the authorities recommended the exclusion of short-acting insulin analogs since there were no data demonstrating superiority over NPH insulin to justify significantly higher prices, the manufacturers introduced significant price reductions to continue being reimbursed.48 A similar approach could be adopted in Brazil, as well as in the state of Minas Gerais, as more biosimilar long-acting analogs become available with limited differences between them, in terms of their clinical effectiveness and safety, and potentially considerable price reductions versus the originators. These are considerations for the future.
We acknowledge there are limitations of this systematic review. It included cohort studies with the intrinsic selection bias of observational studies. There were also differences in the number of participants between the groups and the monitoring period between studies. Nevertheless observational studies generally have greater statistical power and a population closer to the ‘real world’, that is, with broader inclusion criteria, without exclusion of patients with potentially more complicated disease and without the strict limits of RCTs.
The selected data for the meta-analysis can also be influenced by publication bias, that is, the tendency of the published results is systematically different from reality. An analysis of clinical trials registered on the basis of the ClinicalTrial.gov protocol revealed that less than 70% of studies are published.49 The nonpublication of results may be due to the decision of the author or the funder of the study where there are unfavorable findings; alternatively, less interest from publishers of scientific journals where there are negative results or results without statistical significance. The publication bias, with the selection of favorable results, can also influence the data used in meta-analyses.40 To minimize the potential for publication bias, a comprehensive search was conducted, including gray literature and complementary searches. However, in this systematic review analysis of the funnel plot we found asymmetry. Most of the studies showed great precision though, usually performed with large samples, and distributed symmetrically in the upper part of the funnel. Only the study by Kabadi and colleagues31 showed lower precision, located on the outside of the funnel. Another limitation of our meta-analysis was the small number of studies included in the review and the lack of complete and accurate information for inclusion in the quantitative analyses, as few published studies made direct comparison between GLA and DET, which hindered the explanation of sources of heterogeneity. In relation to the sensitivity analysis, the inclusion and exclusion of studies in each comparison did not change the direction of most outcome measures, without significant changes in heterogeneity, with the exception of the study by Kabadi and colleagues,31 which when deleted, changed the direction of the results in the analysis favoring DET. Overall, the scarcity of studies comparing GLA versus DET, and the absence of other analyses with ‘real world’ data, make it difficult to fully compare the results. Nevertheless, we believe our findings are robust, providing direction to the authorities in Brazil and wider.
Conclusion
Although some results are favorable to DET, it has not been possible to identify differences in effectiveness and safety compared with GLA. This would require new long-term studies and better methodologies. Nevertheless, our findings, suggesting limited clinical differences between the different long-acting insulin analogs, can help in the current debate on the inclusion of long-acting analogs, including biosimilars, in the official list of medicines reimbursed in Brazil. The market entry of GLA and other future biosimilars can assist with price negotiations and subsequent listing, including potentially expanding population groups. It is important to note though that for good glycemic control, therapeutic interventions should be accompanied by continuous monitoring of blood glucose, dietary interventions and effective education. These are considerations for the future.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not for-profit sectors.
Conflict of interest statement: The authors declare that there is no conflict of interest.
ORCID iD: Brian Godman  https://orcid.org/0000-0001-6539-6972
https://orcid.org/0000-0001-6539-6972
Contributor Information
Thales B. C. Silva, School of Pharmacy, Federal University of Minas Gerais (UFMG), Minas Gerais, Brazil SUS Collaborating Center for Technology Assessment and Excellence in Health (CCATES), Federal University of Minas Gerais (UFMG), Minas Gerais, Brazil.
Paulo H. R. F. Almeida, School of Pharmacy, Federal University of Minas Gerais (UFMG), Minas Gerais, Brazil SUS Collaborating Center for Technology Assessment and Excellence in Health (CCATES), Federal University of Minas Gerais (UFMG), Minas Gerais, Brazil.
Vania E. Araújo, School of Pharmacy, Federal University of Minas Gerais (UFMG), Minas Gerais, Brazil SUS Collaborating Center for Technology Assessment and Excellence in Health (CCATES), Federal University of Minas Gerais (UFMG), Belo Horizonte, Minas Gerais Brazil; School of Dentistry, Pontifícia Universidade Católica de Minas Gerais (PUCMG), Minas Gerais, Brazil.
Francisco de Assis Acurcio, School of Pharmacy, Federal University of Minas Gerais (UFMG), Minas Gerais, Brazil; SUS Collaborating Center for Technology Assessment and Excellence in Health (CCATES), Federal University of Minas Gerais (UFMG), Minas Gerais, Brazil.
