Abstract
Meglitinides such as repaglinide and nateglinide are useful to treat type 2 diabetes patients who follow a flexible lifestyle. They are short-acting insulin secretagogues and are associated with less risk of hypoglycemia, weight gain and chronic hyperinsulinemia compared with sulfonylureas. Meglitinides are the substrates of cytochrome P450 (CYP) enzymes and organic anion transporting polypeptide 1B1 (OATP1B1 transporter) and the coadministration of the drugs affecting them will result in pharmacokinetic drug interactions. This article focuses on the drug interactions of meglitinides involving CYP enzymes and OATP1B1 transporter. To prevent the risk of hypoglycemic episodes, prescribers and pharmacists must be aware of the adverse drug interactions of meglitinides.
Keywords: drug interactions, CYP2C8, CYP2C9, CYP3A4, nateglinide, OATP1B1 transporter, repaglinide
Introduction
Drug interaction is defined as the interference of effects of one drug by the coadministered drugs, nutrients (food), herbs, alcohol or tobacco smoke.1 The drug interaction results in either increased or decreased beneficial effects or increased adverse effects. The drug interaction leading to undesirable effects, is termed ‘adverse drug interaction’. Polypharmacy, having liver or kidney disease, or a number of underlying chronic disorders elevate the risk of adverse drug interactions.2
Interacting drugs can alter the pharmacokinetic or pharmacodynamic profile of another. Plasma concentration of one drug is either increased or decreased by altering absorption, distribution, metabolism, or excretion of another drug, and this type of interaction is known as pharmacokinetic drug interactions. The pharmacodynamic interactions are those in which the effect of one drug is altered by the presence of another drug at the same receptor or molecular site.3–5 Object drug is the one affected by the interaction, and the drug causing the interaction is termed precipitant drug. The absorption, distribution, metabolism, excretion, or actual clinical effect of the object drug is usually modified by the precipitant drug.6
The risk of adverse drug interactions is higher in diabetes patients, as they coadminister the medications to manage their comorbidities such as dyslipidemia, hypertension, heart disease, depression, infections, etc., along with their antidiabetic medications. A Brazil study comprised 140 diabetes patients who attended a tertiary care outpatient center, indicated a prevalence of 75% of potential drug–drug interactions, of which 20.7% were major interactions.7 And a study from Croatia identified that 80.9% of diabetes patients had at least one potential drug interaction requiring monitoring of therapy.8 Most of the antidiabetic drug interactions may result in hypoglycemia-related complications. Severe hypoglycemia is a life-threatening emergency and can result in seizures, coma and death.9,10
Meglitinides are short-acting insulin secretagogues and they include repaglinide and nateglinide. Repaglinide is a benzoic acid derivative and nateglinide a d-phenylalanine derivative. They are more useful for treating type 2 diabetes mellitus patients having irregular meal times, and act by lowering postprandial glucose primarily. Meglitinides bind to the sulfonylurea receptors (SUR1s) of pancreatic β cells and activate the closure of KATP channels [adenosine triphosphate (ATP)-dependent potassium channels] on the β-cell membrane, resulting in depolarization of the β cell and opening of voltage-gated calcium channels. Elevated intracellular calcium concentrations lead to increased fusion of insulin-stored vesicles with the cell membrane, resulting in release of insulin.11–15 Nateglinide inhibits KATP channels faster than repaglinide and it has shorter duration of action and reduced risk of hypoglycemia in comparison with repaglinide.12 The risk of hypoglycemia, weight gain and chronic hyperinsulinemia is lower with meglitinides compared with sulfonylureas.14,16
Pharmacokinetic drug interactions of meglitinides
Meglitinides are the substrates of cytochrome P450 (CYP) enzymes and organic anion transporting polypeptide 1B1 (OATP1B1 transporter). Repaglinide is metabolised by CYP2C8 and CYP3A4 enzymes17,18 while nateglinide is metabolized primarily by CYP2C9 enzyme and by CYP3A4 enzyme to a much lesser extent.19–21
Organic anion transporting polypeptides (OATPs) are membrane influx transporters and their family consists of 11 members including OATP1B1, OATP1B3 and OATP2B1.22 OATPs are the members of solute-linked carriers (SLCO) superfamily and particularly SLCO21A family; they are ATP-independent polypeptides.23–25
OATP1B1 transporter found in sinusoidal membrane of hepatocytes aids liver uptake of their substrate drugs.26–28 The pharmacokinetics of repaglinide is majorly determined by OATP1B1 transporter.29 Nateglinide is also a substrate of OATP1B1 transporter, which determines the hepatic uptake of nateglinide.30
Drug interactions of repaglinide
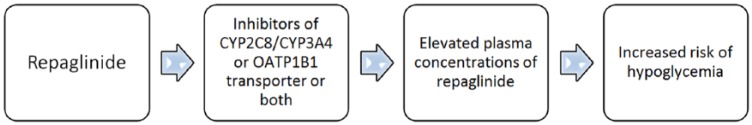
Drugs inducing or inhibiting CYP enzymes (CYP2C8 and CYP3A4) and OATP1B1 transporter do play an important role in the drug interactions of repaglinide (Figure 1 and Table 1).
Figure 1.
Drug interactions of repaglinide.
Drugs such as gemfibrozil, clopidogrel, cyclosporine, trimethoprim, macrolide antibiotics, atorvastatin and nifedipine inhibit CYP2C8/CYP3A4 enzymes, and OATP1B1 transporter can increase the plasma concentrations of repaglinide and likelihood of subsequent hypoglycemia.
Table 1.
