Abstract
The present study used perforated-patch electrophysiology and calcium imaging in GnRH transgenic mouse lines to determine the mechanisms underlying the potent excitatory effects of kisspeptin upon GnRH neurons in the acute brain slice preparation. Kisspeptin (100nM) depolarized (6±1mV) and/or evoked an 87±4% increase in firing rate of 75% of adult GnRH neurons (n=51). No sex differences were found. Analyses of input resistance and current-voltage curves indicated that a heterogenous closure of potassium channels and opening of non-selective cation (NSC) channels was involved in kisspeptin’s depolarizing response. Pharmacological pre-treatment with either barium, a potassium channel blocker, or flufenamic acid, an NSC channel antagonist, reduced the percentage of responding GnRH neurons from 75% to 40% (p<0.05). Pretreatment with both barium and flufenamic acid reduced the response rate to 17% (p<0.05). To examine the intracellular signaling cascade involved, GnRH neurons were treated with antagonists of phospholipase C (PLC), inositol-trisphosphate receptors (IP3R), and ERK1/2 prior kisspeptin exposure. PLC and IP3R antagonism reduced the percentage of responding GnRH neurons from 80% to 15% and 7%, respectively (p<0.001). Real-time calcium imaging showed that kisspeptin evoked an ~10% increase in intracellular calcium levels in GnRH neurons that was followed by a decrease and return to pre-test calcium levels. Further experiments indicated that mechanisms intrinsic to the GnRH neuron are responsible for their prolonged depolarizing response to kisspeptin. These studies indicate that kisspeptin activates GPR54 to initiate a PLC–IP3R-calcium cascade that modulates both potassium and NSC channels to initiate depolarization in GnRH neurons.
In 2003, two groups discovered that GPR54 was essential for puberty and subsequent fertility in humans (1, 2). Since that time, a substantial amount of work has been undertaken to elucidate the signaling pathways and mechanisms through which kisspeptin activates GPR54 to regulate fertility. In all species examined to date, it appears that kisspeptin neurons located within the hypothalamus provide direct excitatory inputs to gonadotropin-releasing hormone (GnRH) neurons. Kisspeptin activates GnRH (3) and luteinizing hormone (LH) (4–6) secretion in vivo, and GnRH neurons are surrounded by kisspeptin fibres (7), express GPR54 mRNA (8, 9), and are activated intensely by kisspeptin (10). Importantly, kisspeptin is unable to stimulate LH secretion in GPR54 knockout mice (3) and kisspeptin knockout mice are infertile (11), demonstrating that kisspeptin and GPR54 are exclusive signaling partners with respect to fertility control. At present, it seems very likely that the excitatory influence of kisspeptin upon GnRH neurons is essential for puberty to proceed (6, 10) and evidence is accumulating for a similar role in ovulation (12–14).
In our previous electrophysiological experiments we reported that kisspeptin was the most potent activator of GnRH neurons yet discovered (10). Patch-clamp recordings of GnRH neurons in the acute brain slice prepared from adult male and female GnRH-GFP transgenic mice demonstrated that a brief application of 10-100nM kisspeptin evoked a prolonged membrane depolarization that was often accompanied by intense firing. Given the key importance of kisspeptin-GPR54 signaling to fertility, and the remarkable effects of kisspeptin on GnRH neuron excitability, it is essential that the intracellular signaling cascade initiated by GPR54 activation in GnRH neurons is established. Early studies on GPR54 expressed in HEK and CHO cells showed that this receptor was likely coupled to Gq/11 and resulted in the activation of calcium from intracellular stores (15, 16). More recent studies examining GnRH release from juvenile female hypothalamic explants in vitro, showed that kisspeptin’s stimulatory effect was blunted by a phospholipase C (PLC) blocker and the calcium store depleter thapsigargin, in addition to ERK1/2 and p39 MAPK antagonists (17). Together, these data suggest that GPR54 utilizes a Gq/11 – PLC – inositol (1,4,5)-trisphosphate (IP3) – Ca2+ pathway to alter cellular activity. The relevance of this pathway to GPR54 activation in GnRH neurons themselves has not been established and, critically, the ion channels modulated to activate GnRH neurons are not known. The present study employed perforated patch-clamp electrophysiology and calcium imaging in GnRH transgenic mouse lines to address these issues.
Materials and Methods
Animals
Adult GnRH–GFP (18) and GnRH-Pericam (19) mice were housed under 12 h light/dark cycles (lights on at 7:00 A.M.) with ad libitum access to food and water. All experimentation was approved by the University of Otago Animal Welfare and Ethics Committee. The cycles of female mice were determined by vaginal smear with females being investigated on diestrus. Due to the nature of perforated-patch electrophysiological recordings, the great majority (>90%) of mice provided only a single acceptable recording. As such, the animal N number is almost identical with the GnRH neuron N number. The same is true for the calcium imaging experiments reported here where the 10 recorded neurons were obtained from 8 individual mice.
Electrophysiology
Gramicidin-perforated patch recordings of GnRH neurons were undertaken at room temperature on 200 μm-thick coronal brain slices, containing the rostral preoptic area, cut with a vibratome (Leica VT1000S). After cutting in high (6 mM) MgCl2 and low (0.5 mM) CaCl2 artificial cerebrospinal fluid (aCSF), slices were incubated in normal aCSF (in mM: 118 NaCl, 3 KCl, 2.5 CaCl2, 1.2 MgCl2, 11 D-glucose, 10 HEPES, 25 NaHCO3) equilibrated with 95% O2-5% CO2 for ~1h at 30ºC and then >20min at room temperature. Slices were transferred to a submerged recording chamber and viewed under a fixed-stage upright microscope (BX51WI; Olympus, Tokyo, Japan). Fluorescent GnRH neurons were identified under brief fluorescence illumination and patched under Nomarski differential interference contrast optics. Patch pipettes with resistances between 3–5.5 MOhms were pulled from glass capillaries (id, 1.18 mm; od, 1.5 mm) with a microelectrode puller (Sutter Instruments, Novato, CA). Gramicidin (Sigma, St. Louis, MO) was dissolved in methanol to a concentration of 10 mg/ml, which was diluted in the pipette solution (in mM: 145 KCl, 5 NaCl, 0.1 CaCl2, 10 HEPES, 0.5 BAPTA, pH 7.35 adjusted by KOH, ~290 mOsmol) to a final concentration of 25~75 μg/ml just before use, then sonicated for 1-2 min. After a giga-seal was established, access resistance was monitored, and experiments in current clamp were begun when access resistance (Ra) reduced to below 100 MOhms (36±1MOhms, n=188). The 188 GnRH neurons included in this report had an average resting membrane potential (RMP) of -55±1mV and Rin of 1478±35MOhms. Signals (voltage and current) were amplified with a Multiclamp 700B (CV7B; Molecular Devices, Foster City, CA) and sampled on-line with the use of a Digidata 1440A interface (Molecular Devices) connected to a personal computer. Signals were filtered (3 or 10 kHz; Bessel filter of Multiclamp 700B) before being digitized at a rate of 1 kHz. Acquisition and subsequent analysis of the acquired data were performed with the Clampex 10 suite of software (Molecular Devices) and Origin pro 7.5 (OriginLab Corporation, Northampton, MA). RMPs were the potentials recorded in current clamp without applying any holding current and were not corrected for the liquid junction potentials of ~-2 mV. The input resistance (Rin) was determined by fitting the linear part of the current-voltage curve (in current clamp from -60 to -90mV). During experiments, Ra was monitored and if found to change by >15%, the cell was discarded.
Electrophysiology analysis
A significant membrane depolarization was defined as being >1.5mV change from the baseline. To compare the kisspeptin-induced changes in the frequency of action potentials, the percentage change in firing frequency was calculated as follows: 100 x ((frequency during test minus frequency during control)/frequency during test). In this way, silent cells induced to fire had a maximum increase in frequency of 100%. Data for the duration of kisspeptin responses was determined from those cells in which membrane potential returned to pre-test levels within 30 min of the kisspeptin application. Some GnRH neurons do not return to baseline levels during the entire recording period which can be >60 min. However, statistically-useful datum collection on the duration of kisspeptin effects becomes limited by the period of time within which the majority of GnRH neurons can be held in an acceptable perforated-patch mode. Based on our previous experience, we have set this to be 30 min as we find that 95% of recordings can be maintained for this period. As kisspeptin can only be applied to any GnRH neuron (or brain slice) once, pharmacological analyses were undertaken by assigning brain sections to control or specific drug treatments and the % of responding cells analyzed using Fisher's Exact Test. Data are expressed as mean±SEM.
