Abstract
Blue honeysuckle is rich in polyphenols, and recently receiving attention because of its potential antioxidant and anti-inflammatory properties. Nonalcoholic fatty liver disease (NAFLD) is the leading cause of chronic liver disease that develops hepatic inflammation and metabolic syndrome. The present study aims to study the effect of blue honeysuckle extract (BHE) on fat deposition and hepatic lipid peroxidation in a high-fat-diet (HFD)-induced mouse model. Mice were fed a normal diet (ND) or a HFD containing 0.5% or 1% of BHE or not for 45 d. Liver sections were stained by hematoxylin-eosin staining. Serum lipids were measured by a clinical analyzer. Insulin was examined by ELISA, and hepatic proteins were detected by Western blotting. Dietary supplementation of BHE dose-dependently suppressed HFD-induced obesity and hepatic fat deposition. Moreover, BHE improved glucose metabolism by increasing insulin sensitivity and attenuated oxidative stress potentially by up-regulating nuclear factor (erythroid-derived 2)-like 2 (Nrf2)-mediated pathway.
Keywords: Blue honeysuckle, Polyphenols, Fatty liver, Lipid peroxidation, Antioxidant
1. Introduction
Cool climate berries are considered to possess many biological functions and benefit to prevent humans against chronic disorders (Rupasinghe et al., 2015). Blue honeysuckle (Lonicera caerulea L.), also known as honeyberry or haskap, is a member of the Caprifoliaceae family that grow naturally in cool temperate Northern Hemisphere such as Siberia of Russia, Hokkaido of Japan (Terahara et al., 1993), and northern China (Jin et al., 2006). The fruit of blue honeysuckle, a berry with oval to long fruit shape and dark navy blue to purple in color, is widely harvested in Russia, China, and Japan, but practically unknown as edible berries in Europe and North America until recent years (Chaovanalikit et al., 2004, Palikova et al., 2008). Blue honeysuckle has been used for treating cancer, inflammation, hepatic complications, influenza, and wounds for thousands of years in East Asian countries (Kaczmarska et al., 2015), and is known for its effects of heat clearing and detoxicating, detumescence, and visual improvement in the folk medicine in China (Dong, 2013, Jin et al., 2006). The biological activities of blue honeysuckle are considered to be polyphenols-dependent based on the antioxidant (Palikova et al., 2009) and anti-inflammatory (Rupasinghe et al., 2015) studies. In laboratory investigations, polyphenols from blue honeysuckle have shown protective effects against inflammation and oxidative stress in many disease models such as lipopolysaccharide (LPS)-induced inflammation (Wang et al., 2016a), ultraviolet-induced skin damage (Vostalova et al., 2013), and abnormal lipid metabolism (Takahashi et al., 2014). Our recent studies revealed that blue honeysuckle extracts (BHE), which mainly composed by –(−)epicatechin (EC) and cyanidin 3-glucoside (C3G), could modulate inflammatory and antioxidant pathways to regulate cytokine network (Wu et al., 2017a), and potentially attenuate liver fibrosis induced by high fat diets and carbon tetrachloride (Wu et al., 2017b).
Liver is one of the most important metabolic organs, and it is critical for digestion especially the breakdown of fat. Nonalcoholic fatty liver disease (NAFLD) is the leading cause of chronic liver disease that develops hepatic inflammation and metabolic syndrome. The prevalence of NAFLD ranges from 20% to 30% in the general population and up to 75% to 100% in obese individuals (Henao-Mejia et al., 2012). Nonalcoholic fatty liver disease comprises nonalcoholic fatty liver (NAFL) and nonalcoholic steatohepatitis (NASH) (Machado and Diehl, 2016). Nonalcoholic fatty liver is reversible and usually remains asymptomatic, but the accumulation of fat can develop NASH leading to cirrhosis, portal hypertension, hepatocellular carcinoma and increased mortality (Rafiq et al., 2009). The factors leading to progression from NAFL to NASH are still poorly understood, but lipid peroxidation is a potential key process that promotes fibrosis according to liver biopsies from human patients (MacDonald et al., 2001).
Based on the information related to blue honeysuckle and the potential pathogenesis of NAFLD, the present study aims to study the effect of BHE on fat deposition and hepatic lipid peroxidation in a high-fat-diet (HFD)-induced mouse model.
2. Materials and methods
2.1. Chemicals and reagents
Lard oil, choline chloride, and methionine were purchased from Sigma–Aldrich, USA. Casein, sucrose, cellulose, mineral mix, vitamin mix, and corn starch were purchased from Oriental Yeast Co., Ltd., Tokyo, Japan. Antibodies against nuclear factor (erythroid-derived 2)-like 2 (Nrf2), heme oxygenase-1 (HO-1), and manganese-dependent superoxide dismutase (MnSOD) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Antibodies against glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and corresponding secondary antibodies were purchased from Cell Signaling Technology (Beverly, MA, USA). Blue honeysuckle extract was extracted as described previously (Wu et al., 2015). In brief, blue honeysuckle was homogenized in 75% aqueous ethanol (250 g/L) for 60 min before filtered under reduced pressure. The filtrates were purified on a column packed with nonionic polystyrene-divinylbenzene resin (D101, Shanghai, China), and then freeze-dried into powder. According to the retention profiles of HPLC analysis at 280 and 520 nm, C3G (59.5%) and EC (25.5%) were the major phenolic components at 280 nm, and other minor anthocyanins including cyanidin 3-rutinoside (1.8%), cyanidin 3,5-diglucoside (1.3%), peonidin 3-glucoside (7.2%), peonidin 3-rutinoside (1.9%), and pelargonidin 3-glucoside (2.3%) were also detected at 520 nm. The quantitative analysis revealed that each milligram of BHE contains 0.37 mg C3G and 0.23 mg EC.