Drug interactions of repaglinide.
| Interacting drugs | Mechanism of interaction | Comments |
|---|---|---|
| Gemfibrozil | Gemfibrozil can increase the plasma concentrations of repaglinide through the inhibition of CYP2C8 enzyme,31,32 OATP1B1 transporter33–35 and UGT1A136 | Concomitant use of repaglinide and gemfibrozil should be avoided;38 other fibrates like bezafibrate or fenofibrate may be recommended in patients taking repaglinide39 |
| Clopidogrel | Clopidogrel can inhibit the CYP2C8-mediated metabolism of repaglinide and elevate the risk of hypoglycemia42,43 | Concomitant use of repaglinide and clopidogrel should be avoided; ticagrelor may be used instead of clopidogrel44 |
| Cyclosporine | Cyclosporine may increase the plasma concentrations of repaglinide and subsequent hypoglycemia by inhibiting OATP1B1 transporter26,52 and CYP3A4 enzyme53,54 | Close monitoring of blood glucose is recommended in patients taking cyclosporine and repaglinide57 |
| Macrolide antibiotics | Macrolide antibiotics inhibit OATP1B1 transporter59 and CYP3A4 enzyme60,61 and increase the plasma concentrations of repaglinide62–64 | The blood glucose levels should monitored in patients taking repaglinide and macrolide antibiotics together;65
azithromycin may be a suitable macrolide for the patients already receiving repaglinide, since it shows least activity against OATP1B1 and CYP3A466 |
| Trimethoprim | Trimethoprim is a potent inhibitor of CYP2C8 enzyme67 | Monitor the blood glucose levels if used concomitantly |
| Atorvastatin | Atorvastatin may increase the plasma concentrations of repaglinide through the inhibition of OATP1B1-mediated hepatic uptake80
and CYP3A4-mediated metabolism of repaglinide81 |
Monitor the blood glucose levels if used concomitantly |
| Nifedipine | Nifedipine is a moderate competitive inhibitor of CYP3A4 enzyme86 | Monitor the blood glucose levels if used concomitantly |
| Rifampicin (Rifampin) | Rifampicin is an inducer of CYP3A488,89 and CYP2C890 enzymes and it may decrease the plasma concentrations and therapeutic efficacy of repaglinide17,91 | Monitor the blood glucose levels if used concomitantly |
| Deferasirox | Deferasirox inhibits CYP3A4 and CYP2C8 enzymes94 | Monitor the blood glucose levels if used concomitantly96 |
Gemfibrozil
Gemfibrozil is an inhibitor of CYP2C8 enzyme.31,32 Gemfibrozil and its glucuronide metabolite also inhibit OATP1B1-transporter-mediated hepatic uptake of repaglinide.33–35 Since repaglinide is the substrate of both CYP2C8 enzyme and OATP1B1 transporter, its concomitant use with gemfibrozil resulted in increased plasma concentrations of repaglinide and subsequent hypoglycemia.36,37 Gemfibrozil is also found to inhibit Uridine Diphosphate (UDP) glucuronosyltransferase 1A1 (UGT1A1) involving in glucuronidation of repaglinide, resulting in additional elevation of plasma concentrations of repaglinide.36
Hence, the patients on repaglinide should avoid using gemfibrozil.38 Hypertriglyceridemia of the patients taking repaglinide could be treated with either bezafibrate or fenofibrate due to their lack of interaction with repaglinide.39
Clopidogrel
Clopidogrel is a second-generation thienopyridine antiplatelet drug and is a P2Y12 receptor antagonist.40 Nowadays, clopidogrel is prescribed most commonly as an antiplatelet drug. The American Diabetes Association (ADA) recommends using clopidogrel as a secondary prevention strategy in diabetes patients with a history of atherosclerotic cardiovascular disease and an intolerance to aspirin therapy.41
Use of clopidogrel in diabetes patients taking repaglinide may result in elevated plasma concentrations of repaglinide, since glucuronide metabolite of clopidogrel is a strong CYP2C8 inhibitor.42,43
To avoid hypoglycemia, it is recommended that repaglinide is not coadministered with clopidogrel. Ticagrelor may be a suitable antiplatelet drug to treat diabetes patients taking repaglinide; or else, patients taking clopidogrel may be prescribed nateglinide rather than repaglinide.44
Cyclosporine
Cyclosporine is an immunosuppressant medication that decreases the production of inflammatory cytokines by T lymphocytes through the blockade of calcineurin’s phosphatase activity by forming the cyclosporine–cyclophilin complex.45
Cyclosporine is less diabetogenic and with less risk of developing post-transplant diabetes mellitus compared with tacrolimus and corticosteroids.46 Hence, cyclosporine may be preferred over tacrolimus to treat diabetes patients needing organ transplantation.47,48 Cyclosporine is identified to reduce the risk of rheumatoid-arthritis-associated atherosclerosis49 and hence it may be useful to treat the patients of rheumatoid arthritis with diabetes. Cyclosporine may also be used in treating the patients of systemic lupus erythematosus (SLE) with diabetes, since it decrease the risk of SLE-associated atherosclerosis.50 In addition, cyclosporine may be a useful treatment option in patients with resistant Churg-Strauss syndrome (CSS).51
