Summary
The mechanisms through which estrogen regulates gonadotropin-releasing hormone (GnRH) neurons to control mammalian ovulation are unknown. We found that estrogen positive feedback to generate the preovulatory gonadotropin surge was normal in estrogen receptor β knockout (ERβ) mutant mice but absent in ERα mutant mice. An ERα-selective compound was sufficient to generate positive feedback in wild-type mice. As GnRH neurons do not express ERα, estrogen positive feedback upon GnRH neurons must be indirect in nature. To establish the cell type responsible, we generated a neuron-specific ERα mutant mouse line. These mice failed to exhibit estrogen positive feedback demonstrating that neurons expressing ERα are critical. We then used GnRH neuron-specific Cre-dependent Pseudorabies virus tracing to show that the ERα-expressing neurons modulating GnRH neurons are located within rostral periventricular regions of the hypothalamus. These studies demonstrate ovulation is driven by estrogen actions upon ERα-expressing neuronal afferents to GnRH neurons.
Introduction
The gonadotropin releasing hormone (GnRH) neurons represent the key output cells of the neuronal network controling fertility in all mammalian species. The GnRH neurons and associated cells that comprise the “GnRH neuronal network” are responsible for integrating multiple internal homeostatic and external environmental signals to ensure appropriate levels of fertility for the individual (Levine, 1997; Herbison, 2006). Arguably, the most important of these signals is that of estrogen, secreted by the gonads to achieve feedback regulation of gonadotropin secretion (Herbison, 1998; Petersen et al., 2003). Throughout most of the menstrual cycle, estrogen suppresses gonadotropin secretion but, at mid-cycle, switches to have a potent stimulatory or “positive feedback” action to evoke the luteinizing hormone (LH) surge that triggers ovulation. Although feedback effects of estrogen are known to occur at the pituitary gland (Shupnik, 1996), actions of estrogen within the brain are accepted as being critical for the generation of the GnRH surge that drives the preovulatory LH surge in all mammals, including primates (Karsch et al., 1997; Herbison, 1998).
Even though estrogen positive feedback is central to mammalian fertility, the underlying mechanism remains poorly understood. Since GnRH neurons express ERβ, but not ERα, it is possible that estrogen acts directly upon them to generate the GnRH surge (Herbison and Pape, 2001; Petersen et al., 2003). However, several lines of evidence indicate that effects of estrogens may be transmitted to GnRH neurons in an indirect manner by ERα– and/or ERβ– expressing neurons, glia or endothelial cells (Rage et al., 1997; Smith and Jennes, 2001; Prevot, 2002; Petersen et al., 2003). The detailed investigation of this mechanism has been hampered by the scattered distribution of the GnRH neurons that makes them difficult to investigate technically. Thus, at present, neither the estrogen receptor subtype (ERα vs. ERβ), nor the critical cell types involved in estrogen positive feedback, have been defined and much controversy surrounds this critical issue. A genetic approach to define which of these two estradiol receptors is crucial for GnRH neuronal activation to induce ovulation, and to characterize its role in specific cells might provide valuable insights. Using mice in which ERα or ERβ have been inactivated, we first demonstrate here that ERα, but not ERβ, is required for estrogen positive feedback to GnRH neurons. Using an ERα-selective ligand in wild-type mice we show that ERα is not only necessary but sufficient to generate estrogen positive feedback. Second, exploiting a novel neuron-specific mutation of the ERα gene we were able to identify neurons, as opposed to other cell types, as critical targets for estradiol action. This clearly establishes that neurons expressing ERα are required for estrogen to activate GnRH neurons. Finally, through use of a GnRH neuron-specific Pseudorabies virus (PRV) tracing approach we have been able to define the location of ERα-expressing neurons projecting to GnRH neurons. These data demonstrate a key role for ERα in mammalian estrogen positive feedback and provide definitive evidence for the “indirect model” of estrogen action whereby estrogen regulates ERα-expressing neuronal afferents to the GnRH neurons to bring about the preovulatory GnRH/LH surge.
Results
Estrogen positive feedback on LH secretion and GnRH neuron activation is absent in ERα mutant and normal in ERβ mutant female mice
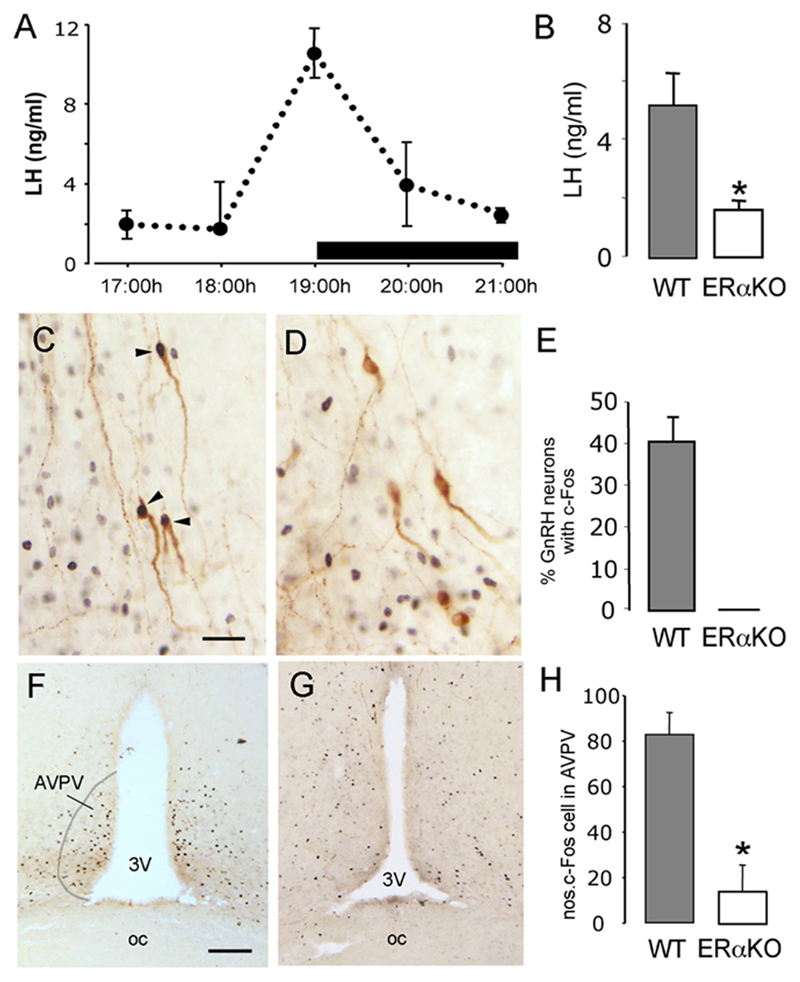
The stimulatory effects of estradiol positive feedback were evaluated using a protocol that enabled the activation status of GnRH neurons to be assessed alongside changes in plasma LH levels. Ovariectomized wild-type C57BL6/J mice given an estradiol capsule followed by an injection of estradiol benzoate were found to exhibit an LH surge centered around 19:00h, on lights out (Fig.1A). In the ERα mutant mice, there was no evidence of an LH surge in any of the mice (n=8) in response to the same estrogen regimen with mean LH levels at 19:00h of 1.6±0.3 ng/ml compared with 5.2±1.0 ng/ml in wild-type control animals (n=7, p<0.05; Fig.1B). In contrast, clear evidence of an LH surge was observed in each of the ovariectomized, estrogen-treated ERβ mutant mice (mean LH levels of 8.6±1.7 ng/ml compared with 8.9±1.5 ng/ml in controls, n= 7 each group) (Fig.2A).
Figure 1. Absence of estrogen positive feedback in ERα mutant mice.
A. Depicts the profile of the LH surge in wild-type ovariectomized mice treated with estrogen. n=4-5 at each time point. B. Mean (+SEM) LH levels in wildtype-littermates (n=7) and ERα mutant mice (n=8), ovariectomized, treated with estrogen and killed at 19:00h, * p<0.05. C-E, Dual-labelling c-Fos (black nuclei) and GnRH (brown cytoplasmic staining) immunocytochemistry in wild-type (C) and ERα mutant (D) mice. Whereas approximately 40% of GnRH neurons express c-Fos (C, arrowheads and E) in wild-type mice, no GnRH neurons express c-Fos in ERα mutant mice (D). F-H, Low-power photomicrographs showing the location of many c-Fos-expressing cells within the AVPV of wild-type mice (F) compared with few in ERα mutant mice (G). The mean (+SEM) number of c-Fos cells per unit area in the AVPV is shown in H. p<0.05. Scale bars represent 30μm in C and 100μm in F.
