Abstract
Pemphigoid gestationis, which is also known as herpes gestationis, is a rare, pregnancy-associated, autoimmune bullous disease. Treatment depends on the severity of the disease for each patient and the safety and use of these drugs during pregnancy and breastfeeding must be taken into consideration to guide their use. We describe the therapeutic response of two cases of pemphigoid gestationis that did not respond to conventional immunosuppressive therapy or adverse effects limited their use. Both patients eventually received treatment with intravenous immunoglobulin therapy, which resulted in clinical remission. This clinical improvement with disappearance of lesions and a reduction in pruritus was paralleled in a decline in Bullous Pemphigoid Disease Activity Index activity scores, which is a validated scoring system to measure the related condition, bullous pemphigoid.
Keywords: Bullous gestationis, herpes gestationis, BP180, intravenous immunoglobulin, bullous pemphigoid, pregnancy, BPDAI, IVIG
Introduction
Pemphigoid gestationis (PG), which is also known as herpes gestationis, is a rare, pregnancy-associated, autoimmune bullous disease. Patients develop intensely pruritic vesiculobullous lesions that involve any part of the body but often the periumbilical area. The intense pruritus causes significant morbidity and interferes with patients' quality of life. Symptoms usually resolve after pregnancy but in some women, the condition and symptoms can persist for months to years (Intong and Murrell, 2011a).
Treatment depends on the severity of the disease for each patient and the safety and use of these drugs during pregnancy and breastfeeding must be taken into consideration to guide their use (Intong and Murrell, 2011a, Intong and Murrell, 2011b). We describe the therapeutic response of two cases of PG that did not respond to conventional immunosuppressive therapy or adverse effects limited their use. Both patients eventually received treatment with intravenous immunoglobulin (IVIG) therapy, which resulted in clinical remission. This clinical improvement with the disappearance of the lesions and a reduction in pruritus was paralleled with a decline in Bullous Pemphigoid Disease Activity Index (BPDAI) activity scores, which is a validated scoring system to measure the related condition, bullous pemphigoid (Wijayanti et al., 2017).
Case 1
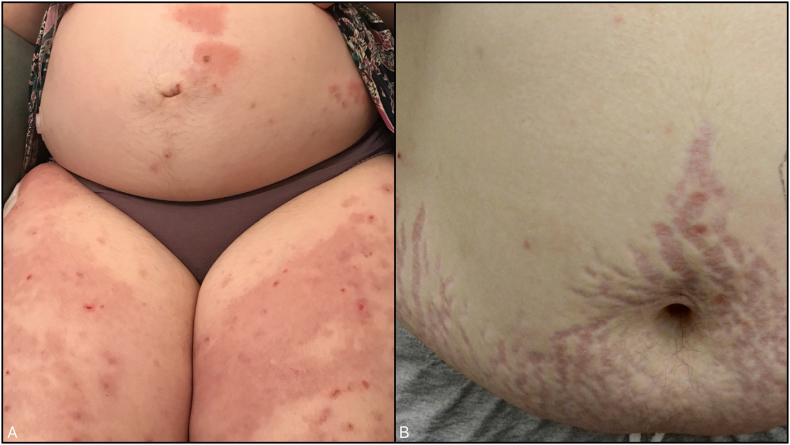
A 28-year-old, pregnant, Caucasian patient developed a pruritic rash at 28 to 40 weeks of gestation. This was her first pregnancy and her medical history included type 1 diabetes mellitus, hyperthyroidism, and asthma. Her medications included a continuous insulin pump, metformin 500 mg three times per day, and ranitidine 150 mg daily. During the examination, pruritic erythematous papules and tense bullae were observed arising on erythematous annular patches and plaques in a periumbilical distribution on her abdomen and thighs (Fig. 1).
Fig. 1.
A) Case 1 after the initiation of treatment, urticarial plaques with bullae on the abdomen and thighs. B) Case 1 with steroid-induced striae as an additional adverse effect of prolonged prednisone use.
Punch biopsies of representative lesions on her thigh were performed for histopathological examination and direct immunofluorescence (DIF) studies. The histopathology demonstrated mild acanthosis and spongiosis with overlying parakeratosis. A subepidermal vesicle was seen with eosinophils and lymphocytes. The upper dermis demonstrated prominent edema that consisted of moderate-to-heavy dermal inflammatory infiltrate with perivascular lymphocytes and interstitial eosinophils. DIF demonstrated a linear deposition of C3 and scant immunoglobulin (Ig) G along the basement membrane zone at the dermo-epidermal junction, which is also in keeping with a diagnosis of PG. Indirect serum investigations were performed and demonstrated C3 and scanty IgG on salt split skin at the basement membrane zone.