Cyclosporine is shown to inhibit the OATP1B1 transporter-mediated hepatic uptake of substrates.26,52 Cyclosporine is also an inhibitor of the CYP3A4 enzyme.53,54 Hence, cyclosporine may elevate the exposure of repaglinide and the risk of hypoglycemia by inhibiting OATP1B1-transporter-mediated hepatic uptake and CYP3A4-enzyme-mediated metabolism of repaglinide.55,56 Close monitoring of blood glucose is recommended in patients taking cyclosporine and repaglinide.57
Macrolide antibiotics
Macrolide antibiotics include erythromycin, clarithromycin and azithromycin. They are widely used to treat respiratory tract infections and skin and soft tissue infections, primarily.58
OATP1B1-mediated hepatic uptake of substrates is inhibited by macrolide antibiotics such as erythromycin, roxithromycin and telithromycin in a concentration-dependent manner.59 Macrolide antibiotics such as erythromycin, clarithromycin and roxithromycin can also inhibit intestinal and hepatic CYP3A4 enzyme.60,61 Concomitant use of repaglinide and clarithromycin or telithromycin resulted in increased plasma concentrations and blood-glucose-lowering effect of repaglinide, which may lead to hypoglycemic risk.62–64
The blood glucose levels should be monitored in patients taking repaglinide and macrolide antibiotics together.65 Azithromycin may be a suitable macrolide for the patients already receiving repaglinide, since it shows least activity against OATP1B1 and CYP3A4.66
Trimethoprim
Trimethoprim is a potent inhibitor of CYP2C8 enzyme at clinically relevant concentrations.67 In healthy subjects, the plasma concentrations of repaglinide found elevated by trimethoprim are probably due to the inhibition of CYP2C8-mediated metabolism of repaglinide.68 The risk of hypoglycemia may be higher in diabetic patients with renal dysfunction and taking repaglinide and trimethoprim concurrently.69
Hydroxymethylglutaryl coenzyme A reductase inhibitors (statins)
Hydroxymethylglutaryl coenzyme A reductase inhibitors or statins are effective lipid-lowering drugs and they decrease the serum cholesterol by inhibiting hepatic cholesterol biosynthesis, resulting in upregulation of hepatic low-density lipoprotein (LDL) receptors and increased clearance of LDL-cholesterol (LDL-C).70 In addition, statins can improve endothelial function and blood flow, enhance the stability of atherosclerotic plaques, decrease oxidative stress and inflammation, inhibit vascular smooth muscle proliferation and platelet aggregation, and reduce vascular inflammation as their cholesterol-independent or ‘pleiotropic’ effects.71–74
Statins are very much effective in secondary prevention of cardiovascular diseases (CVDs) and decrease the mortality in people with pre-existing CVD.75,76 Statins are also useful in the primary prevention of cardiovascular diseases (CVD) and reduce the risk of major cardiovascular events like myocardial infarction, stroke, etc. in people without established cardiovascular disease but with cardiovascular risk factors like diabetes, elevated blood pressure, obesity, etc.77–79
The plasma concentrations of repaglinide might be raised by atorvastatin through the inhibition of OATP1B1-mediated hepatic uptake.80 Atorvastatin may also inhibit CYP3A4-mediated metabolism of repaglinide and increase its activity.81 The bioavailability of oral repaglinide enhanced by fluvastatin might be due to the inhibition of CYP3A4-mediated metabolism of repaglinide.82
Nifedipine
Nifedipine is a dihydropyridine calcium channel blocker and it is useful to treat older patients with systolic hypertension and diabetes.83,84 Nifedipine found to improve diabetes-associated cognitive impairment as a pleiotropic effect.85
Nifedipine is a moderate competitive inhibitor of the CYP3A4 enzyme.86 The oral bioavailability of repaglinide may be elevated by the coadministration of nifedipine which can inhibit CYP3A4-mediated metabolism of repaglinide.87
Rifampicin (rifampin)
Rifampicin is an antitubercular drug and it is a potent inducer of the CYP3A4 enzyme.88,89 Rifampicin can also induce the expression of other CYP enzymes, including CYP2C8.90 Since rifampicin induces both CYP3A4 and CYP2C8 enzymes, it may decrease the plasma concentrations and therapeutic efficacy of repaglinide.17,91
Deferasirox
Deferasirox is the most commonly used oral iron chelator and it is useful for the treatment of chronic iron overload resulting from long-term blood transfusions.92–94 The diabetic patients with β thalassemia may be prescribed deferasirox to treat iron overload.95
Deferasirox is a weak inhibitor of both CYP3A4 and CYP2C8 enzymes,96 which are involved in the metabolism of repaglinide. Concomitant use of deferasirox and repaglinide warrants careful monitoring of glucose levels.97
Drug interactions of nateglinide
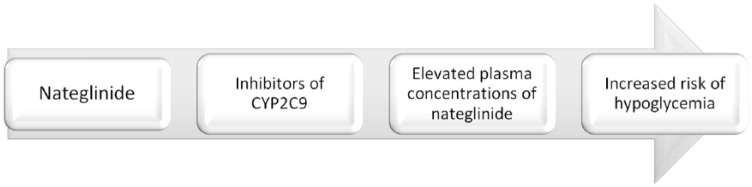
Drugs inhibiting or inducing CYP2C9 enzyme and OATP1B1 transporter do play an important role in the drug interactions of nateglinide (Figure 2 and Table 2).
Figure 2.
Drug interactions of nateglinide.
Nateglinide is a substrate of the CYP2C9 enzyme and drugs like fluconazole and miconazole may increase the plasma concentrations of nateglinide through inhibition of the CYP2C9 enzyme.
Table 2.
