Abstract
There is increasing recognition that estrogen exerts multi-faceted regulatory effects on gonadotropin-releasing hormone (GnRH) neurons. The acute effects of estrogen on calcium dynamics in these cells were examined using a transgenic mouse line that allows real-time measurement of intracellular calcium concentration ([Ca2+]i) in GnRH neurons in the acute brain slice preparation. 17-β-estradiol (E2) at 100pM-100nM was found to activate [Ca2+]i transients in ~40% of GnRH neurons with an ~15 min latency. This effect was not replicated by E2-6-BSA, that limits E2 action to the membrane, 17-α-estradiol, the inactive isomer at classical estrogen receptors (ERs), or G-1 the GPR30 agonist. E2 continued to activate [Ca2+]i transients when transcription was blocked. An ER alpha-selective agonist was equally potent in activating [Ca2+]i transients and E2 remained effective in ERβ knockout x GnRH-pericam mice. E2’s activation of [Ca2+]i transients continued in the presence of tetrodotoxin, that blocks action potential-dependent transmission, but was abolished completely by the further addition of a GABAA receptor antagonist. Exogenous GABA was found to initiate [Ca2+]i transients in GnRH neurons. Whole-cell, voltage-clamp recordings of GnRH-GFP neurons revealed that E2 generated discrete bursts of miniature inhibitory postsynaptic currents with a latency of ~15 min. These observations provide evidence for a new mechanism of non-classical estrogen action within the brain. Estrogen interacts with the classical ERalpha at the level of the GABAergic nerve terminal to regulate action potential-independent GABA release that, in turn, controls postsynaptic calcium dynamics.
Keywords: estrogen, non-classical, estrogen receptor alpha, estrogen receptor beta, GPR30, GnRH, calcium, pericam, GABA
Introduction
It is well established that estrogens bind to nuclear-located estrogen receptors (ERs) to modulate gene expression in a classical manner within the brain. It is also now acknowledged that estrogens exert rapid actions on neurons that cannot be attributed to its classical transcriptional effects (1). However the mechanisms of action underlying these rapid or non-classical estrogen actions are much less clear. Studies have demonstrated that 17-β-estradiol (E2) can rapidly, within seconds or minutes, modulate ion channel activity and neuronal firing, phosphorylate second messenger signalling molecules and alter intracellular calcium concentrations [Ca2+]i within neurons (for reviews see (2, 3)). This suggests diverse and likely cell-specific modes of non-classical E2 actions within the brain. Although much controversy surrounds the nature of the estrogen-binding receptors involved in non-classical actions, it is generally assumed that non-classical effects are postsynaptic in origin.
One of the most important feedback targets for estrogen in the brain is the gonadotropin-releasing hormone (GnRH) neuronal population. These cells represent the final output neurons of the GnRH neuronal network regulating gonadal function and fertility in all mammals (4, 5). In females, in particular, a complex feedback relationship exists between the cyclical changes in circulating levels of E2 and the secretory behaviour of GnRH neurons (6, 7). For the greater part of the ovarian cycle, E2 suppresses the activity of the GnRH neurons. However, at mid-cycle, the high circulating levels of E2 exert a stimulatory action on the GnRH neurons to evoke the GnRH surge that, in turn, initiates ovulation. Whereas it appears that this stimulatory action of E2 on GnRH neurons is mediated by an indirect but nonetheless classical ERα-dependent mode of estrogen action (8, 9), the mechanisms involved in the suppressive effects are unknown (6, 7). Non-classical actions of E2 may contribute to estrogen feedback as E2 has been reported to rapidly modulate the phosphorylation status of intracellular signalling molecules, [Ca2+]i, and the electrical excitability of GnRH neurons (10–13). Further, it was recently reported that estrogen negative feedback on luteinizing hormone secretion is at least partly dependent upon non-classical estrogen signaling (9).
In the present study we employed a new transgenic mouse model (14) that enables real-time measurement of [Ca2+]i in GnRH neurons in situ, to examine whether E2 exerts rapid non-classical effects upon [Ca2+]i dynamics in adult GnRH neurons. These studies have resulted in the identification of a novel mode of non-classical E2 action that involves the direct presynaptic regulation of action potential-independent GABA release.
Materials and Methods
Experimental animals
All experimentation was approved by the University of Otago Animal Welfare and Ethics Committee under application 66/02. Transgenic GnRH-Pericam mice (C57BL6/J x CBA/Ca) were maintained under 12:12 lighting conditions (lights on 07:00h) with food and water available ad libitum. The GnRH-Pericam mice were crossed with ERβ knockout mice (15) to generate GnRH-Pericam; ERβKO-/- mice that were genotyped by PCR as reported previously (8). The stage of estrous cycle was determined by vaginal smear.