Augusto A. Guerra Júnior, School of Pharmacy, Federal University of Minas Gerais (UFMG), Minas Gerais, Brazil SUS Collaborating Center for Technology Assessment and Excellence in Health (CCATES), Federal University of Minas Gerais (UFMG), Minas Gerais, Brazil.
Brian Godman, Strathclyde Institute of Pharmacy and Biomedical Sciences, University of Strathclyde, Glasgow G4 0RE, United Kingdom.
Juliana Alvares, School of Pharmacy, Federal University of Minas Gerais (UFMG), Minas Gerais, Brazil; SUS Collaborating Center for Technology Assessment and Excellence in Health (CCATES), Federal University of Minas Gerais (UFMG), Minas Gerais, Brazil.
References
- 1. United States Renal Data System. International Comparisons. In United States Renal Data System. 2014 USRDS annual data report: epidemiology of kidney disease in the United States. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2014, pp.188–210. [Google Scholar]
- 2. Moxey PW, Gogalniceanu P, Hinchliffe RJ, et al. Lower extremity amputations – a review of global variability in incidence. Diabeti Med 2011; 28: 1144–1153. [DOI] [PubMed] [Google Scholar]
- 3. De Oliveira GL, Guerra Junior AA, Godman B, et al. Cost-effectiveness of vildagliptin for people with type 2 diabetes mellitus in Brazil; findings and implications. Expert Rev Pharmacoecon Outcomes Res 2017; 17: 109–119. [DOI] [PubMed] [Google Scholar]
- 4. International Diabetes Federation. IDF Diabetes Atlas. 7th ed. Brussels: International Diabetes Federation, http://www.diabetesatlas.org/ (2015, accessed 10 December 2017). [Google Scholar]
- 5. Plank J, Bodenlenz M, Sinner F, et al. The double-blind, randomized, dose-response study investigating the pharmacodynamic and pharmacokinetic properties of the long-acting insulin analog detemir. Diabetes Care 2005; 28: 1107–1112. [DOI] [PubMed] [Google Scholar]
- 6. Caires de, Souza AL, De Assis Acurcio F, Guerra Junior AA, et al. Insulin glargine in a Brazilian state: should the government disinvest? An assessment based on a systematic review. Appl Health Econ Health Policy 2014; 12: 19–32. [DOI] [PubMed] [Google Scholar]
- 7. Marra LP, Araújo VE, Silva TBC, et al. Clinical effectiveness and safety of analog glargine in type 1 diabetes: a systematic review and meta-analysis. Diabetes Ther 2016; 7: 241–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Singh SR, Ahmad F, Lal A, et al. Efficacy and safety of insulin analogs for the management of diabetes mellitus: a meta-analysis. CMAJ 2009; 180: 385–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Monami M, Marchionni N, Mannucci E. Long-acting insulin analogs vs NPH human insulin in type 1 diabetes: a meta-analysis. Diabetes Obes Metab 2009; 11: 372–378. [DOI] [PubMed] [Google Scholar]
- 10. Marra LP, Araújo VE, Oliveria GCC, et al. The clinical effectiveness of insulin glargine in patients with type 1 diabetes in Brazil: findings and implications. J Comp Eff Res 2017; 6: 519–527. [DOI] [PubMed] [Google Scholar]
- 11. Almeida PHRF, Silva TBC, De Assis Acurcio F, et al. Quality of life of patients with type 1 diabetes mellitus using insulin analog glargine compared with NPH insulin: a systematic review and policy implications. Patient. Epub ahead of print 10 January 2018. DOI: 10.1007/s40271-017-0291-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tunis SL, Minshall ME, Conner C, et al. Cost effectiveness of insulin detemir compared to NPH insulin for type 1 and type 2 diabetes mellitus in the Canadian payer setting: modeling analysis. Curr Med Res Opin 2009; 25: 1273–1284. [DOI] [PubMed] [Google Scholar]
- 13. CADTH. COMPUS. Long-acting insulin analogs for the treatment of diabetes mellitus: meta-analyses of clinical outcomes, http://www.cadth.ca (2008, accessed 10 December 2017).
- 14. Rede Brasileira de Avaliacão de Tecnologia em Saúde (Brazilian Network for Health Technology Assessment): Boletim Brasileiro de Avaliacão de Tecnologias em Saúde (Brazilian Bulletin of Health Technology Assessment). [Glargine insulin and detemir insulin: the control of type 1 diabetes mellitus], http://bvsms.saude.gov.br/bvs/ct/pdf/brats2010_n13.pdf (2010, accessed 5 December 2017).
- 15. RESOLUÇÃO SES Nº 1761. http://www.saude.mg.gov.br/images/documentos/resolucao_1761.pdf (2009, accessed 14 December 2017).