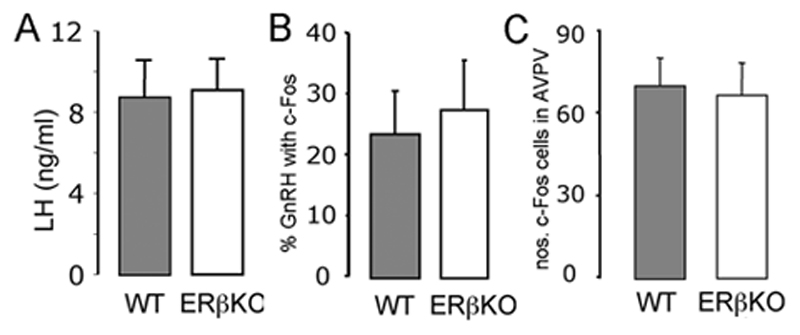
Figure 2. Positive feedback actions of estrogen appear normal in ERβ mutant mice.
A. Mean (+SEM) LH levels in wildtype-littermates (n=7) and ERβ mutant mice (n=7), ovariectomized, treated with estrogen and killed at 19:00h. B. The percentage of GnRH neurons (+SEM) found to express c-Fos in wildtype-littermates (n=7) and ERα mutant mice (n=8), ovariectomized, treated with estrogen and killed at 19:00h. C. The number of c-Fos-expressing neurons detected within the AVPV of wildtype-littermates (n=7) and ERα mutant mice (n=8), ovariectomized, treated with estrogen and killed at 19:00h.
Approximately 40% of the GnRH neuronal population express the immediate early gene c-Fos at the time of GnRH surge and this is believed to be an accurate indicator of those GnRH neurons activated by estrogen to generate the GnRH surge (Hoffman et al., 1993). In addition, there is increasing evidence that neurons within the anteroventral periventricular nucleus (AVPV) of the rostral hypothalamus may be an important target for estrogen in bringing about the GnRH surge (Herbison, 1998; Simerly, 2002). Dual labeling immunocytochemistry (Fig.1C,D) revealed that approximately 40% of GnRH neurons in wild-type mice expressed c-Fos at the time of the estrogen-induced LH surge (Fig.1C,E). The GnRH neurons expressing c-Fos were located preferentially within the preoptic area with dual-labelled GnRH neurons only rarely detected in more rostral areas such as the medial septum (data not shown). In contrast, no GnRH neurons were detected to express c-Fos in any brain region of ERα mutant mice (Fig.1D,E). The distribution and number of GnRH neurons detected in wild-type and ERα mutant females were not different (data not shown). In addition, we found that the numbers of singly-labelled c-Fos-expressing cells located within the AVPV were significantly greater in wild-type mice (Fig.1F) compared with ERα mutant females (p<0.05; Fig.1G,H).
Single and dual-label immunocytochemistry experiments in ERβ mutant females revealed no differences in the number of dual-labelled c-Fos-GnRH neurons compared with wild-type controls (Fig.2B) or in the number of c-Fos-expressing cells detected in the AVPV (Fig.2C). The distribution and number of GnRH neurons in wild-type and ERβ mutant females were not different (data not shown).
An ERα-specific ligand is sufficient to generate positive feedback in wild-type mice
The results with the ERα and β mutant mice indicated that ERα was necessary for estrogen positive feedback. To evaluate whether ERα-pathways were sufficient for positive feedback, wild-type mice were given the ERα-selective compound 16α-LE2 (Hegele-Hartung et al., 2004) as the second estradiol injection alongside control mice receiving the normal estrogen protocol. The second estradiol injection is critical for evoking positive feedback as mice given vehicle control at this time point never exhibit an LH surge. Mice treated with 16α-LE2 (n=5) exhibited an LH surge at 19:00h (LH, 5.9±1.7ng/ml compared with normal estrogen-treated controls 6.5±2.3) and had 49±7% of rostral preoptic area GnRH neurons expressing c-Fos (compared with controls that had 60±11%).
Together, these observations demonstrate that cells expressing ERα are both necessary and sufficient for estrogen positive feedback actions upon GnRH neurons. As GnRH neurons do not express ERα, these results demonstrate that ERα-expressing neuronal, glial or other cell types must mediate estrogen positive feedback actions upon GnRH neurons.
Neuron-specific ERα mutant mice are infertile
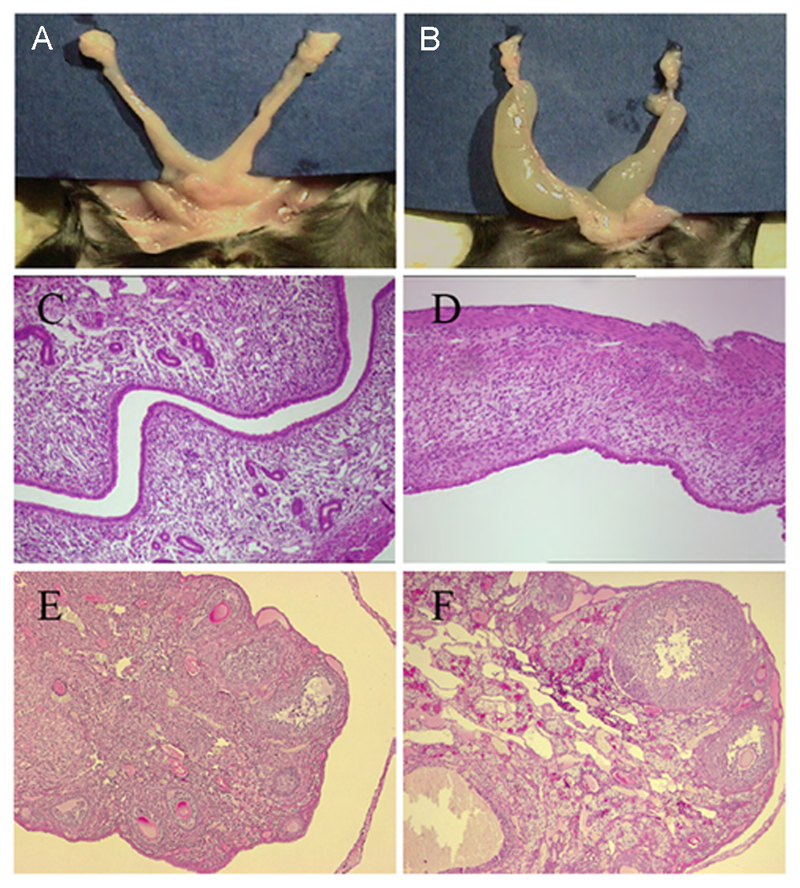
To examine the hypothesis that estrogen acts through ERα-expressing neurons, rather than other cell types, to activate GnRH neurons, neuron-specific ERα mutant mice were developed by breeding ERαflox mice with a CamKiCre transgenic mouse line. Immunocytochemical analyses of ERα expression in the resulting mice showed that ERα immunoreactivity was absent from the hypothalamus (Fig.3 AB) and all other brain regions (Suppl. Fig. 2) of ERαfl/fl;CamKiCre mice. In contrast, ERα protein was detected in the pituitary of all genotypes including female ERαfl/fl;CamKiCre mice (Fig.3 AB insets). Female ERαfl/fl;CamKiCre mice were found to be infertile and, from an age of 5-6 weeks, to exhibit striking abnormalities in their reproductive organs (Fig.4). In the mutants, the uterus was grossly enlarged and filled with liquid (Fig.4B). The endometrium, however, was severely atrophic and lacked all glandular structures. Whereas the endometrial stroma was loose and vascularized in control mice (Fig.4C), it appeared condensed and showed granulocyte infiltration in the mutants (Fig.4D). Histologically, the ovaries of ERαfl/fl;CamKiCre mice showed signs of gonadotropin hyperactivation; in mutant mice, ovaries contained a large number of antral follicles compared with wildtype animals (Fig.4E,F). In the ovarian hilus, theca cells appeared hypertrophic and luteinized in mutant animals, indicating inappropriate stimulation of the ovaries. Furthermore, no corpora lutea were observed in the ovaries of mutants (Fig.4F) suggesting a failure of ovulation. Basal LH levels were not different between diestrous controls (0.91±0.11 ng/ml) and ERαfl/fl;CamKiCre (1.23±0.08 ng/ml) female mice.
