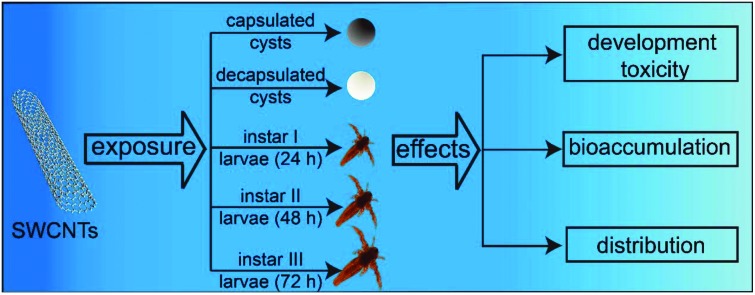
 The developmental toxicity, bioaccumulation and distribution of SWCNTs in Artemia salina.
The developmental toxicity, bioaccumulation and distribution of SWCNTs in Artemia salina.
Abstract
With the increasing production and applications of single walled carbon nanotubes (SWCNTs), concerns about the likelihood of SWCNTs being present in the aquatic environment and the subsequent effects on aquatic organisms are emerging. This work aimed to investigate the developmental toxicity, bioaccumulation and distribution of oxidized SWCNTs (O-SWCNTs) in a marine model organism, Artemia salina (A. salina). The results indicated that the hatching rates of capsulated and decapsulated cysts were decreased as the O-SWCNT concentration increased from 0 to 600 mg L–1 at 12, 18, 24 and 36 h. For instar I, II and III larvae exposure to 600 mg L–1, the mean mortality rates were 36.1%, 57.9% and 45.2%, respectively. Both the body length and swimming speed showed a concentration-dependent decrease after exposure to O-SWCNTs for 24 h. The inhibition of swimming may be caused by (1) the malformation of gills; (2) the attachment of O-SWCNTs on the gills. Reactive oxygen species (ROS) and antioxidant enzyme (catalase, superoxide dismutase and glutathione peroxidase) activities substantially increased following exposure, indicating that the toxic effects were related to oxidative stress. O-SWCNTs can be ingested, accumulated and excreted by A. salina, and distributed in the intestine, lipid vesicles and phagocytes. However, the accumulated O-SWCNTs were not completely excreted by A. salina. Uptake kinetics data showed that the O-SWCNT content increased from 1 to 48 h followed by a decrease from 48 to 72 h in the range from 0.08 to 5.7 mg g–1. The combined results so far indicate that O-SWCNTs have the potential to affect aquatic organisms when released into the marine ecosystems.
1. Introduction
The growing research and diverse applications of nanomaterials have propelled nanotechnology to the forefront of scientific and technological development. Of the nanomaterials associated with the inception and progression of nanotechnology, single-walled carbon nanotubes (SWCNTs) are the noted and important ones. Many potential applications in various fields have been proposed for SWCNTs, including energy storage, composites, sensors and drug delivery areas.1,2 Moreover, advances in SWCNTs’ synthesis, purification and modification are making them suitable for more commercial applications. Although SWCNTs have shown promising characteristics, much debate still exists regarding their environmental risks.3,4 With the rapid increase of related products and applications, SWCNTs can potentially be released from: (1) the polymer nanocomposites’ life cycle; (2) manufacturing, use and disposal stages; and (3) wastewater treatment plants into the environment.3,5 Sun et al. (2016) modeled that the environmental concentration of CNTs was at the “mg kg–1” level, and a higher CNT concentration would be reached when the nanocomposites come to the disposal phase in the future.3 The released SWCNTs will likely enter and concentrate in the aquatic ecosystem along with rainwater, runoff and wastewater,6 raising concerns on the potential risk of SWCNTs to the aquatic ecosystem.
The toxicity, bioaccumulation and fate of SWCNTs in some freshwater organisms have been studied, such as Pseudokirchneriella subcapitata,7Daphnia magna,8Lymnea luteola9 and Danio rerio.10 Besides, the development toxicity of SWCNTs on rare minnow embryos and larvae was also investigated in our previous work.11 The mechanism for the toxicity of CNTs is mainly a combined effect of agglomeration, shading, physical interactions and oxidative stress.9,12,13 For example, Long et al. (2012) investigated the mechanism of CNTs’ toxicity toward Chlorella. They demonstrated that the major contributor at a lower concentration was oxidative stress, whereas the contribution of agglomeration and shading increased when increasing the concentration.12 Moreover, other mechanisms were also verified, such as physical blocking of the gut and disruption in the mobility of organisms.14 Compared with freshwater organisms, there are a smaller number of studies on the toxicity of SWCNTs in marine organisms. It is only in recent years that the effects of SWCNTs on marine alga15 and benthic organisms6,16 have been investigated. Thakkar et al. (2016) examined the effects of SWCNTs on Dunaliella tertiolecta and demonstrated that increasing the SWCNT concentration increased the toxic effects on growth, photosynthesis and oxidative stress (Thakkar et al., 2016).15 Besides, Parks et al. (2013) investigated the toxicity, bioavailability and bioaccumulation of SWCNTs in marine benthic organisms (Ampelisca abdita, Americamysis bahia and Leptocheirus plumulosus). They showed that SWCNTs were bioaccessible to marine benthic organisms but do not appear to accumulate or cause toxicity (Parks et al., 2013).6 CNTs may be trapped and accumulate in the surface microlayers of oceans due to the viscous and surface tension properties of microlayers.17,18 Therefore, the eggs and early life stages of organisms such as zooplankton may be particularly vulnerable to CNTs. However, the information concerning the accumulation, distribution and toxicity of SWCNTs in marine zooplankton is currently limited.