2.2. Mouse model
The animal experiment was conducted following the guidelines of the Animal Care and Use Committee of Kagoshima University (Permission No. A12005). Twenty C57BL/6N mice (male, 5 weeks of age) from Japan SLC Inc. (Shizuoka, Japan) were housed separately in cages with wood shavings bedding under controlled temperature (25 °C) and light (12 h light/day), and had free access to feed and water. The mice were accommodated for 1 week and then randomly divided into 5 groups (n = 4): normal diet (ND) group, ND + BHE1% group, HFD group, HFD + BHE0.5% group, and HFD + BHE1% group. The mice were fed the corresponding diets as described in Appendix Table 1. The experimental period was 45 d, and the weight of mice was measured on d 0, 30, and 45.
2.3. Determination of lipids, glucose, and insulin
The blood of mice was collected from the heart at d 45, and the serum was obtained by centrifugation (1,500 × g, 10 min) after coagulation. Serum levels of triacylglycerol (TG), total cholesterol (T-cho), high density lipoprotein cholesterol (HDL-C) and glucose were measured by using an automated analyzer for clinical chemistry (Arkray, Kyoto, Japan). The level of insulin in serum was measured with an ELISA kit (Thermo Fisher Scientific Inc., Rockford, IL, USA), according to the manufacturer's manual. The homeostasis model analysis of insulin resistance (HOMA-IR) was calculated using the following formula (Matthews et al., 1985):
HOMA-IR = Fasting insulin (mU/L) × Fasting glucose (mg/dL)/405.
2.4. Determination of thiobarbituric acid reactive substances (TBARS)
The level of TBARS in the liver of mice was measured by using an assay kit (BioAssay Systems, Hayward, CA, USA) according to the manufacturer's manual.
2.5. Hepatic histology
The livers of mice in each group were collected and sectioned by using a freezing microtome system (Yamato, Saitama, Japan) follow the manufacturer's instruction, and the liver sections were stained by hematoxylin-eosin (H&E) staining before observed under a fluorescence microscope (Keyence, Tokyo, Japan).
2.6. Protein extraction and Western blotting
Total proteins of the liver were obtained as described previously (Wu et al., 2017b). In brief, equal amounts (around 0.2 g) of liver tissues were homogenized in RIPA buffer (0.1 g/mL) by using a Speed-Mill PLUS homogenizer (Analytik Jena, Jena, Germany). The homogenate was then centrifuged at 13,500 × g for 5 min at 4 °C, and the supernatant proteins were collected. After the measurement of protein concentration, the proteins were boiled in sodium dodecyl sulfate (SDS) sample buffer for 5 min, and then equal amounts of protein (40 μg) were run on a 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gel before electrophoretically transferred to polyvinylidene fluoride (PVDF) membrane (GE Healthcare, Buckinghamshire, UK). The membrane was then incubated with specific primary antibody (antibodies of Nrf2, HO-1, MnSOD, GAPDH) and anti-rabbit HRP-conjugated secondary antibody, following by the detection with the LumiVision PRO system (TAITEC Co., Saitama, Japan).
2.7. Statistical analysis
Results are presented as individual means ± SD, and the levels of proteins are presented as induction folds relative to that in the ND group measured by the densitometry. The significant differences between groups were analyzed by analysis of variance (ANOVA) tests, followed by Fisher's LSD and Duncan's multiple range tests by using the SPSS statistical program (version 19.0, IBM Corp., Armonk, NY, USA). Differences were considered statistically significant at P < 0.05.