Calcium imaging and analysis
This was undertaken as reported previously (19) with slight modifications. In brief, 200 µm-thick coronal brain slices were prepared from adult female GnRH-Pericam mice as detailed above for electrophysiological experiments using the same aCSF. Slices were placed in a tissue chamber perfused with oxygenated aCSF (22±1°C) at a rate of 2-3 ml/min. Cells were imaged using an Olympus BX51 upright microscope (Tokyo, Japan). Excitation was performed by exposing slices to 415 nm wavelength for 200 ms every second, and emission filtered at 525 nm with the use of a Sutter Instruments Lambda DG-4 high-speed filter unit. Images were acquired using Metafluor to control and synchronize the DG-4 and liquid-cooled ORCA-ER CCD camera (Hamamatsu, Japan). Fluorescence intensity (FI) values were exported for a region of interest on the cell and one of the same area on the background, and analyzed with Origin Pro 7.5. The baseline calcium level was obtained by dividing the difference between the FI cell (Fc) and the FI background (Fb) by Fb. [Baseline [Ca2+]i = (Fc-Fb)/Fb]. This was calculated for every measurement and the % change in baseline calcium evoked by kisspeptin determined by the following formula, ((peak evoked [Ca2+]i – mean of 1 min pre-test [Ca2+]i levels)/ mean of 1 min pre-test [Ca2+]i levels) x 100.
Drugs
The different drugs used for experiments are shown in Table 1. All compounds were obtained from Sigma (St. Louis, MA) with the exception of kisspeptin and PD98059 from Calbiochem (San Diego, CA) and TTX from Tocris (Ellisville, MA). Stock solutions were prepared in DMSO at the concentrations indicated and stored at -20ºC: 2-APB (100 mM), kisspeptin (2 mM), PD98059 (20mM), U73122 (5 mM), and U73343 (5 mM). The final DMSO concentrations were less than 0.2% (v/v). Stock solutions of TTX (0.5 mM) and Antide (100 mM) were prepared with distilled water and stored at -20ºC. FFA was diluted in ethanol at 100 mM before use and final ethanol concentration was 0.1% (v/v). All drugs were applied in perfusion solution with final concentration as indicated.
Table 1. List of drugs (in order of appearance in the text).
| Kisspeptin | GPR54 receptor agonist | |
| Antide | GnRH receptor antagonist | |
| Kynurenic acid | broad-spectrum ionotropic glutamate receptor antagonist | |
| Picrotoxin | GABAA receptor antagonist | |
| TTX | tetrodotoxin | voltage-dependent sodium channel blocker |
| Ba2+ | broad-spectrum potassium channel blocker | |
| FFA | flufenamic acid | NSC channel antagonist |
| U73122 | 1-[6-[((17β)-3-Methoxyestra-1,3,5[10]-trien-17-yl)amino]hexyl]-1H-pyrrole-2,5-dione | phospholipase C antagonist |
| U73343 | 1-[6-[((17β)-3-Methoxyestra-1,3,5[10]-trien-17-yl)amino]hexyl]-2,5-pyrrolidinedione | inactive analog of U73122 |
| 2-APB | 2-aminoethoxy diphenyl borate | IP3 receptor antagonist |
| PD98059 | 2-(2-Amino-3-methoxyphenyl)-4H-1-benzopyran-4-one | ERK1/2 antagonist |
Results
To ensure that electrophysiological recordings were made from GnRH neurons in which their intracellular milieu was undisturbed, we utilized current- and voltage-clamp, perforated-patch recordings with Ra <40 MΩ. Our previous study demonstrated that 100nM kisspeptin is an effective and direct activator of GnRH neurons in the acute brain slice preparation (10).
Kisspeptin activates the majority of GnRH neurons in both male and female mice
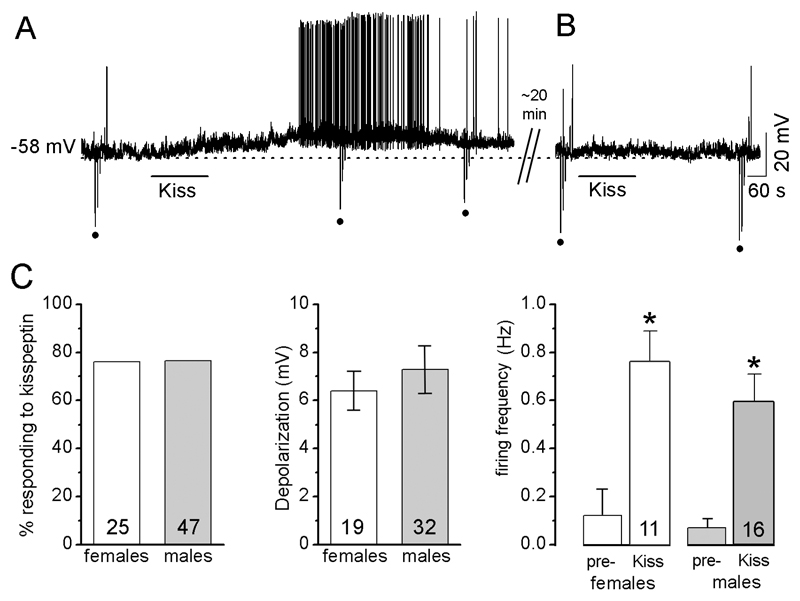
In the first series of experiments, kisspeptin (100 nM for 2-4 min) was applied to 51 GnRH neurons recorded in current clamp and held at their RMP. Of these, 38 (74.5%) responded to kisspeptin with depolarization (6.4±0.9 mV; n=35) and/or an increase in firing of APs (0.12±0.07 to 0.68±0.13 Hz, increase of 87.1±4.3%, n=18). Kisspeptin depolarized GnRH neurons within 90 s of application and, for those GnRH neurons returning to pre-test membrane potential levels within 30 min (see Methods), the depolarization lasted on average for 20.8±2.8 min (n=13; Fig. 1A). Thirteen cells were tested with a subsequent application of 100nM kisspeptin once they had returned to baseline RMP but none responded (Fig. 1B).
Figure 1. Kisspeptin depolarizes GnRH neurons.
Note: for all figures, the upward and downward deflections (dots) from the voltage traces are responses to the injection of hyperpolarizing or depolarizing currents to test membrane resistance. Drug applications are indicated by the horizontal line above or under traces. A, Representative voltage trace showing kisspeptin depolarization of a GnRH neuron with a decrease in Rin. Note, responses to current injections (dots) are smaller during kisspeptin than those during control. B, the same cell approximately 40 min after the initial kisspeptin test does not respond to a second kisspeptin application. C, Histograms showing no differences between diestrous females and males in the % of GnRH neurons that respond to kisspeptin, the degree of depolarization evoked by kisspeptin or the increase in firing frequency evoked by kisspeptin. Pre-= firing rate before kisspeptin. Kiss = firing rate during kisspeptin application * p<0.001 compared with “pre-“, paired-Student-t test. The N numbers are given at the base of each histogram.
No sex differences were found in the effects of kisspeptin on adult GnRH neurons (Fig.1C). We found that 76% of diestrous female GnRH neurons (n=25) responded to kisspeptin compared with 77% in males (n=47). No difference was detected in the evoked depolarization (6.4±0.8 mV in females, n=19; 7.3±1.0 mV in males, n=32) or evoked increase in firing (0.12±0.11 to 0.76±0.14, 90±6% increase in 13 female GnRH neurons; 0.07±0.04 to 0.59±0.12, 90±3% increase in 16 male GnRH neurons)(Fig.1C).
Kisspeptin initiates a prolonged depolarization that depends upon intrinsic properties of GnRH neurons
To assess the mechanisms responsible for the prolonged nature of the kisspeptin-evoked depolarization, we examined the effects of Antide, TTX and amino acid receptor blockers. Antide, the GnRH receptor antagonist, was tested to examine whether GnRH neurons maintain their depolarized state by mutual- or auto-excitation through collateral innervation. We have previously shown that 100 nM Antide completely blocks the effects of GnRH on GnRH neurons (20). TTX was used to examine, more generally, whether action potential-dependent transmission from recurrent feedback loops were involved in the prolonged excitation. Finally, the amino acid receptor blockers kynurenic acid (1 mM, broad-spectrum ionotropic glutamate receptor antagonist) and picrotoxin (0.1 mM, GABAA receptor antagonist) were used as a cocktail to examine the role of action potential-dependent and -independent amino acid transmission.