Artemia salina (A. salina) is a zooplanktonic crustacean found in a variety of seawater systems from lakes to oceans. As one of the most popular live foods for many fishes and aquatic invertebrates, A. salina plays an important role in the energy flow of the food chain.19 It is a non-selective filter feeder and filters plenty of water to get food. Therefore, compared with other aquatic species, it has significant interactions with the aquatic environment and faces higher risk exposure to contaminants. Its intrinsic features make A. salina a popular model organism for toxicological tests, such as cosmopolitan distribution, a short generation time, ease of culture and the commercial availability of its cysts.20,21 Moreover, many endpoints can be selected for toxicological evaluation, including hatching, mortality, swimming, morphology and biomarkers.20A. salina is considered as a suitable model organism in the toxicity assessment of nanoparticles, and has been used to investigate the bioaccumulation and toxicity of some nanoparticles.21,22 Previously, we have utilized A. salina for investigating the developmental toxicity of graphene oxide (GO; Zhu et al., 2017),23 multi-walled carbon nanotubes (MWCNTs; Zhu et al., 2017b)24 and Fe3O4 nanoparticles (Fe3O4-NPs; Zhu et al., 2017c).25 The results indicated that A. salina is a reliable model for elucidating the impact of nanomaterials.
In the study, the toxicity of oxidized SWCNTs (O-SWCNTs) to A. salina was systematically investigated through the evaluation of hatching, mortality and behavioural and biochemical responses. Furthermore, the accumulation and distribution of O-SWCNTs in A. salina was also checked. The study contributes to a better understanding of the effects of O-SWCNTs on marine ecosystems, and lays a foundation for their future exploitation and application.
2. Materials and methods
2.1. Characterization of SWCNTs
SWCNTs (Chengdu Organic Chemicals Co., Ltd, China) were purified and oxidized according to our previous studies.11,26 O-SWCNTs were weighed on aluminum foil and placed in 1 L beakers containing 900 mL of filtered natural seawater (FNSW; 30‰ m/v; pH 8.6). O-SWCNTs were dispersed in filtered natural seawater (FNSW; 30‰ m/v; pH 8.6) to acquire suspensions with nominal concentrations of 25, 50, 100, 200, 400 and 600 mg L–1. The size and shape of O-SWCNTs were characterized using a scanning electron microscope (SEM, Hitachi S-4800, Japan) and a transmission electron microscope (TEM, JEM1200EX, Japan) with accelerating voltages of 15 and 100 kV, respectively. To estimate the hydrodynamic size distribution of O-SWCNTs in FNSW, dynamic light scattering (DLS, Brookhaven BI-200SM, USA) was used. Raman analysis was carried out using an HR800 spectrophotometer (λ = 785 nm; Longjumeau Cedex, France). The elemental compositions and chemical states of pristine SWCNTs (P-SWCNTs) and O-SWCNTs were analyzed using X-ray photoelectron spectroscopy (XPS; PHI-5600, Russia). Besides, the purity of P-SWCNTs and O-SWCNTs was also checked by Raman spectrophotometry and XPS.
2.2. Hatching assay
A. salina cysts (Tianjin, China) were decapsulated according to Sorgeloos et al. (1986).19 In order to study the effects of O-SWCNTs on hatchability, 10 capsulated/decapsulated cysts and 1 mL test suspensions (0, 25, 50, 100, 200, 400, 600 mg L–1) were added into each well of 24-well plates. Each treatment was repeated eight times. The plates were incubated at 28 °C with continuous illumination and shaking. The hatchability of capsulated/decapsulated cysts was checked at 12, 18, 24 and 36 h under a microscope (Olympus Optical Co., Ltd, Tokyo, Japan).
2.3. Mortality of instar I, II and III larvae
Instar I, II and III larvae were obtained as described previously.23,27 Ten A. salina larvae (instar I, II and III) and 1 mL O-SWCNT suspensions (0, 25, 50, 100, 200, 400, 600 mg L–1) were introduced into each well of 24-well plates. Each treatment was repeated eight times. The plates were incubated at 28 °C with a 16 : 8 h light/dark cycle and shaking. During the exposure, the tested larvae were not fed. The number of dead larvae (completely motionless) was counted under the microscope following exposure for 24 h, and the mortality was calculated.
2.4. Morphological and behavioral analysis
Instar I, II and III larvae were treated with O-SWCNT suspensions (0, 25, 50, 100, 200, 400, and 600 mg L–1) as described above. After exposure for 24 h, the surviving larvae were separated, rinsed and transferred into Petri dishes with FNSW. Then, the Petri dishes were placed in a dark box for 10 min to make the larvae reach a uniform spatial distribution and a steady speed. The body length and swimming speed were recorded with a digital camera (Nikon, Japan) fixed on a dissection microscope (Leica, Germany), and analysis of these images was performed using Image Pro Plus software (Media Cybernetics, Bethesda, MD). Twenty larvae in each treatment were checked. The swimming speed was expressed as swimming inhibition that normalized to the average swimming speed of the control. Morphological changes and the attachment of O-SWCNTs were checked using microscopy and SEM.