3. Results
3.1. Blue honeysuckle extract inhibited high-fat-diet-induced obesity
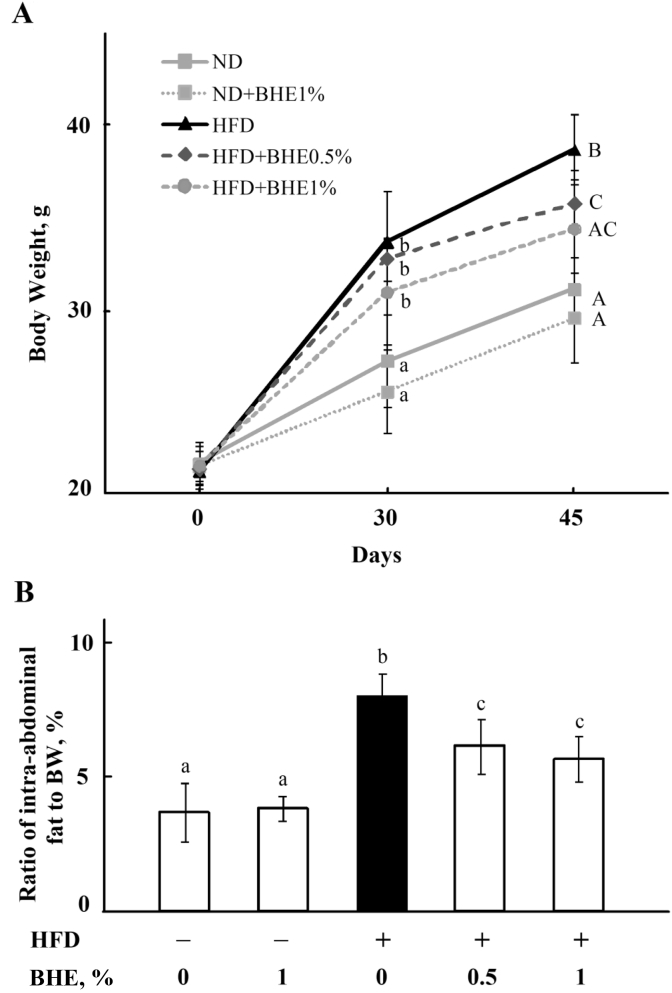
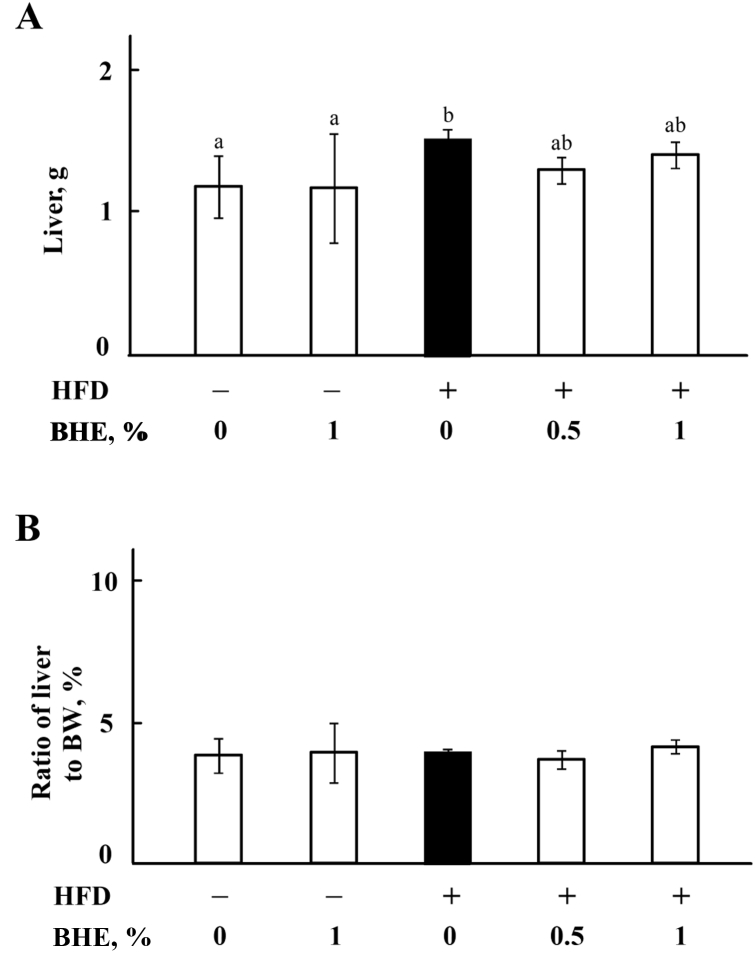
The body weight (BW) of mice is shown in Fig. 1A. There is no significant difference between the initial BW of mice in each group (P > 0.05). However, the BW of HFD-fed mice is higher (P < 0.05) than that of ND-fed mice at d 30 (33.68 ± 2.77 vs. 27.23 ± 2.51 g) and 45 (38.73 ± 1.90 vs. 31.13 ± 1.75 g). The BW of mice fed HFD plus 0.5% or 1% of BHE is lower than mice fed HFD alone at both d 30 and 45, although significant difference has only been observed at d 45 (P < 0.05). As shown in Fig. 1B, the ratio of intra-abdominal fat to BW is also significantly increased (P < 0.05) in HFD-fed mice, but decreased by supplementing with 0.5% or 1% of BHE. There is no significant difference (P > 0.05) between the BW of mice in ND group and ND + BHE1% group. The liver weight of HFD-fed mice is higher (P < 0.05) than that of ND-fed mice (1.47 ± 0.07 vs. 1.15 ± 0.21); however, no significant difference was observed in the ratio of liver to BW among the groups (Appendix Fig. 1).
Fig. 1.
Blue honeysuckle extract (BHE) inhibited high-fat-diet (HFD)-induced obesity in mice. (A) The change in body weight of mice in each group. (B) The ratio of intra-abdominal fat to BW. Data represent means ± SD of 4 mice. Bars with different letters differ significantly (P < 0.05). ND = normal diet.
