Supplemental Digital Content is available in the text.
Keywords: aquaporin 4, blood-brain barrier, ischemia, neuroprotection, pericytes
Abstract
Background and Purpose—
In ischemic stroke, breakdown of the blood-brain barrier (BBB) aggravates brain damage. Pericyte detachment contributes to BBB disruption and neurovascular dysfunction, but little is known about its regulation in stroke. Here, we investigated how loss of RGS5 (regulator of G protein signaling 5) in pericytes affects BBB breakdown in stroke and its consequences.
Method—
We used RGS5 knockout and control mice and applied a permanent middle cerebral occlusion model. We analyzed pericyte numbers, phenotype, and vessel morphology using immunohistochemistry and confocal microscopy. We investigated BBB breakdown by measuring endothelial coverage, tight junctions, and AQP4 (aquaporin 4) in addition to BBB permeability (fluorescent-conjugated dextran extravasation). Tissue hypoxia was assessed with pimonidazole hydrochloride and neuronal death quantified with the terminal deoxynucleotidyl transferase dUTP nick end labeling assay.
Results—
We demonstrate that loss of RGS5 increases pericyte numbers and their endothelial coverage, which is associated with higher capillary density and length, and significantly less BBB damage after stroke. Loss of RGS5 in pericytes results in reduced vascular leakage and preserved tight junctions and AQP4, decreased cerebral hypoxia, and partial neuronal protection in the infarct area.
Conclusions—
Our findings show that loss of RGS5 affects pericyte-related BBB preservation in stroke and identifies RGS5 as an important target for neurovascular protection.
Cerebral ischemia is accompanied by vascular dysfunction because of damage of the blood-brain barrier (BBB).1,2 The BBB is a specialized barrier consisting of endothelial cells, tight junctions, pericytes, astrocytic end-feet processes, and the basement membrane, crucial to establish a highly regulated microenvironment that ensures proper neuronal function.3,4 Disruption of the BBB in stroke causes dramatic changes in the chemical and cellular compositions of this microenvironment,4,5 making the BBB an important target to reduce brain damage in stroke.
Pericytes are an important component of the BBB. They are located between endothelial cells and astrocytes and line the entire microvasculature.5–7 Pericytes support endothelial cell survival and promote normal tight junction development.7 Ischemic injury causes detachment of pericytes from the vascular wall8–10 and their degeneration, leading to progressive capillary reduction and BBB breakdown.11,12 The molecular mechanisms regulating pericyte function and associated BBB alterations under pathological conditions are still poorly understood.
RGS5 (regulator of G-protein signaling 5) is a marker highly expressed in brain pericytes.13,14 It is a member of the RGS family proteins that modulate G protein–coupled receptors15 stimulating GTPase activity and inhibiting signaling downstream of G protein–coupled receptors.16,17 We have previously demonstrated that RGS5 is upregulated in pericytes in response to ischemic injury before detachment from the blood vessels, suggesting a possible role of RGS5 in this process.8 However, little is known about the function of RGS5 in the regulation of pericyte coverage and BBB damage after ischemic stroke.
To investigate the effect of loss of RGS5 in stroke, we induced ischemic stroke in RGS5-knockout mice.18 Our findings identify RGS5 as a potential ischemia-inducible factor in pericytes that negatively regulates pericyte number and endothelial coverage in stroke which in turn leads to BBB preservation and neurovascular protection.
Methods
The data that support the findings of this study are available from the corresponding author on reasonable request.
Animals and Permanent Middle Cerebral Occlusion
All animal experiments followed the principles of laboratory animal care (National Institutes of Health publication No. 86-23, revised 1985), and experiments were approved by the Ethical Committee at Lund University (M146-14). We randomized all mice and applied permanent middle cerebral occlusion as described previously.19 Experiments were performed by investigators blinded to the experimental condition of the animals. For detailed description of the experimental conditions, see the online-only Data Supplement.
Intravenous Injection of Dextran and Its Detection
To detect BBB permeability, 0.1 mL of lysine-fixable 10 kDa dextran conjugated to Alexa 647 10 mg/mL in PBS (Invitrogen) was injected into the tail vein 15 minutes before perfusion. As controls both, intact mice injected with dextran-Alexa 647 and stroke mice that received only PBS were used. Brain sections were analyzed by confocal microscopy (Zeiss LSM510), and extravasated dextran particles in the brain parenchyma were measured using Image J software. For details, please see the online-only Data Supplement.
Immunohistochemistry
One day after permanent middle cerebral occlusion, mice were transcardially perfused with PBS followed by 4% paraformaldehyde and sections prepared as previously described.8 The primary antibodies used were rabbit anti-AQP4 (aquaporin 4; 1:1000, Millipore), chicken anti-GFP (green fluorescent protein; 1:1000, Abcam), rabbit anti-claudin 5 (1:1000, Abcam), mouse anti-NeuN (neuronal nuclei; 1:400, Millipore), rabbit anti-neural/glial antigen 2 (NG2; 1:200, Millipore), rabbit anti–platelet-derived growth factor receptor (PDGFR)-β (1:200, Cell Signaling), anti–platatelet endothelial cell adhesion molecule-1 (CD31; 1:400, BD), rabbit antivascular endothelial-cadherin (1:1000, Abcam), rabbit anti–ZO-1 (zonula occludens 1; 1:500, Fisher), and staining revealed using species-specific fluorophore-conjugated (Streptavidin Alexa 488, Molecular Probes; Cy3 or Cy5, Jackson) or biotin-conjugated secondary antibodies (Jackson). Biotinylated secondary antibodies were revealed using the ABC kit (Vector labs). 4’,6-diamidine-2’-phenylindole (1 μg/mL, Sigma) was used for counterstaining.