2.5. ROS and antioxidant enzyme activities
Approximately 1000 instar I, II and III larvae were added into beakers containing 100 mL O-SWCNT suspensions (0, 25, 50, 100, 200, 400, 600 mg L–1) and incubated at 28 °C with a 16 : 8 h light/dark cycle and shaking. After exposure for 24 h, the larvae (approximately 500) were randomly selected and then immediately reactive oxygen species (ROS) and antioxidant enzymes [catalase (CAT), superoxide dismutase (SOD) and glutathione peroxidase (GPx)] activity measurements were performed. The generation of ROS was measured using dichlorofluorescein-diacetate (DCFH-DA) (Beyotime Biotech, Nantong, China) according to the previous studies.11,23 In brief, the selected larvae were homogenized in 500 μL Tris–HCl buffer (100 mM, pH 7.4) using a glass homogenizer and then centrifuged (12 000g, 15 min). The supernatants (20 μL) were added to 96-well plates and incubated at room temperature for 5 min. Then, 100 μL PBS and 8.3 μL DCFH-DA stock solution (10 mg mL–1) were introduced to each well and incubated at 37 °C for 30 min in the dark. After incubation, the fluorescence was recorded using a microplate reader (Multiskan MK3, Thermo Labsystems Co., Beverly, MA) with excitation and emission at 485 and 530 nm, respectively. CAT, SOD and GPx activities were detected using kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) following the manufacturer's instructions. All of the tests were performed three times.
2.6. Accumulation and distribution of O-SWCNTs
In order to study the uptake and excretion of O-SWCNTs in A. salina, newly hatched larvae were treated with O-SWCNTs (50 mg L–1) for 48 h and then transferred in fresh FNSW to excrete for another 24 h. Five larvae were randomly selected at 0, 48 and 72 h, and thoroughly washed with FNSW. The accumulation of O-SWCNTs in the larvae was checked under a microscope, and images were obtained using a digital camera. Moreover, a TEM was used to observe the distribution of O-SWCNTs in A. salina.
2.7. Uptake kinetics
The newly hatched larvae (approximately 5000) were exposed to O-SWCNTs (50 mg L–1) for 72 h. During the exposure, approximately 300 larvae per time point (1, 3, 5, 7, 9, 12, 15, 18, 24, 30, 36, 48, 60 and 72 h) were randomly sampled and thoroughly washed with distilled water. The larvae were homogenized and pipetted into a square quartz groove (side length: 5 mm; height: 1 mm), and then dried in a vacuum at 80 °C. The contents of O-SWCNTs were quantitatively measured using a Raman spectrophotometer with an excitation wavelength of 785 nm, and calculated from a standard curve. All measurements were carried out three times.
2.8. Statistical analysis
All of the treatments were carried out at least three times, and the data were expressed as mean ± standard deviation (SD). The EC50 values for swimming inhibition were calculated using the Probit method. The SPSS Version 11.0 software package (SPSS Inc., Chicago, IL) was used to perform statistical analysis. Significant differences between the controls and treatments were examined using one-way ANOVA followed by Tukey's test. Significance was accepted at p < 0.05 and extremely significant at p < 0.01.
3. Results and discussion
3.1. Characterization of SWCNTs
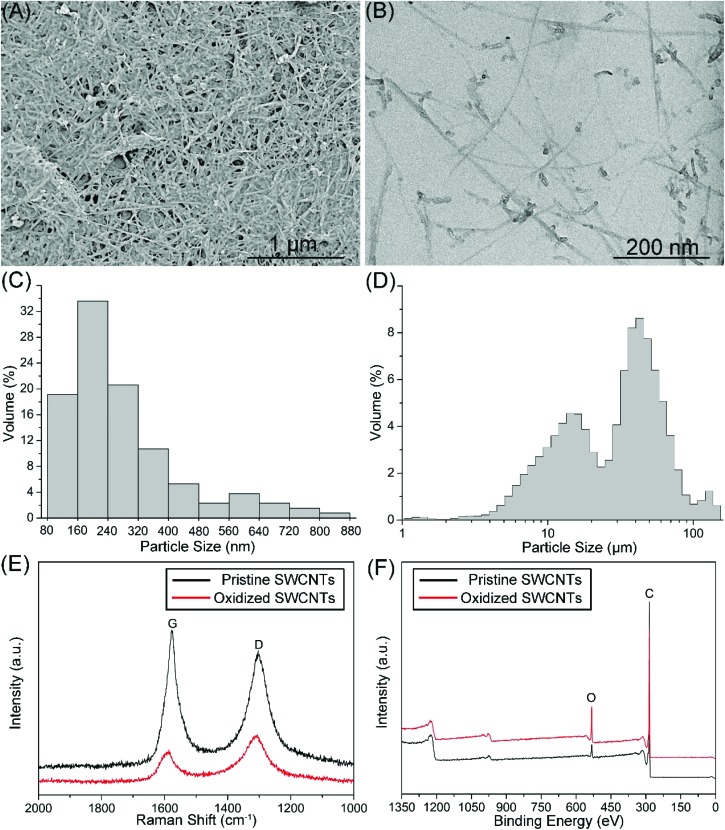
The reported SWCNT toxicity depends on the length, surface modifications, agglomeration state and purity.10,28 For example, Felix et al. (2016)10 investigated the effect of the physicochemical properties of SWCNTs on their toxicity and suggested that surface functionality plays a key role in determining their toxicity. In the study, the length, surface modifications, agglomeration state and purity of SWCNTs were checked. As shown in Fig. 1A and B, O-SWCNTs were fibrous with varying lengths. According to the statistical analysis of TEM images, the average length of O-SWCNTs was 279 nm (Fig. 1C), and shorter than the hydrodynamic size (mean size: 68 μm) that was measured by DLS (Fig. 1D). The result indicates that O-SWCNTs were rapidly aggregated in the FNSW due to the hydration and reduction of electrostatic repulsion.29 However, owing to the anisotropic and fibrous morphology of SWCNTs, the DLS data cannot reveal the exact size. As shown in Fig. 1E, typical G (around 1580 cm–1) and D bands (around 1303 cm–1) of CNTs30 were identified from the Raman spectra of pristine and oxidized SWCNTs. D/G band intensity ratio (ID/IG) can be used to monitor the defects of CNTs as it increases with the increasing defects.30,31 The ID/IG ratios of P-SWCNTs and O-SWCNTs were 0.86 and 1.4, respectively, indicating that the defects of SWCNTs increased following oxidation. Fig. 1F shows the XPS spectra of P-SWCNTs and O-SWCNTs, indicating that the SWCNT surface consisted of carbon (284 eV) and oxygen (532 eV). Surface oxygen contents for P-SWCNTs and O-SWCNTs were 3.6 and 13.9 atomic %, respectively, suggesting that the oxidation was sufficient. Besides, no characteristic peaks of impurities were identified from the Raman and XPS spectra, indicating that P-SWCNTs and O-SWCNTs were free from impurities.