- 16. Siebenhofer-Kroitzsch A, Horvath K, Plank J. Insulin analogues: too much noise about small benefits. CMAJ 2009; 180: 369–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Permsuwan U, Thavorn K, Dilokthornsakul P, et al. Cost-effectiveness of insulin detemir versus insulin glargine for Thai type 2 diabetes from a payer’s perspective. J Med Econ 2017; 20: 991–999. [DOI] [PubMed] [Google Scholar]
- 18. Hasslacher C, Kulozik F, Lorenzo Bermejo J. Treatment with insulin analogs, especially Glargine and Lispro, associates with better renal function and higher hemoglobin levels in type 1 diabetic patients with impaired kidney function. Ther Adv Endocrinol Metab 2016; 7: 166–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zhang T, Lin M, Li W, et al. Comparison of the efficacy and safety of insulin detemir and insulin glargine in hospitalized patients with type 2 diabetes: a randomized crossover trial. Adv Ther 2016; 33: 178–185. [DOI] [PubMed] [Google Scholar]
- 20. Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 2009; 21: 339:b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hartling L, Hamm M, Milne A, et al. Validity and reliability tests between evaluator of quality assessment tools [internet]. Appendix E, decision rules for application of the Newcastle-Ottawa Scale. Rockville, MD: Research and Quality Agency in Health, http://www.ncbi.nlm.nih.gov/books/NBK92291/ (accessed 1 September 2016). [Google Scholar]
- 22. Higgins JPT, Green S. Cochrane handbook for systematic reviews of interventions [Internet]. Version 5.2.0 [updated June 2017]: The Cochrane Collaboration. (2017, accessed 2 May 2018). [Google Scholar]
- 23. Tsujino D, Nishimura R, Morimoto A, et al. The crossover comparison of glycemic variations in Japanese patients with type 1 diabetes receiving insulin glargine versus insulin detemir twice daily using continuous glucose monitoring (CGM): J COLLECTION (Jikei COmparison of Lantus and LEvemir with Cgm is Thinking Insulin OptimizatioN). Diabetes Technol Ther 2012; 14: 596–601. [DOI] [PubMed] [Google Scholar]
- 24. Kurtoglu S Atabek ME Dizdarer C et al.;. PREDICTIVE Turkey Study Group. Insulin detemir improves glycemic control and reduces hypoglycemia in children with type 1 diabetes: findings from the Turkish cohort of the PREDICTIVE observational study. Pediatr Diabetes 2009; 10: 401–407. [DOI] [PubMed] [Google Scholar]
- 25. Philips JC, Scheen AJ. [Insulin detemir in the predictive study: results in patients with type 1 diabetes in the Belgian cohort]. Rev Med Liege 2009; 64: 124–130. [PubMed] [Google Scholar]
- 26. Derosa G, Franzetti I, Querci F, et al. Glucose-lowering effect and glycaemic variability of insulin glargine, insulin detemir and insulin lispro protamine in people with type 1 diabetes. Diabetes Obes Metab 2015; 17: 554–559. [DOI] [PubMed] [Google Scholar]
- 27. Plavšić L, Mitrović K, Todorović S, et al. Glycaemic control and prevalence of hypoglycaemic events in children and adolescents with type 1 diabetes mellitus treated with insulin analogs. Vojnosanit Pregl 2014; 71: 817–820. [PubMed] [Google Scholar]
- 28. Hopkinson HE, Jacques RM, Gardner KJ, et al. Twice - rather than once - daily basal insulin is associated with better glycaemic control in Type 1 diabetes mellitus 12 months after skills - based structured education in insulin self - management. Diabet Med 2015; 32: 1071–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Abali S, Turan S, Atay Z, et al. Higher insulin detemir doses are required for the similar glycemic control: comparison of insulin glargine and detemir in children with type 1 diabetes mellitus. Pediatr Diabetes 2015; 16: 361–366. [DOI] [PubMed] [Google Scholar]
- 30. Haukka J, Hoti F, Erästö P, et al. Evaluation of the incidence and risk of hypoglycemic coma associated with selection of basal insulin in the treatment of diabetes: Finnish register linkage study. Pharmacoepidemiol Drug Saf 2013; 22: 1326–1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kabadi UM. Deleterious outcomes after abrupt transition from insulin glargine insulin detemir to in patients with type 1 diabetes mellitus. Clin Drug Investig 2008; 28: 697–701. [DOI] [PubMed] [Google Scholar]
