Abstract
Objective
Rheumatoid arthritis (RA) is a multifactorial disease; it leads to disabling and painful chronic inflammatory arthritis. Its onset may be delayed or even prevented by modifying the risk factors involved. Many genetic, epigenetic, and environmental factors are implicated in the pathogenesis of RA. The objectives of this case-control study were to assess various risk factors in our population and to compare the same with age- and sex-matched controls.
Methods
We studied 118 cases with RA diagnosed using the EULAR criteria. In total, 581 age- and sex-matched controls were selected. Each individual was administered a separate questionnaire regarding their risk factors (known risk factors were studied). The implicated dietary factors were incorporated in a food frequency questionnaire (FFQ) and administered to both cases and controls. Comparison was made between those who consume an item at a particular frequency, who consume less, and who consume nothing at all. Among those who consume, each group was re-compared. Statistical analysis was conducted using Statistical Package for Social Sciences (IBM Corp.; Armonk, NY, USA).
Results
There was significant relationship for family history, periodontitis, history of chikungunya, and sun exposure (p<0.05). Association with various food items was studied using the FFQ, but the relationship was inconsistent, probably due to consumption of modified diet by the persons with RA. Also, a majority of cases were females and nonsmokers for assessing an association with smoking habits.
Conclusion
In our population, previous infections (e.g., chikungunya and poor oral hygiene with periodontitis) were the prominently observed risk factors. Also, smoking was less common among women, and probably contributed less, as majority of cases were females. For dietary pattern association, a prospective cohort study may be needed.
Keywords: Rheumatoid arthritis, risc factors, periodontitis, chikungunya, environmental
Introduction
Rheumatoid arthritis (RA) is a disabling and painful chronic inflammatory arthritis that can lead to extreme functional disability if not adequately treated; it is also the second commonest disease after to osteoarthritis. It is a multisystem inflammatory disease primarily affecting synovial joints. There are several genetic, epigenetic, and environmental factors implicated in the pathogenesis of RA. These factors vary in different populations, and there are several studies from different populations relating these pathogenic factors, both negative and positive. This suggests that RA is a multifactorial disease, the onset of which may be delayed or even prevented by modifying the risk factors involved. Also, several risk factors, such as genetic factors, may not be modifiable. Many newer risk factors with variable effects are being proposed.
Autoantibodies and cytokines can develop many years prior to the diagnosis of RA. Thus, clinical RA develops in phases. There is an asymptomatic phase of genetic risk over which environmental exposures occur, followed by an immune activation phase, which can be asymptomatic. Autoantibodies and inflammatory markers are found in the serum. This phase may be followed by a phase of active disease, which starts as minor articular symptoms and later results in frank arthritis. Initially, arthritis in many cases is unclassifiable according to the 2010 ACR/European League against Rheumatism (EULAR) criteria. With time, the disease evolves and can then be classified as RA by established criteria (1). Evolution of disease from undifferentiated inflammatory polyarthritis to classical RA may take up to 5 years (2). Thus, a genetically susceptible individual when exposed to environmental risk factors, it triggers autoimmunity with autoantibody production, and further exposures can cause further immune dysregulation and ultimately symptomatic inflammatory arthritis. Many have a long preclinical phase during which autoantibodies develop, and this is the time where we can modify the risk factors and further delay progression. Many environmental and dietary factors influence the disease onset. The environmental factors implicated include smoking habits, vitamin D levels, periodontitis, and previous infection such as chikungunya. Dietary factors include W3 fatty acids, salt intake, fruits and vegetables, coffee and tea, some with predisposition and others with protection. The objective of the present study was to assess different etiologic factors and their association with RA in our population in Calicut, Kerala, India.
Genetic factors
Having a first-degree relative with RA increases the RA risk by 3- to 9-folds compared with that of the general population. It may be due to the influence of shared genetic and/or environmental factors (3). More than 30 novel risk loci are identified by genome association studies in addition to well-established associations with the HLA-DRB1 “shared epitope” (HLA-SE) alleles (4). Interactions between HLA-SE and potentially other risk alleles and environmental factors may contribute to RA pathogenesis (5–7). Models that incorporate genetic, serologic, and lifestyle/environmental factors can predict the risk of developing RA more precisely than any of these factors alone (6, 8).
Environmental factors
Smoking
Smoking is the most studied and implicated preventable risk factor for RA, as proven by many twin, case-control, and cohort studies (9–25). This association was consistent for RF+ RA for male and female smokers, as observed in a meta-analysis (26). A dose-dependent effect of pack-years of smoking was suggested by some studies with a population-attributable risk of 18%–25% (22, 23). Risk for RA exponentially increases among smokers as it interacts with all alleles of the SE (26). There may be geographical variations in the association between smoking, ACPA, and RA, as some studies are against specificity for ACPA+ RA (27–29). Passive smoking and smokeless tobacco do not seem to increase the risk of RA (22, 30).
Periodontitis
Periodontitis is implicated as a risk factor for the development of many immune-related chronic inflammatory diseases. Oral bacteria, such as Porphyromonas gingivalis, are strong markers of disease status. Many studies have shown the association of RA with periodontitis. The role of citrullination and subsequent autoantibody responses as well as the role of mediators released as a result of bacterial colonization have been proposed in the pathogenesis of RA (31, 32). Influx of oxygen-consuming inflammatory cells and the overproduction of reactive oxygen species leads to posttranslational modification (33, 34). There is also enzymatic posttranslational modification (cleavage of extracellular proteins by matrix metalloproteases, bacterial proteases, or citrullination by PAD enzymes (35–37). P. gingivalis expresses both arginine-specific proteases and PAD and may be linked to the inflammatory process in RA (33, 38).
There appears an increased prevalence of periodontitis and a higher rate of tooth loss in patients with RA in comparison with that in the general population, as suggested by several studies (39–45). RA may also be more prevalent among patients with periodontitis (42, 46). Higher antibody titers against P. gingivalis and positive correlation with ACPA have been reported in patients with RA (46, 47).
Vitamin D
Vitamin D deficiency is implicated in the pathogenesis of several autoimmune conditions. It’s role as an immunoregulatory hormone also is documented. Many dietary intake-based prospective studies have failed to show any association between vitamin D and RA, except for the Iowa WHS (48–51). Dietary intake is not the sole determinant of vitamin D status, because it may also be affected by latitude and sunlight exposure. Even with serum vitamin D3 levels, some studies have failed to show any association (52).