Fig. 1. Characterization of O-SWCNTs. SEM (A) and TEM (B) images of O-SWCNTs. Size distributions of O-SWCNTs measured using TEM (C) and DLS (D). Raman (E) and XPS (F) spectra of P-SWCNTs and O-SWCNTs.
3.2. Effects of O-SWCNTs on hatchability
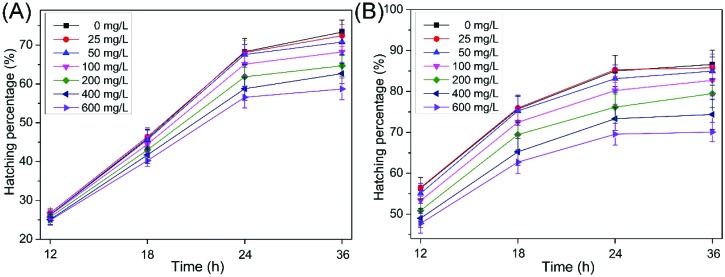
The hatchability of cysts (capsulated and decapsulated) is a sensitive and reliable endpoint for toxicity testing with A. salina, and it has been used in the toxicity assessment of nanoparticles.20,21,32 As shown in Fig. 2A and B, the hatching rates of the capsulated and decapsulated cysts decreased as the concentrations increased from 0 to 600 mg L–1 at 12, 18, 24 and 36 h. They were 57.7% and 66.3% for the capsulated and decapsulated cyst exposure, respectively, to 600 mg L–1 for 36 h, which significantly decreased (p < 0.01) compared to the controls (73.3% and 86.6%, respectively). Previously, we studied the effects of MWCNTs on A. salina cysts.24 The results showed that the hatching rates of the capsulated and decapsulated cysts were 58.9% and 74.3%, respectively, following exposure to 600 mg L–1 for 36 h, indicating that SWCNTs possess a slightly higher toxicity profile than MWCNTs. Compared with the capsulated cysts, the decapsulated cysts showed a higher hatchability due to a lower energy requirement to break out of the decapsulated cysts.19
Fig. 2. Hatching rates of the capsulated (A) and decapsulated (B) cysts exposed to different O-SWCNT concentrations. Values are presented as mean ± SD.
3.3. Acute toxicity test
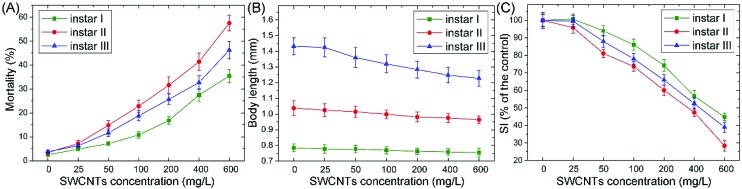
Mortality, morphology and swimming are also reliable endpoints for toxicity testing with A. salina.20,33 Caldwell et al. (2003)33 assessed the toxicity of short chain aldehydes and diatom extracts to A. salina and demonstrated that the hatching assay showed a lower sensitivity compared with mortality assays. As shown in Fig. 3A, mortality ranged from 2% to 5% for instar I, II and III larvae in the controls, suggesting that no lethal effects were induced without feeding even up to 72 h. For instar I, II and III exposure to 600 mg L–1, the mean mortality rates were 36.1%, 57.9% and 45.2%, respectively. The data are higher than that exposed to MWCNTs, which were 33.8%, 55.7% and 40.7% for instar I, II and III, respectively. Jackson et al. (2013)28 reviewed the bioaccumulation and toxicity of CNTs and demonstrated that SWCNTs have a higher toxicity profile than double-/multi-walled CNTs. A similar result is acquired in our study.
Fig. 3. Mortality (A), swimming inhibition (SI; B) and body length (C) for instar I, II and III larvae exposure to different concentrations of O-MWCNTs. Values are presented as mean ± SD.