- 32. Jinno K, Urakami T, Horikawa R, et al. Usefulness of insulin detemir in Japanese children with type 1 diabetes. Pediatr Int 2012; 54: 773–779. [DOI] [PubMed] [Google Scholar]
- 33. Hermansen K, Dornhorst A, Sreenan S. Observational, open-label study of type 1 and type 2 diabetes patients switching from human insulin to insulin analogue basal-bolus regimens: insights from the PREDICTIVE study. Curr Med Res Opin 2009; 25: 2601–2608. [DOI] [PubMed] [Google Scholar]
- 34. Preumont V, Buysschaert M, De Beukelaer S, et al. Insulin detemir in routine clinical practice: the 26-week follow-up in type 1 diabetic patients from the Belgian PREDICTIVE cohort. Acta Clin Belg 2009; 64: 49–55. [DOI] [PubMed] [Google Scholar]
- 35. Yenigun M, Honka M. Switching patients from insulin glargine-based basal-bolus regimens to a once daily insulin detemir-based basal-bolus regimen: results from a subgroup of the PREDICTIVE study. Int J Clin Pract 2009; 63: 425–432. [DOI] [PubMed] [Google Scholar]
- 36. Dornhorst A, Lüddeke HJ, Sreenan S, et al. Safety and efficacy of insulin detemir in clinical practice: 14 - week follow - up data from type 1 and type 2 diabetes patients in the PREDICTIVE TM European cohort. Int J Clin Pract 2007; 61: 523–528. [DOI] [PubMed] [Google Scholar]
- 37. Swinnen SG, Simon AC, Holleman F, et al. Insulin detemir versus insulin glargine for type 2 diabetes mellitus. Cochrane Database Syst Rev 2011; (7): CD006383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. American Diabetes Association (ADA). Standards of medical care in diabetes. Position statement. Diabetes Care 2017; 40: S1–S135.27979885 [Google Scholar]
- 39. Heller S, Koenen S, Bode B. Comparison of insulin detemir and insulin glargine in a basal-bolus regimen, with insulin aspart as the mealtime insulin, in patients with type 1 diabetes: a 52-week, multinational, randomized, open-label, parallel-group, treat-to-target noninferiority trial. Clin Ther 2009; 31: 2086–2097. [DOI] [PubMed] [Google Scholar]
- 40. Bekelman JE, Li Y, Gross CP. Scope and impact of financial conflicts of interest in biomedical research: a systematic review. JAMA 2003; 289: 454–465. [DOI] [PubMed] [Google Scholar]
- 41. Kurtzhals P, Havelund S, Jonassen I, et al. Effect of fatty acids and selected drugs on the albumin binding of a long-acting, acylated insulin analogue. J Pharm Sci 1997; 86: 1365–1368. [DOI] [PubMed] [Google Scholar]
- 42. Hermansen K, Madsbad S, Perrild H, et al. Comparison of the soluble basal insulin analog insulin detemir with NPH insulin: a randomized open crossover trial in type 1 diabetic subjects on basal-bolus therapy. Diabetes Care 2001; 24: 296–301. [DOI] [PubMed] [Google Scholar]
- 43. Pieber TR, Treichel HC, Hompesch B, et al. Comparison of insulin detemir and insulin glargine in subjects with type 1 diabetes using intensive insulin therapy. Diabet Med 2007; 24: 635–642. [DOI] [PubMed] [Google Scholar]
- 44. Wolpert HA. The nuts and bolts of achieving end points with realtime continuous glucose monitoring. Diabetes Care 2008; 31(Suppl. 2): S146–S149. [DOI] [PubMed] [Google Scholar]
- 45. Holden SE, Poole CD, Morgan CL, et al. Evaluation of the incremental cost to the National Health Service of prescribing analogue insulin. BMJ Open 2011; 1: e000258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Diabetes mellitus type 1 and type 2: insulin glargine biosimilar (Abasaglar) - Evidence summary [ESNM64], https://www.nice.org.uk/advice/esnm64/chapter/Key-points-from-the-evidence (2015, Accessed 4 December 2017).
- 47. Maximum Prices of Medicinal Products by Active Substance, Câmara de Regulação do Mercado de Medicamentos-CMED, http://portal.anvisa.gov.br/consulta-lista-de-preco-de-medicamento (2017, accessed 13 December 2017).
- 48.IQWiG Reports–Commission No. A05–02. Rapid-acting insulin analogues in the treatment of diabetes mellitus type 1 [Internet], www.iqwig.de/download/A0502_Abschlussbericht_Kurzwirksame_Insulinanaloga_bei_Diabetes_mellitus_Typ_1.pdf (2007, accessed 13 December 2017).
- 49. Ross JS, Tse T, Zarin DA, et al. Publication of NIH funded trials registered in ClinicalTrials.gov: cross sectional analysis. BMJ 2012; 344(d7292): 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]