3.2. Blue honeysuckle extract reduced fat accumulation in liver
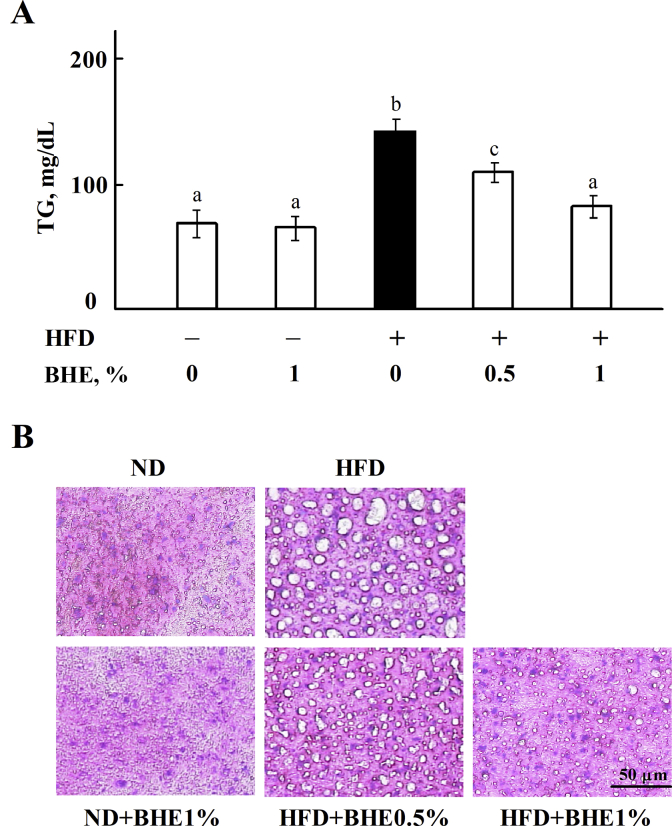
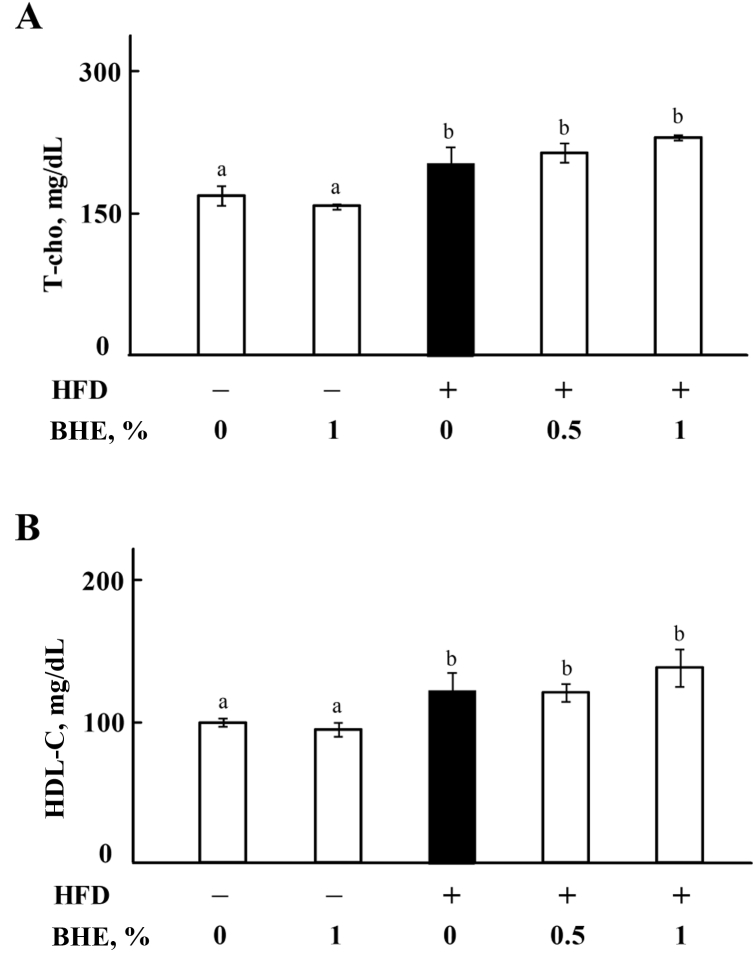
To evaluate the lipid metabolism in mice, serum lipids have been measured by using an automated analyzer for clinical chemistry. As shown in Fig. 2A, the level of TG is significantly (P < 0.05) increased in HFD-fed mice, but reduced by supplementing 0.5% or 1% of BHE in a dose-dependent manner. The levels of T-cho and HDL-C in HFD group are significantly (P < 0.05) higher than those of ND group, but there are no significant differences between HFD and HFD + BHE groups (P > 0.05, Appendix Fig. 2). The liver section is shown in Fig. 2B; HFD caused the accumulation of fat in liver, but obviously decreased by supplementing with 0.5% or 1% of BHE.
Fig. 2.
Blue honeysuckle extract (BHE) reduced the accumulation of fat in liver. (A) The level of triacylglycerol (TG) in serum. (B) Representative liver sections from each group (H&E staining). Data represent means ± SD of 4 mice. Bars with different letters differ significantly (P < 0.05). HFD = high fat diet; ND = normal diet.