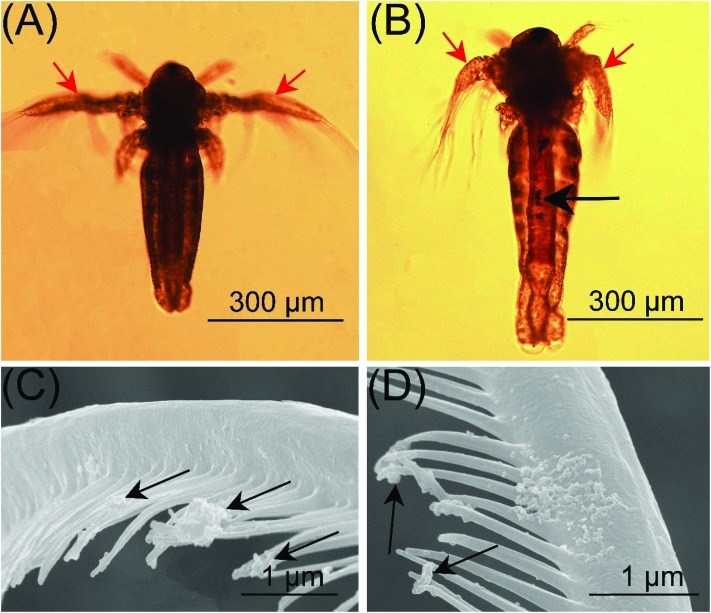
Morphological and ethological responses result from the molecular and physiological aspects of toxicology, and can provide insights into various levels of body tissues. As important morphological and ethological parameters, body length and swimming speed have been widely used in the toxicity assessment of nanoparticles.13,20 In the study, both the body length (Fig. 3B) and swimming speed (Fig. 3C) showed a concentration-dependent decrease after exposure to O-SWCNTs for 24 h. For instar I, II and III, the swimming speed decreased as much as 54.2%, 71.6% and 60.1% in 600 mg L–1 treatments, and the EC50 values for swimming inhibition were 497, 372 and 438 mg L–1, respectively. The inhibition of swimming may be caused by (1) the malformation of gills and (2) the attachment of SWCNTs on the gills. Compared with the control (Fig. 4A), the gills of larvae were malformed after the exposure to SWCNTs (Fig. 4B). Moreover, SWCNTs were extensively attached to the gills, causing gill branches to come together (Fig. 4C) and be poorly developed (Fig. 4D). A similar phenomenon was reported by Mesarič et al., (2015),13 who demonstrated that the high surface adsorption properties of carbon-based nanomaterials were responsible for swimming inhibition.
Fig. 4. Effects of O-SWCNTs on the gills of A. salina. (A) Normal development of gills in the control. (B) Gills were malformed after exposure to SWCNTS. Attachment of O-SWCNTs on the gills, causing gill branches to come together (C) and be poorly developed (D). Red arrows: gills; black arrows: O-SWCNTs.
The toxicity of GO,23 MWCNTs24 and Fe3O4-NPs25 has been investigated in our previous study. Based on the hatching assay, mortality assay and morphological and ethological analysis, it can be concluded that the toxicity of GO, MWCNTs, Fe3O4-NPs and SWCNTs is in the order of GO > Fe3O4-NPs > SWCNTs > MWCNTs.
3.4. Biochemical responses
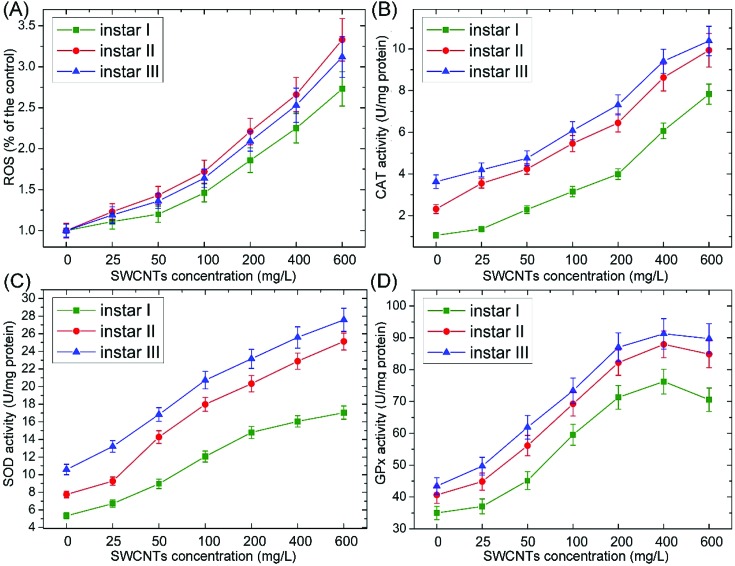
One of the major mechanisms of nanotoxicity is oxidative stress.12,34 Many studies suggested that CNTs can induce oxidative stress by producing ROS and altering the levels of components in the antioxidant defense system, such as CAT, SOD and GPx.9,12,34 CAT, SOD and GPx can catalyze the decomposition of ROS and prevent organisms from the adverse effects of oxidative stress. As shown in Fig. 5, ROS and antioxidant enzyme (CAT, SOD and GPx) activities substantially increased following the exposure to O-SWCNTs, indicating that the toxic effects were related to oxidative stress. The increase of antioxidant enzyme activities was due to the enhanced oxygen free radical production, which could stimulate antioxidant activities to suppress the increased oxidative stress and protect the organism from damage. It is noteworthy that the GPx activity firstly increased and then decreased. The decreased activity of GPx might be due to the overproduction of ROS and the depletion of glutathione.9 Besides, the enzymatic activity decreased at high rates of free radical input, leading to the autocatalysis of the oxidative damage process.35 A similar result was reported by Ali et al. (2015),9 who investigated the toxicity of SWCNTs to Lymnea luteola. They demonstrated that decreased GPx and increased CAT activities were induced following SWCNT exposure, and the toxic effects were related to oxidative stress.
Fig. 5. Changes of ROS (A) and CAT (B), SOD (C) and GPx (D) activities in A. salina larvae (instar I, II and III) following the exposure to different concentrations of O-SWCNTs. Values are presented as mean ± SD.