Real-time calcium imaging in GnRH neurons
Calcium imaging experiments were performed as reported previously (14). Briefly, brains were quickly removed from adult (>50 days old) female GnRH-Pericam mice and placed in ice-cold cutting artificial cerebrospinal fluid (ACSF) containing (in mM): 118 NaCl, 3 KCl, 0.5 CaCl2, 6 MgCl2, 5 HEPES, 25 NaHCO3, 11 D-glucose, pH 7.3 when gassed with 95% O2 and 5% CO2. 150 µm-thick coronal slices were cut with a vibratome and kept in a slice chamber mounted in a heated platform (30±2°C) perfused with prewarmed (28-30°C) oxygenated standard ACSF (118mM NaCl, 3 KCl, 2.5 CaCl2, 1.2 MgCl2, 5 HEPES, 25 NaHCO3, 11 D-glucose, pH 7.3) solution at a rate of 1–2 ml/min. Cells were imaged using an Olympus (Tokyo, Japan) BX51 upright microscope. Excitation was alternately performed at 493 and 415 nm every second, and emission filtered at 525 nm with the use of a Sutter Instruments Lambda DG-4 high-speed filter. Image acquisition was performed using Metafluor to control and synchronize the DG-4 and liquid-cooled ORCA-ER CCD camera (Hamamatsu, Japan). Fluorescence intensity values were exported for a region of interest on the cell and one of the same area on the background, and analysed with custom written software (14).
Drugs were tested by addition to the ACSF: 17β-estradiol, 17α-estradiol, 6-(O-carboxymethyl)oxime:BSA (E2-6-BSA), (all Sigma Aldrich), 3,17-dihydroxy-19-nor-17α-pregna-1,3,5 (10)-triene-21,16α-lactone (16α-LE2, kind gift of Bayer-Schering AG, Berlin), 5,6-dichlorobenzimidazole 1-β-D-ribofuranoside (DRB) and 1-[4-(6-bromobenzo[1,3]dioxol-5-yl)-3a,4,5,9b-tetrahydro-3H-cyclopenta[c]quinolin-8yl]-ethanone (G-1, kind gift of ChemDiv, CA) were dissolved in ethanol (final concentration <=0.001%). Tetrodotoxin (TTX, Tocris), gabazine (Sigma Aldrich), 2-aminoethyl diphenylborinate (2-APB, Sigma) and caffeine (Sigma) were dissolved in water. The E2-6-BSA concentration was calculated considering the molecular weight of BSA plus an average of 35 molecules of E2 per molecule of BSA, giving a total of 75.9 kDa. E2-6-BSA was filtered before use on Microcon YM3 columns (Millipore) to remove unconjugated estradiol (11).
Data analysis
The analysis of calcium traces was performed using a Daubechies 4 wavelet transform to detect peaks and baseline changes as reported previously (14). Individual [Ca2+]i transients were considered significant if their heights were at least 10% above baseline (relative power of >15%). The experimental protocol consisted of imaging the cell for 15 minutes to measure baseline calcium and transient frequency before testing. Initially, 30 cells were imaged for 30 minutes without any treatment and transient frequency in first 15 minutes compared to the second 15 minutes. No significant difference was found in transient frequency between the two periods (mean ± SEM; 0.37 ± 0.10 first period and 0.83 ± 0.24 second period, n=30; paired t-test). In the case of cells that did not show any transients in the control period, we considered them responsive when they showed more than 3 transients after the treatment. For cells that had transients in the control period, a >3-fold increase in transient frequency was considered a response. All statistical analyses were performed using R software (R development Core Team, http://www.R-project.org) and, unless stated otherwise, involved ANOVA followed Student-Newman-Keuls post-hoc tests.