Infections and RA
Many infections, particularly those with articular manifestations, such as Epstein Barr virus, Parvo virus B19, chikungunya virus, and some bacteria, have been implicated as risk factors for RA. Mechanisms by which this predisposition is different in different situations. For example, in case of Epstein Barr virus, it is probably gp110 molecular mimicry; in case of Parvo virus B19, it is alteration of function of FLS; and in certain bacterial infections, it is the cell wall mediated TLR 2 activation which is implicated.
Frequent outbreaks of chikungunya fever with polyarthralgia have been observed, which is also proposed as a risk factor for the future development of RA, as suggested by human as well as animal studies (53–55).
Meat
A diet high in protein is considered an etiology of RA (56). However, many studies are also in the favor of a low-protein diet leading to the improvement of RA symptoms (57–60). The prevalence of RA is higher in countries with greater consumption of red meat (61). Pattison et al. (62) reported the first prospective investigation of red meat and risk for inflammatory polyarthritis and showed that higher intakes of both red meat and total protein increased the risk for IP, whereas meat iron showed no association. However, there are also some studies that have failed to show any association. For example, a large prospective cohort study conducted among subjects of the Nurses’ Health Study failed to demonstrate any association (63).
High-salt diet
Studies in animal models and on human cells have shown an effect of sodium chloride (NaCl) on TH17 cells in promoting inflammation, which may be the reason for its proposed association with RA. However, this association was seen in some studies that were conducted only among smokers with RA (64).
Fruits and vegetables
Consumption of fruits and vegetables has several health benefits, which may be largely related to their antioxidant properties. Vitamins C and E, lycopene, carotenoids, flavonoids, and possibly fructose-mediated urate production are probably important in this regard (65). There are also some studies that suggest that vitamin C and β-kryptoxanthin (a carotenoid) have protective roles, and some studies concerning dietary zinc supplements also exist (66–69). Some studies found no benefit of antioxidants, despite testing multiple parameters of antioxidant content (70, 71). Some observational studies examined whole foods, showing a protective role (72).
Omega-3 fatty acids and fish
Several of studies have shown that omega-3 fatty acids decrease the severity of RA or have a preventive role (73–82). Olive oil consumption is one among the best studied omega-3 fatty acids, and fish oil has proven benefits (83). The prospective Danish Diet, Cancer, and Health population-based cohort study of RA failed to confirm a protective effect of olive oil; however, the consumption of oily fish did, show a trend toward a protective effect (70).
Methods
The present study was a case-control study. Patients who were diagnosed with RA and who satisfied the diagnostic criteria when they visited our OPD were included, whereas age- and sex-matched individuals without RA, irrespective of other parameters and comorbidities, were used as controls.
Informed consent was obtained from all participants. The study was approved by the Institutional Ethics Committee of the KMCT Medical College.
A total of 118 cases and 581 controls were included. Of the 118 cases, 28% were older than 60 y, 57% were aged between 40 and 59 y, and 15% were younger than 40 y. Also, 87% were females and the remaining 13% were males. Almost 29% of the cases had a disease duration of more than 10 y, 33% between 5 and 10 y, and 38% less than 5 y. All patients were under standard treatment for RA with drugs, such as methotrexate, steroids, leflunomide, hydroxychloroquine, and NSAIDs; other comorbidities, such as diabetes, were less common among the cases than the controls. Each individual was administered a separate questionnaire regarding their risk factors (known risk factors were studied). The implicated dietary factors were incorporated in a food frequency questionnaire (FFQ) and were administered to both cases and controls. Comparison was made between those who consume an item at a particular frequency, those who consume less, and those who consume nothing at all. Among those who consume, each group was re-compared. Statistical analysis was conducted using SPSS.
Results
Genetic factors
In the present study, among the 118 cases, 15 (13%) had first-degree relatives with RA, two (0.02%) had second-degree relatives, and only 1 (0.01%) had a third-degree relative with RA. Among the controls, 15 (0.03%) had first-degree relatives, none had second-degree relatives, and two had third-degree relatives (Table 1). The p value was significant, showing a strong positive association, with an OR of 7.64.
Table 1.
Family history
| Cases | Controls | p | |
|---|---|---|---|
| Nil | 100 | 564 | 0.000 |
| First degree | 15 | 15 | |
| Second degree | 2 | 0 | |
| Third degree | 1 | 2 |
Smoking
In our study group, majority of the cases were women and all were nonsmokers. Hence, the association could not be established.
Periodontitis
We compared the incidence of periodontitis among those with RA and an age- and sex-matched general population. The presence of periodontitis was assessed by personal interview and dental examination using probing pocket depth and clinical attachment loss. The extent of periodontitis was not graded. A total of 118 patients with RA and 581 age- and sex-matched controls were observed. Among these cases, 46 had periodontitis (39%); however, among the 581 controls, only 61 (11.73%), with p<0.05 and an OR of 5.84, showed a strong positive association between RA and periodontitis (Table 2).
Table 2.
Periodontitis
| p | ||
|---|---|---|
| Cases | 39% | 0.000 |
| controls | 11.73% |
Sun exposure
We assessed the average sun exposure for at least 1 hour per day in the open sun and the usage of covered clothes in the sun. We compared these results with those of controls (Tables 3 and 4).
Table 3.
Sun exposure
| Cases | Controls | p | |
|---|---|---|---|
| No | 20 | 93 | 0.000 |
| Occasionally | 86 | 264 | |
| Rarely | 12 | 224 |
Table 4.
Wearing covered clothes
| Cases | Controls | p | |
|---|---|---|---|
| No | 78 | 347 | 0.000 |
| Usually | 26 | 117 | |
| occasionally | 14 | 103 | |
| Rarely | 0 | 14 |
Sun exposure and RA were negatively associated, which was statistically significant with (p<0.05). The comparison was based on sun exposure as well as the usage of covered clothes on a routine basis (OR=1.13). This association may be related to vitamin D, compounded by other factors like exercise.
History of chikungunya
We compared the clinical history of chikungunya in patients with RA and in controls. History of chikungunya was assessed by IgM positivity and typical symptoms during the epidemic of chikungunya as per hospital records. There was a significant association between the history of chikungunya and RA. Among all cases, there were 32.2% individuals with a history of chikungunya, whereas among controls, there were only 4.48% with a positive history, the p value being significant (Table 5) and OR of 8.73.
Table 5.
h/o Chikungunya
| Present | Absent | p | |
|---|---|---|---|
| Cases | 32.2% | 67.8% | 0.000 |
| controls | 04.48% | 95.52% |
Meat consumption
We administered separate questionnaires for cases and controls regarding meat consumption, as frequency of servings taken. In our study, we could not find any positive association between meat consumption and RA. Actually, there was a negative correlation (Fig. 1), with an OR<1. The probable reason was that many of our patients with RA were long-term RA patients and were advised to take less amount of meat and a majority of patients were even following this. Thus, this negative association could not be taken as a real one, although the p value was significant.