3.5. Uptake and distribution of O-SWCNTs
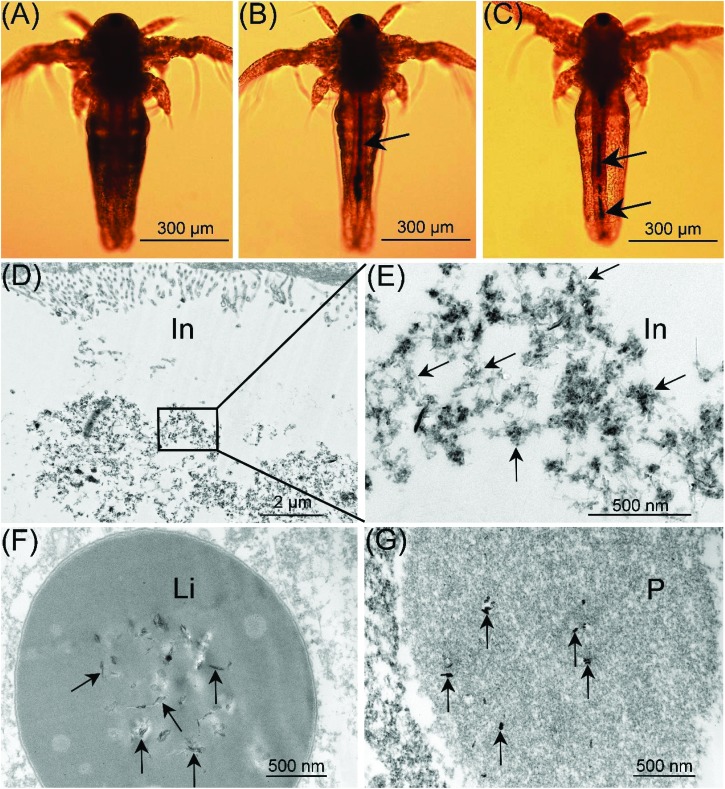
As a non-selective filter feeder, A. salina can ingest particles with a suitable size. Therefore, SWCNTs may be ingested and distributed in some tissues/cells of A. salina due to their small size. As shown in Fig. 6A, the gut of the larvae in the control was empty. After exposure to O-SWCNTs for 48 h, the gut was almost entirely filled with O-SWCNTs manifested by a dark line inside the gut (Fig. 6B). In order to check the excretion of O-SWCNTs, larvae were transferred into fresh FNSW for another 24 h. After the transfer, the accumulated O-SWCNTs were excreted, however they were not completely excreted by A. salina (Fig. 6C). A similar result was reported by Ates et al. (2015),36 who demonstrated that A. salina were unable to eliminate the NPs as rapidly as they accumulated due to the formation of larger aggregates inside the gut. Moreover, Petersen et al. (2009)37 investigated the uptake and depuration of MWCNTs by Daphnia magna. They showed that MWCNTs were accumulated in the gut of Daphnia magna, after exposure for 48 h, and the accumulated MWCNTs were unable to be excreted after being transferred into freshwater for another 24 h. The distribution of O-SWCNTs in A. salina was checked under a TEM. O-SWCNTs were clearly visible within the intestine (in; Fig. 6D and E), lipid vesicle (li; Fig. 6F) and phagocyte (p; Fig. 6G), which was the same as the result reported previously.24
Fig. 6. Uptake and distribution of O-SWCNTs (black arrows) in A. salina. (A) The gut of larvae in the control was empty. (B) After exposure to O-SWCNTs for 48 h, the gut was almost entirely filled with O-SWCNTs manifested by a dark line inside the gut. (C) The accumulated O-SWCNTs were excreted by A. salina following transfer to FNSW for another 24 h; however, they were not completely excreted. Localization of O-SWCNTs in the intestine (in; D and E), lipid vesicle (li; F) and phagocyte (p; G).
3.6. Uptake kinetics
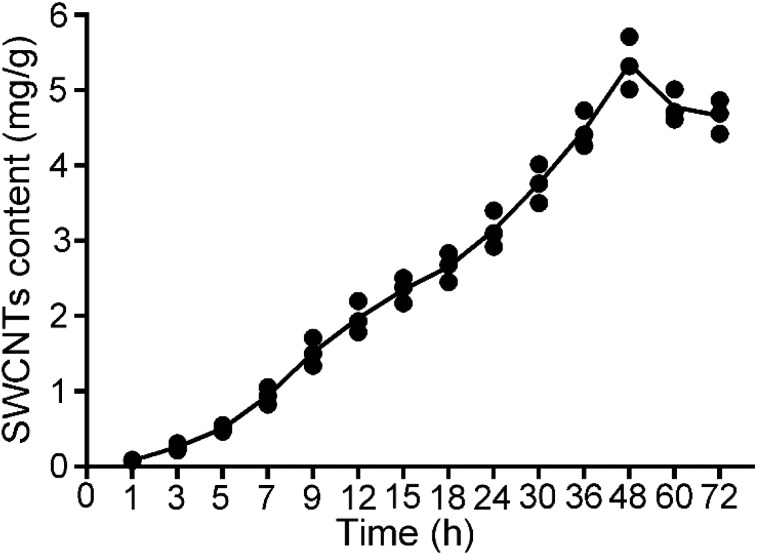
To quantify CNTs in biological tissues, a broad range of techniques have been developed, such as UV/vis/NIR spectroscopy and Raman spectroscopy.38 One of the important selection standards is the limit of detection (LOD). Raman spectroscopy has been verified to be a sensitive, non-destructive and reliable method for the quantification of CNTs because it has a low LOD in biological tissues (0.1–1 mg kg–1).38–40 Previously, the technique has been well performed to quantify MWCNTs in Saccharomyces cerevisiae26 and A. salina.24 In the study, Raman spectroscopy was used to measure the O-SWCNT content based on the dry weight of A. salina. As shown in Fig. 7, the O-SWCNT content increased from 1 to 48 h and then decreased from 48 to 72 h with a range from 0.08 to 5.7 mg g–1. The decrease is probably due to the reduction of the O-SWCNT concentration in suspensions, which may lead to the release of accumulated O-SWCNTs.37,41 The uptake of O-MWCNTs by A. salina has been measured in a previous study.24 The average O-MWCNT content ranged from 5.5 to 28.1 mg g–1, which is higher than the O-SWCNT content.
