 Paraquat (PQ) poisoning is the main medical problem in developing countries and it is spread out worldwide. A similar structure of PQ to MPP+ (1-methyl-4-phenylpyridinium) caused PQ-induced neurotoxicity and injury to renal proximal tubules.
Paraquat (PQ) poisoning is the main medical problem in developing countries and it is spread out worldwide. A similar structure of PQ to MPP+ (1-methyl-4-phenylpyridinium) caused PQ-induced neurotoxicity and injury to renal proximal tubules.
Abstract
Paraquat (PQ) poisoning is principally reported in developing countries. However, most fatalities occur elsewhere due to the induction of multi-organ failure. PQ poisoning can hardly be managed by clinical practice, and no specific antidote has come into existence yet. Here three cases, including 17-, 20-, and 23-year-old men, who were poisoned with PQ, have been reported. Furthermore, the literature regarding biological mechanisms, clinical manifestation, and treatment of PQ-induced toxicity was reviewed. Patients who, either intentionally or accidentally, ingested PQ earlier were initially found to be stable at the emergency department (ED). Therefore, they were discharged from the hospital under a follow-up. However, after several days, the patients were referred to the hospital for the second time and despite cardiovascular resuscitation (CPR) efforts, they suddenly expired. The delayed death following exposure to PQ was reported for inducing gradual progressive pulmonary fibrosis, metabolic acidosis, neurotoxicity, renal failure, and liver injury in poisoned patients. Therefore, PQ-intoxicated patients should be supervised for up to several weeks, and kept in the hospital for a longer period of time. Clinical manifestations and laboratory findings are beneficial markers that act as useful predictors of PQ poisoning.
1. Introduction
Paraquat (PQ, 1,1′-dimethyl-4,4′-bipyridinium) is a highly toxic pro-oxidant that is widely used throughout the world.1 It is used in Europe, Australia, Iran, and the US.2–4 PQ is classified as a fast-acting and non-selective contact herbicide that eradicates a broad range of grasses, persists in the soil for a long time, and is partially inactivated.5 These properties led farmers to use PQ more.6 On the other hand, quaternary nitrogen herbicides, including PQ, diquat (DQ), and difenzoquatare, were difficult to analyze in agriculture.1 Therefore, more exposure to PQ was expected, resulting in severe toxicity in animals and humans.7–9 Human intoxication by PQ occurs by consuming it accidentally or with suicidal intentions. PQ poisoning has occupied the third widely used herbicide worldwide are concerned.7 Toxicokinetic assessment of PQ indicates that it is rapidly, but incompletely, absorbed and then largely eliminated unchanged through urine within 12–24 hours. Its distribution was described by a two-compartment model.10 PQ induces multi-organ failure involving lung, gastrointestinal tract, pancreas, kidney, liver, heart, and brain injury.11–14 Pulmonary fibrosis is the most typical feature of PQ poisoning that is mediated by the oxidative stress (OS) status, and continues from several days to weeks after PQ ingestion.11 However, little is known regarding the toxicity mechanisms of PQ-induced organ failure.
Toxicity of PQ is potentially coordinated with redox activity in order to catalyze two important relative consequences. The first is in accordance with the production of reactive oxygen species (ROS), especially superoxide anions, hydrogen peroxide, and hydroxyl free radicals. These are highly reactive to cellular macromolecules that cause damage. The second is accompanied by oxidation-reducing equivalents such as NADPH, an electron donor, and reduced glutathione (GSH). These are necessary for normal cell functioning.15 In addition to ROS formation, endoplasmic reticulum (ER) induction, apoptosis, mitochondrial damage, and inflammation as evidenced by the regulation of various protein/signaling pathways was reported in relation to PQ-induced toxicity.16–20 This is since OS events were investigated as an underlined mechanism of PQ accumulation in alveolar cells, neurons, and astroglia to cause pneumo- and neuro-toxicity, which lead to death.21 ER stress was mediated by pro-inflammatory mediator toll-like receptor4 (TLR4), edema, and inflammation. This results in neutrophil infiltration and subsequent activation of pro-inflammatory cytokines, such as interleukin-1β (IL-1β) and tumor necrosis factor-α (TNF-α). Cell apoptosis was induced by the up-regulation of the proapoptotic gene (Bax) and down-regulation of the antiapoptotic gene (Bcl-2). Increasing the cleavage of caspase-8 (key mediators of extrinsic apoptosis) and Bid resulted in cytotoxicity.16–18 Furthermore, PQ was interfered with the mitochondrial function via reduction of the adenosine triphosphate levels. This resulted in the activities of complexes I and IV and caused mitochondria toxicity via mitochondrial membrane swelling and depletion of cytochrome C.18,22
The activation of nuclear factor erythroid 2-related factor 2 (Nrf2) signaling pathway, inhibition of nuclear factor kappa B (NF-κB), and JNK/p38 mitogen-activated protein kinase (MAPK) signal pathways have reported the important molecular mechanisms related to PQ-induced toxicity.16,22 The clinical manifestations and severity of PQ poisoning are in accordance with these intracellular mechanisms. Furthermore, administration of PQ has also induced myeloperoxidase (MPO) an abundant proinflammatory enzyme found in neutrophil primary granules and monocyte lysosomes, malondialdehyde (MDA) as a lipid peroxidation (LPO) marker. It reduced the activity of antioxidases enzymes, including superoxide dismutase (SOD), catalase (CAT), and glutathione S-transferases (GSTs).16,23,24
The high mortality rate of PQ poisoning is due to substantial toxicity and lack of specific antidotes or effective treatments. Rapid or delayed death up to several weeks after PQ ingestion, mostly when ingested as concentrated PQ or mixing it with other toxins, may occur. Therefore, we conducted a systematic search using Google Scholar, Scopus, PubMed, and ISI Web of Science. Our research focused on the mechanisms, clinical manifestations, and treatment of PQ. Moreover, we discussed three cases of patients (17, 20, and 23-year-old men) who were earlier exposed to PQ but died later after being admitted to the Hospital Poison Center, with diagnosis of PQ toxicity.