Whole-cell IPSC recordings
Acute brain slices (200μm-thick) were made from female GnRH-EGFP transgenic mice (6-10 weeks old) as described above for calcium imaging experiments. Whole-cell currents were recorded from GnRH neurons at room temperature with the visualization of individual neurons, as reported previously (16). Open resistance was in the range 4–8 MΩ and seal resistance was in the range 1–3 GΩ. Patch pipettes were filled with a solution containing (in mM): 140 KCl, 20 HEPES, 0.5 CaCl2, 5 EGTA and 5 MgATP (pH, 7.2). The electrical activity of GnRH neurons was recorded with a headstage (CV-7B) connected with Multiclamp 700A amplifier. Current signals were filtered at 1 kHz and digitized at 10 kHz using an analogue-digital converter (Digidata 1322A) and pClamp (Version 10.1, Molecular Devices, CA, USA). Upon membrane rupture, GnRH neurons were clamped to -70 mV and allowed to stabilize for 5 min before switching to ACSF (2-3 ml/min) containing 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX, 20 mM), DL-2-amino-5-phosphonovaleric acid (AP5, 20 µM) and TTX (0.5 μM). The experimental protocol was the same as that used for calcium experiments and involved a 15 min pre-treatment period, followed by a 15 min exposure to 100nM E2 or normal ACSF and then a 15 min washout period. Measurements of the amplitude, frequency and the decay time of miniature inhibitory postsynaptic current (mIPSCs) were performed using the Mini Analysis program (Version 6.0, Synaptosoft, NJ, USA). A threshold for detection of mIPSCs was set at 3-fold RMS noise.
Results
E2 regulates [Ca2+]i dynamics in an acute manner in adult GnRH neurons
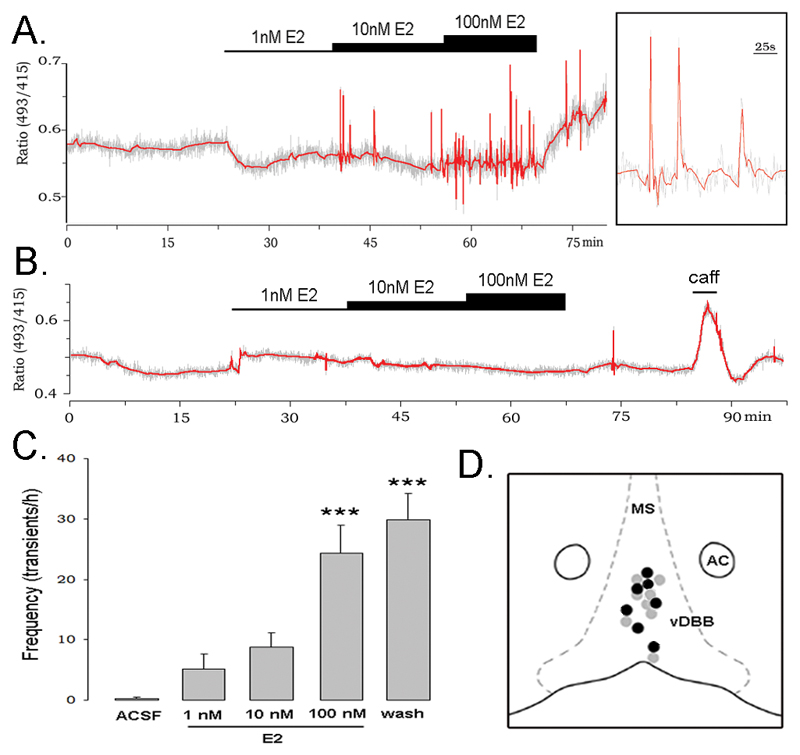
As shown previously for adult male GnRH neurons (14), the great majority of GnRH neurons recorded from adult female mice were found to exhibit no or slow transients (<8 transients/h). In our initial experiment, GnRH neurons located in the rostral preoptic area (rPOA) of female mice exhibiting <8 [Ca2+]i transients/h were tested with an incremental E2 concentration protocol that exposed them to artificial cerebrospinal fluid (ACSF) containing, in sequence, 1nM, 10nM and 100nM E2 for 15 min each, before returning to the E2-free ACSF (Fig.1A). Thirteen of 27 (48%) GnRH neurons from 22 mice responded by increasing their [Ca2+]i transient frequency (Fig.1A). The stimulatory response of individual cells was observed during the 10nM or 100nM E2 treatment period and was prolonged, often continuing throughout the 15 min washout period (Fig.1A,C). As a group, the increase in [Ca2+]i transient frequency achieved statistical significance during 100nM E2 and wash period (Fig.1C; p<0.001; ANOVA). The remaining 14 GnRH neurons did not respond to E2 but were viable as they all responded to a 1-2 min exposure to 30mM caffeine (Fig.1B). The locations of responding and non-responding GnRH neurons were not different (Fig.1D).
Figure 1. Estradiol (E2) stimulates calcium transients in GnRH neurons.