Fig. 7. O-SWCNT contents in A. salina at different time points. Values are presented as mean ± SD.
4. Conclusion
In this study, the effects of O-SWCNTs on different stages of A. salina were systematically evaluated to elucidate their impact on marine ecosystems. The results so far indicated that the acute exposure of A. salina to O-SWCNTs (100–600 mg L–1) results in significant effects on hatching, mortality and behavioural and biochemical parameters. The effects are related to oxidative stress. O-SWCNTs can be ingested, accumulated and excreted by A. salina, and distributed in the intestine, lipid vesicles and phagocytes. However, the accumulated O-SWCNTs were not completely excreted by A. salina following the transfer to FNSW for another 24 h. The study revealed short-term effects of O-SWCNTs on A. salina. For safe and commercial purposes, chronic exposure to environmentally realistic concentrations is necessary.
Conflicts of interest
There are no conflicts of interest to declare.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No. U1701233), the Special Funds for Talents in Northwest A&F University to B. Zhu (Program No. Z111021510), the special grade of the financial support from the China Postdoctoral Science Foundation (Program No. 2016T90956) and the Fundamental Research Funds for the Central Universities (Program No. 2452017078).
References
- De Volder M. F., Tawfick S. H., Baughman R. H., Hart A. J. Science. 2013;339:535–539. doi: 10.1126/science.1222453. [DOI] [PubMed] [Google Scholar]
- Alshehri R., Ilyas A. M., Hasan A., Arnaout A., Ahmed F., Memic A. J. Med. Chem. 2016;59:8149–8167. doi: 10.1021/acs.jmedchem.5b01770. [DOI] [PubMed] [Google Scholar]
- Sun T. Y., Bornhöft N. A., Hungerbühler K., Nowack B. Environ. Sci. Technol. 2016;50:4701–4711. doi: 10.1021/acs.est.5b05828. [DOI] [PubMed] [Google Scholar]
- Ong L.-C., Chung F. F.-L., Tan Y.-F., Leong C.-O. Arch. Toxicol. 2016;90:103–118. doi: 10.1007/s00204-014-1376-6. [DOI] [PubMed] [Google Scholar]
- Petersen E. J., Zhang L., Mattison N. T., O'Carroll D. M., Whelton A. J., Uddin N., Nguyen T., Huang Q., Henry T. B., Holbrook R. D. Environ. Sci. Technol. 2011;45:9837–9856. doi: 10.1021/es201579y. [DOI] [PubMed] [Google Scholar]
- Parks A. N., Portis L. M., Schierz P. A., Washburn K. M., Perron M. M., Burgess R. M., Ho K. T., Chandler G. T., Ferguson P. L. Environ. Toxicol. Chem. 2013;32:1270–1277. doi: 10.1002/etc.2174. [DOI] [PubMed] [Google Scholar]
- Youn S., Wang R., Gao J., Hovespyan A., Ziegler K. J., Bonzongo J.-C. J., Bitton G. Nanotoxicology. 2012;6:161–172. doi: 10.3109/17435390.2011.562329. [DOI] [PubMed] [Google Scholar]
- Edgington A. J., Petersen E. J., Herzing A. A., Podila R., Rao A., Klaine S. J. Nanotoxicology. 2013;8:2–10. doi: 10.3109/17435390.2013.847504. [DOI] [PubMed] [Google Scholar]
- Ali D., Ahmed M., Alarifi S., Ali H. Environ. Toxicol. 2015;30:674–682. doi: 10.1002/tox.21945. [DOI] [PubMed] [Google Scholar]
- Felix L. C., Ede J. D., Snell D. A., Oliveira T. M., Martinez-Rubi Y., Simard B., Luong J. H., Goss G. G. Carbon. 2016;104:78–89. [Google Scholar]
- Zhu B., Liu G. L., Ling F., Song L. S., Wang G. X. Nanotoxicology. 2014;9:1–12. doi: 10.3109/17435390.2014.957253. [DOI] [PubMed] [Google Scholar]
- Long Z., Ji J., Yang K., Lin D., Wu F. Environ. Sci. Technol. 2012;46:8458–8466. doi: 10.1021/es301802g. [DOI] [PubMed] [Google Scholar]
- Mesarič T., Gambardella C., Milivojević T., Faimali M., Drobne D., Falugi C., Makovec D., Jemec A., Sepčić K. Aquat. Toxicol. 2015;163:121–129. doi: 10.1016/j.aquatox.2015.03.014. [DOI] [PubMed] [Google Scholar]
- Kennedy A. J., Hull M. S., Steevens J. A., Dontsova K. M., Chappell M. A., Gunter J. C., Weiss C. A. Environ. Toxicol. Chem. 2008;27:1932–1941. doi: 10.1897/07-624.1. [DOI] [PubMed] [Google Scholar]
- Thakkar M., Mitra S., Wei L. J. Nanomater. 2016;2016:1–9. doi: 10.1155/2016/8380491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galloway T., Lewis C., Dolciotti I., Johnston B. D., Moger J., Regoli F. Environ. Pollut. 2010;158:1748–1755. doi: 10.1016/j.envpol.2009.11.013. [DOI] [PubMed] [Google Scholar]