Figure 1.
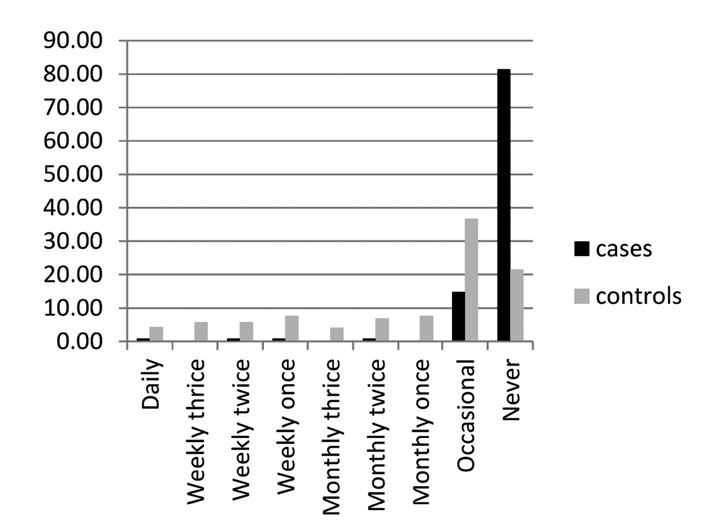
Data on meat consumption (plotted as percentage)
Salt intake
The assessment of salt intake was difficult in our form of dietary practice, and we assessed it using the questionnaire related to excess salt intake in the form of specific food items: salted fish and meat, papads (a food item with high salt content), and pickles, according to number of servings per day/week/month. The comparison did not show any predisposition with salt intake; however, RA was less common in this group with a significant p value (Figs. 2–4). OR also showed a negative correlation. This may not be the real scenario as many of the patients with RA were under strict diet restrictions and low salt intake. To assess this association, a prospective cohort study is probably needed.
Figure 2.
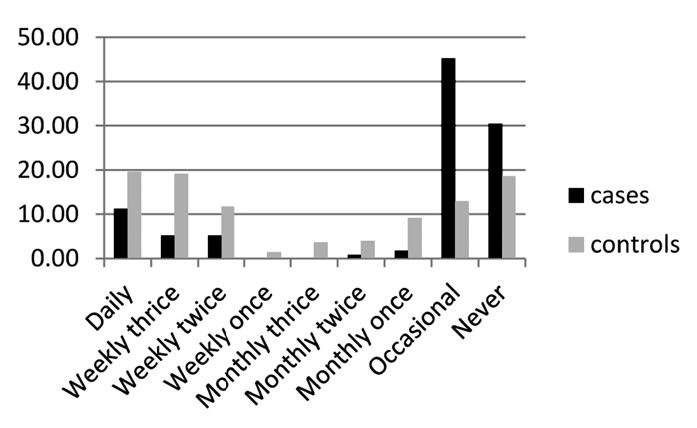
Data on pickles and salted food items (plotted as percentage)
Figure 3.
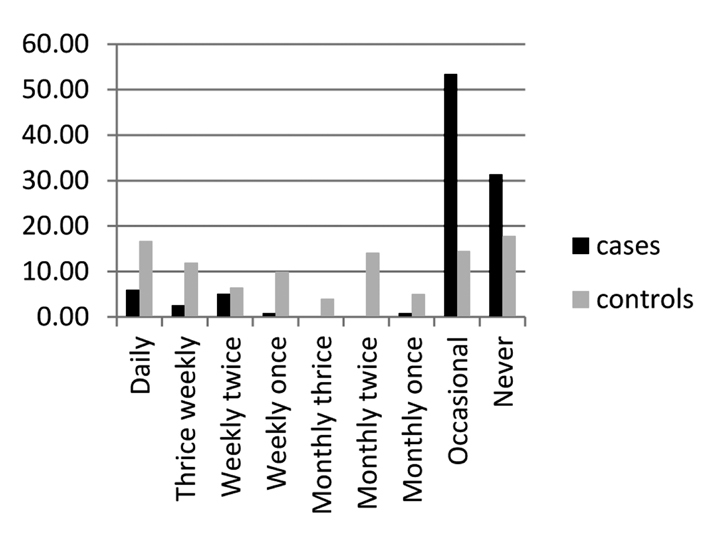
Data on use of papads (plotted as percentage)
Figure 4.
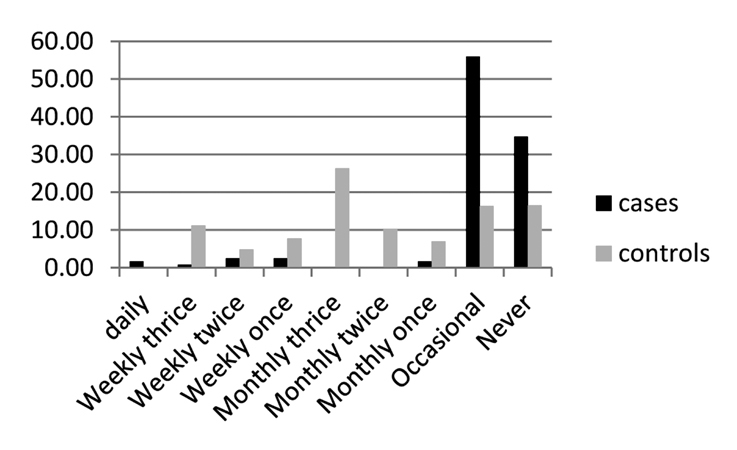
Data on salted dry fish (plotted as percentage)
Vegetables and fruits
By personal interview, the average intake of fruits and vegetables was separately assessed. Consumption among cases and controls was grouped as number of servings per day/week/month. Each group was then compared with the rest of the population and also with the exposed group (Figure 5).
Figure 5.
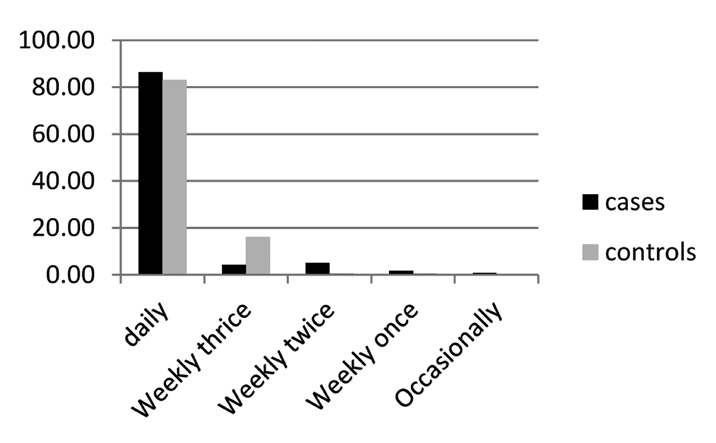
Data on use of vegetables (plotted as percentage)
The association was inconsistent through the group. There appears to be a protective role for vegetables, because the consumption increases with a significant p value; however, this association was not consistent for green leafy vegetables (Table 6).