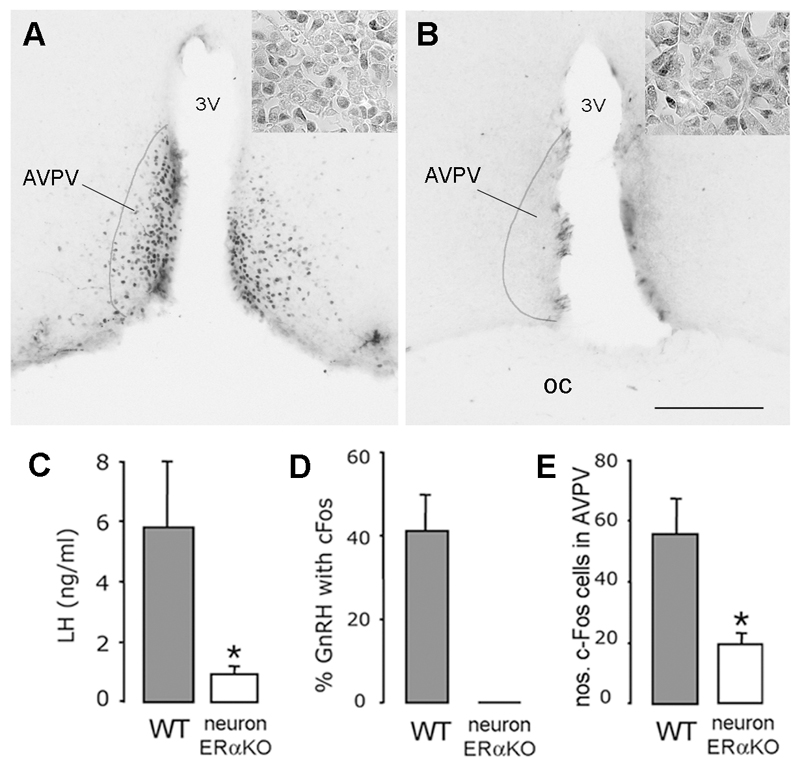
Figure 3. Absence of estrogen positive feedback in neuron-specific ERα mutant mice.
A,B. show ERα expression in the rostral hypothalamus within the anteroventral periventricular nucleus (AVPV) of (A) control (ERαfl/fl) and (B) neuron-specific ERα mutant (ERαfl/fl;CamKiCre) mice. Scale bar is 100μm, 3V= third ventricle, OC = optic chiasm. Insets show equivalent ERα staining in the anterior pituitary of (A) control and (B) neuron-specific ERα mutant mice. C. Mean (+SEM) LH levels in control-littermates (n=5) and ERαfl/fl;CamKiCre mice (n=4), ovariectomized, treated with estrogen and killed at 19:00h. * p<0.05 D. Approximately 40% of GnRH neurons express c-Fos in control mice ovariectomized, treated with estrogen and killed at 19:00h whereas none are found in ERαfl/fl;CamKiCre mice. E. The number of c-Fos-expressing neurons detected within the AVPV of controls and ERαfl/fl;CamKiCre mice, ovariectomized, treated with estrogen and killed at 19:00h. * p<0.05.
Figure 4. Ovarian and uterine phenotype of neuron-specific ERα mutant mice.
A,B. reproductive tract of control ERαfl/fl (A) and mutant ERαfl/fl;CamKiCre (B) mice showing fluid-filled uteri in mutants. C,D H&E staining of uteri from control ERαfl/fl (C) and mutant ERαfl/fl;CamKiCre (D) mice shows atrophy and lack of glandular structures in the mutant. E,F. H&E staining of ovaries from control ERαfl/fl (E) and mutant ERαfl/fl;CamiCre (F) mice shows increased numbers of antral follicles and lack of corpora lutea in the mutant.
Neuron-specific ERα mutant mice are unable to generate estrogen positive feedback
Ovariectomized, estrogen-treated ERαfl/fl;CamKiCre mice (n=6) failed to exhibit an LH surge with mean LH levels at 19:00h of 0.9±0.2 ng/ml compared with 5.8±2.1 ng/ml in littermate controls (n=5; Fig.3C). Immunocytochemical experiments did not detect c-Fos in any GnRH neurons in ERαfl/fl;CamKiCre mice following estrogen treatment. This compared with c-Fos in 41±8% of GnRH neurons in littermate controls (Fig.3D).
As c-Fos protein is detectable for 5-6h in GnRH neurons following the GnRH/Lh surge (Lee et al., 1990) we additionally evaluated c-Fos expression in GnRH neurons at midday of the expected day of the surge and at 1am on the following day to evaluate whether the timing of positive feedback had been altered in ERαfl/fl;CamKiCre mice. We found a complete absence of c-Fos in GnRH neurons at these time points. As we find no evidence for c-Fos in GnRH neurons of ERαfl/fl;CamKiCre at 13:00h, 19:00h or 1am the next day, it seems unlikely that the GnRH neurons receive either an early or delayed positive feedback signal in ERαfl/fl;CamKiCre.
The distribution and number of GnRH neurons in ERαfl/fl;CamKiCre brains was identical to that of controls (data not shown). Whereas numbers of c-Fos-positive cells in a control brain region were not different between control littermates (5.1±2.2 cells/unit area/section) and ERαfl/fl;CamKiCre mice (8.3±1.6 cells/unit area/section), significantly more singly-labelled c-Fos cells were detected within the AVPV of littermate controls compared to ERαfl/fl;CamKiCre mice (p<0.05;Fig.3E). These data closely resemble that of the global ERα mutant mouse and indicate that ERα-expressing neurons are critical for estrogen positive feedback to occur.
The ERα-expressing primary afferents to GnRH neurons are located within periventricular regions of the rostral hypothalamus
The studies above show that ERα-expressing neurons are critical for estrogen positive feedback to GnRH neurons. To identify the locations of these cells within the “GnRH neuronal network”, we have used a Cre-dependent PRV tracing strategy in GnRH-Cre mice (DeFalco et al., 2001; Yoon et al., 2005). In this approach, the Ba2001 PRV is activated only after infecting a Cre-expressing GnRH neuron in vivo. This allows for the now unconditional PRV to replicate and to pass in a retrograde manner to the primary afferents of the GnRH neuron and, subsequently, their own afferents in a time-dependent manner. The retrograde chain of infection can be followed by evaluating GFP expression as the unconditional Ba2001 PRV also expresses GFP in each cell it infects.
Five founder GnRH-Cre lines were generated. Analysis of Cre expression by dual-label immunocytochemistry in adult female mice revealed that one of these lines expressed Cre in a highly selective manner within 97±2% of all GnRH neurons (n=6; Fig.5A). Crossing this line with the ROSA-26 indicator mice (Soriano, 1999) revealed Cre-dependent recombination in 97±1% of GnRH neurons (n=4; not shown).
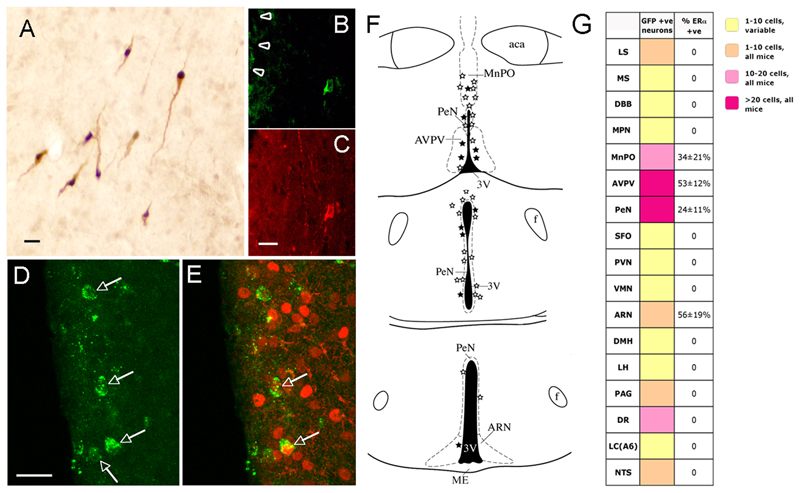
Figure 5. Identification of ERα-expressing primary afferents to GnRH neurons using GnRH-specific viral mediated tracing.