2. Case stories
2.1. Case I
A 17-year-old man with no past medical history was referred to hospital poison center with symptoms of nausea and vomiting after ingesting poisonous grapes. At the very beginning, he underwent gastric lavage. When found stable, he was discharged from the hospital after two days on his request. About 10 days later, the patient developed symptoms, such as body pain, aphthous stomatitis, and oral lesions, which were treated by a physician but to no effect. The patient developed symptoms such as weakness, lethargy, canker sore, painful lesions on the surface of oral candidiasis, redness of the tongue and throat, jaundice, dyspnea, bilateral crackles, crepitation, orrales, bradypnea, and a respiratory rate of 40 breaths per minute at 5 PM. He was transferred to the poisoning emergency section after two days. After being diagnosed with PQ herbicide poisoning, he was admitted to the ward. The patient developed difficulty in breathing and a high level of acetyl cholinesterase enzyme. Therefore, he was transferred to the medical toxicology intensive care unit (MTICU). In the course of hospitalization, the patient had tachycardia. He gasped and then suffered a sharp decline in the percentage of saturated oxygen (O2sat) breathing. The patient was then intubated and connected to a ventilator. However, the percentage of O2sat (possibly because of progressive pulmonary fibrosis) did not improve. Finally, the patient experienced asystole due to a sharp decline of as much as 70% of O2sat and hypotension. Unfortunately, he died after 40 minutes of cardiovascular resuscitation (CPR).
2.2. Case II
A 20-year-old man intentionally ingested 100 ml of poisonous green herbicide solution to commit suicide at 5 PM. He immediately started vomiting severely. At 3 AM, he was sent to a local hospital. On arrival, he was alert and was found stable. The patient had normal stature and was sensitive to light. He had normal mucosa, and the condition of his heart and lungs was normal. As he was found stable, the patient was discharged and asked to follow-up. Three days after, at 12 PM, the patient was referred to the hospital poison center with multiple symptoms. He was diagnosed with PQ poisoning. The signs were nausea, vomiting, drooling, runny nose, tearing or sialorrhoea, dyspnea, bilateral crackles, crepitation, orrales, and pupil mydriasis. The patient was immediately intubated, connected to the ventilation device, and sedated. Unfortunately, the patient suffered from respiratory and cardiac arrest a day after admission at 3 AM and died after 30 minutes of CPR.
2.3. Case III
A 23-year-old man recently attempted suicide with a few sips of PQ poison. The subject, with lethargic general symptoms, was taken on a stretcher to the poisoning department. On admission, the patient showed other symptoms such as dyspnea, burn on lips, oral mucosa, redness of throat, and digestive problems (as was found in the endoscopy). Serum therapy (3 L h–1) was underway. The vital signs were examined (Table 1), and chest X-ray (CXR) and electrocardiograph (ECG) were done.
Table 1. Vital sign parameters in admission to the emergency department (ED).
| Patients vital sign in the ED | Case I | Case II | Case III | Normal range |
| RR (beats per min) | 44 | 20 | 10 | 12–20 |
| PR (beats per min) | 70 | 61 | 53 | 60–100 |
| SBP (mmHg) | 100 | 130 | 90 | 120 |
| DBP (mmHg) | 70 | 90 | 70 | 80 |
| T (°C) | 37.3 | 37 | 37 | 36.1–37.2 |
The oxygen uptake with a mask was underway and the patient was transferred to the MTICU at 5 PM. Despite receiving oxygen with a mask, a sudden drop in the percentage of O2sat to 70 was observed. Due to severe breathing problems, the patient was intubated and sedated. Unfortunately, on the day after admission, the intoxicated patient suffered from asystole at 3 AM and despite supportive cardiovascular measures, he expired after 40 minutes.
3. Discussion
PQ poisoning is the main medical problem in developing countries and it is spread out worldwide. It may be ingested accidentally or in order commit suicide. It fast-acting nature, stability, availability, and affordability, and the lack of effective treatment, make PQ an extremely hazardous substance. It accounts for up to a third of all widely used herbicides worldwide. However, less information was known about the molecular mechanisms underlying PQ-induced toxicity, which continued from several days to weeks after PQ ingestion. Inducing the formation of free radicals or ROS and imbalance of the redox cycle leading to OS, to interact with various molecular or signaling pathways, are the principle toxicity mechanisms of PQ. It is hard to manage PQ toxicity and, unfortunately, most of the intoxicated patients died following PQ exposure.
In this study we concerned three cases, including 17-, 20-, and 23-year-old men, that consumed wide ranges of PQ at 100 ml of poisonous green herbicide solution, a few sips of PQ poison and poisonous grapes who despite the efforts to rescue these patients, they expired. Common formulations of PQ that may be particularly consumed by patients are 20% (w/v). But, concentrated formulations usually need to dilute due to the corrosive properties.10 It has indicated that ingested high dose (more than 50 to 100 ml of liquid concentrate (20%)) of PQ lead to fulminant organ failure, and pulmonary edema, cardiac, renal and hepatic injury and CNS toxicity which can induce convulsion, hypoxia, shock and metabolic acidosis and finally death due to multi organ failure within several hours to a few days occurred.10 Smaller amounts of PQ usually have adverse effects on kidneys and lungs developing over the next 2–6 days.10 Different clinical manifestations according to the amount of ingested PQ can be observed that classified into three categories: mild poisoning (less than 20 mg PQ ion per kg body weight (bw)), that gastrointestinal symptoms with presume development of renal damage may be occurred, but full recovery is possible; severe poisoning (20–40 mg PQ ion per kg bw), that acute renal failure, acute lung injury and advanced pulmonary fibroses, which caused respiratory failure 2–3 weeks after PQ ingestion may be occurred, however, most of patients could still recover; fulminant poisoning (greater than 40 mg PQ ion per kg bw = 20 ml of 20–24% concentrate), that involved multiple organ failure and finally lead to death in all patients within hours to a few days after the PQ ingestion.7 The lethal dose (LD50) of PQ in humans has estimated to 20–40 mg ion per kg of bw, which is equal to 1.2–2.4 US teaspoons of a PQ product with a 30% concentration.25 No exact ingested dose of PQ determined in described cases, however, humans may be at risk to various routes of exposure to PQ and different clinical features and toxicity degrees expected to occur in patients. Therefore, more clinical study according to ingest different doses of PQ should be designed.