Image Processing and Cell Quantification
3,3′-Diaminobenzidine–stained sections were analyzed using Olympus BX53 light microscope, and cells were counted using CellSens digital imaging software. Fluorescent immunostainings were visualized using an epifluorescence microscopy system (Olympus BX53) or confocal microscopes (Zeiss LSM510, Zeiss LSM780). Figures were composed using Photoshop CS5 software. For detailed description and quantification analysis, please refer to the online-only Data Supplement.
For analysis of pericyte number and coverage, only GFP+ cells found on capillaries20,21 that displayed the morphology of typical pericytes with a prominent cell body and elongated processes were analyzed as previously done in studies using the same mouse strain.11,18,22,23
Protein Extraction and Western Blot
Please see the online-only Data Supplement.
Quantification of Gene Expression by Quantitative Polymerase Chain Reaction
Please see the online-only Data Supplement.
Hypoxia Detection
To detect hypoxia in the infarct area after stroke, we applied Hypoxyprobe-1 kit (Hypoxyprobe, Inc, Burlington, MA). Quantification of the hypoxia area was performed using CellSens digital imaging software. For quantification analysis, please refer to the online-only Data Supplement.
TUNEL
Apoptotic cells were assessed by terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay according to the manufacturer instructions (Roche). Please see the online-only Data Supplement.
Statistical Analysis
Statistical analyses were done with Graph Pad Prism version 5.04 software. Data are expressed as mean±SD. Two group comparison was performed by using Student t test and multiple group comparison by 1-way or 2-way ANOVA followed by Tukey post hoc test. For Western blot, after normalization to the actin signal, the ipsilateral expression of each junction protein was compared with the contralateral expression, using a 2-way ANOVA, followed by Tukey post hoc test. Significance was set at P<0.05.
Results
Loss of RGS5 Leads to Increased Pericyte Numbers After Stroke
Pericyte loss is a hallmark for stroke; however, its molecular mechanism remains largely unknown.8,11,12 We first investigated whether deletion of RGS5 changes pericyte numbers after acute ischemic injury.
Under physiological conditions, knockout mice did not show any differences in pericyte numbers, both GFP+ and PDGFRβ+ as compared with their controls heterozygous (HET) and wild type (WT) (Figure IA–IC in the online-only Data Supplement).18 On stroke, however, the number of GFP+ pericytes was 80% higher in the infarct core of knockout mice compared with HET mice (262±27 and 148±9; n=7; Figure 1A and 1B). The number of PDGFRβ+ pericytes was ≈45% higher in the infarct core of knockout mice compared with HET and WT mice (189±12; 129±20; and 123±13; n=5 [knockout] n=4 [HET], n=5 [WT]), whereas there was no difference between HET and WT mice (Figure 1A and 1C).
Figure 1.

Loss of RGS5 (regulator of G-protein signaling 5) leads to increased pericyte numbers after stroke. A, Schematic representation of a coronal brain section showing location of infarct core. Representative images showing GFP+ (green fluorescent protein) pericytes in the infarct core in heterozygous (HET; top) and knockout (KO; bottom) mice; scale bar, 20 μm. Platelet-derived growth factor receptor (PDGFR) β+ pericytes in the infarct core of wild type (WT; top), HET (middle), and KO (bottom) mice; scale bar, 20 μm. B, Higher number of GFP+ pericytes in infarct core in KO mice compared with HET (n=7, **P<0.01, Student t test). C, Quantification of PDGFRβ+ pericytes in the infarct area in KO (n=5), HET (n=4), and WT (n=5) mice (****P<0.0001; ***P<0.001 1-way ANOVA, Tukey post hoc test). Data are expressed as cell numbers per mm2; mean±SD. Ic indicates ischemic core.
None of the GFP+ pericytes were positive for TUNEL (n=300 GFP+ pericytes quantified in total), and GFP+/PDGFRβ+ pericytes of each genotype did not show any characteristics of apoptotic morphology at 1 day after ischemic injury (Figure IIA and IIB in the online-only Data Supplement).
We next investigated whether loss of RGS5 has an impact on the marker expression profile of pericytes. In knockout mice, the number of GFP+ pericytes expressing PDGFRβ+ pericytes in the infarct core was 2-fold higher than in HET mice (122±13 and 66±5; n=6; Figure 2A and 2B). NG2 is another marker for pericytes, in particular activated pericytes.8,24,25 The number of GFP+ pericytes expressing NG2 was >2-fold higher in the infarct core of knockout mice versus HET mice (131±16 and 70±19; n=6; Figure 2C and 2D).
Figure 2.