The patient was initially managed by another dermatologist and initiated 25 mg of prednisone, which was increased to 35 mg/day to control the ongoing blister formation. She also initiated an antihistamine with no additional relief from her pruritus.
She delivered a healthy newborn in February 2017. The patient continued to have flare-ups 10 weeks postpartum and any attempt to reduce the prednisone dose resulted in new lesions. She did not wish to stop breastfeeding to trial treatment with azathioprine or mycophenolate. However, systemic steroid medications alone were ineffective to keep the disease under control and the prednisone resulted in a significant lability to her blood glucose levels as she began to develop severe prednisone-related striae (Fig. 1).
The patient was referred to our clinic for further management of PG that was not responding to systemic steroid medications in the setting of type 1 diabetes. Further investigations of her sera at that time with the Biochip (EuroImmun, Lubeck, Germany) demonstrated indeterminate results on the salt split skin and positive reactivity for BP180 but negative to BP230. The enzyme-linked immunosorbent assay was positive for BP180 but negative for BP230 and well as desmogleins 1 and 3.
At the time of her initial review on prednisone, her BPDAI activity and damage scores were 13 and 4, respectively. Her quality of life score with respect to her blistering disease was measured with the Autoimmune Bullous Quality of Life (ABQOL) and was 29/51. Her treatment ABQOL score, which reflects the toll of treatment, was 29/51. In light of her type 1 diabetes, persistent disease, and desire to continue breastfeeding, IVIG therapy was discussed as a safer alternative. While awaiting approval for IVIG treatment, the patient was commenced on oral cromolyn sodium 100 mg daily to reduce mast cell-derived itch and inflammation as well as erythromycin 400 mg three times a day in view of not being able to receive tetracycline while breastfeeding.
The patient was initiated on IVIG therapy at 2 g/kg divided over 3 days every 4 weeks while her prednisone was at 25 mg/day. She developed a headache after her second dose, which resolved and was managed with prehydration preceding her subsequent doses. A month after the cycles of IVIG, she had had no new lesions and her BPDAI score was improving so her prednisone was weaned in accordance with the Werth prednisone tape (Werth et al., 2008) to 20 mg/day for 2 weeks followed by 17.5 mg/day for 2 weeks. She continued to improve and this clinical improvement in her symptoms of pruritus and disease activity was demonstrated by an improvement of her BPDAI scores (Fig. 2).
Fig. 2.
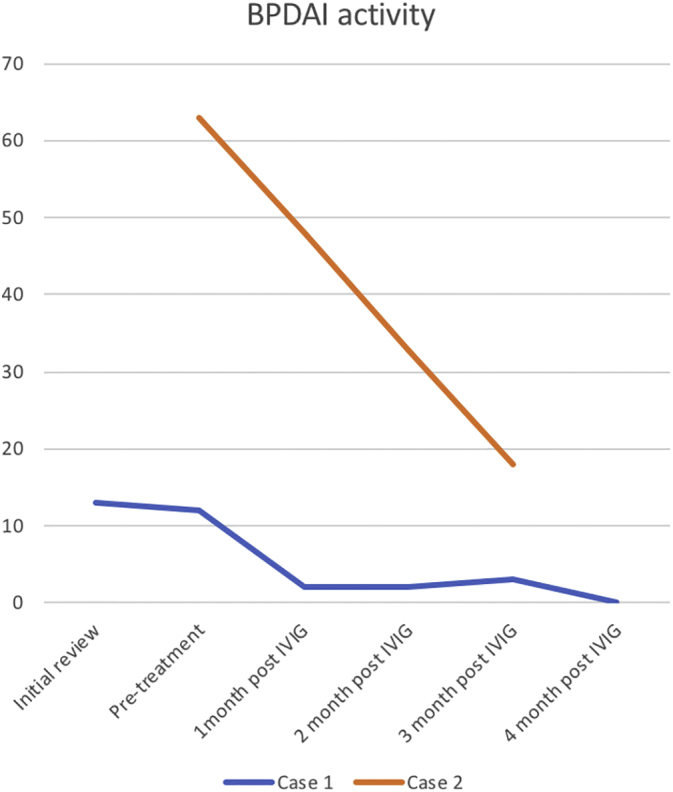
Bullous Pemphigoid Disease Activity Index (BPDAI) activity score. Case 1: Pre-IVIG treatment BPDAI activity score 13, which demonstrates a downward trend to 0 at 4 months post-IVIG. Case 2: Pre-IVIG treatment BPDAI activity score 63, which demonstrates a downward trend to 13 at 3 months post-IVIG.