Drug interactions of nateglinide.
| Interacting drugs | Mechanism of interaction | Comments |
|---|---|---|
| Rifampicin | Rifampicin may decrease plasma concentrations and blood glucose-lowering effect of nateglinide by inducing CYP2C9 enzyme-mediated metabolism of nateglinide101 | Monitor the blood glucose levels |
| Azole antifungals (fluconazole, miconazole) | Fluconazole106 or miconazole107 may increase the plasma concentrations of nateglinide through the inhibition of CYP2C9 enzyme-mediated metabolism of nateglinide | Monitor changes in glycemic control |
Rifampicin
Rifampicin is primarily used in the treatment of tuberculosis. The diabetic patients with tuberculosis would be prescribed with rifampicin along with other anti-TB drugs.98–100
Rifampicin may induce CYP2C9 enzyme-mediated oxidative biotransformation of nateglinide resulting in decreased plasma concentrations and blood-glucose-lowering effect of nateglinide. Monitor the blood glucose levels while the initiation and discontinuation of rifampicin in patients taking nateglinide.101
Azole antifungals
The rate of fungal infections is higher in patients with diabetes.102–104 Fluoconazole is an azole antifungal drug and is recommended in diabetic patients to treat fungal infections, as it is found effective against cutaneous Candidiasis, oropharyngeal Candidiasis (OPC) and vulvovaginal Candidiasis (VVC).105
The plasma concentrations of nateglinide might be enhanced by the coadministration of fluconazole, which can inhibit the CYP2C9 enzyme-mediated metabolism of nateglinide.106 CYP2C9 enzyme-mediated metabolism of nateglinide might also be inhibited by miconazole.107 It is advisable to monitor changes in glycemic control during their concomitant use.
Conclusion
Repaglinide and nateglinide are the substrates of CYP enzymes and OATP1B1 transporter. Repaglinide is metabolized by CYP2C8 and CYP3A4 enzymes and OATP1B1 transporter determines its hepatic uptake. The patients taking repaglinide should avoid using drugs such as gemfibrozil and clopidogrel due to heightened risk of hypoglycemia. Drugs like cyclosporine, macrolide antibiotics, trimethoprim, statins and nifedipine elevate the risk of hypoglycemia in patients taking repaglinide, so blood glucose levels of such patients should be monitored. Concomitant use of repaglinide or nateglinide and rifampicin may result in reduced blood-glucose-lowering effects. To prevent the adverse drug interactions of meglinide antidiabetics, the prescribers and pharmacists must be aware of these effects.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: The authors declare that there is no conflict of interest.
ORCID iD: Naina Mohamed Pakkir Maideen  https://orcid.org/0000-0002-6950-9783
https://orcid.org/0000-0002-6950-9783
Contributor Information
Naina Mohamed Pakkir Maideen, Pharmacology, Dubai Health Authority, PB No: 4545, Dubai, UAE.
Gobinath Manavalan, Department of Chemistry, Ratnam Institute of Pharmacy, Nellore, India.
Kumar Balasubramanian, Department of Pharmacy Practice, Ratnam Institute of Pharmacy, Nellore, India.
References
- 1. Baxter K, Preston CL. (eds). Stockley’s drug interactions. London: Pharmaceutical Press, 2010. [Google Scholar]
- 2. Anastasio GD, Cornell KO, Menscer D. Drug interactions: keeping it straight. Amer Family Phys 1997; 56: 883–888. [PubMed] [Google Scholar]
- 3. Kashuba AD, Bertino JS. Mechanisms of drug interactions I: absorption, metabolism, and excretion. In: Piscitelli S, Rodvold K, Pai M. (eds) Drug interactions in infectious diseases. 2nd ed. Totowa, NJ: Humana Press, 2005, pp.13–39. [Google Scholar]
- 4. D’Arcy PF, McElnay JC, Welling PG. (eds). Mechanisms of drug interactions. Springer Science & Business Media, 2012. [Google Scholar]
- 5. Hussar DA. Mechanisms of drug interactions. J Am Pharm Assoc 1969; 9: 208–213. [DOI] [PubMed] [Google Scholar]
- 6. Ansari JA. Drug interaction and pharmacist. J Young Pharm 2010; 2: 326–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Trevisan DD, Silva JB, Póvoa VC, et al. Prevalence and clinical significance of potential drug-drug interactions in diabetic patients attended in a tertiary care outpatient center, Brazil. Int J Diabetes Dev Ctries 2016; 36: 283–289. [Google Scholar]
- 8. Samardzic I, Bacic-Vrca V. Incidence of potential drug–drug interactions with antidiabetic drugs. Die Pharmazie-Int J Pharma Sci 2015; 70: 410–415. [PubMed] [Google Scholar]
- 9. Miller CD, Phillips LS, Ziemer DC, et al. Hypoglycemia in patients with type 2 diabetes mellitus. Arch Intern Med 2001; 161: 1653–1659. [DOI] [PubMed] [Google Scholar]