A. Restricted expression of Cre (black nuclei) in GnRH neurons (brown cytoplasm) of transgenic GnRH-Cre mice. B,C. A rostral preoptic area GnRH neuron adjacent to the injection site (arrowheads) exhibiting GFP immunoreactivity 48h following injection of Ba2001. B shows GFP immunoreactivity and C is GnRH immunostaining. D. Four neurons (arrows) in the AVPV exhibiting GFP immunoreactivity 72h after Ba2001 injection into the rostral preoptic area. E. Dual-labelling for GFP (green) and ERα (red) reveals that 2 of these cells (arrows) express ERα. F. Schematic brain maps demonstrating GFP-immunoreactive neurons (open stars) and GFP+ERα immunoreactive (filled stars) cells detected in individual 30μm-thick coronal brain sections at three levels in the hypothalamus. Abbreviations, aca, anterior commisure; ARN, arcuate nucleus; AVPV, anteroventral periventricular nucleus; f, fornix; MnPO, median preoptic nucleus; ME, median eminence, PeN, periventricular nucleus; 3V, third ventricle. Scale bar is 15μm in A,C,D. G. Summary of locations of primary afferents to GnRH neurons following stereotaxic injection of Ba2001 PRV into the rPOA of GnRH-Cre mice. Various colors in boxes adjacent to listed brain areas indicate ranges of mean numbers of GFP-positive neurons identified in female mice (n=6). Brain regions in which labelled cells were detected in <5 of the 6 mice are indicated as yellow boxes (variable). Right-most column indicates the mean percentage (±SEM) of GFP-positive neurons expressing ERα immunoreactivity. Abbreviations; ARN, arcuate nucleus; AVPV, anteroventral periventricular nucleus; DBB, diagonal band of Broca; DMH, dorsomedial hypothalamus; DR, dorsal raphe; LC, locus coereulus; LH, lateral hypothalamus; LS, lateral septum; MnPO, median preoptic nucleus; MPN, medial preoptic nucleus; MS, medial septum; NTS, nucleus tractus solitarius; PAG, periaqueductal grey; PeN, periventricular nucleus; PVN, paraventricular nucleus; SFO, subfornical organ; VMN, ventromedial nucleus.
To evaluate the ERα-expressing afferents to the GnRH neuron population that are activated at the time of the GnRH surge (i.e. those in the rostral preoptic area), a single 500nl injection of Ba2001 PRV was given into the rostral preoptic area of adult female mice. Mice examined at 6, 12, 24 and 30h after injection of Ba2001 (n=8) displayed no evidence of GFP expression in any region of the brain. However, 48h later (n=4), we found that between 5 and 9 GnRH neurons located around the injection site expressed GFP (Fig.5B,C) indicating that Cre-mediated recombination had occurred in these cells. No other GFP-expressing cells were detected within the brain at this time point. Seventy-two hours following PRV injection, GFP-expressing cells (representing afferent neurons to rostral preoptic GnRH neurons, Fig.5D) were identified in multiple brain regions. Twenty-four hours later (96h post-injection), we found a much larger distribution and number of GFP-expressing cells. As 24h is required for PRV to move from one order of neurons to the next (Card and Enquist, 1995; Horvath et al., 2002), the neurons identified at 72h post-injection are almost certainly primary afferents to GnRH neurons.
At 72h post-injection (n=6), GFP-expressing neurons were identified in multiple areas of the forebrain and brainstem (Fig.5G). The regions displaying the largest numbers of retrogradely-labeled neurons were the hypothalamic periventricular nucleus (PeN; mean of 36 cells/mouse in a 1:3 series of brain sections), AVPV (23 cells), median preoptic nucleus (MnPO; 14 cells) and dorsal raphe (16 cells). Other brain regions that consistently exhibited GFP-expressing neurons at lower densities were the lateral septum, arcuate nucleus (ARN) and nucleus tractus solitarius (Fig.5G).
Dual-labelling immunocytochemistry (n=6) for GFP and ERα (Fig.5D,E) identified ERα-expressing afferents to be located in four brain regions; the AVPV, MnPO, PeN and ARN (Fig.5F). The percentage of GFP-positive neurons expressing ERα was 53±12%, 34±21%, 24±11% in the AVPV, MnPO and PeN respectively, and 56±19%, for the ARN although we only ever observed 1-2 GFP-expressing cells in the ARN (Fig.5G). To ensure the neuronal identity of PRV-infected cells expressing ERα, immunocytochemical labeling for GFP and ERα was undertaken in combination with Nissl staining. All GFP/ERα-expressing cells were also positive for Nissl (Suppl. Fig. 3). Injections of Ba2001 PRV into the rostral preoptic area of wild-type, non-Cre-expressing mice, or into the striatum of GnRH-Cre mice, resulted in a complete absence of GFP-expressing neurons. These findings demonstrate that ERα-expressing primary afferents to GnRH neurons have their cell bodies located principally within rostral periventricular nuclei of the hypothalamus.
The findings of the PRV study demonstrated that significant estrogen-receptive primary afferents to the GnRH neurons existed in the PeN and MnPO in addition to the AVPV. As such, we returned to the estrogen positive feedback c-Fos studies and examined c-Fos expression in the MnPO and PeN of ERα and ERβ mutant mice. The numbers of c-Fos cells in the PeN of mice treated with estrogen were significantly reduced in ERα mutants (22±3 cells, p<0.05) compared with wild-type mice (40±6 cells per section) but were unchanged in ERβ mutant females. The numbers of c-Fos-expressing cells in the MnPO were not significantly different in either ERα- or ERβ-mutant mice compared with controls. These data indicate that a sub-population of cells within the PeN, as well as the AVPV, are activated by estrogen positive feedback.
Discussion
We have used here a series of ER mutant mouse models and an ER-selective ligand to demonstrate the critical receptor isoform and cell type necessary and sufficient for estrogen to initiate the GnRH/LH surge and ovulation. We show that global ERβ mutant mice exhibit normal patterns of estrogen-induced GnRH activation and LH surge secretion. In contrast, global ERα mutant mice fail to exhibit GnRH neuron activation or LH surge secretion in response to estrogen. As GnRH neurons do not express ERα (Herbison and Pape, 2001), this indicated that positive feedback effects of estrogen must occur in an indirect manner. To define the critical ERα-expressing cell-type in the pathway, neuron-specific ERα mutant mice were generated by crossing a new floxed exon 3 ERα mouse line with CamKIIα-Cre mice. CamKIIα is expressed selectively by forebrain neurons (Ouimet et al., 1984; Burgin et al., 1990) and the CamKIIα-Cre mouse line used here has been shown previously to efficiently delete loxP-flanked target sequences in neurons of the CNS, leading to neuron-specific gene ablation (Casanova et al., 2001; Marsicano et al., 2003). Mice harbouring a neuron-specific ERα deletion were found to be infertile and lack estrogen positive feedback demonstrating that ERα-expressing neurons are critical. To define the location of these neurons we have used a novel conditional Cre-Pseudorabies virus approach to demonstrate that the ERα-expressing primary afferents to GnRH neurons are located primarily within the periventricular nuclei of the rostral hypothalamus. Together, these observations provide conclusive evidence that critical positive feedback actions of estrogen upon GnRH neurons are mediated by ERα-expressing neuronal afferents within the GnRH neuronal network.
It is now recognized that estrogen can modulate the activity of neuronal networks through multiple mechanisms including both slow genomic and rapid actions (McEwen and Alves, 1999). In the case of estrogen positive feedback, however, it seems clear that classical genomic mechanisms underlie the estrogen activation of GnRH neurons. In all species examined, positive feedback only occurs after several hours of estrogen exposure (Legan et al., 1975; Bronson, 1981; Xia et al., 1992; Evans et al., 1997). Furthermore, once the animal has been exposed to estrogen for a sufficient period, it can be removed without having any negative effect upon subsequent GnRH neuron activation. Thus, rapid and immediate actions of estrogen are not likely to be critical for GnRH neuron activation and positive feedback relies upon a classical genomic mechanism. The down-stream genes regulated by estrogen to bring about GnRH neuron activation are not yet established although the progesterone receptor is one candidate known to be regulated potently by estrogen (Levine, 1997; Shughrue et al., 1997). The finding of ERβ mRNA and protein in GnRH neurons of the mouse (Skynner et al., 1999a), the rat (Hrabovszky et al., 2001) and, more recently, the sheep (Skinner and Dufourny, 2005), raised the possibility that estrogen might act directly upon GnRH neurons via this isoform to generate the GnRH/LH surge (Herbison and Pape, 2001). However, in terms of both LH secretion and immediate early gene expression in GnRH neurons, we found that estrogen positive feedback is normal in the global ERβ mutant mouse. This strongly suggests that ERβ-regulated signaling within GnRH neurons is not critical for estrogen’s positive feedback effects upon these cells.