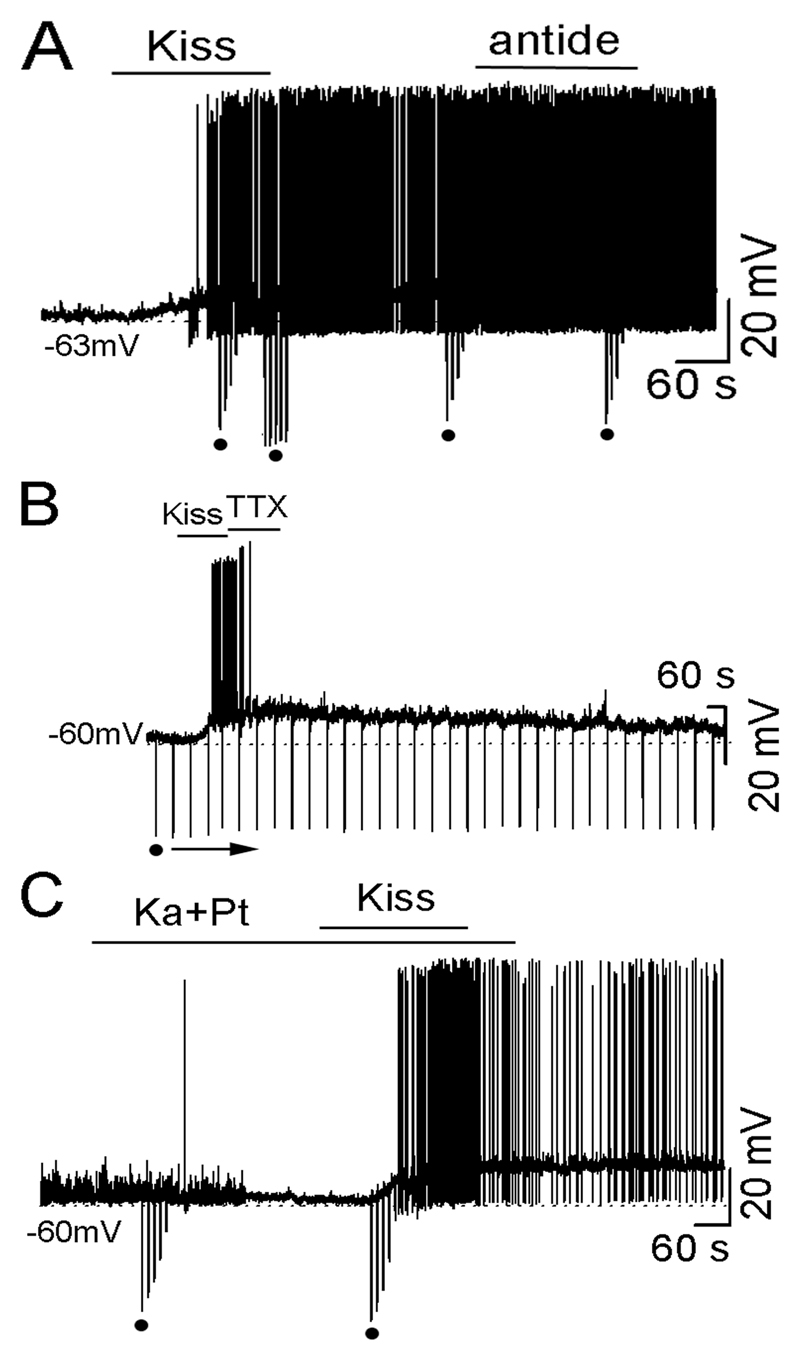
Antide (100 nM for 4 min), applied before (n=10) or after kisspeptin (n=10; Fig. 2A) treatment, did not change the response of GnRH neurons to kisspeptin. Following pretreatment with Antide, kisspeptin depolarized (6.2±1.3mV, duration 20.5±1.3min) 6 of 10 GnRH neurons and increased firing frequency by 84±6%. For cells in which Antide was administered following the kisspeptin activation, no effect on membrane potential or firing rate was found (Fig.2A) and the duration of the response remained unaltered (21.2±1.1 min).
Figure 2. Kisspeptin excites GnRH neurons via an intrinsic mechanism independent upon external neural transmission.
A, representative voltage trace showing kisspeptin-induced depolarization and firing was not affected by antide (100 nM) administered during the kisspeptin activation. B, representative voltage trace showing kisspeptin-induced depolarization was not affected by TTX (0.5 μM administered during the kisspeptin activation. C, representative voltage trace showing kynurenic acid (1 mM) and picrotoxin (0.1 mM) (Ka+Pt) blocking synaptic events but having no effect on the kisspeptin-induced depolarization.
TTX administered to GnRH neurons (n=3) after their activation by kisspeptin was found to block action potential generation but had no effect upon the magnitude or length (23±5 min) of membrane depolarization (Fig.2B).
The amino acid blocker cocktail blocked postsynaptic potentials in GnRH neurons (Fig.2C) but had no effect upon the kisspeptin responses of GnRH neurons. Of 21 GnRH neurons tested, 17 (81%) responded to kisspeptin with a depolarization (8.1±0.9 mV, duration 22.8±2.9 min; n=15) and/or an increase in firing (0.03±0.04 to 0.61±0.10 Hz, increased by 94.9±2.8%, n=9; Fig. 2C).
Together, these observations suggest that the prolonged depolarizing response of GnRH neurons to kisspeptin is an intrinsic mechanism not dependent upon external neural transmission.
Potassium and non-selective cation (NSC) channels underlie kisspeptin’s deplorizing effects on GnRH neurons
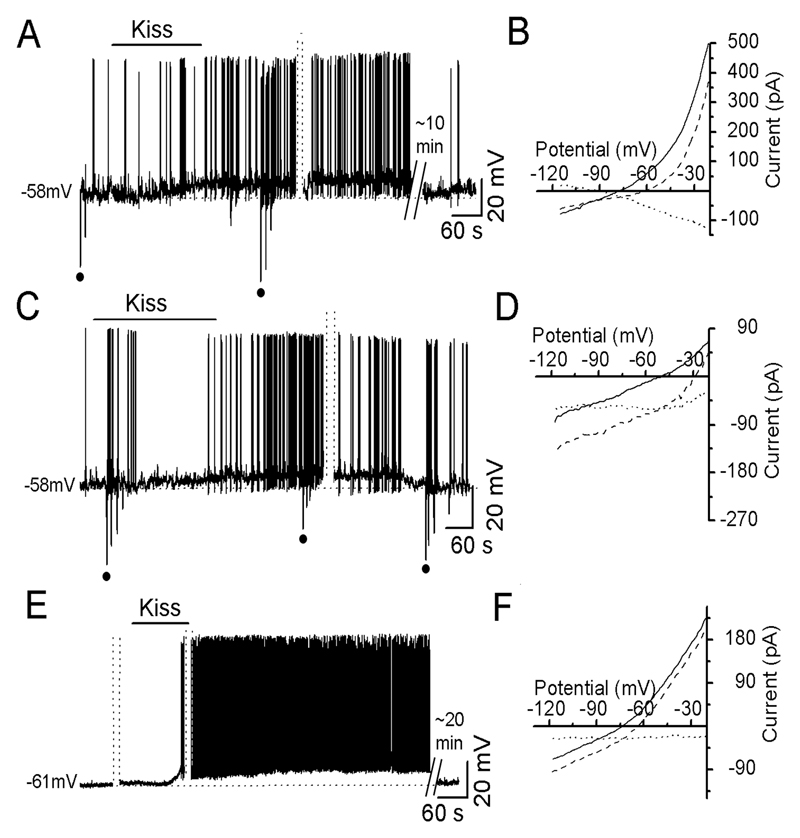
To identify the ion channels underling kisspeptin’s initial depolarizing effects, 40 GnRH neurons held at -60mV in current clamp were tested with 100nM kisspeptin. Before, and at different times after kisspeptin administration, current clamp was switched to voltage clamp to establish current-voltage curves (IV curve). On the basis of Rin change and the reversal potential of the current induced by kisspeptin, we found that GnRH neurons could be classified into three groups:
-
1)
Depolarization with an increase in Rin and reversal potential of -93±2.8 mV (Fig.3AB; n=6). This was suggestive of a depolarizing mechanism involving the closing of K+ channels.
-
2)
Depolarization with a decrease in Rin and reversal potential of -10±5.1 mV (Fig. 3CD; n=7). This response suggested that the depolarization occurred through the opening of NSC channels.
-
3)
Depolarization without a constant change in Rin or reversal potential (Fig.3EF, n=27). This could occur through the opening and/or closing of multiple ion channels.
Figure 3. Kisspeptin depolarizes GnRH neurons by affecting multiple ion channels.
Dotted-parallel lines indicate the switch from current to voltage clamp to establish IV curves (3-5 min duration) and omitted voltage sections are also indicated. A, Representative voltage trace showing kisspeptin depolarizing a GnRH neuron with an increase in Rin. The duration of depolarization is about 26 min. B, IV curves established by running a ramp from -20 to -120mV for 4s in voltage clamp. Solid line: an IV curve obtained before kisspeptin; dashed line: an IV curve during kisspeptin action; dotted line: an IV curve obtained by the point-by-point subtraction of currents during kisspeptin from those of control at corresponding potentials mathematically (the same for E and F). This shows that a kisspeptin-induced current reversed at -95mV. C, Representative voltage trace showing kisspeptin depolarizing a GnRH neuron with a decrease in Rin (responses to current injections (dots) are smaller during kisspeptin than control). D, IV curves and dotted line showing kisspeptin-induced current reversed at about -10 mV (estimated by extrapolating). E, Representative voltage trace showing kisspeptin depolarizing a GnRH neuron without any change in Rin (See IV curves in F). Note the kisspeptin application indication line is shorter than 3 min due to omitted trace during voltage recording. F, IV curves and dotted line showing kisspeptin-induced current did not reverse between -20 and -120 mV.
For all three types of response, the same Rin and IV curve was found regardless of when it was assessed following application of kisspeptin.