- 10. Anderson M, Powell J, Campbell KM, et al. Optimal management of type 2 diabetes in patients with increased risk of hypoglycemia. Diabetes Metab Syndr Obes 2014; 7: 85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hinnen DA. Therapeutic options for the management of postprandial glucose in patients with type 2 diabetes on basal insulin. Clin Diabetes 2015; 33: 175–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Stein SA, Lamos EM, Davis SN. A review of the efficacy and safety of oral antidiabetic drugs. Expert Opin Drug Saf 2013; 12: 153–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Quianzon CC, Cheikh IE. History of current non-insulin medications for diabetes mellitus. J Community Hosp Intern Med Perspect 2012; 2: 19081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Guardado-Mendoza R, Prioletta A, Jiménez-Ceja LM, et al. The role of nateglinide and repaglinide, derivatives of meglitinide, in the treatment of type 2 diabetes mellitus. Arch Med Sci 2013; 9: 936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Landgraf R. Meglitinide analogues in the treatment of type 2 diabetes mellitus. Drugs Aging 2000; 17: 411–425. [DOI] [PubMed] [Google Scholar]
- 16. Lorenzati B, Zucco C, Miglietta S, et al. Oral hypoglycemic drugs: pathophysiological basis of their mechanism of actionoral hypoglycemic drugs: pathophysiological basis of their mechanism of action. Pharmaceuticals 2010; 3: 3005–3020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kajosaari LI, Laitila J, Neuvonen PJ, et al. Metabolism of repaglinide by CYP2C8 and CYP3A4 in vitro: effect of fibrates and rifampicin. Basic Clin Pharmacol Toxicol 2005; 97: 249–256. [DOI] [PubMed] [Google Scholar]
- 18. Bidstrup TB, Bjørnsdottir I, Sidelmann UG, et al. CYP2C8 and CYP3A4 are the principal enzymes involved in the human in vitro biotransformation of the insulin secretagogue repaglinide. British J Clin Pharmacol 2003; 56: 305–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Niemi M, Backman JT, Juntti-Patinen L, et al. Coadministration of gemfibrozil and itraconazole has only a minor effect on the pharmacokinetics of the CYP2C9 and CYP3A4 substrate nateglinide. British J Clin Pharmacol 2005; 60: 208–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kirchheiner J, Meineke I, Müller G, et al. Influence of CYP2C9 and CYP2D6 polymorphisms on the pharmacokinetics of nateglinide in genotyped healthy volunteers. Clin Pharmacokinet 2004; 43: 267–278. [DOI] [PubMed] [Google Scholar]
- 21. Weaver ML, Orwig BA, Rodriguez LC, et al. Pharmacokinetics and metabolism of nateglinide in humans. Drug Metab Dispos 2001; 29: 415–421. [PubMed] [Google Scholar]
- 22. Kalliokoski A, Niemi M. Impact of OATP transporters on pharmacokinetics. Br J Pharmacol 2009; 158: 693–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Girardin F. Membrane transporter proteins: a challenge for CNS drug development. Dialogues Clin Neurosci 2006; 8: 311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Roth M, Obaidat A, Hagenbuch B. OATPs, OATs and OCTs: the organic anion and cation transporters of the SLCO and SLC22A gene superfamilies. Br J Pharmacol 2012; 165: 1260–1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nigam SK. What do drug transporters really do? Nat Rev Drug Discov 2015; 14: 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Shitara Y. Clinical importance of OATP1B1 and OATP1B3 in drug-drug interactions. Drug Metab Pharmacokinet 2011; 26: 220–227. [DOI] [PubMed] [Google Scholar]
- 27. Gui C, Obaidat A, Chaguturu R, et al. Development of a cell-based high-throughput assay to screen for inhibitors of organic anion transporting polypeptides 1B1 and 1B3. Curr Chem Genomics 2010; 4: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kalliokoski A, Niemi M. Impact of OATP transporters on pharmacokinetics. Br J Pharmacol 2009; 158: 693–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Niemi M, Backman JT, Kajosaari LI, et al. Polymorphic organic anion transporting polypeptide 1B1 is a major determinant of repaglinide pharmacokinetics. Clin Pharmacol Ther 2005; 77: 468–478. [DOI] [PubMed] [Google Scholar]
- 30. Takanohashi T, Kubo S, Arisaka H, et al. Contribution of organic anion transporting polypeptide (OATP) 1B1 and OATP1B3 to hepatic uptake of nateglinide, and the prediction of drug–drug interactions via these transporters. J Pharm Pharmacol 2012; 64: 199–206. [DOI] [PubMed] [Google Scholar]
- 31. Honkalammi J, Niemi M, Neuvonen PJ, et al. Mechanism-based inactivation of CYP2C8 by gemfibrozil occurs rapidly in humans. Clin Pharmacol Ther 2011; 89: 579–586. [DOI] [PubMed] [Google Scholar]
- 32. Honkalammi J, Niemi M, Neuvonen PJ, et al. Dose-dependent interaction between gemfibrozil and repaglinide in humans: strong inhibition of CYP2C8 with subtherapeutic gemfibrozil doses. Drug Metab Dispos 2011; 39: 1977–1986. [DOI] [PubMed] [Google Scholar]
- 33. Kudo T, Hisaka A, Sugiyama Y, et al. Analysis of the repaglinide concentration increase produced by gemfibrozil and itraconazole based on the inhibition of the hepatic uptake transporter and metabolic enzymes. Drug Metab Dispos 2012; 41: 362–371. [DOI] [PubMed] [Google Scholar]
- 34. Varma MV, Lin J, Bi YA, et al. Quantitative rationalization of gemfibrozil drug interactions: consideration of transporters-enzyme interplay and the role of circulating metabolite gemfibrozil 1-O-β-glucuronide. Drug Metab Dispos 2015; 43: 1108–1118. [DOI] [PubMed] [Google Scholar]