When she reached treatment with 7.5 mg prednisone, there was a slight relapse in her PG and she increased the prednisone dose back to 10 mg/day and then tapered by 1 mg every 2 weeks. In November 2017, she ceased breastfeeding and was switched from erythromycin to doxycycline 100 mg twice a day. In January 2018, the patient had not had relapses of PG, was on 2 mg/day of prednisone, doxycycline, and her IVIG was reduced by one third (over 2 days per month). Because she felt nauseated with the doxycycline, this treatment was ceased and mycophenolate mofetil commenced at 750 mg twice a day to wean the IVIG therapy. In addition to an improvement of her PG, her glucose control also improved with the reduction in prednisone therapy. Her ABQOL and treatment ABQOL scores have remained high due to the toll that the disease and its treatment have taken on her.
Case 2
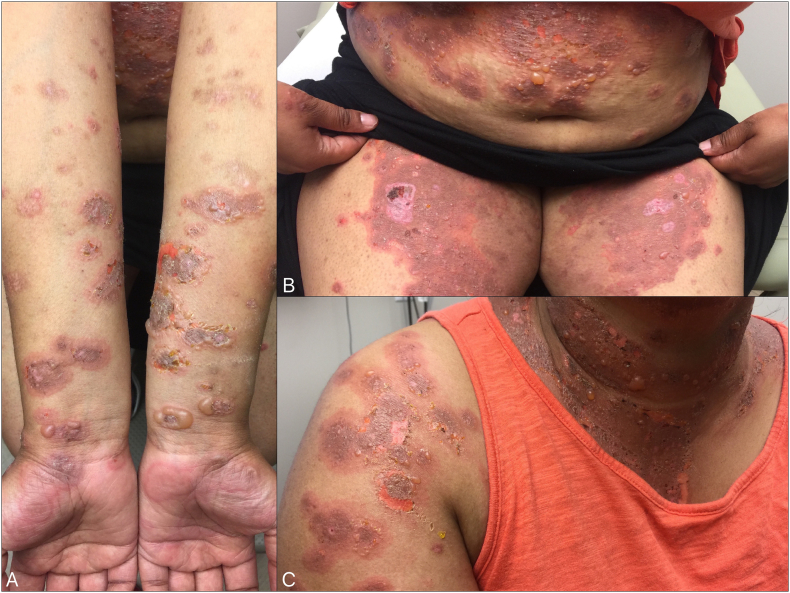
A 37-year-old, South-Asian, Indian, female patient presented with a 6-week history of a pruritic urticarial annular and targetoid plaques on her extremities and abdomen. She was prescribed clobetasol cream, cetirizine 20 mg daily, fexofenadine 180 mg daily, and diphenhydramine 50 mg at night. Punch biopsies were performed and the results showed a dermal hypersensitivity with subepidermal clefting. Direct immunofluorescence showed IgG and C3 positivity along the dermoepidermal junction. The patient returned for a follow-up visit 2 weeks later with diffuse tense bullae. She reported a miscarriage of a fetus at approximately 14 weeks of gestation. She was subsequently evaluated by obstetrics and underwent a dilation and curettage procedure for retained fetal parts (Fig. 3).
Fig. 3.
Pretreatment images of Case 2 while on 1 mg/kg prednisone daily and azathioprine 2.5 mg/kg for 3 weeks with ongoing new bullae formation and erythematous dusky plaques exquisitely tender to touch and pruritic. A) Palms and forearms with annular erythematous plaques and tense bullae. B) Abdomen and upper thighs with annular erythematous plaques and tense bullae and erosions. C) Tense bullae, erosions, and erythematous plaques on the neck that extend up to the face, chest, and arms.