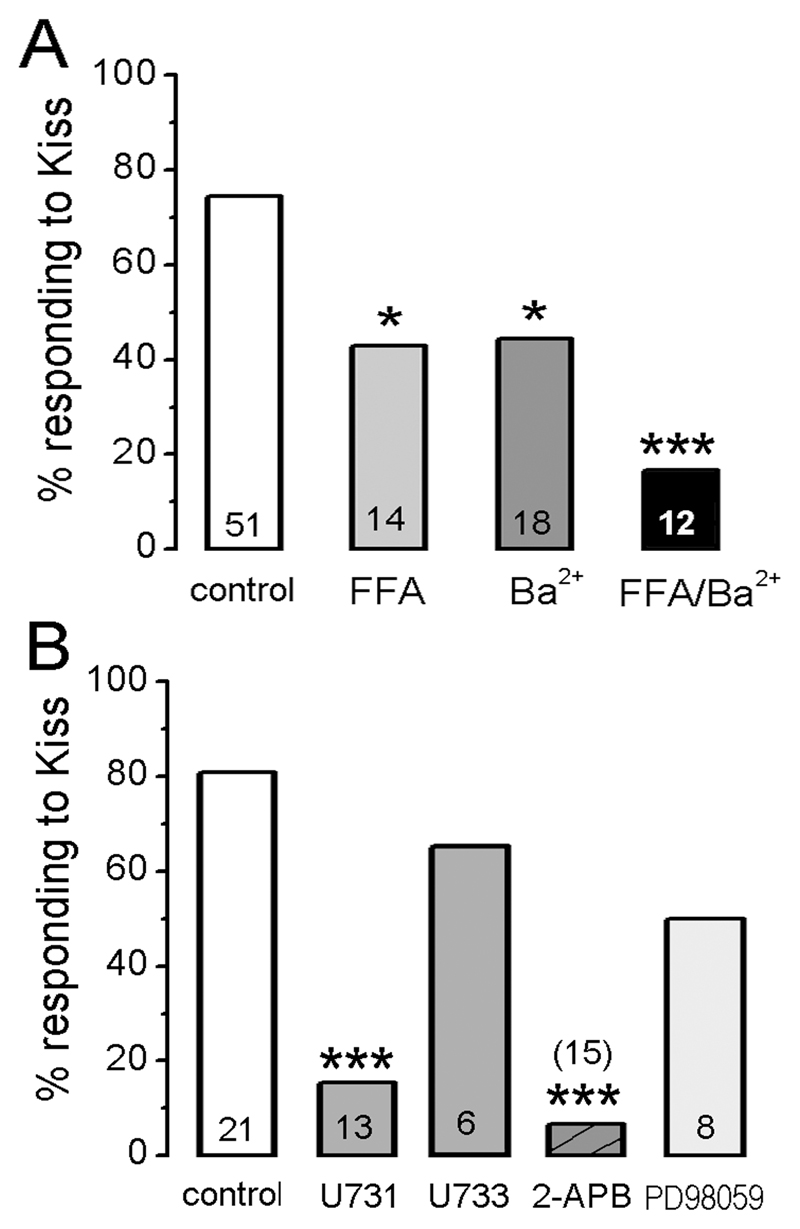
As these observations suggested that potassium and NSC channels were involved in the kisspeptin response we undertook a pharmacological analysis of kisspeptin action using Ba2+, a broad spectrum potassium channel blocker, and 0.1mM flufenamic acid (FFA), an NSC channel antagonist (21, 22). As kisspeptin can only be applied once to a GnRH neuron, brain slices were attributed to control (no drug), Ba2+ alone, FFA alone, or both Ba2++ FFA treatments. The percentage of responding cells was determined and tested for significant differences by Fisher's Exact Test.
Controls
In total, 38 of 51 (75%) control GnRH neurons given no pharmacological pre-treatment responded to kisspeptin.
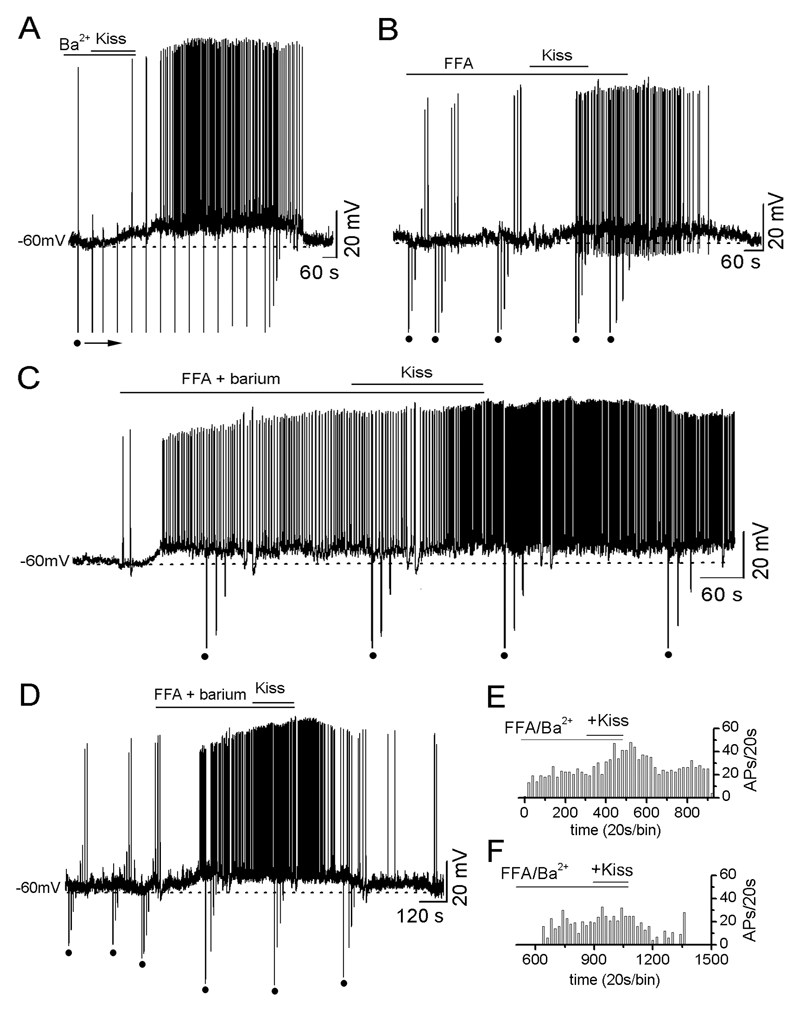
Ba2+ applied to 27 GnRH neurons was found to depolarize most cells (n=23) by itself (7.5±1.1 mV depolarization with a 78±7% increase in firing) suggesting that most all GnRH neurons have tonically-active Ba2+-sensitive potassium channels restraining their excitability. Kisspeptin (100nM) applied in the presence of Ba2+ (with or without the membrane potential being held back to control levels) still depolarized 8 of 18 (44%) GnRH neurons (n=18; Fig. 4A) by 5.9±1.3 mV (n=8) and increased firing frequency by 38±14% (n=8). The other 10 GnRH neurons showed no response to kisspeptin. The decrease in the % of GnRH neurons depolarized by kisspeptin in the presence of Ba2+ (44% versus controls 75%) was significantly different (p<0.05; Fig.5A).
Figure 4. Ba2+ and FFA decrease the number of kisspeptin-responsive GnRH neurons.
A, Representative voltage trace showing kisspeptin depolarizing a GnRH neuron in the presence of Ba2+ with an increase in Rin. The depolarization induced by 3 min pre-application of 1 mM Ba2+ was removed by injected current (not shown) and then 100nM kisspeptin applied as indicated. B, A representative voltage trace showing kisspeptin depolarizing another GnRH neuron in the presence of 0.1mM FFA with an increase in Rin. Note that 5 min pre-application of FFA did not change membrane potential. C, A voltage trace showing one of the two GnRH neurons still responding to kisspeptin in the presence of FFA+Ba2+. Pre-application of FFA+Ba2+ depolarized the cell with an increase in Rin. Note that kisspeptin had no further effect on membrane depolarization but increased the frequency of action potentials (see E). D, Representative voltage trace showing typical effect of kisspeptin being unable to activate GnRH neurons in the presence of Ba2++FFA. In this case the Ba2++FFA depolarize the cell and make it fire but kisspeptin has no further effect. E & F, Ratemeter histograms of action potential firing rate (20s bins) for the two cells shown in C and D, respectively.
Figure 5. Histograms showing the percentage of GnRH neurons responding to kisspeptin in the presence of different antagonists.
A. Ion channel experiments. B. Intracellular signaling cascade experiments. control = cells tested with kisspeptin without antagonists; U731 = U73122; U733 = U73343. Total number of cell tested is given at base of each histogram. * p<0.05 and *** p<0.001 compared with control (Fischer’s exact test).
FFA itself was found to have no effects upon the majority (85%) of recorded GnRH neurons (Fig.4B) with the remaining 15% showing either small depolarizations or hyperpolarizations. When kisspeptin was applied following FFA, 6 of 14 (43%) of GnRH neurons responded in the normal manner with an increase in membrane potential (5.1±0.7 mV; n=5) and/or an increase in firing frequency of 59±22% (n=4; Fig.4B). The remaining 8 GnRH neurons showed no response to kisspeptin. The decrease in the % of GnRH neurons depolarized by kisspeptin in the presence of FFA was significantly different compared with controls (p<0.05; Fig.5A).