It has been reported that 93% of fatalities from PQ intoxication are suicides which take place in developing countries.26 It was withdrawn from the European Union in July 2007.2 Data were collected at the Poison Control Center in Marseille during the nine-year period (4.5 years before and after the PQ ban in Europe). The results indicated that the mortality rate of intentional ingestion was approximately 50% including 34 suicide attempts and 15 deaths. Furthermore, it was reported that the total number of poisonings recorded after the PQ ban (38 vs. 33 after the ban) reduced because of the reduction in the number of unintentional exposures (21 vs. 16 after the ban). However, it was further reported that there was no manifested change in number of the suicide attempts involving PQ.2 Therefore, PQ poisoning continues to be considered to be growing in the world.
PQ may cause poisoning through oral contact (often suicide), inhalation, and dermal (mainly occupational) routes.27 Two of the reported cases intentionally ingested PQ in order to attempt suicide, whereas the other patient was accidentally exposed to PQ. Therefore, usage of proper protective clothing, mask, gloves, and adherence to correct spraying practices are steps that ensure one has sufficiently protected himself before applying pesticides (both in the arena of agriculture and industry). However, PQ, when ingested, can cause fatality in animals and humans.2 Diluted PQ used for spraying (farmers) is considered less toxic than pure PQ, which (during mixing or loading or consuming intentionally) carries the greatest risk of poisoning.
The clinical manifestations mainly included nausea, vomiting, aphthous stomatitis, weakness, lethargy, oral canker sores, painful lesions, tongue and throat redness, jaundice, dyspnea, bilateral crackles, crepitation, orrales, tachypnea, fever, sialorrhoea, pupil mydriasis, oral and lips burn, body pain, and digestive problems (as reported). Epilepsy and acute pancreatitis are also induced by ingesting 20 ml of PQ (20% W/V), which was related to PQ-produced OS or free radicals.28 Nausea, vomiting, and a severe substernal burning sensation are indicated after accidental poisoning caused by ingesting approximately 100 ml of PQ (60% solution) concentration.29 Nausea, vomiting, discomfort, and neutropenia were observed in a 15-year-old girl, who ingested a small quantity of wine full of PQ.30 Similarly, 10 symptoms including cough, diarrhea, eye irritation, headache, nausea, rhinitis, throat irritation, breathing trouble, unusual tiredness, and wheezing were found to be significantly elevated after PQ exposure.31 Furthermore, hepatitis (66.7%), shock (50.0%), hypoxemia (33.3%), respiratory failure (33.3%), abdominal pain, (33.3%), acute renal failure (33.3%), gastrointestinal tract bleeding (33.3%), nausea/vomiting (16.7%), and seizures (16.7%) were also reported in pediatric patients following PQ poisoning.32 Oral mucosa ulceration with developed acute non-oliguric renal failure and acute liver injury were observed in patients who intentionally ingested PQ. One such patient arrived at the hospital after four hours and, unfortunately, expired despite receiving standard supportive treatment.33 These clinical features demonstrated the life-threatening effects of PQ on the gastrointestinal tract, lungs, liver, kidney, heart, and other organs. Therefore, clinical manifestations in addition to odor perception help to primarily diagnose PQ poisoning and these should be monitored during PQ intoxication.
Laboratory parameters obtained from cases were indicated in Table 2. As shown in Table 2, urea and creatinine (Cr) are indicators of kidney function; AST, ALT, and ALP are indicators of liver function; and fasting blood sugar (FBS), LDH, CPK, WBC, Hb, Hct, Plt, protein urea, PT, and PTT are indicators of various organs’ functioning.
Table 2. Laboratory data of the patients at admission in the hospital.
| Laboratory parameters (normal range) | Case I | Case II | Case III |
| Fasting blood sugar (60–110 mg dL–1) | 120 | 146 | 137 |
| Urea (15–45 mg dL–1) | 81 | 40 | 166 |
| Creatinine (0.7–1.4 mg dL–1) | 1.2 | 2.5 | 9.5 |
| Na (135–150 mEq L–1) | 141 | 141 | 132 |
| K (3.2–5.5 mEq L–1) | 5 | 4.2 | 3.9 |
| Phosphorus (2.5–4.8 mg dL–1) | 3 | ND | ND |
| Calcium (8.5–10.5 mg dL–1) | 8.5 | 9.2 | 0.47 |
| SGOT or AST (up to 37 U L–1) | 96 | ND | 215 |
| SGPT or ALT (up to 40 U L–1) | 220 | ND | 374 |
| ALP (180–1200 U L–1) | ND | ND | 282 |
| LDH (140–280 U L–1) | 2140 | 906 | 2133 |
| Creatinine phosphokinase (24–195 U L–1) | 70 | 928 | 553 |
| Red blood cell (μ L–1) | 5.65 | 6.19 | 3.66 |
| White blood cell (4–10 × 103 μ L–1) | 16.8 | 15.5 | 15.4 |
| Hemoglobin (12–14 g dL–1) | 15.1 | 15.8 | 11.8 |
| Hematocrit (30–45%) | 46.2 | 48.2 | 35 |
| Platelet (150–350 × 103 μ L–1) | 444 | 186 | 271 |
| Protein urea | 6 | ND | 166 |
| Prothrombin time (12–14 seconds) | 14 | 21.1 | 16.9 |
| Partial thromboplastin time (24–36 seconds) | 27 | 36 | 45 |
| International normalized ratio (up to 1) | 1.4 | 3.07 | 1.6 |