The global ERα mutant mice are infertile and exhibit an anovulatory ovarian phenotype with polycystic follicles and an absence of corpora lutea (Couse et al., 1999; Couse and Korach, 1999). We provide evidence here that the failure of estrogen positive feedback on the GnRH neuron network is very likely to underlie the anovulatory phenotype of the global ERα mutant mice. Estrogen administration to ovariectomized global ERα mutant mice failed to generate an LH surge or evidence of GnRH neuron activation. This provided direct evidence of the critical importance of ERα-expressing cells in positive feedback. In keeping with this observation, the ERα-selective compound 16α-LE2 (Hegele-Hartung et al., 2004) was found to be capable of eliciting normal positive feedback in wild-type mice. This indicates that ERα activation is both necessary and sufficient for positive feedback to occur. As the GnRH neurons do not themselves express ERα (Herbison and Pape, 2001), these observations in ERα mutant mice suggested that other ERα-expressing cell types within the GnRH neuronal network were critical for positive feedback to occur. Several different modes of indirect estrogen input to the GnRH neurons have been proposed and include effects mediated by vascular endothelial cells, tanycytes, glial cells, and interneurons (Rage et al., 1997; Herbison, 1998; Smith and Jennes, 2001; Prevot, 2002; Petersen et al., 2003). Using a Cre-loxP strategy that generates a neuron-specific ERα knockout, we have been able to demonstrate that, of these possibilities, it is ERα-expressing neurons within the network that are critical for estrogen positive feedback. Other cell types expressing ERα may play a role in positive feedback but are insufficient, by themselves, to activate the GnRH neurons to generate the GnRH/LH surge. Thus, the present genetic dissection of the estrogen positive feedback mechanism identifies clearly both the critical ER isoform and cell type involved.
The absence of estrogen positive feedback in neuron-specific ERα mutant mice is entirely compatible with their infertile reproductive phenotype. Ovarian histology shows an absence of corpora lutea and abundance of antral follicles supporting the failure of the ovulatory mechanism. In contrast to the global ERα mutant line, there was no evidence in neuron-specific ERα mutant mice for a polycystic hemorrhagic ovarian phenotype generated by excessive gonadotrophin secretion (Couse et al., 1999). Indeed, basal LH levels are not elevated in neuron-specific ERα mutant mice suggesting that pituitary estrogen negative feedback is probably sufficient to restrain gonadotrophin secretion. Another prominent feature of the neuron-specific ERα mutant mice is that of dilated, fluid-filled uteri reminiscent of that found on proestrus. Unlike in the global ERα mutant mouse (Couse and Korach, 1999; Dupont et al., 2000), the uteri of neuron-specific ERα mutant mice remain sensitive to estrogen and may be exposed to a continual high level of estrogen originating from the large numbers of developing follicles. Thus, the reproductive phenotype of the neuron-specific ERα mutant mouse is compatible with that of a mouse in which estrogen negative feedback is competent but positive feedback is absent. This would enable relatively normal basal gonadotrophin secretion, sufficient to promote follicular growth and estrogen production, to exist in association with a complete failure of ovulation.
Studies in the rat have implicated the AVPV as an estrogen-sensitive brain region involved in the positive feedback mechanism (Wiegand and Terasawa, 1982; Herbison, 1998; Simerly, 2002). The present results provide definitive evidence for this hypothesis and also suggest that the locations of estrogen-sensitive neurons involved in estrogen positive feedback include the preoptic PeN immediately caudal to the AVPV. To determine the locations of ERα-expressing afferents to GnRH neurons we generated a GnRH-Cre transgenic mouse for use with a novel Cre-dependent PRV retrograde tracing strategy (DeFalco et al., 2001). By establishing a time-course of viral infection and restricting our PRV injection sites to the rostral preoptic area, where GnRH neurons expressing c-Fos at the time of positive feedback reside, we traced out the primary afferents to the sub-population of GnRH neurons activated by estrogen to create the preovulatory GnRH surge. Primary afferent inputs to GnRH neurons were found to originate from a variety of predominantly mid-line hypothalamic and brainstem locations. However, the sub-populations of ERα-expressing afferents were found to be tightly clustered within the rostral preoptic area. In addition to a strong projection from ERα-expressing neurons in the AVPV, we also identified populations of ERα-primary afferents originating from the PeN and, nearby the GnRH somata, in the MnPO. Our c-Fos studies support the functional relevance of both AVPV and PeN neurons in estrogen positive feedback as a strong correlation existed between levels of c-Fos in these brain regions and GnRH neuron activation and the occurrence of an LH surge. A similar observation has been made in the rat where c-Fos is expressed by AVPV/PeN neurons in response to estrogen positive feedback (Le et al., 1999).
Brought together, the evidence for the AVPV and PeN as sites of primary afferents mediating estrogen positive feedback to rodent GnRH neurons is now very strong; (1) lesions of the AVPV/ventral PeN abolish positive feedback (Wiegand and Terasawa, 1982), (2) implantation of anti-estrogens in the AVPV/PeN abolish positive feedback (Petersen and Barraclough, 1989), (3) AVPV and PeN neurons provide inputs to GnRH neurons (present study and (Horvath et al., 1993; Gu and Simerly, 1997; Simonian et al., 1999)), (4) neurons expressing ERα are critical for estrogen positive feedback (present study), (5) AVPV and PeN neurons express ERα and are activated by estrogen positive feedback (present study and (Le et al., 1999)), and (6) AVPV/PeN neurons expressing ERα project directly to GnRH neurons (present study).
Together, these studies clarify the mechanism of estrogen positive feedback to GnRH neurons by providing definitive evidence that it is ERα, and not ERβ, that is critical and that a neuron-specific ablation of ERα renders mice infertile with a complete ablation of estrogen positive feedback. Thus, estrogen acts indirectly upon GnRH neurons to bring about positive feedback driving ovulation and the ERα-expressing neurons mediating this pathway are located in periventricular regions. Future studies involving the genetic ablation of ERα in specific neuronal phenotypes will now be required to define the hierarchy of periventricular neurons mediating estrogen feedback to GnRH neurons.
Experimental Procedures
Generation of an ERα flox (ERαfl) mouse line
A conditional allele of the mouse Esr1 allele was generated. The targeting construct (pERaflox.tkneo.DTA) (Suppl. Fig.1A, top panel) was based on a 9 kB BamHI fragment representing the genomic sequence of exon 3 and surrounding introns of the mouse Esr1 gene (Suppl. Fig.1A, second panel). This fragment, obtained from the RPCI21 mouse genomic library (Vente et al., 1999), was modified using homologous recombination in E. Coli (Zhang et al., 1998) to carry a loxP site 5´ to exon 3, a PGKtkneo cassette flanked by frt sites and one loxP site in the 3´ direction of exon 3. E14 embryonic stem cells were transfected with the linearised targeting construct and selected for construct integration (Tronche et al., 1999). G418-resistant clones were characterised by Southern blot using external genomic probes from the Estra locus (Suppl. Fig.1B). Clones that had undergone homologous recombination were transiently transfected with the expression plasmid pCAGGS-Flpe (Schaft et al., 2001) and selected with 1 µM Gancyclovir to isolate subclones that have lost the selection cassette after Flp recombination. This was verified by Southern blot analysis (Suppl. Fig.1C). From the resulting embryonic stem cell clones, chimeric mice were generated by blastocyst injection and uterine transfer. By breeding these chimeras to C57BL/6 mice, the ERαfl mouse line was established. To generate a neuron-specific ERα knockout mouse, ERαfl mice were bred to CamKIIα-iCre BAC transgenic mice (referred to as CamKiCre mice) (Casanova et al., 2001). CamKIIα is expressed around birth in almost all forebrain neurons but not glial cells (Ouimet et al., 1984; Burgin et al., 1990). This resulted in the generation of ERαfl/fl;CamKiCre mice with heterozygous ERα+/fl; CamKiCre mice, and ERαfl/fl mice serving as controls.