Ba2+ and FFA applied together were found to depolarize GnRH neurons in the same way described for Ba2+ alone (Fig.4C). Kisspeptin (100nM) applied in the presence of Ba2++FFA depolarized and/or increased the firing rate of 2 out of 12 (17%) GnRH neurons (Fig.4C,E) with the remainder being unresponsive (Fig.4D,F). The decrease in the % of GnRH neurons depolarized by kisspeptin in the presence of Ba2++FFA was significantly different compared with controls (p<0.05; Fig.5A).
Kisspeptin requires phospholipase C (PLC) and IP3 receptors to activate GnRH neurons
To evaluate the intracellular signaling pathway responsible for kisspeptin’s depolarizing actions, a pharmacological approach was employed by using blockers of phospholipase C (U73122, and its inactive analog U73343) (23), IP3 receptors (2-APB) (24) and ERK1/2 (PD98059) (25). To try and isolate effects of these compounds to GnRH neurons, while retaining the ability to observe action potentials, these experiments were undertaken in the presence of the amino acid receptor antagonists kynurenic acid and picrotoxin. As kisspeptin can only be applied once to a GnRH neuron, brain slices were attributed to control (no drug), U73122, U73343, 2-APB or PD98059 treatments and the percentage of responding cells determined and tested by Fisher's Exact Test.
Controls
In total, 17 of 21 (80%) control GnRH neurons pre-treated with kynurenic acid and picrotoxin responded to kisspeptin. The cocktail of amino acid blockers themselves resulted in depolarizations in ~15% of GnRH neurons.
U73122
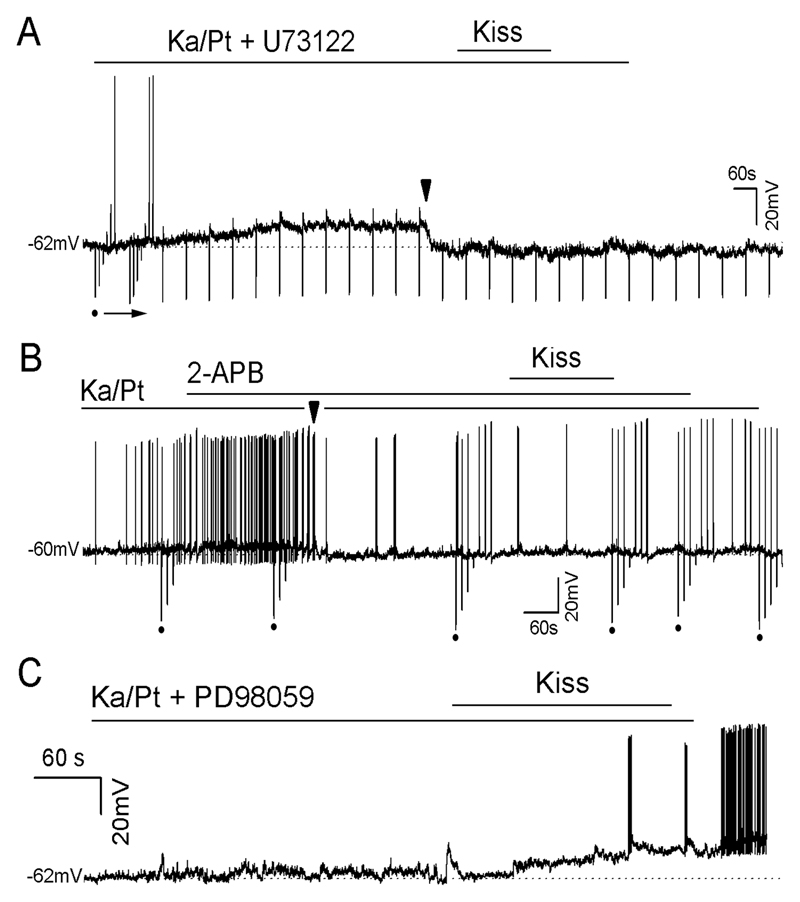
Pretreatment (8-15 min) with 10 μM U73122 resulted in 2 of 13 GnRH neurons (15%) responding to kisspeptin with depolarization or increased firing with the remainder being unresponsive (Fig.6A). This response rate was significantly different compared with controls (Fig.5B; p<0.001). Treatment with U73343, the inactive analog, resulted in 4 of 6 (67%; p>0.05) GnRH neurons responding to kisspeptin (Fig.5B).
Figure 6. Intracellular signaling casecad involved in mediating kisspeptin depolarization of GnRH neurons.
All experiments were undertaken in the presence of the amino acid blockers 1mM kynurenic acid and 0.1mM picrotoxin (Ka/Pt). A, Representative voltage trace showing a GnRH neuron that fails to respond to 100nM kisspeptin following pre-treatment with 10μM U73122. Note that the Ka/Pt/U73122 cocktail progressively depolarized the cell and that it was held back to control RMP level by injecting current at the arrowhead. B, Representative example of a GnRH neuron that fails to be activated by kisspeptin following 10 min pretreament with 100μM 2-APB. Note that Ka/Pt depolarized the cell progressively and that it was held back to its control RMP by injecting current (arrowhead). C, Representative example of a voltage trace of a GnRH neuron that was activated by kisspeptin despite pretreatment with 20μM PD98059.
2-APB
Pretreatment (10 min) with 100 μM 2-APB had no consistent effect on GnRH neuron membrane potential but resulted in only 1 of 15 (7%) GnRH neurons responding to kisspeptin with remainder being unresponsive (Fig.5B). This response rate was significantly different to that of controls (p<0.001).
PD98059
Pretreatment (5 min) with 20 μM PD98059, that again had no consistent effect on membrane potential itself, resulted in 4 of 8 (50%) of GnRH neurons responding to kisspeptin with depolarization (8.6±2.5mV, n=4) and an increase in firing (Fig.6C). This was not significantly different compared with controls (Fig.5B).
Kisspeptin initiates an increase then decrease in intracellular calcium concentrations [Ca2+]i
As the electrophysiology results suggested that kisspeptin was likely to activate an increase in [Ca2+]i through PLC activating IP3Rs, we used GnRH-Pericam mice to examine the effect of kisspeptin upon [Ca2+]i in GnRH neurons. The GnRH-pericam transgenic line allows real-time imaging of [Ca2+]i in GnRH neurons in the acute brain slice preparation (19).
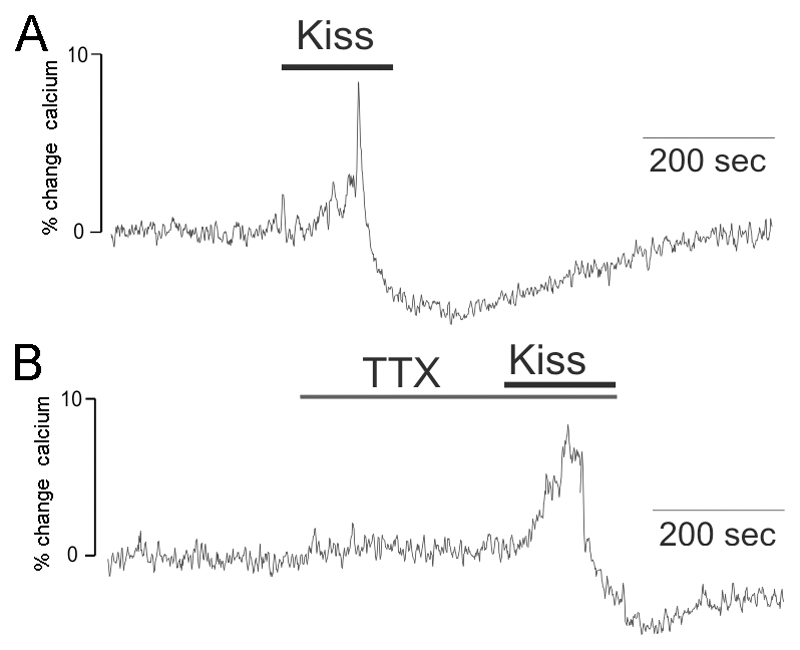
Kisspeptin at 100nM was found to evoke a rise in [Ca2+]i immediately following entry into the bath in 6 of 6 female GnRH neurons (Fig.7A). An increase in [Ca2+]i peaking at 11±3% above pre-test baseline levels lasted for 2-3 minutes and was then followed by a sudden drop in [Ca2+]i before levels gradually returned to basal concentrations (Fig.7A). Four female GnRH neurons were tested with kisspeptin in the presence of 0.5 μM TTX and 3 responded (75%) with a similar profile including an 11±5% baseline increase in [Ca2+]i (Fig.7B). The remaining cells did not display any change in [Ca2+]i.
Figure 7. Kisspeptin alters intracellular calcium levels in GnRH neurons.