Table 6.
Vegetable intake
| Daily | 102/118 | 482/581 | 0.353 |
|---|---|---|---|
| Weekly thrice | 5/118 | 93/581 | 0.000 |
| Weekly twice | 6/118 | 3/581 | 0.455 |
| Weekly once | 2/118 | 3/581 | |
| Occasionally | 1/118 |
There also appears a trend toward protective roles for fruit; however, the association was not statistically significant (Table 7).
Table 7.
Fruit intake
| Cases % | Controls % | p (comparison with less/non exposed) | p (comparison among exposed) | |
|---|---|---|---|---|
| D3 | 0 | 1 | 0.318 | 0.020 |
| D2 | 0 | 4 | 0.011 | |
| D1 | 22 | 22 | 0.992 | |
| W3 | 25 | 28 | 0.278 | 0.270 |
| W2 | 25 | 12 | 0.001 | 0.001 |
| W1 | 6 | 4 | 0.176 | 0.581 |
| M3 | 0 | 4 | 0.027 | 0.009 |
| M2 | 3 | 5 | 0.362 | 0.135 |
| M1 | 2 | 1 | 0.397 | 0.426 |
| O | 13 | 10 | 0.042 | 0.277 |
| N | 4 | 9 | 0.049 | 0.037 |
D3: thrice daily; D2: twice daily; D1: once daily; W3: thrice weekly; W2: twice weekly; W1: once weekly; M3: thrice monthly; M2: twice monthly; M1: once monthly; O: occasional; N: never (all in terms of at least one serving)
Fish consumption
We studied fish consumption among the patients with RA and age-matched controls as the number of servings per day/month/week. Regarding fish, there was a stronger association throughout the group, with a more significant p value (Table 8). Comparison was made on the basis of daily consumption as well as whether consuming or not. Either way, a positive relationship was observed (OR=1.4 and 1.43, respectively).
Table 8.
Fish consumption
| Cases % | Controls % | p (comparison with less/non exposed) | p (comparison among exposed) | |
|---|---|---|---|---|
| D3 | 0 | 1 | 0.318 | 0.020 |
| D2 | 0 | 4 | 0.011 | |
| D1 | 22 | 22 | 0.992 | |
| W3 | 25 | 28 | 0.278 | 0.270 |
| W2 | 25 | 12 | 0.001 | 0.001 |
| W1 | 6 | 4 | 0.176 | 0.581 |
| M3 | 0 | 4 | 0.027 | 0.009 |
| M2 | 3 | 5 | 0.362 | 0.135 |
| M1 | 2 | 1 | 0.397 | 0.426 |
| O | 13 | 10 | 0.042 | 0.277 |
| N | 4 | 9 | 0.049 | 0.037 |
D3: thrice daily; D2: twice daily; D1: once daily; W3: thrice weekly; W2: twice weekly; W1: once weekly; M3: thrice monthly; M2: twice monthly; M1 once monthly; O: occasional; N: never (all in terms of at least one serving)
Oily food and fried items
Consumption of oily food and fried bakery items was also studied in the same manner. Majority of the population used vegetable oil for cooking, which also included coconut oil (Tables 9 and 10). In fact, there was a negative association with OR<1. This may be due to modified long-term dietary habits followed by patients on the physician’s advice.
Table 9.
Consumption of bakery items
| Cases % | Controls % | p (comparison with less/non exposed) | p (comparison among exposed) | |
|---|---|---|---|---|
| D3 | 0.5 | 0.277 | 0.536 | |
| D2 | 0.9 | 0.353 | ||
| D1 | 4.3 | 5.7 | 0.361 | |
| W3 | 0.9 | 24.6 | 0.000 | 0.000 |
| W2 | 3.4 | 8.6 | 0.000 | 0.032 |
| W1 | 6.9 | 4.6 | 0.937 | 0.035 |
| M3 | 3.6 | 0.003 | 0.012 | |
| M2 | 1.7 | 2.8 | 0.136 | 0.400 |
| M1 | 0.9 | 9.5 | 0.000 | 0.001 |
| O | 62.9 | 11 | 0.000 | 0.000 |
| N | 19 | 28 | 0.039 | 0.032 |
D3: thrice daily; D2: twice daily; D1: once daily; W3: thrice weekly; W2: twice weekly; W1: once weekly; M3: thrice monthly; M2: twice monthly; M1 once monthly; O: occasional; N: never (all in terms of at least one serving)
Table 10.
Oily food/fried item intake
| Cases % | Controls % | p (comparison with less/non exposed) | p (comparison among exposed) | |
|---|---|---|---|---|
| D3 | 0.2 | 0.000 | 0.000 | |
| D2 | 11.2 | 0.000 | ||
| D1 | 17.9 | 36.8 | 0.000 | |
| W3 | 5.1 | 22.5 | 0.000 | 0.211 |
| W2 | 5.1 | 9.3 | 0.000 | 0.193 |
| W1 | 3.4 | 1.9 | 0.164 | 0.713 |
| M3 | 0.3 | 0.321 | 0.077 | |
| M2 | 2.6 | 0.000 | 0.080 | |
| M1 | 2.6 | 0.7 | 0.553 | 0.000 |
| O | 47.9 | 5 | 0.000 | 0.008 |
| N | 17.9 | 9.5 | 0.007 | 0.000 |
D3: thrice daily; D2: twice daily; D1: once daily; W3: thrice weekly; W2: twice weekly; W1: once weekly; M3: thrice monthly; M2: twice monthly; M1 once monthly; O: occasional; N: never (all in terms of at least one serving)
In conclusion, omega-3/PUFAs have an inconsistent association as obtained from daily oil usage; however, fish intake, particularly on a daily basis, has shown a protective effect against RA. Other parameters investigated were tea, coffee, alcohol, pan chewing, egg, milk, etc. These variables did not show any association with RA.
Discussion
There is a positive association with a family history of RA among close relatives as seen in other studies. Regarding the environmental factors studied, there is a definite correlation with the history of chikungunya. Another association was obtained with sun exposure, which is negative. Vitamin D levels were not assessed. Periodontitis was another factor found to be significantly associated with RA.