All these were found to be above the normal range in the reported cases. These results demonstrated a complicated situation and indicated damage to various organs in PQ-poisoned patients. It was demonstrated that ingestion of PQ had induced rapid death from multi-organ failure, involving the circulatory system or delayed death from lung fibrosis.34 It was reported that patients who had ingested almost 40 ml of PQ solution (24%) often die rapidly due to multiple organ failure within the next few hours or days.35 The other study investigated developed pulmonary fibrosis that occurred due to PQ ingestion (approximately 50 ml of a solution containing 13% PQ and 7% DQ [about 6650 mg of PQ and 3500 mg of DQ, respectively]).34 Toxicity was reported to be more principally found in the lungs, resulting in death due to progressive pulmonary fibrosis, and injury in the liver, kidney, brain, and heart.19 The generation of free radicals and ROS to induce OS status, and various molecular mechanisms such as caspase cleavage, MMP-9, PPAR-γ, DDX3/GSK3; NF-κB, JNK/p38 MAPK, Nrf2/Nox4 redox balance, and TGF-β1/Smad3 signaling pathway were the main reasons causing lung toxicity and fibrosis.9,12,16,17,23,24 PQ induced acute liver injury (AKI) via induction of mitochondrial damage, LPO, and secretion of pro-inflammatory molecules. These include iNOS, TNF-α IL-1β, and lessening the antioxidant capacity by reducing phase I and phase II xenobiotic metabolizing enzymes. These enzymes include cytochrome P450 (CYP) and GST, GP-X, MPO, and CAT activities, GSH level and increasing ROS leading to an increase in serum AST, ALT, and ALP activities and total bilirubin as liver damage biomarkers.18,36–38 Approximately 47% of the patients, intoxicated by PQ, suffered from mild and transient toxic liver and kidney.35,39 These patients were younger and suffered from acute respiratory and renal failure. A retrospective study found that this occurs less in patients without hepatitis. Thus, the hospitalization period was longer in patients with hepatitis than patients without hepatitis.35 Furthermore, it has been recorded that many of PQ-intoxicated patients experienced AKI and indicated a higher mortality rate than patients without AKI (70.1% and 40%, respectively), which often requires hemodialysis.39 As shown in Table 2, two patients showed proteinuria as a biomarker of AKI. Albuminuria was used to evaluate the kidney function and effects on biomarker cutoffs for diagnosis and outcome prediction in PQ-intoxicated patients.40 Albuminuria was indicated in 34 out of 50 patients and urinary neutrophil gelatinase-associated lipocalin (uNGAL), urinary cystatin C (uCysC), uClusterin, Urine β-2 microglobulin (Uβ2M), and urinary kidney injury molecule-1 (uKIM-1) levels, as markers to diagnose functional AKI. These were significantly higher in albuminuric patients as compared to non-albuminuric patients.40 These injury biomarkers were elevated within 24 hours after ingestion of PQ as compared to patients without AKI and healthy controls. This could be applied to guide early intervention for rescuing renal damage within 16–24 hours.41 Albuminuria was correlated to PQ-induced nephrotoxicity and causes death.40 Nucleotide-binding domain, leucine-rich repeat containing protein 3 (NLRP3) inflammasome activation regulated by pro-inflammatory cytokine NF-κB and death-associated protein kinase (DAPK) were molecular mechanisms that have been associated with PQ-induced AKI.23 Therefore, proteinuria could be used in the prediction of PQ-induced nephrotoxicity. Urea and Cr were also elevated following PQ intoxication. Age, serum Cr, hours (since ingestion), and plasma PQ concentration were scrutinized. This was done to analyze the prediction of survival in PQ-poisoning patients.42 This is since survivors were younger and had lower PQ concentrations (1.44 ± 8.77 μg ml–1vs. 80.33 ± 123.15 μg ml–1) than non-survivors.42 Although it indicated that serum/urine Cr was not a good marker of AKI after PQ intoxication, kidney injury should be evaluated using more specific biomarkers.41
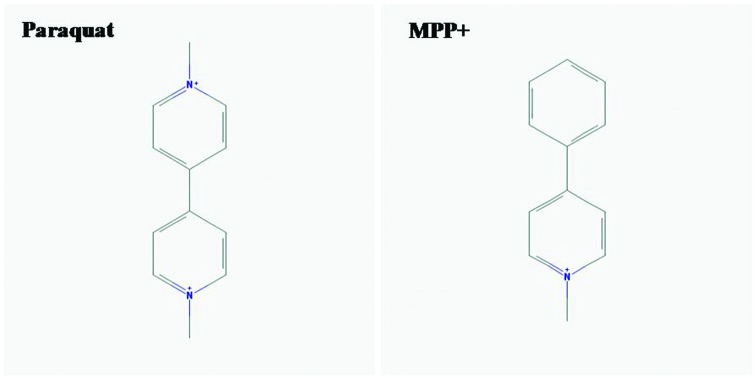
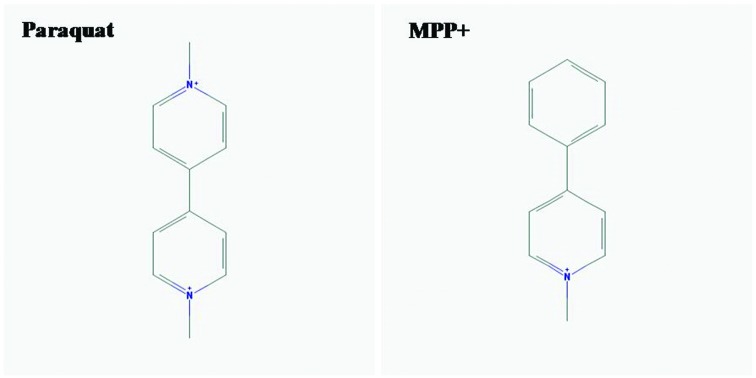
Increasing urine N-acetyl-beta-d-glucosaminidase (NAG) was also used as an early biomarker for AKI and a predictor of mortality in patients with acute PQ intoxication.43 AKI, due to rhabdomyolysis, was also described in PQ-intoxicated patients and, therefore, should be more concerning.44 Parkinson's disease (PD) is a neurodegenerative disease of the nervous system. This is characterized by progressive tremor, bradykinesia, rigidity, and postural instability. This also indicates a link with PQ intoxication. It demonstrated that mitochondrial dysfunction and increasing of OS events were pathophysiologic mechanisms involved in PD-induced by PQ.45 Furthermore, as shown in Fig. 1, a similar structure of PQ to MPP+ (1-methyl-4-phenylpyridinium) caused PQ-induced neurotoxicity and injury to renal proximal tubules.46,47
Fig. 1. Chemical structure of paraquat (PQ) and MPP + (1-methyl-4-phenylpyridinium) drawled with PubChem.