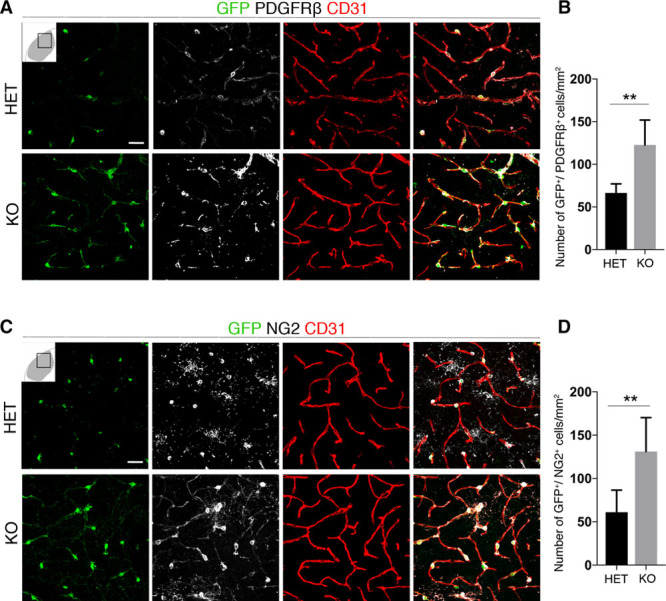
Increased platelet-derived growth factor receptor (PDGFR) β and neural/glial antigen 2 (NG2) expression in pericytes in RGS5 (regulator of G-protein signaling 5)-knockout (KO) mice after stroke. A, Confocal images show GFP+ (green fluorescent protein) pericytes around blood vessel (CD31, red) expressing PDGFRβ (gray) in heterozygous (HET) and KO mice; scale bar, 20 μm. B, Quantification of GFP+ pericytes expressing PDGFRβ in HET and KO mice (n=6, **P<0.01 Student t test). C, Confocal images show GFP+ pericytes expressing NG2 (gray), around blood vessel (CD31, red) in HET and KO mice; scale bar, 20 μm. D, Quantification of GFP+ pericytes expressing NG2 in HET and KO mice (n=6, **P<0.01 Student t test). Data are expressed as cell numbers per mm2; mean±SD.
Our findings show that loss of RGS5 in pericytes results in an increase in numbers of pericytes, as well as increased both NG2 and PDGFβ expression in GFP+ pericytes at 1 day after stroke.
Loss of RGS5 Is Associated With Higher Pericyte Coverage and Vascular Density After Stroke
In stroke, pericyte detachment from the capillaries is associated with an increase in RGS5 expression in pericytes.8 When assessing GFP+ pericyte coverage of blood vessels 1 day after stroke (Figure 3A), we found a 3-fold higher pericyte coverage in the infarct core of knockout mice compared with HET mice (62.5%±2.5% and 19%±3.8%; n=5; Figure 3A).
Figure 3.
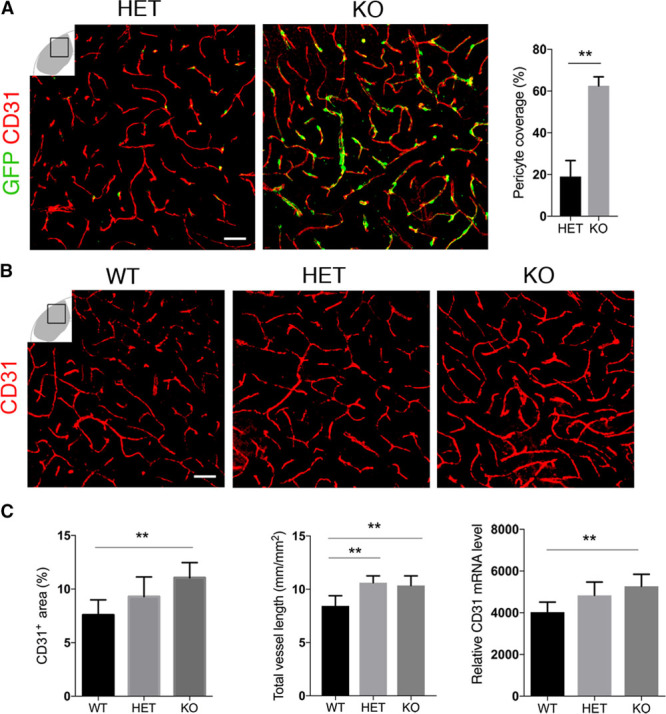
Loss of RGS5 (regulator of G-protein signaling 5) is associated with higher pericyte coverage and vascular density after stroke. A, Confocal images show GFP+ (green fluorescent protein) pericyte coverage around the blood vessels (CD31, red) in heterozygous (HET) and knockout (KO) mice; scale bar, 20 μm. Quantification of GFP+ pericyte coverage in HET and KO mice (n=5 *P<0.05, Student t test). Data are expressed as mean±SD. B, Endothelial cell staining (CD31, red) shows higher vessel density in KO vs HET and wild type (WT) mice; scale bar, 10 μm. C, Quantification shows significantly higher blood vessel density in KO vs HET and WT mice. Data expressed as percentage of capillary area; mean±SD (n=6 [WT], n=8 [HET], n=10 [KO],**P<0.01, 1-way ANOVA; left). Quantification of total blood vessel length shows significant increase in KO compared with WT mice. Data expressed in μm; mean±SD (n=7 [WT], n=4 [HET], n=6 [KO], **P<0.01, 1-way ANOVA; middle). Quantitative reverse transcription polymerase chain reaction shows higher mRNA levels of CD31 in the infarct core of KO mice vs WT; mean±SD (n=7 [KO and HET], n=5 [WT], **P<0.01, 1-way ANOVA; right).
We next examined changes in capillary density in the infarct core as pericytes have been shown to promote endothelial cell survival.26 Under physiological conditions, capillary density was not affected in knockout mice compared with HET and WT (Figure IC in the online-only Data Supplement). However, the capillary density was significantly higher in the infarct core of knockout mice compared with HET and WT mice (11.06±1.4; 9.29±1.8; and 7.58±1.4; n=10 [knockout], n=8 [HET] n=6 [WT]; Figure 3B and 3C). When we quantified CD31 capillaries in the infarct core, we found that the total vessel length was 22% higher in knockout mice compared with WT (10.36±0.89, 10.62±0.63, and 8.44±0.95 mm/mm2; n=6 [knockout], n=4 [HET], n=7 [WT]; Figure 3C). The higher capillary density was also reflected in a 30% increase in CD31 mRNA in the infarct core of knockout mice in comparison to WT (5271±216, 4837±634, and 4028±216; n=7 [knockout], n=7 [HET], n=5 [WT]; Figure 3C).