3.3. Blue honeysuckle extract attenuated high-fat-diet-induced insulin resistance
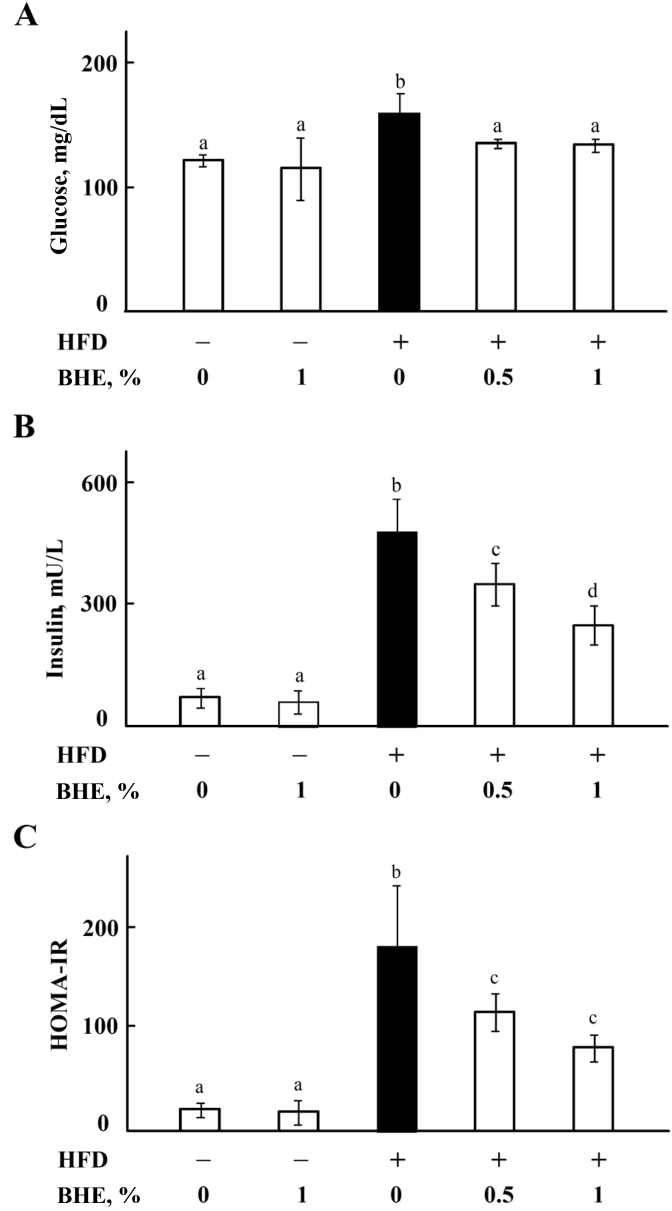
Insulin resistance is closely related to steatosis in NAFLD (Fabbrini et al., 2010) and, thus, the levels of glucose and insulin have been measured in mice serum. As shown in Fig. 3, HFD significantly (P < 0.05) increased both glucose (A) and insulin (B) levels, as well as the HOMA-IR index (C), whereas supplement with 0.5% or 1% of BHE in diets reduced levels of glucose, insulin and HOMA-IR in a dose-dependent manner. There is no significant difference (P > 0.05) in the levels of glucose, insulin and HOMA-IR between ND group and ND + BHE1% group.
Fig. 3.
Blue honeysuckle extract (BHE) attenuated high-fat-diet (HFD)-induced insulin resistance. (A) The level of glucose (Glu) in serum. (B) The level of insulin in serum. (C) HOMA-IR index. Data represent means ± SD of 4 mice. Bars with different letters differ significantly (P < 0.05).
3.4. Blue honeysuckle extract improved hepatic antioxidant capacity
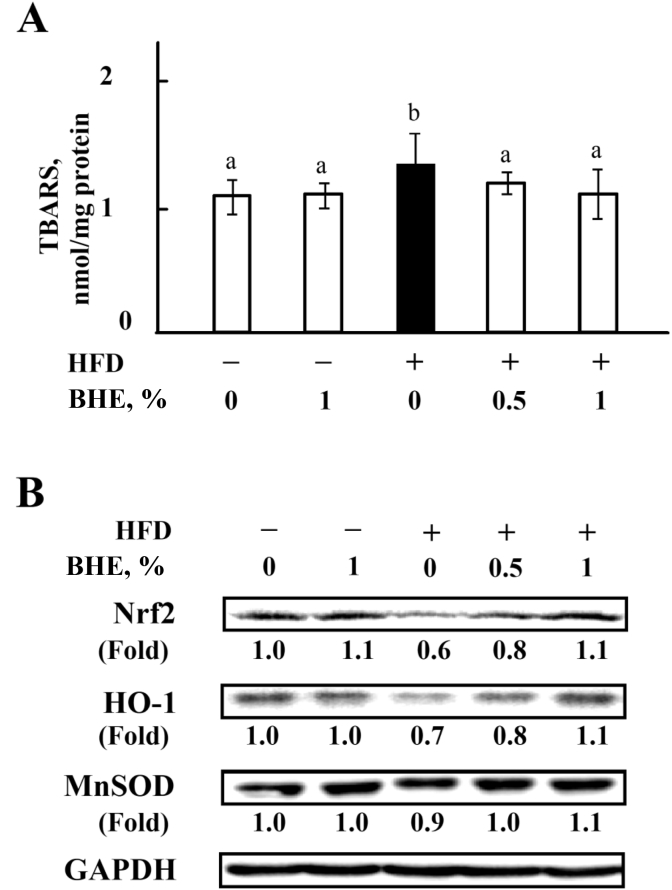
Since oxidative stress-mediated lipid peroxidation is an important factor that promotes the progression of NAFLD (Rolo et al., 2012), the level of TBARS, an indicator to represent the products of lipid peroxidation, has been measured in liver. As shown in Fig. 4A, the level of TBARS is significantly (P < 0.05) increased in HFD-fed mice, but recovered by supplementing with 0.5% or 1% of BHE. To further evaluate the oxidative status in liver, the levels of Nrf2, HO-1, and MnSOD have been detected. Fig. 4B indicates that the levels of Nrf2, HO-1 and MnSOD in the liver of HFD-fed mice are decreased to 60% (P < 0.05), 70% (P < 0.05) and 90% of ND level, respectively. Supplement with 0.5% or 1% of BHE in diets recovered the levels of all the antioxidant proteins in liver.