PQ also induced gliotoxic via protein kinase Cdelta (PKCdelta) that emphasized the important role of PKCdelta in PQ-induced glial cell death.48 OS events and the metabolic processes caused by PQ exposure induced excitotoxicity, α-synuclein aggregate formation, autophagy, alteration of dopamine catabolism, and inactivation of tyrosine hydroxylase. These were in accordance with the loss of dopaminergic cells and the developing of PD.49 In addition, epilepsy or convulsion was investigated in clinical case reports and studies.28,29,50 Therefore, PQ-induced neurotoxicity is a matter of concern and should be prevented or treated in intoxicated patients. These findings indicated that PQ poisoning patients should be followed up for a long time after discharge from a hospital.
Cardiotoxicity was also investigated in an experimental study following PQ exposure. Various molecular mechanisms were reported as underlying this effect. This was because the ablation of Akt2 had protected against increasing myocytes resting intracellular Ca2+, cardiac contractile dysfunction, and apoptosis, possibly through the regulation of Nrf2 activation and myocardial mitochondrial homeostasis.51 Cardiotoxicity was important to manage as most of the patients with severe PQ intoxication died following multiple-organ failure involving the circulatory (cardiovascular shock) and respiratory systems.34 Hypercholesterolemia was also associated with increased OS events. OS, which was induced by the PQ, increased cellular dysfunction in endothelial and smooth muscle cells and reduced arterial healing.52 Therefore, the administration of antioxidants could accelerate endothelial cell healing and attenuated hyperplasia, and it reduced the vessel thrombogenicity.52 Furthermore, xanthine oxidase (XO) was also investigated to be responsible for the toxicity of PQ on artery endothelial cells through intracellular O2– production and the subsequent formation of other free radicals. Thus, XO inhibitors such as allopurinol could attenuate artery injury, especially in the pulmonary artery endothelial cells.53 Therefore, vital signs including blood pressure, heart rate, and electrolyte imbalance should be monitored in these patients.
Laboratory arterial blood gas (ABG) was shown in Table 3. Determining ABG could be used as a powerful prediction marker for poisoning with a toxic substance.54–56
Table 3. Arterial blood gas (ABG) data of the patients at admission to the hospital.
| Laboratory parameters (normal range) | Case I | Case II | Case III |
| pH (range 7.35 to 7.45) | 7.51 | 7.18 | 7.47 |
| PCO2 (38 to 42 mmHg) | 34.1 | 30.6 | 24.8 |
| HCO3 (22 to 28 mEq L–1) | 27.6 | 11.8 | 18.3 |
| PO2 (80–100 mmHg) | 40.2 | 59.7 | 49.1 |
| O2sat (94% to 100%) | 80.9 | 84.4 | 87.4 |
It was indicated that arterial oxygen desaturation was a more sensitive test to evaluate the respiratory effects of long-term and low-dose exposure to PQ spraying among workers.57 It reported a decline in forced vital capacity and forced expiratory volume in one second, following PQ-applying farmers.58 As shown in Table 3, lower pH was observed in case II, and lower PCO2 was revealed in cases I, II, and III, as well as lower HCO3 was observed in cases II and III. These results indicated that PQ-intoxicated patients had complicated acute respiratory alkalosis, and thereafter a shift to metabolic acidosis. It was investigated that PQ-intoxicated patients suffering from AKI spent a longer time upon arriving in the hospital. They demonstrate significantly lower PCO2, higher PO2, and lower HCO3 during admission. However, these patients show significantly lower PO2, higher alveolar-arterial O2 difference, and higher sequential organ failure 48 hours after admission, as compared with patients without AKI.39 Therefore, acute hepatitis, the time taken to arrive at the hospital, and PO2 at admission were powerful predictors of AKI in PQ-intoxicated patients. In another study, it was investigated that PO2 was significantly lower in the patients who died than in the patients who survived 48 hours after admission, following PQ intoxication.59 Therefore, PQ-poisoned patients might experience respiratory alkalosis or metabolic acidosis. In addition, it demonstrated that toxic dose, the time lapse from poisoning to gastric lavage, WBC, ALT, SCr at admission, PO2, PCO2, and arterial lactate 48 hours after admission were the predictor risk factors indicating the prognosis of acute PQ poisoning.59
The high mortality of PQ was due to inherent toxicity and the lack of specific antidotes or effective treatments. However, because PQ-induced ROS and OS events and inflammation underlying various molecular mechanisms, thus antioxidant such as acetylcysteine and salicylate, as scavenging of free radical, anti-inflammatory and NF-κB inhibitory effects and immunosuppressants such as dexamethasone, cyclophosphamide (CP) and methylprednisolone (MP) was applied.10,11 There have been some successful cases of using pulse treatment with two 1 g doses of CP and three 1 g doses of MP. The doses were given daily for three consecutive days or 15 mg kg–1 day–1 MP for three days in patients with moderate to severe PQ poisoning. It was noticed that there was a significantly lower mortality risk in these cases.60,61 However, the risk of leucopenia during prolonged MP therapy after pulse treatment is a matter of concern.61 According to the molecular mechanisms involved in PQ intoxication, rapamycin, resveratrol, and tetracycline can protect against PQ-induced organ toxicity.20 Tetracycline and resveratrol were investigated to ameliorate OS and inflammatory markers in the lungs, brain, and liver.19 Treatments including oral Fuller's earth, forced diuresis, hemofiltration, corticosteroids, N-acetylcysteine (NAC), MP, CP, vitamin E, colchicine, and nitric oxide (NO) inhalation were investigated and positive outcomes were seen in PQ-intoxicated patients.34 Treatment with sequential whole gastric and bowel irrigation included oral usage of montmorillonite powder (60 g), followed by gastric lavage with bicarbonate liquid (2.5%). This was followed by activated charcoal (30 g), montmorillonite powder (30 g), and polyethylene glycol electrolyte lavage solution, in order to remove intestinal toxins once a day for five days. This was found to be effective too.62 The incidence of hypokalemia, elevation of serum amylase, ALT, TB, BUN, Cr, lactate, and decreasing of PO2 were seen to be significantly declined following this treatment in PQ-poisoning patients.62 The beneficial use of hyperbaric oxygen treatment in attenuating PQ-induced lung injury was also reported in an experimental study.63 Natural source products such as thymoquinone can protect against PQ-induced lung fibrosis or hepatotoxicity.64,65
Plasma perfusion effectively decreased the plasma PQ rate and was considered as a promising treatment in PQ-poisoning patients.66 In severe cases of PQ poisoning, hemoperfusion, hemodialysis, and plasma exchange were required. Hemodialysis was usually started on the fourth day after admission because of uremia.67 Anemia, thrombocytopenia, and LDH showed signs of improvement following plasma exchange.67 Thus, the risk of thrombocytopenia, anemia, and hemolytic uremic syndrome should be taken into consideration in treating PQ-intoxicated patients.67 Although several therapies are available that improve a PQ-poisoned patients' conditions, many of the patients were provided with poor prognoses and, therefore, they expired.