Note: in all traces, the grey represents the actual recording trace and the red line the Daubechies 4 wavelet transform. A. Representative example of a GnRH neuron in which the E2 treatment protocol activated Ca2+ transients. Inset shows a magnification of the 3 peaks indicated by the arrow. B. GnRH neuron that was unresponsive to the E2 treatment but responded to 30mM caffeine (caff). C. Effect of E2 on Ca2+ transient frequency in responding GnRH neurons given as mean+SEM transient frequency over 15 min time bins including washout period (wash) (n=13; *** p<0.001). D. Schematic diagram of the locations of individual rostral preoptic area GnRH neurons that were stimulated (black dot) or unaffected (grey dot) by E2. As many GnRH neurons overlap in location, not all cells are shown. MS = medial septum, AC = anterior commissure, DBB = diagonal band of Broca.
Previous studies have demonstrated that interactions may occur between classical and non-classical E2 actions; specifically, that classical genomic regulation may impact upon the rapid effects of estrogen on neurons (17). However, we found that there was no effect of the stage of estrous cycle upon the ability of E2 to activate [Ca2+]i transients in GnRH neurons. The percentage response rate of GnRH neurons to E2 was 50%, 55% and 44% in diestrous (n=11), proestrous (n=9) and estrous (n=18) mice, respectively.
In addition to effects of E2 on [Ca2+]i transient frequency, some GnRH neurons also exhibited baseline changes in [Ca2+]i immediately upon application of E2 (Fig.1A). However, there was no relationship between changes in baseline [Ca2+]i and changes in transient frequency and, overall, no consistent variation in baseline [Ca2+]i was observed.
Dose and time dependency of E2 action upon [Ca2+]i transients
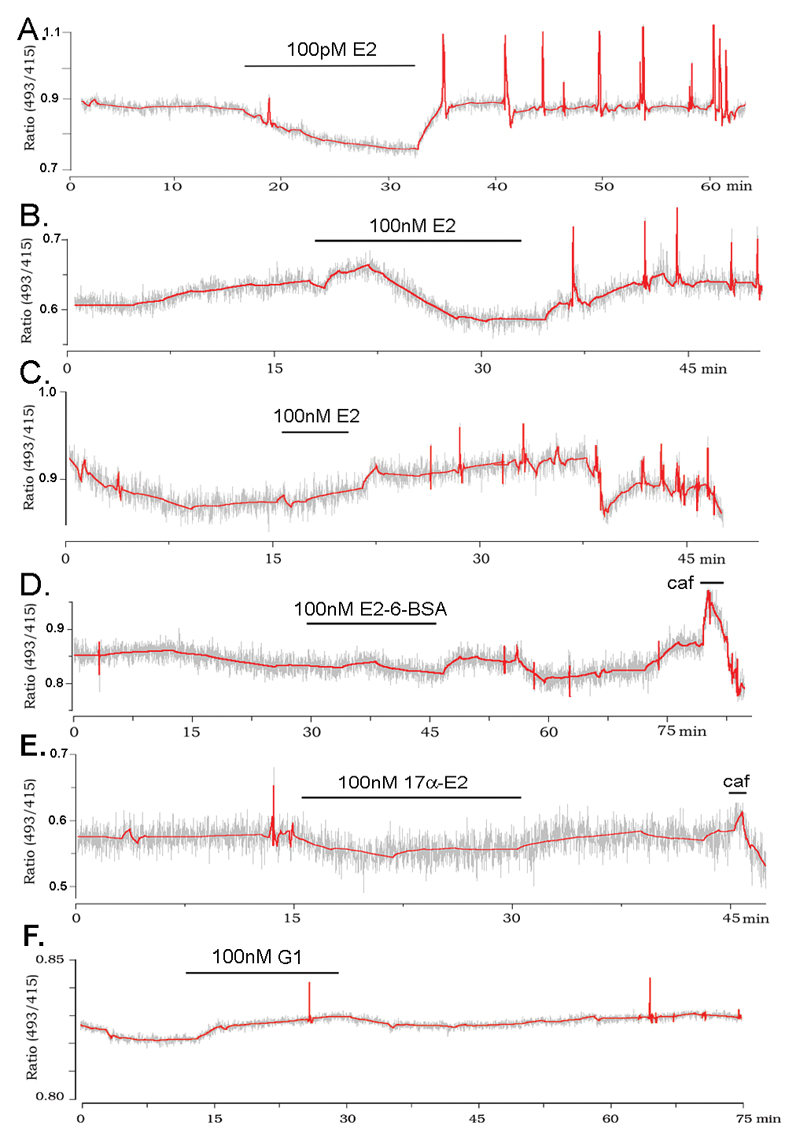
The results of the incremental E2 concentration protocol suggested that the stimulatory response may require many minutes to occur. To address this issue, as well as the concentration dependence of [Ca2+]i activation, experiments were undertaken in which individual GnRH neurons were exposed to single 100pM, 1nM, 10nM or 100nM concentrations of E2 for a 15 min period. All concentrations were found to be effective in stimulating [Ca2+]i transients with a common ~15 min delay (Fig.2AB) [100pM, 3/13 cells responded with delay 15.4±2.4 min; 1nM, 6/13 cells responded with delay 13.6±3.0 min; 10nM, 4 of 8 cells responded with delay 17.9±3.9 min; 100nM, 6/13 cells responded with delay 14.7±2.7 min].