- 35. Karlgren M, Ahlin G, Bergström CA, et al. In vitro and in silico strategies to identify OATP1B1 inhibitors and predict clinical drug–drug interactions. Pharm Res 2012; 29: 411–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Gan J, Chen W, Shen H, et al. Repaglinide-gemfibrozil drug interaction: inhibition of repaglinide glucuronidation as a potential additional contributing mechanism. Br J Clin Pharmacol 2010; 70: 870–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Niemi M, Backman JT, Neuvonen M, et al. Effects of gemfibrozil, itraconazole, and their combination on the pharmacokinetics and pharmacodynamics of repaglinide: potentially hazardous interaction between gemfibrozil and repaglinide. Diabetologia 2003; 46: 347–351. [DOI] [PubMed] [Google Scholar]
- 38. Grant JS, Graven LJ. Progressing from metformin to sulfonylureas or meglitinides. Workplace Health Saf 2016; 64: 433–439. [DOI] [PubMed] [Google Scholar]
- 39. Kajosaari LI, Backman JT, Neuvonen M, et al. Lack of effect of bezafibrate and fenofibrate on the pharmacokinetics and pharmacodynamics of repaglinide. Br J Clin Pharmacol 2004; 58: 390–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Jiang XL, Samant S, Lesko LJ, et al. Clinical pharmacokinetics and pharmacodynamics of clopidogrel. Clin Pharmacokinet 2015; 54: 147–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. American Diabetes Association. Standards of medical care in diabetes—2016 abridged for primary care providers. Clin Diab 2016; 34: 3–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kim SJ, Yoshikado T, Ieiri I, et al. Clarification of the mechanism of clopidogrel-mediated drug-drug interaction in a clinical cassette small-dose study and its prediction based on in vitro information. Drug Metab Dispos 2016; 44: 1622–1632. [DOI] [PubMed] [Google Scholar]
- 43. Tornio A, Filppula AM, Kailari O, et al. Glucuronidation converts clopidogrel to a strong time-dependent inhibitor of CYP2C8: a phase II metabolite as a perpetrator of drug–drug interactions. Clin Pharmacol Ther 2014; 96: 498–507. [DOI] [PubMed] [Google Scholar]
- 44. Wang ZY, Chen M, Zhu LL, et al. Pharmacokinetic drug interactions with clopidogrel: updated review and risk management in combination therapy. Ther Clin Risk Manag 2015; 11: 449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Matsuda S, Koyasu S. Mechanisms of action of cyclosporine. Immunopharmacology 2000; 47: 119–125. [DOI] [PubMed] [Google Scholar]
- 46. Vincenti F, Friman S, Scheuermann E, et al. Results of an international, randomized trial comparing glucose metabolism disorders and outcome with cyclosporine versus tacrolimus. Am J Transplant 2007; 7: 1506–1514. [DOI] [PubMed] [Google Scholar]
- 47. Dumortier J, Bernard S, Bouffard Y, et al. Conversion from tacrolimus to cyclosporine in liver transplanted patients with diabetes mellitus. Liver Transpl 2006; 12: 659–664. [DOI] [PubMed] [Google Scholar]
- 48. Ghisdal L, Bouchta NB, Broeders N, et al. Conversion from tacrolimus to cyclosporine A for new-onset diabetes after transplantation: a single-centre experience in renal transplanted patients and review of the literature. Transpl Int 2008; 21: 146–151. [DOI] [PubMed] [Google Scholar]
- 49. Kisiel B, Kruszewski R, Juszkiewicz A, et al. Methotrexate, cyclosporine A, and biologics protect against atherosclerosis in rheumatoid arthritis. J Immunol Res 2015; 2015: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Oryoji K, Kiyohara C, Horiuchi T, et al. Reduced carotid intima–media thickness in systemic lupus erythematosus patients treated with cyclosporine A. Mod Rheumatol 2014; 24: 86–92. [DOI] [PubMed] [Google Scholar]
- 51. McDermott E, Powell R. Cyclosporin in the treatment of Churg-Strauss syndrome. Ann Rheum Dis 1998; 57: 258–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Shitara Y, Itoh T, Sato H, et al. Inhibition of transporter-mediated hepatic uptake as a mechanism for drug-drug interaction between cerivastatin and cyclosporin A. J Pharmacol Exp Ther 2003; 304: 610–616. [DOI] [PubMed] [Google Scholar]
- 53. Gertz M, Cartwright CM, Hobbs MJ, et al. Cyclosporine inhibition of hepatic and intestinal CYP3A4, uptake and efflux transporters: application of PBPK modeling in the assessment of drug-drug interaction potential. Pharm Res 2013; 30: 761–780. [DOI] [PubMed] [Google Scholar]
- 54. Amundsen R, Åsberg A, Ohm IK, et al. Cyclosporine A-and tacrolimus-mediated inhibition of CYP3A4 and CYP3A5 in vitro. Drug Metab Dispos 2012; 40: 655–661. [DOI] [PubMed] [Google Scholar]
- 55. Backman JT, Kajosaari LI, Niemi M, et al. Cyclosporine A increases plasma concentrations and effects of repaglinide. Am J Transplant 2006; 6: 2221–2222. [DOI] [PubMed] [Google Scholar]
- 56. Kajosaari LI, Niemi M, Neuvonen M, et al. Cyclosporine markedly raises the plasma concentrations of repaglinide. Clin Pharmacol Ther 2005; 78: 388–399. [DOI] [PubMed] [Google Scholar]
- 57. Türk T, Witzke O. Pharmacological interaction between cyclosporine A and repaglinide. Is it clinically relevant? Am J Transplant 2006; 6: 2223. [DOI] [PubMed] [Google Scholar]
- 58. Zuckerman JM, Qamar F, Bono BR. Review of macrolides (azithromycin, clarithromycin), ketolids (telithromycin) and glycylcyclines (tigecycline). Med Clin North Am 2011; 95: 761–791. [DOI] [PubMed] [Google Scholar]