Generation of a GnRH-cre transgenic mouse
The GnRH-Cre mouse was generated in the same manner as reported previously(Skynner et al., 1999b) using a ~12kb transgene incorporating all the introns and exons of the GnRH gene, 5.5 kb of upstream (5') and 3.5 kb of downstream (3') flanking sequence, and a SmaI site engineered by site-directed mutagenesis in exon II between sequences encoding amino acids 2 and 3 of the GnRH decapeptide. An expression cassette consisting of (in 5' to 3' order) a synthetic intron, nuclear-localizing signal, CRE recombinase and a polyadenylation (polyA) sequence was then inserted at the SmaI site to produce the transgene. Transgenic mice were produced by pronuclear injection and identified by PCR analysis of genomic DNA isolated from tail biopsies.
Animals and estradiol-induced LH surge protocol
All animal experimental procedures were approved by the University of Otago Animal Ethics Committee and the Regierungspräsidium Karlsruhe. Animals were housed in groups of 3-4/cage with food and water freely available and a 12:12 lighting schedule (lights on 07:00h, off 19:00h). Genotyping of ERα mutant and ERβ mutant mice was undertaken as described previously (Abraham et al., 2003). Adult (>60 days of age) wild-type and ER mutant female C57BL6/J mice were anesthetized with Halothane, ovariectomized and an estradiol-filled Silastic capsule implanted subcutaneously. Estradiol capsules were made according to the protocol of Bronson (Bronson, 1981), and involved filling Silastic tubing (1.0 mm internal, 2.1mm external diameter; Dow Corning, Michigan) with Silastic medical-grade adhesive (Dow Corning) containing 0.1mg 17-β-estradiol (Sigma, Missouri)/ml adhesive. Each mouse was given an ~ 1cm length of Silastic tubing (1μg estradiol/20g body weight). To prevent infection in brain-specific ERα knockout mice (see below), ERαfl/fl;CamKiCre mice and controls were given daily s.c. injections of 5 µl/g Baytril 0.1 % (Bayer, Leverkusen, Germany) for five consecutive days, starting two days pre-ovariectomy (OVX). Six days after OVX, mice received a subcutaneous injection of estradiol benzoate (1μg/20g body weight, Intervet, Castle Hill, Australia) at 09:00h. On the following day, animals were killed with an i.p. overdose of pentobarbitone and trunk blood collected between 17:00 and 21:00h for LH radioimmunoassay. The sensitivity of the radioimmunoassay was 0.4 ng/ml and had an intra-assay coefficient of variation of 12.1%.
Five separate LH surge experiments were undertaken. In the first, wild-type mice were treated as above and groups of 4-5 mice killed at 17:00h, 18:00h, 19:00h, 20:00h and 21:00 to ascertain the profile of the LH surge. As this showed that 19:00h represented to time of peak LH secretion, further experiments were undertaken by killing mice at 19:00h. In the second experiment, ERα mutant and wild-type littermates (N=7-8 each group) were treated as above, anesthetized with pentobarbitone, and a 0.3ml blood sample obtained from the right atrium before perfusion of the mouse through the left ventricle with 15ml 4% paraformaldehyde fixative solution. Brains were then removed and post-fixed for 90 min at room temperature in 4% paraformaldehyde before being placed in a 30% sucrose/Tris-buffered saline (TBS) solution overnight. The third experiment involved the same procedure but using ERβ mutant mice and wild-type littermates (n=7 each group). The fourth experiment used wild-type adult female mice given the estrogen-replacement protocol but with half of the mice receiving the ERα-selective agonist 3,17-dihydroxy-19-nor-17α-pregna-1,3,5 (10)-triene-21,16α-lactone (16α-LE2; 0.8μg/20g body weight, s.c.;kind gift of Schering AG, Berlin, Germany) instead of the estradiol benzoate injection. At this dose, 16α-LE2 is highly selective for ERα in vivo(Hegele-Hartung et al., 2004) The fifth experiment involved neuron-specific ERα knockout mice (n=4; ERαfl/fl;CamKiCre genotype) and control littermates (n=4-5; ERα+/fl;CamKiCre, ERαfl/fl and ERα+/fl genotypes) treated as above. To evaluate possible altered timing of positive feedback and the LH surge in these mice, animals were perfused at 13:00h, 19:00h and 01:00h with the addition that pituitaries were collected alongside the brains. In each experiment, individual control and ER mutant mice were perfused in an alternate manner.
Immunohistochemistry
Single labeling ERα immunohistochemistry was performed using either the monoclonal rat H222 antibody (1.3μg/ml, gift of Abbott Laboratories, IL) for free-floating brain sections or a polyclonal rabbit antibody (1 μg/ml, MC-20, SC-542, Santa Cruz Biotechnology, CA) for the pituitary. Brains sections were cut in the coronal plane at 30µm on a freezing microtome and processed as free-floating sections using biotinylated anti-rat IgGs followed by the VECTASTAIN ABC system (Vector Laboratories) and nickel-diaminobenzidine as the chromagen. Fixed pituitaries were dehydrated, embedded in paraffin wax, and cut into 6-µm-sections for slide-mounted immunohistochemistry using biotinylated anti-rabbit IgGs and the VECTASTAIN ABC system with diaminobenzidine.
Dual-labeling c-Fos and GnRH immunocytochemistry was undertaken as described previously (Herbison et al., 1995). Briefly, the rostral forebrain was cut into three sets of 30μm-thick coronal sections and one set immunostained for c-Fos (Li et al., 1999) using a polyclonal rabbit anti-cFos antibody (1:10,000, SC52, Santa Cruz Biotechnology Inc., Santa Cruz, CA) followed by biotinylated anti-rabbit IgGs (1:200; Vector, Burlingame, CA) and Vector Elite avidin-peroxidase (1:100), and revealed using nickel-diaminobenzedine hydrochloride. A second sequential staining using a rabbit anti-GnRH antibody (1:40,000; LR1, gift of R. Benoit, Montreal) and peroxidase-labelled anti-rabbit immunoglobulins (1:400; Vector Labs) revealed by diaminobenzidine hydrochloride alone was used to visualize the GnRH neurons. The removal of either primary antibodies resulted in a complete absence of the respective immunoreactivity.
Analysis was undertaken by counting the numbers of single-labelled (brown cytoplasm only) and dual-labelled (brown cytoplasm and black nucleus) GnRH neurons. The distribution and number of dual labelled GnRH neurons were counted in the regions of the medial septum, rostral preoptic area and anterior hypothalamic area represented by plates 22-24, 25-27 and 28-31 respectively, of the Franklin and Paxinos brain atlas (Franklin and Paxinos, 1997). In addition, the number of c-Fos-labelled cells identified within the AVPV, PeN and MePN were counted. Cell counts in the AVPV were determined by placing a right-angle triangle (160μm base x 400μm side) over the AVPV (plate 29) and counting all immunoreactive nuclei bilaterally on two sections from each mouse. The same procedure was undertaken for the MnPO by placing a square (300μm x 300μm) over the midline MnPO (plate 27) and counting the numbers of c-Fos-immunoreactive cells in 2 sections from each mouse. Counts in the PeN were undertaken in the same way by placing a rectangle (60μm x 800μm) next to the third ventricle and the level of plate 31. Values for each mouse were used to determine mean counts and these used to generate means+SEM values for each group. All data were analysed by non-parametric Mann-Whitney U tests with a p<0.05 considered significant.