Loss of RGS5 in Pericytes Maintains BBB Integrity After Stroke
The number of pericytes and their endothelial coverage are important for the maintenance of endothelial tight junction, adherens proteins, and BBB integrity.6,7,27
Tight junctions in the brain endothelial cells maintain BBB integrity and consist of different proteins, such as claudins, cadherins, and occludins.28 We first examined the expression pattern of ZO-1, claudin-5, and vascular endothelial-cadherin in capillaries of the infarct core. Whereas in WT mice, the pattern of tight junctions staining in the infarct core was clearly disrupted, and knockout mice showed a relatively homogenous distribution (Figure 4A). Consistently, protein levels of claudin-5, ZO-1, and vascular endothelial-cadherin were reduced in the infarct core of WT mice but not in knockout mice even though the reduction of vascular endothelial-cadherin did not reach significance (Figure 4B).
Figure 4.

Loss of RGS5 (regulator of G-protein signaling 5) in pericytes preserves tight junctions (TJs) and AQP4 (aquaporin 4). A, Confocal images show costaining of TJs markers ZO-1 (zonula occludens 1), and claudin-5, and vascular endothelial (VE)-cadherin (cyan) with CD31 (red) in the infarct core of wild type (WT) and knockout (KO) mice, scale bar, 5 μm. B, Representative Western blots and quantification of ZO-1, claudin-5, and VE-cadherin in the infarct area of WT and KO mice. Contralateral hemisphere of each group served as a control. Data expressed in band intensity; mean±SD (n=3 per group for ZO-1, n=5 per group for claudin-5 and VE-cadherin, *P<0.05, 2-way ANOVA, Tukey post hoc test). C, Representative confocal pictures show AQP4 expression in the infarct area of WT and KO mice; scale bar, 200 μm. D, Quantification of the areas lacking AQP4 expression was significantly higher in WT mice as compared with KO mice; mean±SD (n=3, **P<0.01, Student t test).
AQP4 is a water channel protein expressed on astrocytic end-feet and plays an important role in BBB integrity.29 Ischemic injury caused loss of AQP4 in the infarct core of both WT and knockout mice (Figure 4C) that was significantly higher in WT compared with knockout mice (4.88±0.12 and 2.16±0.04 mm2/section, n=3;Figure 4D).
We next investigated BBB permeability after stroke and found that the fluorescent tracer leaked out from vessels and accumulated in the parenchyma of the infarct area. Compared with WT mice, in knockout mice the amount of extravascular dextran was reduced by 3.5-fold (0.73±0.02 versus 0.26±0.03; n=3 group; Figure 5A and 5B).
Figure 5.

Maintained blood-brain barrier integrity in RGS5 (regulator of G-protein signaling 5)-knockout (KO) mice in stroke. A, Distribution of 10 kDa dextran-tracer (cyan) in the infarct area in wild type (WT) and KO mice. Dextran is mainly intravascularly distributed in CD31-positive blood vessels (red) in KO mice, whereas it leaks more into the brain parenchyma in WT mice. Right, Higher magnification of framed areas in WT and KO mice; scale bars, 50 μm (left), 20 μm (middle), and 10 μm (right). B, Quantification of extravasation of the 10 kDa dextran of KO mice vs WT after stroke; mean±SD (n=3 ***P<0.001, Student t test).
RGS5 Loss Leads to Reduced Hypoxia and Increased Neuronal Survival After Stroke
In ischemic stroke, neuronal damage is a result of BBB leakage and hypoxia.30 To further evaluate the effect of RGS5-related changes, we next analyzed the infarct volume, hypoxia levels, and neuronal death in the infarct core. There were no significant differences in infarct size and edema between WT and knockout mice (Figure IIIA–IIID in the online-only Data Supplement). However, we found a significant decrease in hypoxic areas (pimonidazole-positive areas) in the infarct area of knockout in comparison to WT mice (0.033±0.006 and 0.067±0.003 mm2/section; n=3; Figure 6A and 6B). We observed that NeuN-expressing neurons were positive for pimonidazole in the peri-infarct area (Figure 6A, bottom). Using TUNEL, we detected apoptotic cells in the entire infarct core in both genotypes (Figure 6C). Compared with WT mice, the number of TUNEL-positive cells was significantly reduced by 50% in the infarct core in knockout mice (609±111.4 and 287±19.8; n=3; Figure 6D, top). We next analyzed whether neuronal apoptosis decreased in the infarct core of knockout mice compared with WT mice (Figure 6C, bottom). We found that the percentage of NeuN+ cells expressing TUNEL was 2-fold higher in the infarct core of WT mice than knockout mice (48±3.41 and 25±4.03; Figure 6D, bottom).
Figure 6.