Her initial BPDAI activity score was 143 with extensive bullae and erythema that affected the majority of her body surface area. Her pruritus score was 30 of 30 and she was significantly distressed. The patient was initiated on prednisone 1 mg/kg daily and azathioprine at a dose of 2.5 mg/kg. The patient continued to develop new vesiculobullous lesions and had severe intractable pruritus over the next 6 weeks. She was admitted to the hospital to receive intravenous steroid and antihistamine medications for 10 days. Due to her ongoing symptoms, an ultrasound was performed to rule out additional retained fetal or placental tissue. As she continued to flare, rituximab was administered but had to be discontinued after an anaphylactic response. Omalizumab was trialed but also failed to prevent new bullae formation.
The patient was then commenced on IVIG therapy at 2 g/kg over 3 days every 4 weeks. An improvement of her pruritus and bullae formation was observed after her second IVIG session. One month after commencing treatment, her BPDAI activity score reduced to 48. After 3 months of treatment, her BPDAI activity score reduced to 13 and her pruritus score improved significantly to 9 (Fig. 2). She has continued this course of IVIG therapy over 12 weeks with marked and sustained improvement of her PG.
Discussion
PG is a rare, subepidermal, autoimmune bullous disease that occurs during pregnancy or early postpartum period (Katz et al., 1977). PG is the third most common bullous disease after pemphigus and bullous pemphigoid. PG has an incidence of 1 in 50,000 pregnancies (Jenkins et al., 1999). Pruritus during pregnancy is very common and bile acids should always be checked and cholestasis of pregnancy should be considered as a differential. If these are normal, one potential cause where a rash has not yet appeared is evolving PG. This commonly affects women during the second or third trimester and has a rapid onset of intensely pruritic, urticarial-like patches and plaques with vesiculobullous lesions that affect the periumbilical area and then spread to other areas of the skin (Daneshpazhooh et al., 2012, Jenkins et al., 1999, Schmidt and Zillikens, 2013). The average duration postpartum is 4 to 60 weeks but disease activity has been reported to last up to 12 years postpartum. Hence, some cases of PG behave like bullous pemphigoid.
PG is characterized by protein- and tissue-bound IgG autoantibodies that bind to the NC16A domain of BP180 antigen and is located in the hemidesmosomes of the dermo-epidermal junction, which is the same autoantigen that is found in most cases of bullous pemphigoid. The diagnosis of PG is the identification of a subepidermal blister on histopathology and C3, IgA, IgG, IgM, and fibrinogen deposition in the basement membrane on DIF studies (Intong and Murrell, 2011a, Katz et al., 1977).
Autoantibodies to BP180 have been detected in the first trimester of pregnancy at double the level of pregnant controls but still within the normal range before PG develops clinically, by which time the antibody levels are well above normal. PG has also been reported at least twice before as occurring after termination of pregnancy (Huilaja et al., 2008, Tbatoua et al., 2012). BP180 is present in fetal membranes and promotes the transmigration of trophoblasts from the placenta into the tissue (Huilaja et al., 2015). How the autoimmune response to BP180 occurs is still unknown, but its presentation in the context of paternal major histocompatibility complex has been proposed (Shimanovich et al., 2002).
Treatment depends on the severity of the disease for each patient. The safety and use of these treatments during pregnancy and breastfeeding must be taken into consideration to guide their use. Topical corticosteroid therapy may be sufficient in milder cases but systemic corticosteroid therapy may be necessary in more severe cases. Other management includes antiinflammatory antibiotic medications and immunosuppressive therapy (Intong and Murrell, 2011b, Schmidt and Zillikens, 2013). In cases of PG that do not respond to conventional treatment options or the side effect profile limits conventional options, IVIG therapy has been reported as an effective therapeutic option (Gan et al., 2012, Intong and Murrell, 2011b, Nguyen et al., 2015). IVIG therapy was demonstrated to be effective in our two cases of PG.
The recurrence of PG during subsequent pregnancies is likely and often with an earlier onset and more severe course. Flare ups of the disease have also been reported in patients during menstruation and with oral contraceptive use (Jenkins et al., 1999).
Conclusions
PG is a rare but a differential diagnosis when dealing with pregnant women who present with vesiculobullous lesions is important. This highlights the importance of early recognition, an accurate and timely diagnosis, and successful treatment using IVIG therapy of a rare but distressing autoimmune disease such as PG.
Footnotes
Conflicts of interest: None.