Conditional Pseudorabies virus tracing
The conditional PRV strain, Ba2001, was generated as described previously(DeFalco et al., 2001) (3.8 X 108 pfu/ml) and stored at -80C until use. Intracerebral stereotaxic injections of Ba2001 were made in Avertin-anaesthetized adult female GnRH-Cre mice. A Hamilton syringe was used to deliver 500nl of Ba2001 into the rostral preoptic area (coordinates: 0.5mm bregma, 0mm lateral, -5.1mm dorsal-ventral) at a rate of 20nl/min. The needle was left in place for 5 minutes following injection. Control injections were made into the preoptic area of wild-type mice and into the striatum of GnRH-Cre mice. Mice were killed at various time points after injection and brain tissue was prepared for immunohistochemical processing as described above. Thirty micrometer thick sections underwent dual-labelling for either GFP (to detect PRV infected cells) and GnRH, or GFP and ERα. The following antibodies were used: chicken anti-GFP (1:2,500; Chemicon), rabbit anti-GFP (1:5,000, Molecular Probes), rabbit anti-GnRH antibody (1:40,000; LR1, gift of R. Benoit, Montreal), and rabbit anti-ERα (1:10,000, Upstate). GFP/GnRH dual labelling was visualized using anti-rabbit IgGs followed by streptavidin 568 (1:200; Molecular Probes) and anti-chicken FITC (1:200; Jackson Immunolabs). GFP/ERα dual labelling was visualized with biotinylated anti-rabbit IgGs followed by streptavidin 568 (1:200; Molecular Probes), and anti-chicken FITC (1:200; Jackson Immunolabs). Every section throughout the brain of 72h post-injection female mice (n=6) was analysed for GFP/GnRH dual labelling using an Olympus BX51 epifluorescence microscope. Dual labelling for GFP/ERα was analysed in every third section throughout the forebrain (n=5). GFP/ERα/Nissl staining was undertaken by including a final incubation step in Neurotrace 435/455 blue fluorescent Nissl stain (1:100, Molecular Probes).
Supplementary Material
Acknowledgements
This research was supported by the Wellcome Trust, Royal Society of New Zealand Marsden Fund, “Deutsche Forschungsgemeinschaft” through Collaborative Research Centres 405, 488 and 636,, FOR 302, GRK 791/1.02, GRK 484, and Sachbeihilfe Schu 51/7-2, by the “Fonds der Chemischen Industrie”, the European Community through grant QLG1-CT-2001-01574, the Bundesministerium für Bildung und Forschung (BMBF) through NGFN grants FZK 01GS01117 and KGCV1/01GS0416, German-Polish cooperation project 01GZ0310 and project number 0313074C (systems biology), and by the Alexander von Humboldt-Stiftung through the Max-Planck-Forschungspreis für Internationale Kooperation, and by the Division of Intramural Research, NIEHS/NIH. CAP is a Research Fellow of the Sjogren’s Syndrome Foundation. Joachim Elzer is thanked for help with generation of the ERα mutant mice, and Katja Prelle, Schering AG Berlin, for support of the 16aLE2 study.
References
- Abraham IM, Han K, Todman MG, Korach KS, Herbison AE. Estrogen receptor β mediates rapid estrogen actions on gonadotropin-releasing hormone neurons in vivo. J Neurosci. 2003;23:5771–5777. doi: 10.1523/JNEUROSCI.23-13-05771.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronson FH. The regulation of luteinizing hormone secretion by estrogen: relationships among negative feedback, surge potential, and male stimulation in juvenile, peripubertal, and adult female mice. Endocrinology. 1981;108:506–516. doi: 10.1210/endo-108-2-506. [DOI] [PubMed] [Google Scholar]
- Burgin KE, Waxham MN, Rickling S, Westgate SA, Mobley WC, Kelly PT. In situ hybridization histochemistry of Ca2+/calmodulin-dependent protein kinase in developing rat brain. J Neurosci. 1990;10:1788–1798. doi: 10.1523/JNEUROSCI.10-06-01788.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Card JP, Enquist LW. Neurovirulence of pseudorabies virus. Crit Rev Neurobiol. 1995;9:137–162. [PubMed] [Google Scholar]
- Casanova E, Fehsenfeld S, Mantamadiotis T, Lemberger T, Greiner E, Stewart AF, Schutz G. A CamKIIalpha iCre BAC allows brain-specific gene inactivation. Genesis. 2001;31:37–42. doi: 10.1002/gene.1078. [DOI] [PubMed] [Google Scholar]
- Couse JF, Bunch DO, Lindzey J, Schomberg DW, Korach KS. Prevention of the polycystic ovarian phenotype and characterization of ovulatory capacity in the estrogen receptor-alpha knockout mouse. Endocrinology. 1999;140:5855–5865. doi: 10.1210/endo.140.12.7222. [DOI] [PubMed] [Google Scholar]
- Couse JF, Korach KS. Estrogen receptor null mice: what have we learned and where will they lead us? Endocr Rev. 1999;20:358–417. doi: 10.1210/edrv.20.3.0370. [DOI] [PubMed] [Google Scholar]
- DeFalco J, Tomishima M, Liu H, Zhao C, Cai X, Marth JD, Enquist L, Friedman JM. Virus-assisted mapping of neural inputs to a feeding center in the hypothalamus. Science. 2001;291:2608–2613. doi: 10.1126/science.1056602. [DOI] [PubMed] [Google Scholar]
- Dupont S, Krust A, Gansmuller A, Dierich A, Chambon P, Mark M. Effect of single and compound knockouts of estrogen receptors alpha (ERalpha) and beta (ERbeta) on mouse reproductive phenotypes. Development. 2000;127:4277–4291. doi: 10.1242/dev.127.19.4277. [DOI] [PubMed] [Google Scholar]
- Evans NP, Dahl GE, Padmanabhan V, Thrun LA, Karsch FJ. Estradiol requirements for induction and maintenance of the gonadotropin-releasing hormone surge: implications for neuroendocrine processing of the estradiol signal. Endocrinology. 1997;138:5408–5414. doi: 10.1210/endo.138.12.5558. [DOI] [PubMed] [Google Scholar]
- Franklin KBJ, Paxinos G. The Mouse Brain in Stereotaxic Coordinates. San Diego: Academic Press; 1997. [Google Scholar]
- Gu GB, Simerly RB. Projections of the sexually dimorphic anteroventral periventricular nucleus in the female rat. J Comp Neurol. 1997;384:142–164. [PubMed] [Google Scholar]
- Hegele-Hartung C, Siebel P, Peters O, Kosemund D, Muller G, Hillisch A, Walter A, Kraetzschmar J, Fritzemeier KH. Impact of isotype-selective estrogen receptor agonists on ovarian function. Proc Natl Acad Sci U S A. 2004;101:5129–5134. doi: 10.1073/pnas.0306720101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbison AE. Multimodal influence of estrogen upon gonadotropin-releasing hormone neurons. Endocr Rev. 1998;19:302–330. doi: 10.1210/edrv.19.3.0332. [DOI] [PubMed] [Google Scholar]
- Herbison AE. Physiology of the GnRH neuronal network. In: Neill JD, editor. Knobil and Neill's Physiology of Reproduction. San Diego: Academic Press; 2006. pp. 1415–1482. [Google Scholar]
- Herbison AE, King IS, Tan KKC, Dye S. Increased fos expression in preoptic calcitonin gene-telated peptide (CGRP) neurones following mating but not the luteinizing hormone surge in female rats. J Neuroendocrinol. 1995;7:377–385. doi: 10.1111/j.1365-2826.1995.tb00772.x. [DOI] [PubMed] [Google Scholar]
- Herbison AE, Pape JR. New evidence for estrogen receptors in gonadotropin-releasing hormone neurons. Front Neuroendocrinol. 2001;22:292–308. doi: 10.1006/frne.2001.0219. [DOI] [PubMed] [Google Scholar]
- Hoffman GE, Smith MS, Verbalis JG. c-Fos and related immediate early gene products as markers of activity in neuroendocrine systems. Front Neuroendocrinol. 1993;14:173–213. doi: 10.1006/frne.1993.1006. [DOI] [PubMed] [Google Scholar]
- Horvath S, Kis Z, Boldogkoi Z, Nogradi A, Toldi J. Oestrogen-dependent tracing in the rat CNS after pseudorabies virus infection. Eur J Neurosci. 2002;15:937–943. doi: 10.1046/j.1460-9568.2002.01923.x. [DOI] [PubMed] [Google Scholar]
- Horvath TL, Naftolin F, Leranth C. Luteinizing hormone-releasing hormone and gamma-aminobutyric acid neurons in the medial preoptic area are synaptic targets of dopamine axons originating in anterior periventricular areas. Journal of Neuroendocrinology. 1993;5:71–79. doi: 10.1111/j.1365-2826.1993.tb00365.x. [DOI] [PubMed] [Google Scholar]