Regarding the food items studied, the consumption of vegetables and fruits seems to have a protective role; however, the significance is inconsistent. Another factor with a protective effect was fish intake.
This study was not supported by any financial aid. The authors report no conflicts of interest.
Study limitations
Genetic and environmental factors were easily comparable between the groups; however, many of the patients were long-term RA patients and it was difficult to obtain their dietary habits prior to disease development. Rather, many of them were advised by the treating clinician regarding the dietary modifications and the need to maintain an ideal body weight. Thus, many reported modified sorts of dietary habits. This was probably the reason for the lack of association with many dietary elements. For obtaining such details, a prospective cohort study is needed.
Footnotes
Ethics Committee Approval: Ethics committee approval was received for this study from the Ethics Committee of the KMCT Medical College.
Informed Consent: Written informed consent was obtained all the patients who participated in this study.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept - B.J.P.; Design - R.P.; Supervision - B.J.P.; Resources - R.P.; Materials - R.P.; Data Collection and/or Processing R.P.; Analysis and/or Interpretation R.P.; Literature Review R.P., B.J.P.; Writing Manuscript - B.J.P., R.P.; Critical Review - R.P.
Conflict of Interest: The authors have no conflict of interest to declare.
Financial Disclosure: The authors declared that this study has received no financial support.
References
- 1.Aletaha D, Neogi T, Silman AJ, Funovits J, Felson DT, Bingham CO. Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum. 2010;62:2569–81. doi: 10.1002/art.27584. [DOI] [PubMed] [Google Scholar]
- 2.Symmons DP, Silman AJ. The Norfolk Arthritis Register (NOAR) Clin Exp Rheumatol. 2003;21:S94–9. [PubMed] [Google Scholar]
- 3.Hemminki K, Li X, Sundquist J, Sundquist K. Familial associations of rheumatoid arthritis with autoimmune diseases and related conditions. Arthritis Rheum. 2009;60:661–8. doi: 10.1002/art.24328. [DOI] [PubMed] [Google Scholar]
- 4.Stahl EA, Raychaudhuri S, Remmers EF, Xie G, Eyre S, Thomson BP. Genome-wide association study meta-analysis identifies seven new rheumatoid arthritis risk loci. Nat Genet. 2010;42:508–14. doi: 10.1038/ng.582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Costenbader KH, Chang SC, De Vivo I, Plenge R, Karlson EW. Genetic polymorphisms in PTPN22, PADI-4, and CTLA-4 and risk for rheumatoid arthritis in two longitudinal cohort studies: evidence of gene-environment interactions with heavy cigarette smoking. Arthritis Res Ther. 2008;10:R52. doi: 10.1186/ar2421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Karlson EW, Chibnik LB, Kraft P, Cui J, Keenan BT, Ding B, et al. Cumulative association of 22 genetic variants with seropositive rheumatoid arthritis risk. Ann Rheum Dis. 2010;69:1077–85. doi: 10.1136/ard.2009.120170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mahdi H, Fisher BA, Kallberg H, Plant D, Malmström V, Rönnelid J, et al. Specific interaction between genotype, smoking and autoimmunity to citrullinated alpha-enolase in the etiology of rheumatoid arthritis. Nat Genet. 2009;41:1319–24. doi: 10.1038/ng.480. [DOI] [PubMed] [Google Scholar]
- 8.Karlson EW, Chang SC, Cui J, Chibnik LB, Fraser PA, De Vivo I, et al. Gene-environment interaction between HLA-DRB1 shared epitope and heavy cigarette smoking in predicting incident rheumatoid arthritis. Ann Rheum Dis. 2010;69:54–60. doi: 10.1136/ard.2008.102962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Silman AJ, Newman J, MacGregor AJ. Cigarette smoking increases the risk of rheumatoid arthritis. Results from a nationwide study of disease-discordant twins. Arthritis Rheum. 1996;39:732–5. doi: 10.1002/art.1780390504. [DOI] [PubMed] [Google Scholar]
- 10.Hazes JM, Dijkmans BA, Vandenbroucke JP, deVries RR, Cats A. Lifestyle and the risk of rheumatoid arthritis: cigarette smoking and alcohol consumption. Ann Rheum Dis. 1990;49:980–2. doi: 10.1136/ard.49.12.980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hutchinson D, Shepstone L, Moots R, Lear JT, Lynch MP. Heavy cigarette smoking is strongly associated with rheumatoid arthritis (RA), particularly in patients without a family history of RA. Ann Rheum Dis. 2001;60:223–7. doi: 10.1136/ard.60.3.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Krishnan E. Smoking, gender and rheumatoid arthritis-epidemiological clues to etiology. Results from the behavioral risk factor surveillance system. Joint Bone Spine. 2003;70:496–502. doi: 10.1016/S1297-319X(03)00141-6. [DOI] [PubMed] [Google Scholar]
- 13.Krishnan E, Sokka T, Hannonen P. Smoking-gender interaction and risk for rheumatoid arthritis. Arthritis Res Ther. 2003;5:R158–62. doi: 10.1186/ar750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Olsson AR, Skogh T, Wingren G. Comorbidity and lifestyle, reproductive factors, and environmental exposures associated with rheumatoid arthritis. Ann Rheum Dis. 2001;60:934–9. doi: 10.1136/ard.60.10.934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Olsson AR, Skogh T, Wingren G. Aetiological factors of importance for the development of rheumatoid arthritis. Scand J Rheumatol. 2004;33:300–6. doi: 10.1080/03009740310004748. [DOI] [PubMed] [Google Scholar]
- 16.Padyukov L, Silva C, Stolt P, Alfredsson L, Klareskog L. A gene-environment interaction between smoking and shared epitope genes in HLA-DR provides a high risk of seropositive rheumatoid arthritis. Arthritis Rheum. 2004;50:3085–92. doi: 10.1002/art.20553. [DOI] [PubMed] [Google Scholar]