Fig. 4.
Blue honeysuckle extract (BHE) improved hepatic antioxidant capacity. (A) The level of thiobarbituric acid reactive substances (TBARS) in liver. (B) The representative blots of nuclear factor (erythroid-derived 2)-like 2 (Nrf2), heme oxygenase-1 (HO-1), and manganese-dependent superoxide dismutase (MnSOD) protein in liver by Western blotting. The induction folds of the proteins were calculated as the intensity of the treatment relative to that of control normalized to glyceraldehyde-3-phosphate dehydrogenase (GAPDH) by densitometry. Data represent means ± SD of 4 mice, and the blots are representatives of 4 samples. Bars with different letters differ significantly (P < 0.05). HFD = high fat diet.
4. Discussion
Epidemiological studies and associated meta-analyses provide growing evidence that long term consumption of polyphenol-rich diets offered protective effects against the development of chronic or degenerative diseases such as cardiovascular diseases, cancers, diabetes, osteoporosis and neurodegenerative diseases (Arts and Hollman, 2005, Graf et al., 2005). Blue honeysuckle extract is rich in polyphenols, and our previous study has shown that BHE exerts potential protective effects on hepatic inflammation and fibrosis by regulating both antioxidant and anti-inflammatory pathways in NAFLD (Wu et al., 2017b). The present study aims to clarify the protective effect of BHE on HFD-induced fatty liver in mice, which is a prelude to the progressive NASH.
The accumulation of fat in hepatocytes, which also defined as hepatic steatosis, is the typical histological feature of NAFLD (Kleiner et al., 2005), and TG is the most conspicuous type of fat in fatty livers. Thus, the range of TG accumulation is considered as the basis for grading the severity of steatosis in NAFLD (Machado and Diehl, 2016). In this study, HFD caused fat accumulation in liver and significantly increased the level of TG in mice, and BHE reduced hepatic fat deposition and TG level. Hyperinsulinemia associated with hepatic insulin resistance, which has been reported to play an important role in the progression of hepatic fibrosis, is a hallmark feature of NAFLD (Cai et al., 2017). The level of insulin in serum is dramatically increased in HFD-fed mice in the present study; however, the glucose level has not been reduced correspondingly, instead, it is increased significantly. Supplementation with BHE in HFD decreased the levels of both glucose and insulin in serum. The HOMA-IR, a valuable index to reflect insulin resistance (Matthews et al., 1985), is also significantly increased in HFD group, but is decreased by supplementing BHE. Thus, BHE can potentially increase insulin sensitivity to improve lipid and glucose metabolism.
Oxidative stress-mediated lipid peroxidation is considered as one of the most important factors that promote the progression of NAFLD (Rolo et al., 2012). Nrf2-mediated antioxidant response pathway is identified as an important mechanism that promotes cellular defense against oxidative stress and inflammatory response (Hashimoto et al., 2016, Mo et al., 2014). Manganese-dependent superoxide dismutase has been reported as an important enzyme to reduce hepatic mitochondrial oxidative stress (Mansouri et al., 2010). In this study, HFD significantly increased the product of lipid peroxidation (TBARS), and decreased the levels of Nrf2 and HO-1 (one of the key downstream enzymes of Nrf2). In contrast, BHE recovered the levels of both Nrf2 and HO-1 in liver, and subsequently decreased the level of TBARS. Other studies also revealed that C3G and EC, the major phenolic components in BHE, can activate Nrf2/HO-1 pathway to counteract oxidative stress (Shah et al., 2010, Wang et al., 2016b). Both HFD and BHE showed limited effects on the level of MnSOD in the liver of mice. Thus, BHE potentially attenuated lipid peroxidation in liver by up-regulating Nrf2-mediated antioxidant pathway.
5. Conclusion
In summary, dietary supplementation of BHE dose-dependently inhibited HFD-induced obesity and hepatic fat deposition. Moreover, BHE improved glucose metabolism by increasing the sensitivity of insulin, and attenuated oxidative stress potentially by up-regulating Nrf2-mediated pathway. These results demonstrated that BHE can inhibit HFD-induced hepatic lipid peroxidation by improving insulin sensitivity and Nrf2-mediated antioxidant pathway.
Conflict of interest
We declare that we have no financial and personal relationships with other people or organizations that can inappropriately influence our work. There is no professional or other personal interest of any nature or kind in any product, service and/or company that could be construed as influencing the content of this paper.
Acknowledgements
This work was partially supported by National Natural Science Foundation of China (No. 31741115) and Core Research Program 1515 of Hunan Agricultural University.
Footnotes
Peer review under responsibility of Chinese Association of Animal Science and Veterinary Medicine.
Contributor Information
Jianhua He, Email: jianhuahy@hunau.net.
Shusong Wu, Email: wush688@126.com.
Appendix.
Appendix Fig. 1.
Effect of blue honeysuckle extract (BHE) on liver weight of mice. (A) The weight of liver. (B) The ratio of liver weight to BW. Data represent means ± SD. Bars with different letters differ significantly (P < 0.05). HFD = high fat diet.
Appendix Fig. 2.