References
- Daneshpazhooh M., Chams-Davatchi C., Payandemehr P., Nassiri S., Valikhani M., Safai-Naraghi Z. Spectrum of autoimmune bullous diseases in Iran: A 10-year review. Int J Dermatol. 2012;51(1):35–41. doi: 10.1111/j.1365-4632.2011.04946.x. [DOI] [PubMed] [Google Scholar]
- Gan D.C., Welsh B., Webster M. Successful treatment of a severe persistent case of pemphigoid gestationis with antepartum and postpartum intravenous immunoglobulin followed by azathioprine. Australas J Dermatol. 2012;53(1):66–69. doi: 10.1111/j.1440-0960.2011.00854.x. [DOI] [PubMed] [Google Scholar]
- Huilaja L., Hurskainen T., Autio-Harmainen H., Hofmann S.C., Sormunen R., Rasanen J. Pemphigoid gestationis autoantigen, transmembrane collagen XVII, promotes the migration of cytotrophoblastic cells of placenta and is a structural component of fetal membranes. Matrix Biol. 2008;27(3):190–200. doi: 10.1016/j.matbio.2007.10.007. [DOI] [PubMed] [Google Scholar]
- Huilaja L., Surcel H.M., Bloigu A., Tasanen K. Elevated serum levels of BP180 antibodies in the first trimester of pregnancy precede gestational pemphigoid and remain elevated for a long time after remission of the disease. Acta Derm Venereol. 2015;95(7):843–844. doi: 10.2340/00015555-2088. [DOI] [PubMed] [Google Scholar]
- Intong L.R.A., Murrell D.F. Pemphigoid gestationis: Current management. Dermatol Clin. 2011;29(4):621–628. doi: 10.1016/j.det.2011.06.013. [DOI] [PubMed] [Google Scholar]
- Intong L.R.A., Murrell D.F. Pemphigoid gestationis: Pathogenesis and clinical features. Dermatol Clin. 2011;29(3):447–452. doi: 10.1016/j.det.2011.03.002. [DOI] [PubMed] [Google Scholar]
- Jenkins R.E., Hern S., Black M.M. Clinical features and management of 87 patients with pemphigoid gestationis. Clin Exp Dermatol. 1999;24(4):255–259. doi: 10.1046/j.1365-2230.1999.00472.x. [DOI] [PubMed] [Google Scholar]
- Katz A., Minto J.O., Toole J.W., Medwidsky W. Immunopathologic study of herpes gestationis in mother and infant. Arch Dermatol. 1977;113(8):1069–1072. [PubMed] [Google Scholar]
- Nguyen T., Alraqum E., Razzaque Ahmed A. Positive clinical outcome with IVIg as monotherapy in recurrent pemphigoid gestationis. Int Immunopharmacol. 2015;26(1):1–3. doi: 10.1016/j.intimp.2015.02.038. [DOI] [PubMed] [Google Scholar]
- Schmidt E., Zillikens D. Pemphigoid diseases. Lancet. 2013;381(9863):320–332. doi: 10.1016/S0140-6736(12)61140-4. [DOI] [PubMed] [Google Scholar]
- Shimanovich I., Bröcker E.B., Zillikens D. Pemphigoid gestationis: New insights into the pathogenesis lead to novel diagnostic tools. BJOG. 2002;109(9):970–976. doi: 10.1111/j.1471-0528.2002.01016.x. [DOI] [PubMed] [Google Scholar]
- Tbatoua F., Studera M., Dellestableb P., Hurietc V., Cunya J.F., Barbauda A. Pemphigoïde gestationnelle post-abortum. Ann Dermatol Venereol. 2012;3751(11):699–787. doi: 10.1016/j.annder.2012.06.047. [DOI] [PubMed] [Google Scholar]
- Werth V.P., Fivenson D., Pandya A.G., Chen D., Rico M.J., Albrecht J. Multicenter randomized, double-blind, placebo-controlled, clinical trial of dapsone as a glucocorticoid-sparing agent in maintenance-phase pemphigus vulgaris. Arch Dermatol. 2008;144(1):25–32. doi: 10.1001/archderm.144.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wijayanti A., Zhao C.Y., Boettiger D., Chiang Y.Z., Ishii N., Hashimoto T. The reliability, validity and responsiveness of two disease scores (BPDAI and ABSIS) for bullous pemphigoid: Which one to use? Acta Derm Venereol. 2017;97(1):24–31. doi: 10.2340/00015555-2473. [DOI] [PubMed] [Google Scholar]