Traces showing the effects of 100nM kisspeptin upon [Ca2+]i in GnRH neurons of GnRH-Pericam transgenic mice. The trace shows the % change in [Ca2+]i calculated by determining [Ca2+]i every second and comparing it to the mean [Ca2+]i levels in the 1 min prior to the testing period. A. A representative trace showing a GnRH neuron responding to kisspeptin with an increase in [Ca2+]i followed by a sudden decline and slow recovery. B. A GnRH neuron pre-treated with tetrodotoxin (TTX) showing the same response to 100nM kisspeptin.
Discussion
Kisspeptin is a remarkably potent activator of GnRH neurons. As noted in our initial study (10), and also reported recently by others (26), a short application of 10-100nM kisspeptin generates a 5-10 mV depolarization that is prolonged, lasting for at least 20 min. However, this response appears to desensitize quickly as GnRH neurons no longer respond to kisspeptin 20-30 min after the first application. Given the potent effects of kisspeptin on GnRH neuron excitability, this desensitization may be important in preventing GnRH neurons from over-excitation. It is not known how long this period of desensitization lasts, but it is important to note that hourly kisspeptin administration can initiate LH pulses over several hours in the monkey (27). This suggests that the GPR54 receptor expressed by GnRH neurons can be re-activated after 60 min in vivo. It will be interesting in future studies to determine the mechanism of GPR54 receptor desensitization in GnRH neurons.
Although sex differences exist in the expression of kisspeptin protein and mRNA within the rostral preoptic area (28, 29), we have not found any sex differences in the percentage of GnRH neurons responsive to kisspeptin, or in the dynamics of their response. This suggests that GPR54 receptor expression by GnRH neurons is not sexually differentiated. As such, any sexually dimorphic features of kisspeptin-GPR54 signaling with respect to GnRH neurons most likely result from post-synaptic differences such as kisspeptin innervation (7).
Using conventional voltage-clamp procedures, we found that many GnRH neurons exhibited a marked kisspeptin-induced depolarization without any apparent change in their Rin or IV relationship. Although ~ 15% of cells exhibited changes in Rin with IV curves characteristic of potassium channel closure, and another ~ 15% exhibited features consistent with the opening of a NSC channel, the remainder of GnRH neurons exhibited no change in Rin or IV relationship during kisspeptin-evoked depolarization. To probe this further, we used barium as a relatively broad-spectrum potassium channel antagonist and found that it was able to block ~40% of the normal number of GnRH neurons from responding to kisspeptin. Similarly, NSC channel blockade with FFA resulted in a significant ~40% reduction in the number of responding GnRH neurons. This suggested that barium-sensitive potassium and NSC channels were necessary for kisspeptin-evoked depolarization in many more than the 15% of GnRH neurons indicated by the IV studies. When both antagonists were added together, we found that nearly 80% of the kisspeptin-responding GnRH neurons were unable to be activated (17% of GnRH neurons responded compared with 75% for controls). Thus, kisspeptin-evoked depolarizations in the great majority of GnRH neurons showing no Rin or IV change require potassium and/or NSC channels.
The most plausible explanation for these results is that GPR54 activation results in the inhibition of potassium channels as well as the activation of a NSC channel (Fig.8). As such, the effects of both channels effectively cancel each other out in terms of Rin and result in a depolarization without any IV reversal. Interestingly, the exact same scenario has been suggested to account for the depolarizing actions of muscarinic receptor activation in locus coeruleus and CA1 hippocampal neurons (30, 31). In those cases, acetylcholine was found to evoke a depolarization without a change in Rin and, as found here, two different IV relationships suggestive of potassium channel closure and NSC channel opening were encountered. The GnRH neurons rarely respond as a homogenous population (32) and the present results would suggest a variable balance of potassium versus NSC channel dependence for the kisspeptin responses in individual GnRH neurons. Nevertheless, it is clear that by removing both channels, the great majority of GnRH neurons are prohibited from responding to kisspeptin. The small population of GnRH neurons that respond to kisspeptin in the presence of barium and FFA may represent the activation of yet another ion channel class following GPR54 activation. It seems unlikely that the heterogeneity in GnRH neuron response arises from the GPR54 receptor itself as nearly all GnRH neurons in the adult mouse are reported to express GPR54 mRNA (10).
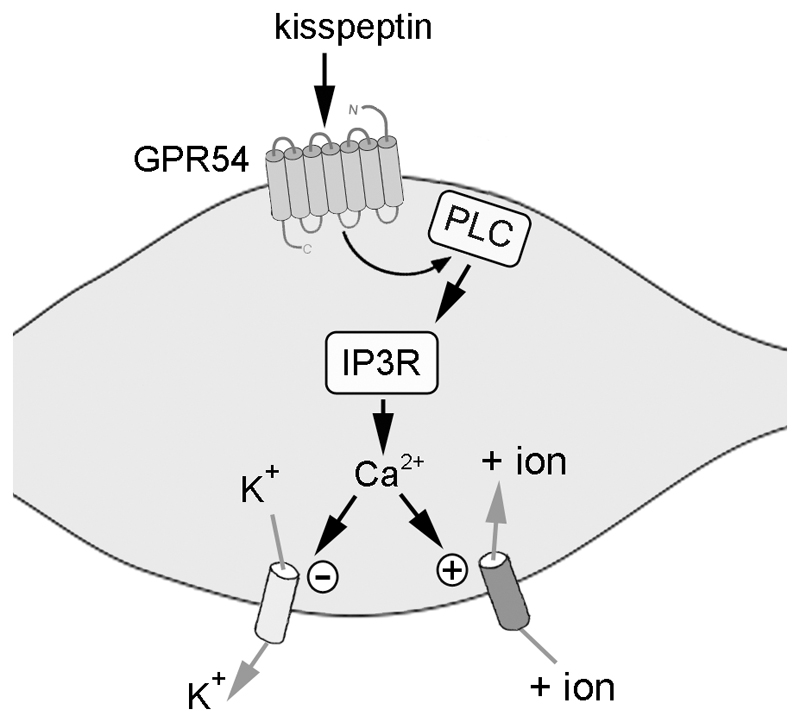
Figure 8. Schematic diagram showing how kisspeptin activates GnRH neurons.
Kisspeptin binds to GPR54 and this results in the activation of PLC that in turn results in the release of calcium from stores following stimulation of IP3R. The released calcium then closes a calcium-sensitive potassium channel and/or opens a calcium-sensitive non-selective cation channel.
The prolonged nature of the kisspeptin-induced depolarization and accompanying intense firing is unique to GPR54 activation in GnRH neurons, and likely to be important physiologically. Although the prolonged nature of the GnRH response does not appear to fit well with a role for kisspeptin in initiating individual GnRH pulses, it would seem to match the prolonged pattern of GnRH neuron activation suspected to be necessary for ovulation (33, 34). Several studies have suggested that kisspeptin may be involved in the generation of the preovulatory GnRH/LH surge (12–14). For this reason, we were interested in examining the mechanism behind the prolonged depolarization evoked by kisspeptin. We speculated that this may arise from recurrent feedback loops involving GnRH itself or other neurotransmitters. Electron microscopy studies have documented the presence of GnRH terminals synapsing on GnRH neurons (35) and GnRH exerts a depolarizing action upon GnRH neurons (20). Thus, it was plausible that, once activated, GnRH neurons may maintain their activation through collateral reciprocal- or even auto-feedback. However, we failed to provide any evidence for this possibility as Antide, the GnRH receptor antagonist, had no effect upon the kisspeptin-evoked depolarization. Similarly, and in agreement with the Antide result, TTX had no effect suggesting that recurrent feedback loops and afferent inputs are not necessary. As, GnRH neurons are subjected to considerable action potential-independent amino acid transmission (36), we also examined the effect of glutamate and GABA receptor blockers but, again, found the kisspeptin response to be unchanged. Together, these observations clearly document that the prolonged depolarizing response of GnRH neurons to kisspeptin does not require extrinsic neural components. The identity of the intrinsic mechanism maintaining the kisspeptin depolarization is unknown but it is interesting to note the transient (2-3 min) nature of the [Ca2+]i rise evoked by kisspeptin suggesting that it may not rely upon prolonged changes in [Ca2+]i.
The intracellular pathway underlying the effects of kisspeptin on GnRH neurons is shown here to involve a PLC - IP3R – calcium cascade. The PLC antagonist U73122 was very effective in blocking GnRH neurons from responding to kisspeptin, whereas its inactive isomer U73343 had no significant effect. Similarly, 2-APB was also very potent in suppressing kisspeptin actions. In accord with these pharmacological studies showing the importance of PLC and IP3Rs, experiments in Pericam-GnRH neurons demonstrated that kisspeptin quickly elevated [Ca2+]i in GnRH neurons. Curiously, the kisspeptin-evoked increase in [Ca2+]i was not maintained but terminated after 2-3 minutes by a sudden fall that resulted in a period of depressed [Ca2+]i levels before returning to normal. We believe that this response is not, however, unique to the GPR54 cascade as the exact same profile has been observed following the caffeine-evoked increase in [Ca2+]i from calcium stores within GnRH neurons (19). It may, therefore, represent a protective, calcium re-setting mechanism triggered by any sudden increase in [Ca2+]i within adult GnRH neurons.