- 17.Stolt P, Bengtsson C, Nordmark B, Lindblad S, Lundberg I, Klareskog L, et al. Quantification of the influence of cigarette smoking on rheumatoid arthritis: results from a population-based case-control study, using incident cases. Ann Rheum Dis. 2003;62:835–41. doi: 10.1136/ard.62.9.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Symmons DP, Bankhead CR, Harrison BJ, Brennan P, Barrett EM, Scott DG, et al. Blood transfusion, smoking, and obesity as risk factors for the development of rheumatoid arthritis: results from a primary care-based incident case-control study in Norfolk, England. Arthritis Rheum. 1997;40:1955–61. doi: 10.1002/art.1780401106. [DOI] [PubMed] [Google Scholar]
- 19.Uhlig T, Hagen KB, Kvien TK. Current tobacco smoking, formal education, and the risk of rheumatoid arthritis. J Rheumatol. 1999;26:47–54. [PubMed] [Google Scholar]
- 20.Voigt LF, Koepsell TD, Nelson JL, Dugowson CE, Daling JR. Smoking, obesity, alcohol consumption, and the risk of rheumatoid arthritis. Epidemiology. 1994;5:525–32. [PubMed] [Google Scholar]
- 21.Vessey MP, Villard-Mackintosh L, Yeates D. Oral contraceptives, cigarette smoking and other factors in relation to arthritis. Contraception. 1987;35:457–64. doi: 10.1016/0010-7824(87)90082-5. [DOI] [PubMed] [Google Scholar]
- 22.Costenbader KH, Feskanich D, Mandl LA, Karlson EW. Smoking intensity, duration, and cessation, and the risk of rheumatoid arthritis in women. Am J Med. 2006;119:503–9. doi: 10.1016/j.amjmed.2005.09.053. [DOI] [PubMed] [Google Scholar]
- 23.Criswell LA, Merlino LA, Cerhan JR, Mikuls TR, Mudano AS, Burma M, et al. Cigarette smoking and the risk of rheumatoid arthritis among postmenopausal women: results from the Iowa Women’s Health Study. Am J Med. 2002;112:465–71. doi: 10.1016/S0002-9343(02)01051-3. [DOI] [PubMed] [Google Scholar]
- 24.Heliövaara M, Aho K, Aromaa A, Knekt P, Reunanen A. Smoking and risk of rheumatoid arthritis. J Rheumatol. 1993;20:1830–5. [PubMed] [Google Scholar]
- 25.Karlson EW, Lee IM, Cook NR, Manson JE, Buring JE, Hennekens CH. A retrospective cohort study of cigarette smoking and risk of rheumatoid arthritis in female health professionals. Arthritis Rheum. 1999;42:910–7. doi: 10.1002/1529-0131(199905)42:5<910::AID-ANR9>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 26.Klareskog L, Stolt P, Lundberg K, Bengtsson C, Grunewald J, Rönnelid J, et al. A new model for an etiology of rheumatoid arthritis: smoking may trigger HLA-DR (shared epitope)-restricted immune reactions to autoantigens modified by citrullination. Arthritis Rheum. 2006;54:38–46. doi: 10.1002/art.21575. [DOI] [PubMed] [Google Scholar]
- 27.Lee HS, Irigoyen P, Kern M, Lee A, Batliwalla F, Lee A, et al. Interaction between smoking, the shared epitope, and anti-cyclic citrullinated peptide: a mixed picture in three large North American rheumatoid arthritis cohorts. Arthritis Rheum. 2007;56:1745–53. doi: 10.1002/art.22703. [DOI] [PubMed] [Google Scholar]
- 28.Bang SY, Lee KH, Cho SK, Lee HS, Lee KW, Bae SC. Smoking increases rheumatoid arthritis susceptibility in individuals carrying the HLA-DRB1 shared epitope, regardless of rheumatoid factor or anti-cyclic citrullinated peptide antibody status. Arthritis Rheum. 2010;62:369–77. doi: 10.1002/art.27272. [DOI] [PubMed] [Google Scholar]
- 29.Mikuls TR, Sayles H, Yu F, Levan T, Gould KA, Thiele GM, et al. Associations of cigarette smoking with rheumatoid arthritis in African Americans. Arthritis Rheum. 2010;62:3560–8. doi: 10.1002/art.27716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Carlens C, Hergens MP, Grunewald J, Ekbom A, Höglund CO, Askling J, et al. Smoking, use of moist snuff, and risk of chronic inflammatory diseases. Am J Respir Crit Care Med. 2010;181:1217–22. doi: 10.1164/rccm.200909-1338OC. [DOI] [PubMed] [Google Scholar]
- 31.Agnihotri R, Gaur S. Rheumatoid arthritis in the elderly and its relationship with periodontitis: are view. Geriatr Gerontol Int. 2014;14:8–22. doi: 10.1111/ggi.12062. [DOI] [PubMed] [Google Scholar]
- 32.Bingham CO, 3rd, Moni M. Periodontal disease and rheumatoid arthritis: the evidence accumulates for complex pathobiologic interactions. Curr Opin Rheumatol. 2013;25:345–53. doi: 10.1097/BOR.0b013e32835fb8ec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hendler A, Mulli TK, Hughes FJ, Perrett D, Bombardieri M, Houri-Haddad Y, et al. Involvement of autoimmunity in the pathogenesis of aggressive periodontitis. J Dent Res. 2010;89:1389–94. doi: 10.1177/0022034510381903. [DOI] [PubMed] [Google Scholar]
- 34.Waddington RJ, Moseley R, Embery G. Reactive oxygen species: a potential role in the pathogenesis of periodontal diseases. Oral Dis. 2000;6:138–51. doi: 10.1111/j.1601-0825.2000.tb00325.x. [DOI] [PubMed] [Google Scholar]
- 35.Murphy G, Nagase H. Reappraising metalloproteinasesin rheumatoid arthritis and osteoarthritis: destruction or repair? Nat Clin Pract Rheumatol. 2008;4:128–35. doi: 10.1038/ncprheum0727. [DOI] [PubMed] [Google Scholar]
- 36.Moelants EA, Loozen G, Mortier A, Martens E, Opdenakker G, Mizgalska D, et al. Citrullination and proteolytic processing of chemokines by Porphyromonas gingivalis. Infect Immun. 2014;82:2511–19. doi: 10.1128/IAI.01624-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.van Venrooij WJ, Vossenaar ER, Zendman AJ. Anti-CCP antibodies: the new rheumatoid factorin the serology of rheumatoid arthritis. Autoimmun Rev. 2004;1:S17–9. [PubMed] [Google Scholar]