4. Conclusions
Poisoning by PQ herbicide was a principle medical problem in developing countries. Due to its availability and affordability, it continued to occur elsewhere. The OS events and various underlined molecular mechanisms were responsible for rapid death from multisystem failure and cardiovascular shock or delayed death from progressive pulmonary fibrosis. Clinical features, including nausea, vomiting, oral lesions, dyspnea, and tachypnea may be observed following PQ poisoning. Antioxidant, immunosuppressant, early application of gastrointestinal lavage, activated charcoal (if taken in time), manitol, hemoperfusion, or hemodialysis can be used to reduce plasma PQ concentration. This may help the patients to recover and normalize the injury biomarkers in serial blood and urine samples. Biomarkers such as PO2, PCO2, SCr, AST, ALT, and ALP were used as predictor factors for PQ poisoning. Therefore, clinical manifestations and laboratory findings scanned organ functions that were recommended to be measured. However, there was no specific antidote, and despite the presence of supportive treatments, the case fatality was very high in toxicology centers. Therefore, delayed death occurred in PQ poisoning and regarding to continue as describe mechanisms, and there should be a follow-up for these patients over a long period. These properties make PQ a hazardous substance. No published human trials were reported in any survey. Furthermore, clinical trials in this regard need to be designed.
Conflicts of interest
No potential conflicts of interest declared.
Acknowledgments
Our thanks are due to Mr Atari who works at archive center of Loghman Hakim general teaching hospital for his corporations to find out the patients file.
References
- Pateiro-Moure M., Martínez-Carballo E., Arias-Estévez M., Simal-Gándara J. J. Chromatogr., A. 2008;1196–1197:110–116. doi: 10.1016/j.chroma.2008.03.081. [DOI] [PubMed] [Google Scholar]
- Kervégant M., Merigot L., Glaizal M., Schmitt C., Tichadou L., de Haro L. J. Med. Toxicol. 2013;9:144–147. doi: 10.1007/s13181-012-0283-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor P. J., Salm P., Pillans P. I. J. Anal. Toxicol. 2001;6:456–460. doi: 10.1093/jat/25.6.456. [DOI] [PubMed] [Google Scholar]
- Fortenberry G. Z., Beckman J., Schwartz A., Prado J. B., Graham L. S., Higgins S., Lackovic M., Mulay P., Bojes H., Waltz J., Mitchell Y., Leinenkugel K., Oriel M. S., Evans E., Calvert G. M. Environ. Res. 2016;146:191–199. doi: 10.1016/j.envres.2016.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coats Jr. G. E., Funderburk H. H., Lawrence J. M., Davis D. E. Weed Res. 2006;1:58–66. [Google Scholar]
- Huggins D. R., Reganold J. P. Sci. Am. 2008;1:70–77. doi: 10.1038/scientificamerican0708-70. [DOI] [PubMed] [Google Scholar]
- Sabzghabaee A. M., Eizadi-Mood N., Montazeri K., Yaraghi A., Golabi M. Singapore Med. J. 2010;6:496–500. [PubMed] [Google Scholar]
- Shen H., Wu N., Wang Y., Zhao H., Zhang L., Li T., Zhao M. Int. Immunopharmacol. 2017;46:16–22. doi: 10.1016/j.intimp.2017.02.020. [DOI] [PubMed] [Google Scholar]
- Tyagi N., Dash D., Singh R. Inflammopharmacology. 2016;6:335–345. doi: 10.1007/s10787-016-0286-z. [DOI] [PubMed] [Google Scholar]
- Gawarammana I. B., Buckley N. A. Br. J. Clin. Pharmacol. 2011;5:745–757. doi: 10.1111/j.1365-2125.2011.04026.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suntres Z. E. Toxicology. 2002;1:65–77. doi: 10.1016/s0300-483x(02)00382-7. [DOI] [PubMed] [Google Scholar]
- Lei Y., Li X., Yuan F., Liu L., Zhang J., Yang Y., Zhao J., Han Y., Ren J., Fu X. Environ. Toxicol. 2017;2:656–668. doi: 10.1002/tox.22267. [DOI] [PubMed] [Google Scholar]
- Zhang X. F., Thompson M., Xu Y. H. Lab. Invest. 2016;5:496–507. doi: 10.1038/labinvest.2015.161. [DOI] [PubMed] [Google Scholar]
- Gil H. W., Yang J. O., Lee E. Y., Hong S. Y. Clin. Toxicol. 2009;4:308–311. doi: 10.1080/15563650902834497. [DOI] [PubMed] [Google Scholar]
- Bus J. S., Gibson J. E. Environ. Health Perspect. 1984;55:37–46. doi: 10.1289/ehp.845537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X., Shen H., Wang Y., Zhao M. BioMed. Res. Int. 2017;2017:4652695. doi: 10.1155/2017/4652695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hathaichoti S., Visitnonthachai D., Ngamsiri P., Niyomchan A., Tsogtbayar O., Wisessaowapak C., Watcharasit P., Satayavivad J. Toxicol. In Vitro. 2017;42:123–129. doi: 10.1016/j.tiv.2017.04.016. [DOI] [PubMed] [Google Scholar]