- 59. Seithel A, Eberl S, Singer K, et al. The influence of macrolide antibiotics on the uptake of organic anions and drugs mediated by OATP1B1 and OATP1B3. Drug Metab Dispos 2007; 35: 779–786. [DOI] [PubMed] [Google Scholar]
- 60. Quinney SK, Malireddy SR, Vuppalanchi R, et al. Rate of onset of inhibition of gut-wall and hepatic CYP3A by clarithromycin. Eur J Clin Pharmacol 2013; 69: 439–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Westphal JF. Macrolide–induced clinically relevant drug interactions with cytochrome P-450A (CYP) 3A4: an update focused on clarithromycin, azithromycin and dirithromycin. Br J Clin Pharmacol 2000; 50: 285–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Niemi M, Neuvonen PJ, Kivistö KT. The cytochrome P4503A4 inhibitor clarithromycin increases the plasma concentrations and effects of repaglinide. Clin Pharmacol Ther 2001; 70: 58–65. [DOI] [PubMed] [Google Scholar]
- 63. Plosker GL, Figgitt DP. Repaglinide: a pharmacoeconomic review of its use in type 2 diabetes mellitus. Pharmacoeconomics 2004; 22: 389–411. [DOI] [PubMed] [Google Scholar]
- 64. Kajosaari LI, Niemi M, Backman JT, et al. Telithromycin, but not montelukast, increases the plasma concentrations and effects of the cytochrome P450 3A4 and 2C8 substrate repaglinide. Clin Pharmacol Ther 2006; 79: 231–242. [DOI] [PubMed] [Google Scholar]
- 65. Khamaisi M, Leitersdorf E. Severe hypoglycemia from clarithromycin-repaglinide drug interaction. Pharmacotherapy 2008; 28: 682–684. [DOI] [PubMed] [Google Scholar]
- 66. Wright AJ, Gomes T, Mamdani MM, et al. The risk of hypotension following co-prescription of macrolide antibiotics and calcium-channel blockers. Can Med Assoc J 2011; 183: 303–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Wen X, Wang JS, Backman JT, et al. Trimethoprim and sulfamethoxazole are selective inhibitors of CYP2C8 and CYP2C9, respectively. Drug Metab Dispos 2002; 30: 631–635. [DOI] [PubMed] [Google Scholar]
- 68. Niemi M, Kajosaari LI, Neuvonen M, et al. The CYP2C8 inhibitor trimethoprim increases the plasma concentrations of repaglinide in healthy subjects. Br J Clin Pharmacol 2004; 57: 441–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Roustit M, Blondel E, Villier C, et al. Symptomatic hypoglycemia associated with trimethoprim/sulfamethoxazole and repaglinide in a diabetic patient. Ann Pharmacother 2010; 44: 764–767. [DOI] [PubMed] [Google Scholar]
- 70. Stancu C, Sima A. Statins: mechanism of action and effects. J Cell Mol Med 2001; 5: 378–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Liao JK, Laufs U. Pleiotropic effects of statins. Annu Rev Pharmacol Toxicol 2005; 45: 89–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Marzilli M. Pleiotropic effects of statins: evidence for benefits beyond LDL-cholesterol lowering. Am J Cardiovasc Drugs 2010; 10: 3–9. [DOI] [PubMed] [Google Scholar]
- 73. Kavalipati N, Shah J, Ramakrishan A, et al. Pleiotropic effects of statins. Indian J Endocr Metab 2015; 19: 554–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Oesterle A, Laufs U, Liao JK. Pleiotropic effects of statins on the cardiovascular system. Circ Res 2017; 120: 229–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Afilalo J, Duque G, Steele R, et al. Statins for secondary prevention in elderly patients: a hierarchical bayesian meta-analysis. J Am Coll Cardiol 2008; 51: 37–45. [DOI] [PubMed] [Google Scholar]
- 76. Athyros VG, Papageorgiou AA, Mercouris BR, et al. Treatment with atorvastatin to the National Cholesterol Educational Program goal versus ‘usual’ care in secondary coronary heart disease prevention. Curr Med Res Opin 2002; 18: 220–228. [DOI] [PubMed] [Google Scholar]
- 77. Brugts JJ, Yetgin T, Hoeks SE, et al. The benefits of statins in people without established cardiovascular disease but with cardiovascular risk factors: meta-analysis of randomised controlled trials. BMJ 2009; 338: b2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Taylor F, Ward K, Moore TH, et al. Statins for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev 2011; (1): CD004816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Ebrahim S, Taylor FC, Brindle P. Statins for the primary prevention of cardiovascular disease. BMJ 2014; 348: g280. [DOI] [PubMed] [Google Scholar]
- 80. Kalliokoski A, Backman JT, Kurkinen KJ, et al. Effects of gemfibrozil and atorvastatin on the pharmacokinetics of repaglinide in relation to SLCO1B1 polymorphism. Clin Pharmacol Ther 2008; 84: 488–496. [DOI] [PubMed] [Google Scholar]
- 81. Sekhar MC, Reddy PJ. Influence of atorvastatin on the pharmacodynamic and pharmacokinetic activity of repaglinide in rats and rabbits. Mol Cell Biochem 2012; 364: 159–164. [DOI] [PubMed] [Google Scholar]
- 82. Lee CK, Choi JS, Bang JS. Effects of fluvastatin on the pharmacokinetics of repaglinide: possible role of CYP3A4 and P-glycoprotein inhibition by fluvastatin. Korean J Physiol Pharmacol 2013; 17: 245–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Tuomilehto J, Rastenyte D, Birkenhäger WH, et al. Effects of calcium-channel blockade in older patients with diabetes and systolic hypertension. N Engl J Med 1999; 340: 677–684. [DOI] [PubMed] [Google Scholar]