- Klaine S. J., Alvarez P. J., Batley G. E., Fernandes T. F., Handy R. D., Lyon D. Y., Mahendra S., McLaughlin M. J., Lead J. R. Environ. Toxicol. Chem. 2008;27:1825–1851. doi: 10.1897/08-090.1. [DOI] [PubMed] [Google Scholar]
- Wurl O., Obbard J. P. Mar. Pollut. Bull. 2004;48:1016. doi: 10.1016/j.marpolbul.2004.03.016. [DOI] [PubMed] [Google Scholar]
- Sorgeloos P., Lavens P., Leger P., Tackaert W. and Versichele D., Manual for the culture and use of brine shrimp Artemia in aquaculture, 1986. [Google Scholar]
- Libralato G., Prato E., Migliore L., Cicero A. M., Manfra L. Ecol. Indic. 2016;69:35–49. [Google Scholar]
- Libralato G. Mar. Environ. Res. 2014;101:38–43. doi: 10.1016/j.marenvres.2014.08.002. [DOI] [PubMed] [Google Scholar]
- Rajabi S., Ramazani A., Hamidi M., Naji T. Daru, J. Pharm. Sci. 2015;23:20. doi: 10.1186/s40199-015-0105-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu S., Luo F., Chen W., Zhu B., Wang G. Sci. Total Environ. 2017;595:101–109. doi: 10.1016/j.scitotenv.2017.03.224. [DOI] [PubMed] [Google Scholar]
- Zhu S., Luo F., Tu X., Chen W. C., Zhu B., Wang G. X. Environ. Pollut. 2017;229:679. doi: 10.1016/j.envpol.2017.07.020. [DOI] [PubMed] [Google Scholar]
- Zhu S., Xue M. Y., Luo F., Chen W. C., Zhu B., Wang G. X. Environ. Pollut. 2017;230:683. doi: 10.1016/j.envpol.2017.06.065. [DOI] [PubMed] [Google Scholar]
- Zhu S., Zhu B., Huang A., Hu Y., Wang G., Ling F. J. Hazard. Mater. 2016;318:650–662. doi: 10.1016/j.jhazmat.2016.07.049. [DOI] [PubMed] [Google Scholar]
- Sorgeloos P., Wielen R. V. D., Persoone G. Ecotoxicol. Environ. Saf. 1979;2:249–255. doi: 10.1016/s0147-6513(78)80003-7. [DOI] [PubMed] [Google Scholar]
- Jackson P., Jacobsen N. R., Baun A., Birkedal R., Kühnel D., Jensen K. A., Vogel U., Wallin H. Chem. Cent. J. 2013;7:154. doi: 10.1186/1752-153X-7-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston H. J., Hutchison G. R., Christensen F. M., Peters S., Hankin S., Aschberger K., Stone V. Nanotoxicology. 2010;4:207–246. doi: 10.3109/17435390903569639. [DOI] [PubMed] [Google Scholar]
- Dresselhaus M. S., Dresselhaus G., Saito R., Jorio A. Phys. Rep. 2005;409:47–99. [Google Scholar]
- Neves V., Heister E., Costa S., Tîlmaciu C., Borowiak-Palen E., Giusca C. E., Flahaut E., Soula B., Coley H. M., McFadden J. Adv. Funct. Mater. 2010;20:3272–3279. [Google Scholar]
- Arulvasu C., Jennifer S. M., Prabhu D., Chandhirasekar D. Sci. World J. 2014;2014:256919. doi: 10.1155/2014/256919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell G. S., Bentley M. G., Olive P. J. W. Toxicon. 2003;42:301–306. doi: 10.1016/s0041-0101(03)00147-8. [DOI] [PubMed] [Google Scholar]
- Møller P., Christophersen D. V., Jensen D. M., Kermanizadeh A., Roursgaard M., Jacobsen N. R., Hemmingsen J. G., Danielsen P. H., Cao Y., Jantzen K. Arch. Toxicol. 2014;88:1939–1964. doi: 10.1007/s00204-014-1356-x. [DOI] [PubMed] [Google Scholar]
- Escobar J., Rubio M., Lissi E. Free Radicals Biol. Med. 1996;20:285–290. doi: 10.1016/0891-5849(95)02037-3. [DOI] [PubMed] [Google Scholar]
- Ates M., Demir V., Arslan Z., Daniels J., Farah I. O., Bogatu C. Environ. Toxicol. 2015;30:109–118. doi: 10.1002/tox.21917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen E. J., Akkanen J., Kukkonen J. V., Weber Jr. W. J. Environ. Sci. Technol. 2009;43:2969–2975. doi: 10.1021/es8029363. [DOI] [PubMed] [Google Scholar]
- Petersen E. J., Florescervantes D. X., Bucheli T. D., Elliott L. C. C., Fagan J. A., Gogos A., Hanna S., Kägi R., Mansfield E., Bustos A. R. M. Environ. Sci. Technol. 2016;50:4587. doi: 10.1021/acs.est.5b05647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertulli C., Beeson H. J., Hasan T., Huang Y. Y. S. Nanotechnology. 2013;24:265102. doi: 10.1088/0957-4484/24/26/265102. [DOI] [PubMed] [Google Scholar]
- Salzmann C. G., Chu B. T., Tobias G., Llewellyn S. A., Green M. L. Carbon. 2007;45:907–912. [Google Scholar]
- Guo X., Dong S., Petersen E. J., Gao S., Huang Q., Mao L. Environ. Sci. Technol. 2013;47:12524. doi: 10.1021/es403230u. [DOI] [PubMed] [Google Scholar]