- Han J., Zhang Z., Yang S., Wang J., Yang X., Tan D. Food Chem. Toxicol. 2014;70:100–106. doi: 10.1016/j.fct.2014.04.038. [DOI] [PubMed] [Google Scholar]
- Huang Y., He Q. Environ. Toxicol. Pharmacol. 2017;52:62–68. doi: 10.1016/j.etap.2017.01.023. [DOI] [PubMed] [Google Scholar]
- Huang M., Yang H., Zhu L., Li H., Zhou J., Zhou Z. Environ. Toxicol. 2016;11:1620–1626. doi: 10.1002/tox.22166. [DOI] [PubMed] [Google Scholar]
- Satpute R. M., Pawar P. P., Puttewar S., Sawale S. D., Ambhore P. D. Hum. Exp. Toxicol. 2017;36:1303–1314. doi: 10.1177/0960327116688070. [DOI] [PubMed] [Google Scholar]
- Xu Y., Tai W., Qu X., Wu W., Li Z., Deng S., Vongphouttha C., Dong Z. Biochem. Biophys. Res. Commun. 2017;17:31206–31208. doi: 10.1016/j.bbrc.2017.06.074. [DOI] [PubMed] [Google Scholar]
- Liu B., Cao B., Zhang D., Xiao N., Chen H., Li G. Q., Peng S. C., Wei L. Q. Toxicol. Appl. Pharmacol. 2016;309:111–120. doi: 10.1016/j.taap.2016.08.004. [DOI] [PubMed] [Google Scholar]
- Li A., Liu Y., Zhai L., Wang L., Lin Z., Wang S. Inflammation. 2016;2:928–937. doi: 10.1007/s10753-016-0326-2. [DOI] [PubMed] [Google Scholar]
- Vale J. A., Meredith T. J., Buckley B. M. Hum. Toxicol. 1987;6:41–47. doi: 10.1177/096032718700600107. [DOI] [PubMed] [Google Scholar]
- Dinham B. Pesticide News. 1996;32:20–21. [Google Scholar]
- Baharuddin M. R., Sahid I. B., Noor M. A., Sulaiman N., Othman F. J. Environ. Sci. Health, Part B. 2011;7:600–607. doi: 10.1080/03601234.2011.589309. [DOI] [PubMed] [Google Scholar]
- Dong H., Peng X., Qiu Z. Ann. Transl. Med. 2017;7:163. doi: 10.21037/atm.2017.03.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davarpanah M. A., Hosseinzadeh F., Mohammadi S. S. Iran Red Crescent. Med. J. 2015;10:e19373. doi: 10.5812/ircmj.19373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papanikolaou N., Paspatis G., Dermitzakis A., Tzortzakakis E., Charalambous E., Tsatsakis A. M. Hum. Exp. Toxicol. 2001;11:597–599. doi: 10.1191/096032701718620891. [DOI] [PubMed] [Google Scholar]
- Ames R. G., Howd R. A., Doherty L. Arch. Environ. Health. 1993;1:47–52. doi: 10.1080/00039896.1993.9938392. [DOI] [PubMed] [Google Scholar]
- Hsieh Y. W., Lin J. L., Lee S. Y., Weng C. H., Yang H. Y., Liu S. H., Wang I. K., Liang C. C., Chang C. T., Yen T. H. Pediatr. Emerg. Care. 2013;4:487–491. doi: 10.1097/PEC.0b013e31828a347e. [DOI] [PubMed] [Google Scholar]
- Olson D. P., Diaz J. A., Jereda J. D. Med. Sci. Monit. 2010;12:CS153–CS156. [PubMed] [Google Scholar]
- Eisenman A., Armali Z., Raikhlin-Eisenkraft B., Bentur L., Bentur Y., Guralnik L., Enat R. J. Toxicol., Clin. Toxicol. 1998;6:575–584. doi: 10.3109/15563659809028051. [DOI] [PubMed] [Google Scholar]
- Yang C. J., Lin J. L., Lin-Tan D. T., Weng C. H., Hsu C. W., Lee S. Y., Lee S. H., Chang C. M., Lin W. R., Yen T. H. Liver Int. 2012;9:1400–1406. doi: 10.1111/j.1478-3231.2012.02829.x. [DOI] [PubMed] [Google Scholar]
- Shi Q., Song X., Fu J., Su C., Xia X., Song E., Song Y. Int. Immunopharmacol. 2015;2:722–729. doi: 10.1016/j.intimp.2015.09.003. [DOI] [PubMed] [Google Scholar]
- Costa M. D., de Freitas M. L., Dalmolin L., Oliveira L. P., Fleck M. A., Pagliarini P., Acker C., Roman S. S., Brandão R. Environ. Toxicol. Pharmacol. 2013;3:750–758. doi: 10.1016/j.etap.2013.07.009. [DOI] [PubMed] [Google Scholar]
- Ahmad I., Shukla S., Kumar A., Singh B. K., Kumar V., Chauhan A. K., Singh D., Pandey H. P., Singh C. Chem.-Biol. Interact. 2013;1–3:9–18. doi: 10.1016/j.cbi.2012.10.027. [DOI] [PubMed] [Google Scholar]
- Weng C. H., Chen H. H., Hu C. C., Huang W. H., Hsu C. W., Fu J. F., Lin W. R., Wang I. K., Yen T. H. Oncotarget. 2017;8:51345–51354. doi: 10.18632/oncotarget.17975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohamed F., Buckley N. A., Wl Pickering J., Wunnapuk K., Dissanayake S., Chathuranga U., Gawarammana I., Jayamanne S., Endre Z. H. BMC Nephrol. 2017;1:122. doi: 10.1186/s12882-017-0532-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohamed F., Buckley N. A., Jayamanne S., Pickering J. W., Peake P., Palangasinghe C., Wijerathna T., Ratnayake I., Shihana F., Endre Z. H. Toxicol. Lett. 2015;2:140–150. doi: 10.1016/j.toxlet.2015.06.008. [DOI] [PubMed] [Google Scholar]