- 84. Konzem SL, Devore VS, Bauer DW. Controlling hypertension in patients with diabetes. Am Fam Physician 2002; 66: 1209–1214. [PubMed] [Google Scholar]
- 85. Tsukuda K, Mogi M, Li JM, et al. Diabetes-associated cognitive impairment is improved by a calcium channel blocker, nifedipine. Hypertension 2008; 51: 528–533. [DOI] [PubMed] [Google Scholar]
- 86. Wrighton SA, Ring BJ. Inhibition of human CYP3A catalyzed 1’-hydroxy midazolam formation by ketoconazole, nifedipine, erythromycin, cimetidine, and nizatidine. Pharm Res 1994; 11: 921–924. [DOI] [PubMed] [Google Scholar]
- 87. Choi JS, Choi I, Choi DH. Effects of nifedipine on the pharmacokinetics of repaglinide in rats: possible role of CYP3A4 and P-glycoprotein inhibition by nifedipine. Pharmacol Rep 2013; 65: 1422–1430. [DOI] [PubMed] [Google Scholar]
- 88. Yamashita F, Sasa Y, Yoshida S, et al. Modeling of rifampicin-induced CYP3A4 activation dynamics for the prediction of clinical drug-drug interactions from in vitro data. PLoS One 2013; 8: e70330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Venkatesan K. Pharmacokinetic drug interactions with rifampicin. Clin Pharmacokinet 1992; 22: 47–65. [DOI] [PubMed] [Google Scholar]
- 90. Rae JM, Johnson MD, Lippman ME, et al. Rifampin is a selective, pleiotropic inducer of drug metabolism genes in human hepatocytes: studies with cDNA and oligonucleotide expression arrays. J Pharmacol Exp Ther 2001; 299: 849–857. [PubMed] [Google Scholar]
- 91. Niemi M, Backman JT, Neuvonen M, et al. Rifampin decreases the plasma concentrations and effects of repaglinide. Clin Pharmacol Ther 2000; 68: 495–500. [DOI] [PubMed] [Google Scholar]
- 92. Cappellini MD, Cohen A, Piga A, et al. A phase 3 study of deferasirox (ICL670), a once-daily oral iron chelator, in patients with β-thalassemia. Blood 2006; 107: 3455–3462. [DOI] [PubMed] [Google Scholar]
- 93. Piga A, Galanello R, Forni GL, et al. Randomized phase II trial of deferasirox (Exjade, ICL670), a once-daily, orally-administered iron chelator, in comparison to deferoxamine in thalassemia patients with transfusional iron overload. Haematologica 2006; 91: 873–880. [PubMed] [Google Scholar]
- 94. Taher A, Cappellini MD. Update on the use of deferasirox in the management of iron overload. Ther Clin Risk Manag 2009; 5: 857–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Lioudaki E, Whyte M. Acute cardiac decompensation in a patient with beta-thalassemia and diabetes mellitus following cessation of chelation therapy. Clin Case Rep 2016; 4: 992–996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Skerjanec A, Wang J, Maren K, et al. Investigation of the pharmacokinetic interactions of deferasirox, a once-daily oral iron chelator, with midazolam, rifampin, and repaglinide in healthy volunteers. J Clin Pharmacol 2010; 50: 205–213. [DOI] [PubMed] [Google Scholar]
- 97. Tanaka C. Clinical pharmacology of deferasirox. Clin Pharmacokinet 2014; 53: 679–694. [DOI] [PubMed] [Google Scholar]
- 98. Ruslami R, Aarnoutse RE, Alisjahbana B, et al. Implications of the global increase of diabetes for tuberculosis control and patient care. Trop Med Int Health 2010; 15: 1289–1299. [DOI] [PubMed] [Google Scholar]
- 99. Nijland HM, Ruslami R, Stalenhoef JE, et al. Exposure to rifampicin is strongly reduced in patients with tuberculosis and type 2 diabetes. Clin Infect Dis 2006; 43: 848–854. [DOI] [PubMed] [Google Scholar]
- 100. Chang MJ, Chae JW, Yun HY, et al. Effects of type 2 diabetes mellitus on the population pharmacokinetics of rifampin in tuberculosis patients. Tuberculosis 2015; 95: 54–59. [DOI] [PubMed] [Google Scholar]
- 101. Niemi M, Backman JT, Neuvonen M, et al. Effect of rifampicin on the pharmacokinetics and pharmacodynamics of nateglinide in healthy subjects. Br J Clin Pharmacol 2003; 56: 427–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Al-Attas SA, Amro SO. Candidal colonization, strain diversity, and antifungal susceptibility among adult diabetic patients. Ann Saudi Med 2010; 30: 101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Kumar BV, Padshetty NS, Bai KY, et al. Prevalence of Candida in the oral cavity of diabetic subjects. J Assoc Physicians India 2005; 53: 599–602. [PubMed] [Google Scholar]
- 104. Willis AM, Coulter WA, Fulton CR, et al. Oral candidal carriage and infection in insulin-treated diabetic patients. Diabet Med 1999; 16: 675–679. [DOI] [PubMed] [Google Scholar]
- 105. Penk A, Pittrow L. Therapeutic experience with fluconazole in the treatment of fungal infections in diabetic patients. Mycoses 1999; 42: 97–100. [PubMed] [Google Scholar]
- 106. Niemi M, Neuvonen M, Juntti-Patinen L, et al. Effect of fluconazole on the pharmacokinetics and pharmacodynamics of nateglinide. Clin Pharmacol Ther 2003; 74: 25–31. [DOI] [PubMed] [Google Scholar]
- 107. Takanohashi T, Koizumi T, Mihara R, et al. Prediction of the metabolic interaction of nateglinide with other drugs based on in vitro studies. Drug Metab Pharmacokinet 2007; 22: 409–418. [DOI] [PubMed] [Google Scholar]