Effect of blue honeysuckle extract (BHE) on serum lipids of mice. (A) The level of total cholesterol (T-cho) in serum. (B) The level of high density lipoprotein cholesterol (HDL-C) in serum. Data represent means ± SD. Bars with different letters differ significantly (P < 0.05). HFD = high fat diet.
Appendix Table 1.
Dietary composition of each group.
| Components, % | ND | ND + BHE1% | HFD | HFD + BHE0.5% | HFD + BHE1% |
|---|---|---|---|---|---|
| Lard | 6 | 6 | 40 | 40 | 40 |
| Casein | 21 | 21 | 21 | 21 | 21 |
| Sucrose | 10 | 10 | 10 | 10 | 10 |
| Cellulose | 4 | 4 | 4 | 4 | 4 |
| Mineral mix | 3.5 | 3.5 | 3.5 | 3.5 | 3.5 |
| Vitamin mix | 1 | 1 | 1 | 1 | 1 |
| Choline chloride | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 |
| Methionine | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 |
| Corn starch | 54 | 53 | 20 | 19.5 | 19 |
| BHE | 0 | 1 | 0 | 0.5 | 1 |
| Total | 100 | 100 | 100 | 100 | 100 |
| Gross energy, kcal/100 g | 377.94 | 374.13 | 555.08 | 553.18 | 551.27 |
BHE = blue honeysuckle extract; ND = normal diet; HFD = high fat diet.
References
- Arts I.C., Hollman P.C. Polyphenols and disease risk in epidemiologic studies. Am J Clin Nutr. 2005;81(1 Suppl):317S–325S. doi: 10.1093/ajcn/81.1.317S. [DOI] [PubMed] [Google Scholar]
- Cai C.X., Buddha H., Castelino-Prabhu S., Zhang Z., Britton R.S., Bacon B.R. Activation of insulin-PI3K/Akt-p70S6K pathway in hepatic stellate cells contributes to fibrosis in nonalcoholic steatohepatitis. Dig Dis Sci. 2017;62(4):968–978. doi: 10.1007/s10620-017-4470-9. [DOI] [PubMed] [Google Scholar]
- Chaovanalikit A., Thompson M.M., Wrolstad R.E. Characterization and quantification of anthocyanins and polyphenolics in BluehHoneysuckle (Lonicera caerulea L.) J Agric Food Chem. 2004;52(4):848–852. doi: 10.1021/jf030509o. [DOI] [PubMed] [Google Scholar]
- Dong J. The relationship between traditional Chinese medicine and modern medicine. Evid Based Complement Alternat Med. 2013;2013:1–10. doi: 10.1155/2013/153148. 153148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabbrini E., Sullivan S., Klein S. Obesity and nonalcoholic fatty liver disease: biochemical, metabolic, and clinical implications. Hepatology. 2010;51(2):679–689. doi: 10.1002/hep.23280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graf B.A., Milbury P.E., Blumberg J.B. Flavonols, flavones, flavanones, and human health: epidemiological evidence. J Med Food. 2005;8(3):281–290. doi: 10.1089/jmf.2005.8.281. [DOI] [PubMed] [Google Scholar]
- Hashimoto K., Simmons A.N., Kajino-Sakamoto R., Tsuji Y., Ninomiya-Tsuji J. TAK1 regulates the Nrf2 antioxidant system through modulating p62/SQSTM1. Antioxid Redox Signal. 2016;25(17):953–964. doi: 10.1089/ars.2016.6663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henao-Mejia J., Elinav E., Jin C., Hao L., Mehal W.Z., Strowig T. Inflammasome-mediated dysbiosis regulates progression of NAFLD and obesity. Nature. 2012;482(7384):179–185. doi: 10.1038/nature10809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin X.H., Ohgami K., Shiratori K., Suzuki Y., Koyama Y., Yoshida K. Effects of blue honeysuckle (Lonicera caerulea L.) extract on lipopolysaccharide-induced inflammation in vitro and in vivo. Exp Eye Res. 2006;82(5):860–867. doi: 10.1016/j.exer.2005.10.024. [DOI] [PubMed] [Google Scholar]
- Kaczmarska E., GawroŃSki J., Dyduch-SiemiŃSka M., Najda A., Marecki W., ŻEbrowska J. Genetic diversity and chemical characterization of selected polish and Russian cultivars and clones of blue honeysuckle (Lonicera caerulea) Turk J Agric For. 2015;39:394–402. [Google Scholar]
- Kleiner D.E., Brunt E.M., Van Natta M., Behling C., Contos M.J., Cummings O.W. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41(6):1313–1321. doi: 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- MacDonald G.A., Bridle K.R., Ward P.J., Walker N.I., Houglum K., George D.K. Lipid peroxidation in hepatic steatosis in humans is associated with hepatic fibrosis and occurs predominately in Acinar zone 3. J Gastroenterol Hepatol. 2001;16(6):599–606. doi: 10.1046/j.1440-1746.2001.02445.x. [DOI] [PubMed] [Google Scholar]
- Machado M.V., Diehl A.M. Pathogenesis of nonalcoholic steatohepatitis. Gastroenterology. 2016;150(8):1769–1777. doi: 10.1053/j.gastro.2016.02.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansouri A., Tarhuni A., Larosche I., Reyl-Desmars F., Demeilliers C., Degoul F. MnSOD overexpression prevents liver mitochondrial DNA depletion after an Alcohol binge but worsens this effect after prolonged Alcohol consumption in mice. Dig Dis. 2010;28(6):756–775. doi: 10.1159/000324284. [DOI] [PubMed] [Google Scholar]