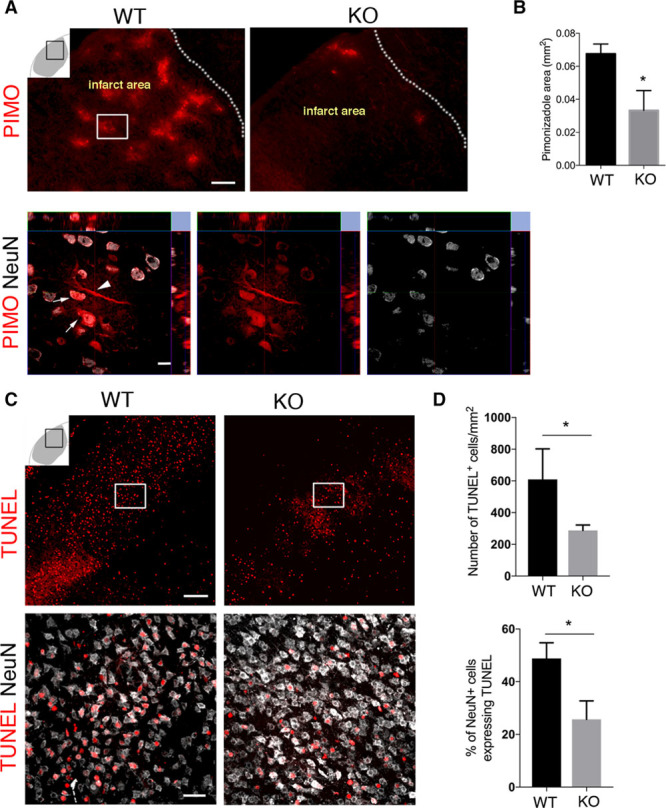
RGS5 (regulator of G-protein signaling 5) loss in pericytes is associated with reduced hypoxia and increased neuronal survival after stroke. A, Representative confocal images showing pimonidazole (PIMO) staining in the infarct area of wild type (WT) and knockout (KO) mice; scale, 50 μm. Arrows indicate NeuN expressing neurons positive for PIMO in the framed area in (A), scale bar, 10 μm. B, Quantification of PIMO-positive area in the infarct area of WT and KO mice; mean±SD (n=3, *P<0.05, Student t test). C, Confocal images showing TUNEL+ (terminal deoxynucleotidyl transferase dUTP nick end labeling) cells (top; scale bar, 50 μm) and TUNEL+ neurons (NeuN, bottom; scale bar, 20 μm) in WT and KO mice. D, Quantification shows significantly less TUNEL+ cells in KO vs WT mice; mean±SD (n=3, *P<0.05 Student t test). The percentage of NeuN cells colabeling with TUNEL is significantly lower in KO than WT mice; mean±SD (n=3, *P<0.05, Student t test).
Discussion
Our findings show that RGS5 loss does not significantly affect cerebral pericyte numbers under physiological conditions, but when exposed to ischemic stroke, RGS5-knockout mice showed an increased number of pericytes in the infarct core. Both RGS5 and PDGFRβ are expressed not only by developing pericytes in the perinatal brain but also in the vascular bed in tumors.31–33 PDGFRβ is known to mediate pericyte survival.5,34 PDGFRβ binds PDGF-BB and thereby facilitates recruitment of pericytes to endothelial cells.35 PDGF-BB might downregulate RGS536 pointing to their opposing functions. In addition, RGS5 may also play a role in the migration of pericytes by regulating their response to migratory factory cues, such as PDGF or sphinogosine-1-phosphate.14,37,38 Pericytes lacking RGS5 in the infarct core were highly positive for NG2 that serves as an activated pericyte marker but also plays an important role in pericyte recruitment, survival, and migration.22,25 Therefore, the increase in pericyte numbers in knockout mice may be a result of increased survival, possibly because of the fact that pericytes lacking RGS5 do not loose their contact with the blood vessel wall to the same extent as in WT mice.
RGS5 has been shown to modulate tumor vessel morphology, where lack of RGS5 led to a reduction in tumor hypoxia, normalization of the vasculature, and subsequently improved tumor tissue destruction.39 Similarly, we found that loss of RGS5 in pericytes preserves capillary density7,31 in ischemic stroke, most likely by improving endothelial cell survival and thereby increasing capillary density.5,7,40
Tight junctions have an important role in the maintenance of the BBB and their unique expression in the brain correlates with BBB permeability.28 In acute stroke, there is degradation of tight junctions resulting in loss of vascular integrity.41 We show that loss of RGS5 in pericytes led to preservation of different tight junctions on ischemic stroke. The increased coverage by pericytes most likely improved both their contact and interaction with the damaged capillaries, leading to maintenance of the BBB by promoting better preservation of tight junctions.6
In addition, we found preserved levels of AQP4 in the infarct core of knockout mice, where we also observed higher pericyte coverage of capillaries, consistent with a crucial role of pericytes in the regulation of astrocytic polarity.7
Our findings of reduced BBB leakage in mice with higher pericyte coverage are in line with previous reports in a transgenic mouse model of pericyte loss showing that BBB leakiness is closely associated with decreased pericyte coverage of cerebral microvessels.7 Progressive pericyte loss has also been shown to increase vascular permeability because of higher rates of transcytosis in endothelial cells.42 However, we cannot completely exclude the possibility that the observed decreased vascular permeability in RGS5-knockout mice may be because of changes in vasodilation signaling that maintains blood pressure independently of changes in pericyte coverage.18,43
Importantly, loss of RGS5 is significantly associated with reduced apoptotic neuronal death in the infarct core, possibly because of the decreased hypoxia and improved vascularization or decreased extravasation of toxic molecules. Impaired BBB function increases hypoxia levels, which are closely linked with neuronal damage.44–46 Given that loss of RGS5 in pericytes leads to partial BBB and vessel protection in the infarct area, this may result in reduced hypoxia levels in knockout mice after stroke. Also, pericytes are known to regulate neurovascular functions necessary for neuronal survival5 and may potentially supply neurotrophic or immunmodulatory support47,48 into the injured ischemic area and thereby reduce neuronal damage.40,49
Conclusions
In the present study, we have established the role of RGS5 in BBB maintenance in stroke and recognize RGS5 as a potential target in pericytes for pharmacotherapeutic intervention to protect the BBB. This opens new therapeutic opportunities to prevent aggravation of brain damage in stroke.