- Hrabovszky E, Steinhauser A, Barabas K, Shughrue PJ, Petersen SL, Merchenthaler I, Liposits Z. Estrogen receptor-b immunoreactivity in luteinizing hormone-releasing hormone neurons of the rat brain. Endocrinology. 2001;142:3261–3264. doi: 10.1210/endo.142.7.8176. [DOI] [PubMed] [Google Scholar]
- Karsch FJ, Bowen JM, Caraty A, Evans NP, Moenter SM. Gonadotropin-releasing hormone requirements for ovulation. Biol Reprod. 1997;56:303–309. doi: 10.1095/biolreprod56.2.303. [DOI] [PubMed] [Google Scholar]
- Le WW, Berghorn KA, Rassnick S, Hoffman GE. Periventricular preoptic area neurons coactivated with luteinizing hormone (LH)-releasing hormone (LHRH) neurons at the time of the LH surge are LHRH afferents. Endocrinology. 1999;140:510–519. doi: 10.1210/endo.140.1.6403. [DOI] [PubMed] [Google Scholar]
- Lee WS, Smith MS, Hoffman GE. Luteinizing hormone-releasing hormone neurons express Fos protein during the proestrous surge of luteinizing hormone. Proc Natl Acad Sci U S A. 1990;87:5163–5167. doi: 10.1073/pnas.87.13.5163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legan SJ, Coon GA, Karsch FJ. Role of estrogen as initiator of daily LH surges in the ovariectomized rat. Endocrinology. 1975;96:50–56. doi: 10.1210/endo-96-1-50. [DOI] [PubMed] [Google Scholar]
- Levine JE. New concepts of the neuroendocrine regulation of gonadotropin surges in rats. Biology of Reproduction. 1997;56:293–302. doi: 10.1095/biolreprod56.2.293. [DOI] [PubMed] [Google Scholar]
- Li C, Chen P, Smith MS. Identification of neuronal input to the arcuate nucleus (ARH) activated during lactation: implications in the activation of neuropeptide Y neurons. Brain Res. 1999;824:267–276. doi: 10.1016/s0006-8993(99)01217-2. [DOI] [PubMed] [Google Scholar]
- Marsicano G, Goodenough S, Monory K, Hermann H, Eder M, Cannich A, Azad SC, Cascio MG, Gutierrez SO, van der Stelt M, et al. CB1 cannabinoid receptors and on-demand defense against excitotoxicity. Science. 2003;302:84–88. doi: 10.1126/science.1088208. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Alves SE. Estrogen actions in the central nervous system. Endocr Revs. 1999;20:279–307. doi: 10.1210/edrv.20.3.0365. [DOI] [PubMed] [Google Scholar]
- Ouimet CC, McGuinness TL, Greengard P. Immunocytochemical localization of calcium/calmodulin-dependent protein kinase II in rat brain. Proc Natl Acad Sci U S A. 1984;81:5604–5608. doi: 10.1073/pnas.81.17.5604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen SL, Barraclough CA. Suppression of spontaneous LH surges in estrogen-treated ovariectomized rats by microimplants of antiestrogens into the preoptic brain. Brain Research. 1989;484:279–289. doi: 10.1016/0006-8993(89)90371-5. [DOI] [PubMed] [Google Scholar]
- Petersen SL, Ottem EN, Carpenter CD. Direct and indirect regulation of gonadotropin-releasing hormone neurons by estradiol. Biol Reprod. 2003;69:1771–1778. doi: 10.1095/biolreprod.103.019745. [DOI] [PubMed] [Google Scholar]
- Prevot V. Glial-neuronal-endothelial interactions are involved in the control of GnRH secretion. J Neuroendocrinol. 2002;14:247–255. doi: 10.1046/j.0007-1331.2001.00772.x. [DOI] [PubMed] [Google Scholar]
- Rage F, Lee BJ, Ma YJ, Ojeda SR. Estradiol enhances prostaglandin E2 receptor gene expression in luteinizing hormone-releasing hormone (LHRH) neurons and facilitates the LHRH response to PGE2 by activating a glia-to-neuron signaling pathway. J Neurosci. 1997;17:9145–9156. doi: 10.1523/JNEUROSCI.17-23-09145.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaft J, Ashery-Padan R, van der Hoeven F, Gruss P, Stewart AF. Efficient FLP recombination in mouse ES cells and oocytes. Genesis. 2001;31:6–10. doi: 10.1002/gene.1076. [DOI] [PubMed] [Google Scholar]
- Shughrue PJ, Lane MV, Merchenthaler I. Regulation of progesterone receptor messenger ribonucleic acid in the rat medial preoptic nucleus by estrogenic and antiestrogenic compounds: an in situ hybridization study. Endocrinology. 1997;138:5476–84. doi: 10.1210/endo.138.12.5595. [DOI] [PubMed] [Google Scholar]
- Shupnik MA. Gonadal hormone feedback on pituitary gonadotropin genes. Trends in Endocrinology and Metabolism. 1996;7:272–276. doi: 10.1016/s1043-2760(96)00124-5. [DOI] [PubMed] [Google Scholar]
- Simerly RB. Wired for reproduction: organization and development of sexually dimorphic circuits in the mammalian forebrain. Annu Rev Neurosci. 2002;25:507–536. doi: 10.1146/annurev.neuro.25.112701.142745. [DOI] [PubMed] [Google Scholar]
- Simonian SX, Spratt DP, Herbison AE. Identification and characterization of estrogen receptor a-containing neurons projecting to the vicinity of the gonadotropin-releasing hormone perikarya in the rostral preoptic area of the rat. J Comp Neurol. 1999;411:346–358. doi: 10.1002/(sici)1096-9861(19990823)411:2<346::aid-cne13>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Skinner DC, Dufourny L. Oestrogen receptor beta-immunoreactive neurones in the ovine hypothalamus: distribution and colocalisation with gonadotropin-releasing hormone. J Neuroendocrinol. 2005;17:29–39. doi: 10.1111/j.1365-2826.2005.01271.x. [DOI] [PubMed] [Google Scholar]
- Skynner MJ, Sim JS, Herbison AE. Detection of estrogen receptor α and β messenger ribonucleic acids in adult gonadotropin-releasing hormone neurons. Endocrinology. 1999a;140:5195–5201. doi: 10.1210/endo.140.11.7146. [DOI] [PubMed] [Google Scholar]
- Skynner MJ, Slater R, Sim JA, Allen ND, Herbison AE. Promoter transgenics reveal multiple gonadotropin-releasing hormone-1-expressing cell populations of different embryological origin in mouse brain. J Neurosci. 1999b;19:5955–5966. doi: 10.1523/JNEUROSCI.19-14-05955.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MJ, Jennes L. Neural signals that regulate GnRH neurones directly during the oestrous cycle. Reproduction. 2001;122:1–10. doi: 10.1530/rep.0.1220001. [DOI] [PubMed] [Google Scholar]
- Soriano P. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat Genet. 1999;21:70–71. doi: 10.1038/5007. [DOI] [PubMed] [Google Scholar]
- Tronche F, Kellendonk C, Kretz O, Gass P, Anlag K, Orban PC, Bock R, Klein R, Schutz G. Disruption of the glucocorticoid receptor gene in the nervous system results in reduced anxiety. Nat Genet. 1999;23:99–103. doi: 10.1038/12703. [DOI] [PubMed] [Google Scholar]
- Vente A, Korn B, Zehetner G, Poustka A, Lehrach H. Distribution and early development of microarray technology in Europe. Nat Genet. 1999;22:22. doi: 10.1038/8734. [DOI] [PubMed] [Google Scholar]
- Wiegand SJ, Terasawa E. Discrete lesions reveal functional heterogeneity of suprachiasmatic structures in regulation of gonadotropin secretion in the female rat. Neuroendocrinology. 1982;34:395–404. doi: 10.1159/000123335. [DOI] [PubMed] [Google Scholar]
- Xia L, Van Vugt D, Alston EJ, Luckhaus J, Ferin M. A surge of gonadotropin-releasing hormone accompanies the estradiol-induced gonadotropin surge in the rhesus monkey. Endocrinology. 1992;131:2812–2820. doi: 10.1210/endo.131.6.1446619. [DOI] [PubMed] [Google Scholar]
- Yoon H, Enquist LW, Dulac C. Olfactory inputs to hypothalamic neurons controlling reproduction and fertility. Cell. 2005;123:669–682. doi: 10.1016/j.cell.2005.08.039. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Buchholz F, Muyrers JP, Stewart AF. A new logic for DNA engineering using recombination in Escherichia coli. Nat Genet. 1998;20:123–128. doi: 10.1038/2417. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.