- Sun I. O., Shin S. H., Yoon H. J., Lee K. Y. Kidney Res. Clin. Pract. 2016;2:102–106. doi: 10.1016/j.krcp.2016.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi X. B., He J. L., Lu Y. Q. Zhonghua Lao Dong Wei Sheng Zhi Ye Bing ZaZhi. 2013;3:223–224. [PubMed] [Google Scholar]
- Xue Q., Wang X. Zhonghua Lao Dong Wei Sheng Zhi Ye Bing ZaZhi. 2015;11:855–856. [PubMed] [Google Scholar]
- Tanner C. M., Kamel F., Ross G. W., Hoppin J. A., Goldman S. M., Korell M., Marras C., Bhudhikanok G. S., Kasten M., Chade A. R., Comyns K., Richards M. B., Meng C., Priestley B., Fernandez H. H., Cambi F., Umbach D. M., Blair A., Sandler D. P., Langston J. W. Environ. Health Perspect. 2011;6:866–872. doi: 10.1289/ehp.1002839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winnik B., Barr D. B., Thiruchelvam M., Montesano M. A., Richfield E. K., Buckley B. Anal. Bioanal. Chem. 2009;1:195–201. doi: 10.1007/s00216-009-2929-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groves C. E., Morales M. N., Gandolfi A. J., Dantzler W. H., Wright S. H. J. Pharmacol. Exp. Ther. 1995;2:926–932. [PubMed] [Google Scholar]
- Kim S., Hwang J., Lee W. H., Hwang D. Y., Suk K. J. Neurosci. Res. 2008;9:2062–2070. doi: 10.1002/jnr.21643. [DOI] [PubMed] [Google Scholar]
- Zhang X. F., Thompson M., Xu Y. H. Lab. Invest. 2016;5:496–507. doi: 10.1038/labinvest.2015.161. [DOI] [PubMed] [Google Scholar]
- Huang C., Zhang X., Jiang Y., Li G., Wang H., Tang X., Wang Q. Toxicol. Ind. Health. 2013;8:722–727. doi: 10.1177/0748233712442712. [DOI] [PubMed] [Google Scholar]
- Wang S., Zhu X., Xiong L., Ren J. Toxicol. Lett. 2017;269:1–14. doi: 10.1016/j.toxlet.2017.01.009. [DOI] [PubMed] [Google Scholar]
- Rosenbaum M. A., Miyazaki K., Graham L. M. J. Vasc. Surg. 2012;2:489–496. doi: 10.1016/j.jvs.2011.07.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai M., Yamagami K., Kitazawa Y., Takeyama N., Tanaka T. Pharmacol. Toxicol. 1995;1:36–40. doi: 10.1111/j.1600-0773.1995.tb01911.x. [DOI] [PubMed] [Google Scholar]
- Rahimi H. R., Soltaninejad K., Shadnia S. J. Res. Med. Sci. 2014;9:855–859. [PMC free article] [PubMed] [Google Scholar]
- Agin K., Hassanian-Moghaddam H., Shadnia S., Rahimi H. R. Environ. Toxicol. Pharmacol. 2016;45:15–19. doi: 10.1016/j.etap.2016.05.001. [DOI] [PubMed] [Google Scholar]
- Rahimi H. R., Agin K., Shadnia S., Hassanian-Moghaddam H., Oghazian M. B. Toxicol. Mech. Methods. 2015;1:42–47. doi: 10.3109/15376516.2014.975388. [DOI] [PubMed] [Google Scholar]
- Dalvie M. A., White N., Raine R., Myers J. E., London L., Thompson M., Christiani D. C. Occup. Environ. Med. 1999;6:391–396. doi: 10.1136/oem.56.6.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cha E. S., Lee Y. K., Moon E. K., Kim Y. B., Lee Y. J., Jeong W. C., Cho E. Y., Lee I. J., Hur J., Ha M., Lee W. J. Occup. Environ. Med. 2012;6:398–403. doi: 10.1136/oemed-2011-100244. [DOI] [PubMed] [Google Scholar]
- Fengjun J., Wen Z., Taoning W., Yaying Y., Kai K., Liu M. Zhonghua Wei Zhong Bing JiJiu Yi Xue. 2015;11:906–910. [PubMed] [Google Scholar]
- Newstead C. G. Thorax. 1996;7:659–660. doi: 10.1136/thx.51.7.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao J., Feng S., Wang J., Yang S., Li Y. Medicine. 2017;25:e7244. doi: 10.1097/MD.0000000000007244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao B., Dai J., Li J., Xiao L., Sun B., Liu N., Zhang Y., Jian X. Zhonghua Lao Dong Wei Sheng Zhi Ye Bing ZaZhi. 2015;3:213–215. [PubMed] [Google Scholar]
- Akcılar R., Akcılar A., Şimşek H., Koçak F. E., Koçak C., Yümün G., Bayat Z. Int. J. Clin. Exp. Pathol. 2015;10:13034–13042. [PMC free article] [PubMed] [Google Scholar]
- Pourgholamhossein F., Sharififar F., Rasooli R., Pourgholi L., Nakhaeipour F., Samareh-Fekri H., Iranpour M., Mandegary A. Environ. Toxicol. Pharmacol. 2016;45:340–345. doi: 10.1016/j.etap.2016.06.019. [DOI] [PubMed] [Google Scholar]
- Nili-Ahmadabadi A., Tavakoli F., Hasanzadeh G., Rahimi H., Sabzevari O. Daru. 2011;4:282–287. [PMC free article] [PubMed] [Google Scholar]
- Li G. Q., Li Y. M., Wei L. Q., Liu Y., Zhang Y. H. Am. J. Med. Sci. 2014;3:195–203. doi: 10.1097/MAJ.0000000000000235. [DOI] [PubMed] [Google Scholar]
- Jang H. N., Bae E. J., Hwang K., Kang Y., Yun S., Cho H. S., Chang S. H., Park D. J. J. Clin. Apher. 2014;3:183–186. doi: 10.1002/jca.21310. [DOI] [PubMed] [Google Scholar]