Acknowledgments
We thank Alicja Flasch for excellent technical assistance.
Sources of Funding
This work supported by the Swedish Medical Research Council, the Swedish Stroke Foundation, the Crafoord Foundation, and the Olle Engkvist Foundation.
Disclosures
None.
Supplementary Material
Footnotes
The online-only Data Supplement is available with this article at https://www.ahajournals.org/doi/suppl/10.1161/STROKEAHA.118.020124.
References
- 1.Sandoval KE, Witt KA. Blood-brain barrier tight junction permeability and ischemic stroke. Neurobiol Dis. 2008;32:200–219. doi: 10.1016/j.nbd.2008.08.005. doi: 10.1016/j.nbd.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 2.Fischer S, Clauss M, Wiesnet M, Renz D, Schaper W, Karliczek GF. Hypoxia induces permeability in brain microvessel endothelial cells via VEGF and NO. Am J Physiol. 1999;276(4)(pt 1):C812–C820. doi: 10.1152/ajpcell.1999.276.4.C812. [DOI] [PubMed] [Google Scholar]
- 3.Girouard H, Iadecola C. Neurovascular coupling in the normal brain and in hypertension, stroke, and Alzheimer disease. J Appl Physiol (1985) 2006;100:328–335. doi: 10.1152/japplphysiol.00966.2005. doi: 10.1152/japplphysiol.00966.2005. [DOI] [PubMed] [Google Scholar]
- 4.Zlokovic BV, Zhang C, Liu D, Fernandez J, Griffin JH, Chopp M. Functional recovery after embolic stroke in rodents by activated protein C. Ann Neurol. 2005;58:474–477. doi: 10.1002/ana.20602. doi: 10.1002/ana.20602. [DOI] [PubMed] [Google Scholar]
- 5.Bell RD, Winkler EA, Sagare AP, Singh I, LaRue B, Deane R, et al. Pericytes control key neurovascular functions and neuronal phenotype in the adult brain and during brain aging. Neuron. 2010;68:409–427. doi: 10.1016/j.neuron.2010.09.043. doi: 10.1016/j.neuron.2010.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Daneman R, Zhou L, Kebede AA, Barres BA. Pericytes are required for blood-brain barrier integrity during embryogenesis. Nature. 2010;468:562–566. doi: 10.1038/nature09513. doi: 10.1038/nature09513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Armulik A, Genové G, Mäe M, Nisancioglu MH, Wallgard E, Niaudet C, et al. Pericytes regulate the blood-brain barrier. Nature. 2010;468:557–561. doi: 10.1038/nature09522. doi: 10.1038/nature09522. [DOI] [PubMed] [Google Scholar]
- 8.Guclu M, Demirogullari B, Barun S, Ozen IO, Karakus SC, Poyraz A, et al. The effects of melatonin on intestinal adaptation in a rat model of short bowel syndrome. Eur J Pediatr Surg. 2014;24:150–157. doi: 10.1055/s-0033-1343081. doi: 10.1055/s-0033-1343081. [DOI] [PubMed] [Google Scholar]
- 9.Melgar MA, Rafols J, Gloss D, Diaz FG. Postischemic reperfusion: ultrastructural blood-brain barrier and hemodynamic correlative changes in an awake model of transient forebrain ischemia. Neurosurgery. 2005;56:571–581. doi: 10.1227/01.neu.0000154702.23664.3d. [DOI] [PubMed] [Google Scholar]
- 10.Gonul E, Duz B, Kahraman S, Kayali H, Kubar A, Timurkaynak E. Early pericyte response to brain hypoxia in cats: an ultrastructural study. Microvasc Res. 2002;64:116–119. doi: 10.1006/mvre.2002.2413. doi: 10.1006/mvre.2002.2413. [DOI] [PubMed] [Google Scholar]
- 11.Fernández-Klett F, Potas JR, Hilpert D, Blazej K, Radke J, Huck J, et al. Early loss of pericytes and perivascular stromal cell-induced scar formation after stroke. J Cereb Blood Flow Metab. 2013;33:428–439. doi: 10.1038/jcbfm.2012.187. doi: 10.1038/jcbfm.2012.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hall CN, Reynell C, Gesslein B, Hamilton NB, Mishra A, Sutherland BA, et al. Capillary pericytes regulate cerebral blood flow in health and disease. Nature. 2014;508:55–60. doi: 10.1038/nature13165. doi: 10.1038/nature13165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bondjers C, Kalén M, Hellström M, Scheidl SJ, Abramsson A, Renner O, et al. Transcription profiling of platelet-derived growth factor-B-deficient mouse embryos identifies RGS5 as a novel marker for pericytes and vascular smooth muscle cells. Am J Pathol. 2003;162:721–729. doi: 10.1016/S0002-9440(10)63868-0. doi: 10.1016/S0002-9440(10)63868-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bansal R, Magge S, Winkler S. Specific inhibitor of FGF receptor signaling: FGF-2-mediated effects on proliferation, differentiation, and MAPK activation are inhibited by PD173074 in oligodendrocyte-lineage cells. J Neurosci Res. 2003;74:486–493. doi: 10.1002/jnr.10773. doi: 10.1002/jnr.10773. [DOI] [PubMed] [Google Scholar]
- 15.De Vries L, Zheng B, Fischer T, Elenko E, Farquhar MG. The regulator of G protein signaling family. Annu Rev Pharmacol Toxicol. 2000;40:235–271. doi: 10.1146/annurev.pharmtox.40.1.235. doi: 10.1146/annurev.pharmtox.40.1.235. [DOI] [PubMed] [Google Scholar]