- Matthews D.R., Hosker J.P., Rudenski A.S., Naylor B.A., Treacher D.F., Turner R.C. Homeostasis model Assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- Mo C., Wang L., Zhang J., Numazawa S., Tang H., Tang X. The crosstalk between Nrf2 and AMPK signal pathways is important for the anti-inflammatory effect of berberine in LPS-stimulated macrophages and endotoxin-shocked mice. Antioxid Redox Signal. 2014;20(4):574–588. doi: 10.1089/ars.2012.5116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palikova I., Heinrich J., Bednar P., Marhol P., Kren V., Cvak L. Constituents and Antimicrobial properties of blue honeysuckle: a novel source for phenolic antioxidants. J Agric Food Chem. 2008;56(24):11883–11889. doi: 10.1021/jf8026233. [DOI] [PubMed] [Google Scholar]
- Palikova I., Valentova K., Oborna I., Ulrichova J. Protectivity of blue honeysuckle extract against oxidative human endothelial cells and rat hepatocyte damage. J Agric Food Chem. 2009;57(15):6584–6589. doi: 10.1021/jf9003994. [DOI] [PubMed] [Google Scholar]
- Rafiq N., Bai C., Fang Y., Srishord M., McCullough A., Gramlich T. Long-term follow-up of patients with nonalcoholic fatty liver. Clin Gastroenterol Hepatol. 2009;7(2):234–238. doi: 10.1016/j.cgh.2008.11.005. [DOI] [PubMed] [Google Scholar]
- Rolo A.P., Teodoro J.S., Palmeira C.M. Role of oxidative stress in the pathogenesis of nonalcoholic steatohepatitis. Free Radic Biol Med. 2012;52(1):59–69. doi: 10.1016/j.freeradbiomed.2011.10.003. [DOI] [PubMed] [Google Scholar]
- Rupasinghe H.P., Boehm M.M., Sekhon-Loodu S., Parmar I., Bors B., Jamieson A.R. Anti-inflammatory activity of haskap cultivars is polyphenols-dependent. Biomolecules. 2015;5(2):1079–1098. doi: 10.3390/biom5021079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah Z.A., Li R.C., Ahmad A.S., Kensler T.W., Yamamoto M., Biswal S. The flavanol (-)-epicatechin prevents stroke damage through the nrf2/HO1 pathway. J Cereb Blood Flow Metab. 2010;30(12):1951–1961. doi: 10.1038/jcbfm.2010.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi A., Okazaki Y., Nakamoto A., Watanabe S., Sakaguchi H., Tagashira Y. Dietary Anthocyanin-rich haskap phytochemicals inhibit postprandial hyperlipidemia and hyperglycemia in rats. J Oleo Sci. 2014;63(3):201–209. doi: 10.5650/jos.ess13196. [DOI] [PubMed] [Google Scholar]
- Terahara N., Sakanashi T., Tsukui A. Anthocyanins from the berries of haskaap, Lonicera caerulea L. J home Econ Jpn. 1993;44(3):197–201. [Google Scholar]
- Vostalova J., Galandakova A., Palikova I., Ulrichova J., Dolezal D., Lichnovska R. Lonicera caerulea fruits reduce UVA-induced damage in hairless mice. J Photochem Photobiol. 2013;B 128:1–11. doi: 10.1016/j.jphotobiol.2013.07.024. [DOI] [PubMed] [Google Scholar]
- Wang Y., Li B., Ma Y., Wang X., Zhang X., Zhang Q. Lonicera caerulea berry extract attenuates lipopolysaccharide induced inflammation in BRL-3A cells: oxidative stress, energy metabolism, hepatic function. J Funct Foods. 2016;24:1–10. doi: 10.1039/c6fo00627b. [DOI] [PubMed] [Google Scholar]
- Wang Y., Huo Y., Zhao L., Lu F., Wang O., Yang X. Cyanidin-3-glucoside and its phenolic acid metabolites attenuate visible light-induced retinal degeneration in vivo via activation of nrf2/HO-1 pathway and NF-kappab suppression. Mol Nutr Food Res. 2016;60:1564–1577. doi: 10.1002/mnfr.201501048. [DOI] [PubMed] [Google Scholar]
- Wu S., He X., Wu X., Qin S., He J., Zhang S. Inhibitory effects of blue honeysuckle (Lonicera caerulea L) on adjuvant-induced arthritis in rats: crosstalk of anti-inflammatory and antioxidant effects. J Funct Foods. 2015;17:514–523. [Google Scholar]
- Wu S., Yano S., Chen J., Hisanaga A., Sakao K., He X. Polyphenols from Lonicera caerulea L. Berry inhibit LPS-induced inflammation through dual modulation of inflammatory and antioxidant mediators. J Agric Food Chem. 2017;65(25):5133–5141. doi: 10.1021/acs.jafc.7b01599. [DOI] [PubMed] [Google Scholar]
- Wu S., Yano S., Hisanaga A., He X., He J., Sakao K. Polyphenols from Lonicera caerulea L. berry attenuate experimental nonalcoholic steatohepatitis by inhibiting proinflammatory cytokines productions and lipid peroxidation. Mol Nutr Food Res. 2017;61(4):1–8. doi: 10.1002/mnfr.201600858. [DOI] [PubMed] [Google Scholar]