- 38.McGraw WT, Potempa J, Farley D, Travis J. Purification, characterization, and sequence analysis of a potential virulence factor from Porphyromonasgingivalis, peptidylargininedeiminase. Infect Immun. 1999;67:3248–56. doi: 10.1128/iai.67.7.3248-3256.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.de Pablo P, Dietrich T, McAlindon TE. Association of periodontal disease and tooth loss with rheumatoid arthritis in the US population. J Rheumatol. 2008;35:70–6. [PubMed] [Google Scholar]
- 40.Dissick A, Redman RS, Jones M, Rangan BV, Reimold A, Griffiths GR, et al. Association of periodontitis with rheumatoid arthritis: apilot study. J Periodontol. 2010;81:223–30. doi: 10.1902/jop.2009.090309. [DOI] [PubMed] [Google Scholar]
- 41.Rosamma J, Sreeraj R, Sameera GN, Binoy JP. Association between chronic periodontitis and rheumatoid arthritis: a hospital based case-control study. Rheumatol Int. 2013;33:103–9. doi: 10.1007/s00296-011-2284-1. [DOI] [PubMed] [Google Scholar]
- 42.Tolo K, Jorkjend L. Serum antibodies and loss of periodontal bone in patients with rheumatoid arthritis. J Clin Periodontol. 1990;17:288–91. doi: 10.1111/j.1600-051X.1990.tb01091.x. [DOI] [PubMed] [Google Scholar]
- 43.Mercado FB, Marshall RI, Klestov AC, Bartold PM. Relationship between rheumatoid arthritis and periodontitis. J Periodontol. 2001;72:779–87. doi: 10.1902/jop.2001.72.6.779. [DOI] [PubMed] [Google Scholar]
- 44.Mercado F, Marshall RI, Klestov AC, Bartold PM. Is there a relationship between rheumatoid arthritis and periodontal disease? J Clin Periodontol. 2000;27:267–72. doi: 10.1034/j.1600-051x.2000.027004267.x. [DOI] [PubMed] [Google Scholar]
- 45.Nesse W, Abbas F, van der Ploeg I, Spijkervet FK, Dijkstra PU, Vissink A. Periodontal inflamed surface area: quantifying inflammatory burden. J Clin Periodontol. 2008;35:668–73. doi: 10.1111/j.1600-051X.2008.01249.x. [DOI] [PubMed] [Google Scholar]
- 46.McGraw WT, Potempa J, Farley D, Travis J. Purification, characterization, and sequence analysis of a potential virulence factor from porphyromonasgingivalis, peptidylarginine deiminase. Infect Immun. 1999;67:3248–56. doi: 10.1128/iai.67.7.3248-3256.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rosenstein ED, Greenwald RA, Kushner LJ, Weissmann G. Hypothesis: the humoral immune response to oral bacteria provides astimulus for the development of rheumatoid arthritis. Inflammation. 2004;28:311–18. doi: 10.1007/s10753-004-6641-z. [DOI] [PubMed] [Google Scholar]
- 48.Kostoglou-Athanassiou I, Athanassiou P, Lyraki A, Raftakis I, Antoniadis C. Vitamin D and rheumatoid arthritis. Ther Adv Endocrinol Metab. 2012;3:181–7. doi: 10.1177/2042018812471070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–81. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 50.Merlino LA, Curtis J, Mikuls TR, Cerhan JR, Criswell LA, Saag KG. Vitamin D intake is inversely associated with rheumatoid arthritis: results from the Iowa Women’s Health Study. Arthritis Rheum. 2004;50:72–7. doi: 10.1002/art.11434. [DOI] [PubMed] [Google Scholar]
- 51.Costenbader KH, Feskanich D, Holmes M, Karlson EW, Benito-Garcia E. Vitamin D intake and risks of systemic lupus erythematosus and rheumatoid arthritis in women. Ann Rheum Dis. 2008;67:530–5. doi: 10.1136/ard.2007.072736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nielen MM, van Schaardenburg SD, Lems WF, van de Stadt RJ, de Koning MH, Reesink HW, et al. Vitamin D deficiency does not increase the risk of rheumatoid arthritis: comment on the article by Merlino et al. Arthritis Rheum. 2006;54:3719–20. doi: 10.1002/art.22191. [DOI] [PubMed] [Google Scholar]
- 53.Bouquillard E, Combe B. Rheumatoid arthritis after Chikungunya fever: a prospective follow-up study of 21 cases. Ann Rheumatic Dis. 2009;68:1505–6. doi: 10.1136/ard.2008.097626. [DOI] [PubMed] [Google Scholar]
- 54.Nakaya HI, Gardner J, Poo YS, Major L, Pulendran B, Suhrbier A. Gene profiling of Chikungunya virus arthritis in a mouse model reveals significant overlap with rheumatoid arthritis. Arthritis Rheum. 2012;64:3553–63. doi: 10.1002/art.34631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Binoy JP, Geetha P, Shanu PM, Emil JT. Clinical Profile and Long Term Sequaele of Chikungunya fever. Indian J Rheumatol. 2011;1:12–9. [Google Scholar]
- 56.Buchanan WW, Laurent RM. Rheumatoid arthritis: an example of ecological succession? Can Bull Med Hist. 1990;7:77–91. doi: 10.3138/cbmh.7.1.77. [DOI] [PubMed] [Google Scholar]
- 57.Sköldstam L. Fasting and vegan diet in rheumatoid arthritis. Scand J Rheumatol. 1986;15:219–221. doi: 10.3109/03009748609102091. [DOI] [PubMed] [Google Scholar]
- 58.Kjeldsen-Kragh J, Haugen M, Borchgrevink CF, Laerum E, Eek M, Mowinkel P, et al. Controlled trial of fasting and one-year vegetarian diet in rheumatoid arthritis. Lancet. 1991;338:899–902. doi: 10.1016/0140-6736(91)91770-U. [DOI] [PubMed] [Google Scholar]
- 59.Kjeldsen-Kragh J, Haugen M, Forre O, Laache H, Malt UF. Vegetarian diet for patients with rheumatoid arthritis: can the clinical effects be explained by the psychological characteristics of the patients? Br J Rheumatol. 1994;33:569–75. doi: 10.1093/rheumatology/33.6.569. [DOI] [PubMed] [Google Scholar]
- 60.Nenonen MT, Helve TA, Rauma AL, Hanninen OO. Uncooked, lactobacilli-rich, vegan food and rheumatoid arthritis. Br J Rheumatol. 1998;37:274–81. doi: 10.1093/rheumatology/37.3.274. [DOI] [PubMed] [Google Scholar]
- 61.Grant WB. The role of meat in the expression of rheumatoid arthritis. Br J Nutr. 2000;84:589–95. doi: 10.1017/S0007114500001926. [DOI] [PubMed] [Google Scholar]
- 62.Pattison DJ, Symmons DP, Lunt M, Welch A, Luben R, Bingham SA, et al. Dietary risk factors for the development of inflammatory polyarthritis: evidence for a role of high level of red meat consumption. Arthritis Rheum. 2004;50:3804–12. doi: 10.1002/art.20731. [DOI] [PubMed] [Google Scholar]