- 16.Roman DL, Traynor JR. Regulators of G protein signaling (RGS) proteins as drug targets: modulating G-protein-coupled receptor (GPCR) signal transduction. J Med Chem. 2011;54:7433–7440. doi: 10.1021/jm101572n. doi: 10.1021/jm101572n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Neubig RR. Regulators of G protein signaling (RGS proteins): novel central nervous system drug targets. J Pept Res. 2002;60:312–316. doi: 10.1034/j.1399-3011.2002.21064.x. [DOI] [PubMed] [Google Scholar]
- 18.Nisancioglu MH, Mahoney WM, Jr, Kimmel DD, Schwartz SM, Betsholtz C, Genové G. Generation and characterization of rgs5 mutant mice. Mol Cell Biol. 2008;28:2324–2331. doi: 10.1128/MCB.01252-07. doi: 10.1128/MCB.01252-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gregersen R, Lambertsen K, Finsen B. Microglia and macrophages are the major source of tumor necrosis factor in permanent middle cerebral artery occlusion in mice. J Cereb Blood Flow Metab. 2000;20:53–65. doi: 10.1097/00004647-200001000-00009. doi: 10.1097/00004647-200001000-00009. [DOI] [PubMed] [Google Scholar]
- 20.Nikolakopoulou AM, Zhao Z, Montagne A, Zlokovic BV. Regional early and progressive loss of brain pericytes but not vascular smooth muscle cells in adult mice with disrupted platelet-derived growth factor receptor-β signaling. PLoS One. 2017;12:e0176225. doi: 10.1371/journal.pone.0176225. doi: 10.1371/journal.pone.0176225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grant RI, Hartmann DA, Underly RG, Berthiaume AA, Bhat NR, Shih AY. Organizational hierarchy and structural diversity of microvascular pericytes in adult mouse cortex [published online January 1, 2017]. J Cereb Blood Flow Metab. doi: 10.1177/0271678X17732229. doi: 10.1177/0271678X17732229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Özen I, Deierborg T, Miharada K, Padel T, Englund E, Genové G, et al. Brain pericytes acquire a microglial phenotype after stroke. Acta Neuropathol. 2014;128:381–396. doi: 10.1007/s00401-014-1295-x. doi: 10.1007/s00401-014-1295-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Svensson A, Özen I, Genové G, Paul G, Bengzon J. Endogenous brain pericytes are widely activated and contribute to mouse glioma microvasculature. PLoS One. 2015;10:e0123553. doi: 10.1371/journal.pone.0123553. doi: 10.1371/journal.pone.0123553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ozerdem U. Targeting neovascular pericytes in neurofibromatosis type 1. Angiogenesis. 2004;7:307–311. doi: 10.1007/s10456-004-6643-3. doi: 10.1007/s10456-004-6643-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.You WK, Yotsumoto F, Sakimura K, Adams RH, Stallcup WB. NG2 proteoglycan promotes tumor vascularization via integrin-dependent effects on pericyte function. Angiogenesis. 2014;17:61–76. doi: 10.1007/s10456-013-9378-1. doi: 10.1007/s10456-013-9378-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Franco M, Roswall P, Cortez E, Hanahan D, Pietras K. Pericytes promote endothelial cell survival through induction of autocrine VEGF-A signaling and Bcl-w expression. Blood. 2011;118:2906–2917. doi: 10.1182/blood-2011-01-331694. doi: 10.1182/blood-2011-01-331694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shepro D, Morel NM. Pericyte physiology. FASEB J. 1993;7:1031–1038. doi: 10.1096/fasebj.7.11.8370472. [DOI] [PubMed] [Google Scholar]
- 28.Wolburg H, Lippoldt A. Tight junctions of the blood-brain barrier: development, composition and regulation. Vascul Pharmacol. 2002;38:323–337. doi: 10.1016/s1537-1891(02)00200-8. [DOI] [PubMed] [Google Scholar]
- 29.Abbott NJ, Rönnbäck L, Hansson E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat Rev Neurosci. 2006;7:41–53. doi: 10.1038/nrn1824. doi: 10.1038/nrn1824. [DOI] [PubMed] [Google Scholar]
- 30.Ferdinand P, Roffe C. Hypoxia after stroke: a review of experimental and clinical evidence. Exp Transl Stroke Med. 2016;8:9. doi: 10.1186/s13231-016-0023-0. doi: 10.1186/s13231-016-0023-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Berger M, Bergers G, Arnold B, Hämmerling GJ, Ganss R. Regulator of G-protein signaling-5 induction in pericytes coincides with active vessel remodeling during neovascularization. Blood. 2005;105:1094–1101. doi: 10.1182/blood-2004-06-2315. doi: 10.1182/blood-2004-06-2315. [DOI] [PubMed] [Google Scholar]
- 32.Alliot F, Rutin J, Leenen PJ, Pessac B. Pericytes and periendothelial cells of brain parenchyma vessels co-express aminopeptidase N, aminopeptidase A, and nestin. J Neurosci Res. 1999;58:367–378. [PubMed] [Google Scholar]