The present pharmacological observations in GnRH neurons are in agreement with those found recently for juvenile hypothalamic explants where U73122 and thapsigargin, a calcium store uptake blocker, were effective in suppressing kisspeptin-evoked GnRH secretion (17). In contrast to those experiments, however, the ERK1/2 inhibitor PD98059 was not found to block kisspeptin actions in GnRH neurons suggesting that ERK1/2 signaling elsewhere in the hypothalamus contributes to GnRH release in hypothalamic explants. Interestingly, the effects of kisspeptin in the dentate gyrus of the hippocampus are dependent upon ERK1/2, demonstrating the existence of cell-specific GPR54 signaling cascades within the brain.
Together, we believe that these results provide compelling evidence that kisspeptin binding to GPR54 in GnRH neurons results in the activation of a PLC - IP3R cascade to release calcium from internal stores. The link between the calcium rise and the initial membrane depolarization is very likely to depend upon calcium sensitive- potassium and NSC channels. The precise nature of the barium-sensitive potassium channel underlying this effect is not known. Barium-sensitive, calcium-dependent potassium channels have been reported (37, 38) and calcium-activated small and large conductance potassium channels, SK and BK respectively, exist in rat and mouse GnRH neurons (39–41). The closure of the SK channel in GnRH neurons would evoke a depolarization (42) consistent with the effects observed for kisspeptin.
Calcium-activated NSC channels have not been demonstrated to exist in GnRH neurons prior to this report. They comprise a large family of channels well known to be involved in neuronal excitability and the generation of maintained depolarizing responses (22). At RMP, we found that FFA had no consistent effect on membrane excitability itself suggesting that NSC channels are not tonically active. Whereas NSC channels underlie depolarizing afterpotentials in hypothalamic magnocellular neurons (21, 43), they are not involved in generating afterpotentials in GnRH neurons (44). However, we find here that FFA was able to abolish completely the kisspeptin response in a sub-population of GnRH neurons indicating the necessity for likely calcium-activated NSC channels for kisspeptin responses in these cells.
In summary, using peforated-patch electrophysiology and calcium imaging, we show that kisppetin exerts its remarkable depolarizing effects upon GnRH neurons by activating a GPR54-PLC-IP3R-calcium cascade that jointly closes potassium channels and opens NSC channels in the majority of cells (Fig.8). This signaling cascade, that appears to quickly desensitize, initiates a membrane depolarization that is then prolonged by mechanisms intrinsic to the GnRH neuron. Together these observations indicate the presence of a novel GPCR signaling mechanism responsible for the unique and essential role of kisspeptin in the neural control of fertility.
Acknowledgements
We thank Dr. Phil Heyward for advice and discussions related to the electrophysiological experiments. These studies were supported by the New Zealand Marsden Fund and New Zealand Health Research Council
Footnotes
Disclosure Statement: The authors have nothing to disclose
References
- 1.de Roux N, Genin E, Carel JC, Matsuda F, Chaussain JL, Milgrom E. Hypogonadotropic hypogonadism due to loss of function of the KiSS1-derived peptide receptor GPR54. Proc Natl Acad Sci U S A. 2003;100:10972–10976. doi: 10.1073/pnas.1834399100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Seminara SB, Messager S, Chatzidaki EE, Thresher RR, Acierno JS, Jr, Shagoury JK, Bo-Abbas Y, Kuohung W, Schwinof KM, Hendrick AG, Zahn D, et al. The GPR54 gene as a regulator of puberty. N Engl J Med. 2003;349:1614–1627. doi: 10.1056/NEJMoa035322. [DOI] [PubMed] [Google Scholar]
- 3.Messager S, Chatzidaki EE, Ma D, Hendrick AG, Zahn D, Dixon J, Thresher RR, Malinge I, Lomet D, Carlton MB, Colledge WH, et al. Kisspeptin directly stimulates gonadotropin-releasing hormone release via G protein-coupled receptor 54. Proc Natl Acad Sci U S A. 2005;102:1761–1766. doi: 10.1073/pnas.0409330102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Navarro VM, Castellano JM, Fernandez-Fernandez R, Barreiro ML, Roa J, Sanchez-Criado JE, Aguilar E, Dieguez C, Pinilla L, Tena-Sempere M. Developmental and hormonally regulated messenger ribonucleic acid expression of KiSS-1 and its putative receptor, GPR54, in rat hypothalamus and potent luteinizing hormone-releasing activity of KiSS-1 peptide. Endocrinology. 2004;145:4565–4574. doi: 10.1210/en.2004-0413. [DOI] [PubMed] [Google Scholar]
- 5.Gottsch ML, Cunningham MJ, Smith JT, Popa SM, Acohido BV, Crowley WF, Seminara S, Clifton DK, Steiner RA. A role for kisspeptins in the regulation of gonadotropin secretion in the mouse. Endocrinology. 2004;145:4073–4077. doi: 10.1210/en.2004-0431. [DOI] [PubMed] [Google Scholar]
- 6.Shahab M, Mastronardi C, Seminara SB, Crowley WF, Ojeda SR, Plant TM. Increased hypothalamic GPR54 signaling: A potential mechanism for initiation of puberty in primates. Proc Natl Acad Sci U S A. 2005;102:2129–2134. doi: 10.1073/pnas.0409822102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clarkson J, Herbison AE. Postnatal development of kisspeptin neurons in mouse hypothalamus; sexual dimorphism and projections to gonadotropin-releasing hormone neurons. Endocrinology. 2006;147:5817–5825. doi: 10.1210/en.2006-0787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Parhar IS, Ogawa S, Sakuma Y. Laser-captured single digoxigenin-labeled neurons of gonadotropin-releasing hormone types reveal a novel G protein-coupled receptor (GPR54) during maturation in cichlid fish. Endocrinology. 2004;145:3613–3618. doi: 10.1210/en.2004-0395. [DOI] [PubMed] [Google Scholar]
- 9.Irwig MS, Fraley GS, Smith JT, Acohido BV, Popa SM, Cunningham MJ, Gottsch ML, Clifton DK, Steiner RA. Kisspeptin activation of gonadotropin releasing hormone neurons and regulation of KiSS-1 mRNA in the male rat. Neuroendocrinology. 2004;80:264–272. doi: 10.1159/000083140. [DOI] [PubMed] [Google Scholar]
- 10.Han SK, Gottsch ML, Lee KJ, Popa SM, Smith JT, Jakawich SK, Clifton DK, Steiner RA, Herbison AE. Activation of gonadotropin-releasing hormone (GnRH) neurons by kisspeptin as a neuroendocrine switch for the onset of puberty. J Neurosci. 2005;25:11349–11356. doi: 10.1523/JNEUROSCI.3328-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.d'Anglemont de Tassigny X, Fagg LA, Dixon JP, Day K, Leitch HG, Hendrick AG, Zahn D, Franceschini I, Caraty A, Carlton MB, Aparicio SA, et al. Hypogonadotropic hypogonadism in mice lacking a functional Kiss1 gene. Proc Natl Acad Sci U S A. 2007;104:10714–10719. doi: 10.1073/pnas.0704114104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kinoshita M, Tsukamura H, Adachi S, Matsui H, Uenoyama Y, Iwata K, Yamada S, Inoue K, Ohtaki T, Matsumoto H, Maeda K. Involvement of central metastin in the regulation of preovulatory luteinizing hormone surge and estrous cyclicity in female rats. Endocrinology. 2005;146:4431–4436. doi: 10.1210/en.2005-0195. [DOI] [PubMed] [Google Scholar]
- 13.Smith JT, Popa SM, Clifton DK, Hoffman GE, Steiner RA. Kiss1 neurons in the forebrain as central processors for generating the preovulatory luteinizing hormone surge. J Neurosci. 2006;26:6687–6694. doi: 10.1523/JNEUROSCI.1618-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Adachi S, Yamada S, Takatsu Y, Matsui H, Kinoshita M, Takase K, Sugiura H, Ohtaki T, Matsumoto H, Uenoyama Y, Tsukamura H, et al. Involvement of anteroventral periventricular metastin/kisspeptin neurons in estrogen positive feedback action on luteinizing hormone release in female rats. The Journal of reproduction and development. 2007;53:367–378. doi: 10.1262/jrd.18146. [DOI] [PubMed] [Google Scholar]
- 15.Kotani M, Detheux M, Vandenbogaerde A, Communi D, Vanderwinden JM, Le Poul E, Brezillon S, Tyldesley R, Suarez-Huerta N, Vandeput F, Blanpain C, et al. The metastasis suppressor gene KiSS-1 encodes kisspeptins, the natural ligands of the orphan G protein-coupled receptor GPR54. J Biol Chem. 2001;276:34631–34636. doi: 10.1074/jbc.M104847200. [DOI] [PubMed] [Google Scholar]