- 63.Benito-Garcia E, Feskanich D, Hu BF, Mandl AL, Karlson WE. Protein, iron, and meat consumption and risk for rheumatoid arthritis: a prospective cohort study. Arthritis Res Ther. 2007;9:R16. doi: 10.1186/ar2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sundström B, Johansson I, Rantapää-Dahlqvist S. Interaction between dietary sodium and smoking increases the risk for rheumatoid arthritis: results from a nested case-control study. Rheumatology. 2015;54:487–93. doi: 10.1093/rheumatology/keu330. [DOI] [PubMed] [Google Scholar]
- 65.Lotito SB, Frei B. Consumption of flavonoid-rich foods and increased plasma antioxidant capacity in humans: cause, consequence, or epiphenomenon? Free Radic Biol Med. 2006;41:1727–46. doi: 10.1016/j.freeradbiomed.2006.04.033. [DOI] [PubMed] [Google Scholar]
- 66.Pattison DJ, Harrison RA, Symmons DP. The role of diet in susceptibility to rheumatoid arthritis: a systematic review. J Rheumatol. 2004;31:1310–9. [PubMed] [Google Scholar]
- 67.Pattison DJ, Silman AJ, Goodson NJ, Lunt M, Bunn D, Luben R, et al. Vitamin C and the risk of developing inflammatory polyarthritis: prospective nested case-control study. Ann Rheum Dis. 2004;63:843–7. doi: 10.1136/ard.2003.016097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pattison DJ, Symmons DP, Lunt M, Welch A, Bingham SA, Day E, et al. Dietary beta-cryptoxanthin and inflammatory polyarthritis: results from a population-based prospective study. Am J Clin Nutr. 2005;82:451–5. doi: 10.1093/ajcn/82.2.451. [DOI] [PubMed] [Google Scholar]
- 69.Cerhan JR, Saag KG, Merlino LA, Mikuls TR, Criswell LA. Antioxidant micronutrients and risk of rheumatoid arthritis in a cohort of older women. Am J Epidemiol. 2003;157:345–54. doi: 10.1093/aje/kwf205. [DOI] [PubMed] [Google Scholar]
- 70.Pedersen M, Stripp C, Klarlund M, Olsen SF, Tjonneland AM, Frisch M. Diet and risk of rheumatoid arthritis in a prospective cohort. J Rheumatol. 2005;32:1249–52. [PubMed] [Google Scholar]
- 71.Costenbader KH, Kang JH, Karlson EW. Antioxidant intake and risks of rheumatoid arthritis and systemic lupus erythematosus in women. Am J Epidemiol. 2010;172:205–16. doi: 10.1093/aje/kwq089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Linos A, Kaklamani VG, Kaklamani E, Koumantaki Y, Giziaki E, Papazoglou S, et al. Dietary factors in relation to rheumatoid arthritis: a role for olive oil and cooked vegetables? Am J Clin Nutr. 1999;70:1077–82. doi: 10.1093/ajcn/70.6.1077. [DOI] [PubMed] [Google Scholar]
- 73.Volker D, Fitzgerald P, Major G, Garg M. Efficacy of fish oil concentrate in the treatment of rheumatoid arthritis. J Rheumatol. 2000;27:2343–6. [PubMed] [Google Scholar]
- 74.Remans PH, Sont JK, Wagenaar LW, Wouters-Wesseling W, Zuijderduin WM, Jongma A, et al. Nutrient supplementation with polyunsaturated fatty acids and micronutrients in rheumatoid arthritis: clinical and biochemical effects. Eur J Clin Nutr. 2004;58:839–45. doi: 10.1038/sj.ejcn.1601883. [DOI] [PubMed] [Google Scholar]
- 75.Geusens P, Wouters C, Nijs J, Jiang Y, Dequeker J. Long-term effect of omega-3 fatty acid supplementation in active rheumatoid arthritis. A 12-month, double-blind, controlled study. Arthritis Rheum. 1994;37:824–9. doi: 10.1002/art.1780370608. [DOI] [PubMed] [Google Scholar]
- 76.Nielsen GL, Faarvang KL, Thomsen BS, Jensen LT, Hansen TM, Lervang HH, et al. The effects of dietary supplementation with n-3 polyunsaturated fatty acids in patients with rheumatoid arthritis: a randomized, double blind trial. Eur J Clin Invest. 1992;22:687–91. doi: 10.1111/j.1365-2362.1992.tb01431.x. [DOI] [PubMed] [Google Scholar]
- 77.van der Tempel H, Tulleken JE, Limburg PC, Muskiet FA, van Rijswijk MH. Effects of fish oil supplementation in rheumatoid arthritis. Ann Rheum Dis. 1990;49:76–80. doi: 10.1136/ard.49.2.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kremer JM, Jubiz W, Michalek A, Rynes RI, Bartholomew LE, Bigaouette J, et al. Fish-oil fatty acid supplementation in active rheumatoid arthritis. A double-blinded, controlled, crossover study. Ann Intern Med. 1987;106:497–503. doi: 10.7326/0003-4819-106-4-497. [DOI] [PubMed] [Google Scholar]
- 79.Kremer JM, Bigauoette J, Michalek AV, Lininger L, Huyck C, Timchalk MA, et al. Effects of manipulation of dietary fatty acids on clinical manifestations of rheumatoid arthritis. Lancet. 1985;1:184–7. doi: 10.1016/S0140-6736(85)92024-0. [DOI] [PubMed] [Google Scholar]
- 80.Cleland LG, James MJ. Rheumatoid arthritis and the balance of dietary N-6 and N-3 essential fatty acids. Br J Rheumatol. 1997;36:513–4. doi: 10.1093/rheumatology/36.5.513. [DOI] [PubMed] [Google Scholar]
- 81.Recht L, Helin P, Rasmussen JO, Jacobsen J, Lithman T, Schersten B. Hand handicap and rheumatoid arthritis in a fish-eating society (the Faroe Islands) J Intern Med. 1990;227:49–55. doi: 10.1111/j.1365-2796.1990.tb00118.x. [DOI] [PubMed] [Google Scholar]
- 82.Horrobin DF. Low prevalences of coronary heart disease (CHD), psoriasis, asthma and rheumatoid arthritis in Eskimos: are they caused by high dietary intake of eicosapentaenoic acid (EPA), a genetic variation of essential fatty acid (EFA) metabolism or a combination of both? Med Hypotheses. 1987;22:421–8. doi: 10.1016/0306-9877(87)90037-5. [DOI] [PubMed] [Google Scholar]
- 83.Shapiro JA, Koepsell TD, Voigt LF, Dugowson CE, Kestin M, Nelson JL. Diet and rheumatoid arthritis in women: a possible protective effect of fish consumption. Epidemiology. 1996;7:256–63. doi: 10.1097/00001648-199605000-00007. [DOI] [PubMed] [Google Scholar]