- 33.Bergers G, Song S. The role of pericytes in blood-vessel formation and maintenance. Neuro Oncol. 2005;7:452–464. doi: 10.1215/S1152851705000232. doi: 10.1215/S1152851705000232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hellström M, Kalén M, Lindahl P, Abramsson A, Betsholtz C. Role of PDGF-B and PDGFR-beta in recruitment of vascular smooth muscle cells and pericytes during embryonic blood vessel formation in the mouse. Development. 1999;126:3047–3055. doi: 10.1242/dev.126.14.3047. [DOI] [PubMed] [Google Scholar]
- 35.Abramsson A, Lindblom P, Betsholtz C. Endothelial and nonendothelial sources of PDGF-B regulate pericyte recruitment and influence vascular pattern formation in tumors. J Clin Invest. 2003;112:1142–1151. doi: 10.1172/JCI18549. doi: 10.1172/JCI18549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gunaje JJ, Bahrami AJ, Schwartz SM, Daum G, Mahoney WM., Jr PDGF-dependent regulation of regulator of G protein signaling-5 expression and vascular smooth muscle cell functionality. Am J Physiol Cell Physiol. 2011;301:C478–C489. doi: 10.1152/ajpcell.00348.2010. doi: 10.1152/ajpcell.00348.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hobson JP, Rosenfeldt HM, Barak LS, Olivera A, Poulton S, Caron MG, et al. Role of the sphingosine-1-phosphate receptor EDG-1 in PDGF-induced cell motility. Science. 2001;291:1800–1803. doi: 10.1126/science.1057559. doi: 10.1126/science.1057559. [DOI] [PubMed] [Google Scholar]
- 38.Rosenfeldt HM, Hobson JP, Milstien S, Spiegel S. The sphingosine-1-phosphate receptor EDG-1 is essential for platelet-derived growth factor-induced cell motility. Biochem Soc Trans. 2001;29(pt 6):836–839. doi: 10.1042/0300-5127:0290836. [DOI] [PubMed] [Google Scholar]
- 39.Hamzah J, Jugold M, Kiessling F, Rigby P, Manzur M, Marti HH, et al. Vascular normalization in Rgs5-deficient tumours promotes immune destruction. Nature. 2008;453:410–414. doi: 10.1038/nature06868. doi: 10.1038/nature06868. [DOI] [PubMed] [Google Scholar]
- 40.Kashiwamura Y, Sano Y, Abe M, Shimizu F, Haruki H, Maeda T, et al. Hydrocortisone enhances the function of the blood-nerve barrier through the up-regulation of claudin-5. Neurochem Res. 2011;36:849–855. doi: 10.1007/s11064-011-0413-6. doi: 10.1007/s11064-011-0413-6. [DOI] [PubMed] [Google Scholar]
- 41.Nour M, Scalzo F, Liebeskind DS. Ischemia-reperfusion injury in stroke. Interv Neurol. 2013;1:185–199. doi: 10.1159/000353125. doi: 10.1159/000353125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ben-Zvi A, Lacoste B, Kur E, Andreone BJ, Mayshar Y, Yan H, et al. Mfsd2a is critical for the formation and function of the blood-brain barrier. Nature. 2014;509:507–511. doi: 10.1038/nature13324. doi: 10.1038/nature13324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cho H, Park C, Hwang IY, Han SB, Schimel D, Despres D, et al. Rgs5 targeting leads to chronic low blood pressure and a lean body habitus. Mol Cell Biol. 2008;28:2590–2597. doi: 10.1128/MCB.01889-07. doi: 10.1128/MCB.01889-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kaur C, Ling EA. Blood brain barrier in hypoxic-ischemic conditions. Curr Neurovasc Res. 2008;5:71–81. doi: 10.2174/156720208783565645. [DOI] [PubMed] [Google Scholar]
- 45.Halterman MW, Miller CC, Federoff HJ. Hypoxia-inducible factor-1alpha mediates hypoxia-induced delayed neuronal death that involves p53. J Neurosci. 1999;19:6818–6824. doi: 10.1523/JNEUROSCI.19-16-06818.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Brouns R, Wauters A, De Surgeloose D, Mariën P, De Deyn PP. Biochemical markers for blood-brain barrier dysfunction in acute ischemic stroke correlate with evolution and outcome. Eur Neurol. 2011;65:23–31. doi: 10.1159/000321965. doi: 10.1159/000321965. [DOI] [PubMed] [Google Scholar]
- 47.Gaceb A, Ozen I, Padel T, Barbariga M, Paul G. Pericytes secrete pro-regenerative molecules in response to platelet-derived growth factor-bb. J Cereb Blood Flow Metab. 2018;38:45–57. doi: 10.1177/0271678X17719645. doi: 10.1177/0271678X17719645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Domitrović R, Jakovac H, Marchesi VV, Šain I, Romić Ž, Rahelić D. Preventive and therapeutic effects of oleuropein against carbon tetrachloride-induced liver damage in mice. Pharmacol Res. 2012;65:451–464. doi: 10.1016/j.phrs.2011.12.005. doi: 10.1016/j.phrs.2011.12.005. [DOI] [PubMed] [Google Scholar]
- 49.Ishitsuka K, Ago T, Arimura K, Nakamura K, Tokami H, Makihara N, et al. Neurotrophin production in brain pericytes during hypoxia: a role of pericytes for neuroprotection. Microvasc Res. 2012;83:352–359. doi: 10.1016/j.mvr.2012.02.009. doi: 10.1016/j.mvr.2012.02.009. [DOI] [PubMed] [Google Scholar]