- 16.Muir AI, Chamberlain L, Elshourbagy NA, Michalovich D, Moore DJ, Calamari A, Szekeres PG, Sarau HM, Chambers JK, Murdock P, Steplewski K, et al. AXOR12, a novel human G protein-coupled receptor, activated by the peptide KiSS-1. J Biol Chem. 2001;276:28969–28975. doi: 10.1074/jbc.M102743200. [DOI] [PubMed] [Google Scholar]
- 17.Castellano JM, Navarro VM, Fernandez-Fernandez R, Castano JP, Malagon MM, Aguilar E, Dieguez C, Magni P, Pinilla L, Tena-Sempere M. Ontogeny and mechanisms of action for the stimulatory effect of kisspeptin on gonadotropin-releasing hormone system of the rat. Mol Cell Endocrinol. 2006;257–258:75–83. doi: 10.1016/j.mce.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 18.Spergel DJ, Kruth U, Hanley DF, Sprengel R, Seeburg PH. GABA-and glutamate-activated channels in green fluorescent protein-tagged gonadotropin-releasing hormone neurone in transgenic mice. J Neurosci. 1999;19:2037–2050. doi: 10.1523/JNEUROSCI.19-06-02037.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jasoni CL, Todman MG, Strumia MM, Herbison AE. Cell type-specific expression of a genetically encoded calcium indicator reveals intrinsic calcium oscillations in adult gonadotropin-releasing hormone neurons. J Neurosci. 2007;27:860–867. doi: 10.1523/JNEUROSCI.3579-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Todman MG, Han SK, Herbison AE. Profiling neurotransmitter receptor expression in mouse gonadotropin-releasing hormone neurons using green fluorescent protein-promoter transgenics and microarrays. Neuroscience. 2005;132:703–712. doi: 10.1016/j.neuroscience.2005.01.035. [DOI] [PubMed] [Google Scholar]
- 21.Ghamari-Langroudi M, Bourque CW. Flufenamic acid blocks depolarizing afterpotentials and phasic firing in rat supraoptic neurones. J Physiol. 2002;545:537–542. doi: 10.1113/jphysiol.2002.033589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Partridge LD, Muller TH, Swandulla D. Calcium-activated non-selective channels in the nervous system. Brain Res Rev. 1994;19:319–325. doi: 10.1016/0165-0173(94)90017-5. [DOI] [PubMed] [Google Scholar]
- 23.Bleasdale JE, Thakur NR, Gremban RS, Bundy GL, Fitzpatrick FA, Smith RJ, Bunting S. Selective inhibition of receptor-coupled phospholipase C-dependent processes in human platelets and polymorphonuclear neutrophils. J Pharmacol Exp Ther. 1990;255:756–768. [PubMed] [Google Scholar]
- 24.Maruyama T, Kanaji T, Nakade S, Kanno T, Mikoshiba K. 2APB, 2-aminoethoxydiphenyl borate, a membrane-penetrable modulator of Ins(1,4,5)P3-induced Ca2+ release. Journal of biochemistry. 1997;122:498–505. doi: 10.1093/oxfordjournals.jbchem.a021780. [DOI] [PubMed] [Google Scholar]
- 25.Alessi DR, Cuenda A, Cohen P, Dudley DT, Saltiel AR. PD 098059 is a specific inhibitor of the activation of mitogen-activated protein kinase kinase in vitro and in vivo. J Biol Chem. 1995;270:27489–27494. doi: 10.1074/jbc.270.46.27489. [DOI] [PubMed] [Google Scholar]
- 26.Pielecka-Fortuna J, Chu Z, Moenter SM. Kisspeptin acts directly and indirectly to increase GnRH neuron activity and its effects are modulated by estradiol. Endocrinology. 2008 doi: 10.1210/en.2007-1365. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Plant TM, Ramaswamy S, Dipietro MJ. Repetitive activation of hypothalamic G protein-coupled receptor 54 with intravenous pulses of kisspeptin in the juvenile monkey (Macaca mulatta) elicits a sustained train of gonadotropin-releasing hormone discharges. Endocrinology. 2006;147:1007–1013. doi: 10.1210/en.2005-1261. [DOI] [PubMed] [Google Scholar]
- 28.Clarkson J, Herbison AE. Development of GABA and glutamate signaling at the GnRH neuron in relation to puberty. Mol Cell Endocrinol. 2006;254–255:32–38. doi: 10.1016/j.mce.2006.04.036. [DOI] [PubMed] [Google Scholar]
- 29.Kauffman AS, Gottsch ML, Roa J, Byquist AC, Crown A, Clifton DK, Hoffman GE, Steiner RA, Tena-Sempere M. Sexual Differentiation of Kiss1 Gene Expression in the Brain of the Rat. Endocrinology. 2007 doi: 10.1210/en.2006-1540. [DOI] [PubMed] [Google Scholar]
- 30.McQuiston AR, Madison DV. Muscarinic receptor activity has multiple effects on the resting membrane potentials of CA1 hippocampal interneurons. J Neurosci. 1999;19:5693–5702. doi: 10.1523/JNEUROSCI.19-14-05693.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shen KZ, North RA. Muscarine increases cation conductance and decreases potassium conductance in rat locus coeruleus neurones. J Physiol. 1992;455:471–485. doi: 10.1113/jphysiol.1992.sp019312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Herbison AE. Physiology of the GnRH neuronal network. In: Neill JD, editor. Knobil and Neill's Physiology of Reproduction. 3rd ed. San Diego: Academic Press; 2006. pp. 1415–1482. [Google Scholar]
- 33.Karsch FJ, Bowen JM, Caraty A, Evans NP, Moenter SM. Gonadotropin-releasing hormone requirements for ovulation. Biol Reprod. 1997;56:303–309. doi: 10.1095/biolreprod56.2.303. [DOI] [PubMed] [Google Scholar]
- 34.Caraty A, Delaleu B, Chesneau D, Fabre-Nys C. Sequential role of e2 and GnRH for the expression of estrous behavior in ewes. Endocrinology. 2002;143:139–145. doi: 10.1210/endo.143.1.8605. [DOI] [PubMed] [Google Scholar]
- 35.Silverman A, Livne I, Witkin JW. The gonadotrophin-releasing hormone (GnRH), neuronal systems: immunocytochemistry and in situ hybridization. In: Knobil E, Neill JD, editors. The Physiology of Reproduction. 2 ed. New York: Raven; 1994. pp. 1683–1706. [Google Scholar]
- 36.Sim JA, Skynner MJ, Pape J-R, Herbison AE. Late postnatal reorganization of GABAA receptor signalling in native GnRH neurons. Eur J Neurosci. 2000;12:3497–3504. doi: 10.1046/j.1460-9568.2000.00261.x. [DOI] [PubMed] [Google Scholar]
- 37.Hehl S, Moser C, Weik R, Neumcke B. Internal Ca2+ ions inactivate and modify ATP-sensitive potassium channels in adult mouse skeletal muscle. Biochimica et biophysica acta. 1994;1190:257–263. doi: 10.1016/0005-2736(94)90082-5. [DOI] [PubMed] [Google Scholar]
- 38.Selyanko AA, Sim JA. Ca2+-inhibited non-inactivating K+ channels in cultured rat hippocampal pyramidal neurones. J Physiol. 1998;510(Pt 1):71–91. doi: 10.1111/j.1469-7793.1998.071bz.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hiraizumi Y, Nishimura I, Ishii H, Tanaka N, Takeshita T, Sakuma Y, Kato M. Rat GnRH Neurons Exhibit Large Conductance Voltage- and Ca2+-activated K+ (BK) Currents and Express BK Channel mRNAs. J Physiol Sci. 2008;58:21–29. doi: 10.2170/physiolsci.RP013207. [DOI] [PubMed] [Google Scholar]
- 40.Kato M, Tanaka N, Usui S, Sakuma Y. The SK channel blocker apamin inhibits slow afterhyperpolarization currents in rat gonadotropin-releasing hormone neurones. J Physiol. 2006;574:431–442. doi: 10.1113/jphysiol.2006.110155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Spergel DJ. Calcium and small-conductance calcium-activated potassium channels in gonadotropin-releasing hormone neurons before, during, and after puberty. Endocrinology. 2007;148:2383–2390. doi: 10.1210/en.2006-1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu X, Herbison AE. Small-conductance calcium-activated potassium (SK) channels control excitability and firing dynamics in gonadotropin-releasing hormone (GnRH) neurons. Endocrinology. 2008 doi: 10.1210/en.2007-1631. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Teruyama R, Armstrong WE. Calcium-dependent fast depolarizing afterpotentials in vasopressin neurons in the rat supraoptic nucleus. J Neurophysiol. 2007;98:2612–2621. doi: 10.1152/jn.00599.2007. [DOI] [PubMed] [Google Scholar]
- 44.Chu Z, Moenter SM. Physiologic regulation of a tetrodotoxin-sensitive sodium influx that mediates a slow afterdepolarization potential in gonadotropin-releasing hormone neurons: possible implications for the central regulation of fertility. J Neurosci. 2006;26:11961–11973. doi: 10.1523/JNEUROSCI.3171-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]