Figure 2. The stimulatory effect of estradiol is mediated by an intracellular receptor and does not involve GPR30.
A,B Representative examples of GnRH neurons responding to 15min exposure to 100pM (A) or 100nM (B) E2. C Representative example of a GnRH neuron responding to 5 min exposure to 100nM E2. D,E Representative traces of GnRH neurons that did not respond to 100 nM E2-6-BSA (D) 100 nM 17α-estradiol (E) or 100nM G-1 (F). All of these three cells responded to a caffeine (caf) stimulus at the end of the experiment.
The necessity for 15 min of E2 exposure to activate [Ca2+]i transients was tested by treating cells with 100nM E2 for only 5 min. In this case, 3 of 7 neurons from 5 animals responded (Fig.2C) and the latency for effect (from the beginning of the E2 exposure; 13.2±1.1 min) was not different to that found with 15 min E2 treatments.
These experiments showed that concentrations as low as 100pM are effective in activating [Ca2+]i transients in GnRH neurons, and that there is an ~15 min latency for this effect to occur.
E2 stimulates [Ca2+]i transients in GnRH neurons through an ERα-dependent mechanism
To evaluate the site and specificity of the E2 effect, GnRH neurons were treated with E2-6-BSA, that limits E2 action to the cell membrane, and 17α-estradiol, the inactive isomer of E2 at classical ERs. Neither of these compounds, tested at 100nM concentration for 15 minutes, had any effects upon [Ca2+]i in GnRH neurons (n=6 from 6 animals for E2-6-BSA, 7 from 7 animals for 17α-estradiol, Fig. 2D,E) although the cells remained responsive to caffeine (mean ± SEM % increase in baseline [Ca2+]i; E2 = 15.7 ± 2.5%. 17 alpha = 10.4 ± 3.1%, E2-6-BSA = 14.8 ± 1.8%). GPR30 has recently been suggested to be a novel estrogen-binding molecule (18). Application of the GPR30 agonist G-1 (100nM, (19)) did not have any effect on [Ca2+]i in 7 GnRH neurons from 6 animals (Fig 2F).
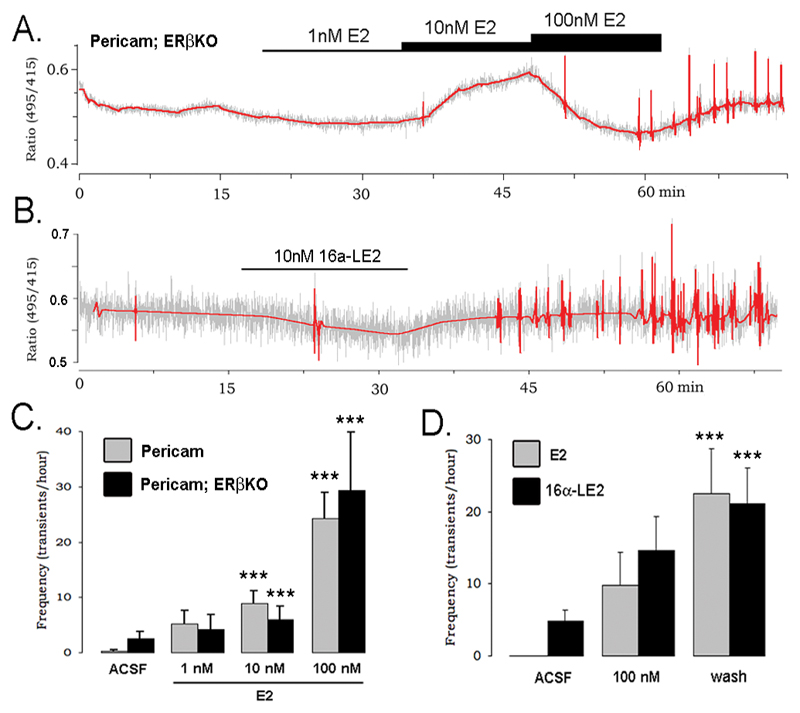
To evaluate the role of classical ERs in this acute effect, recordings were made from 16 GnRH neurons obtained from 10 GnRH-pericam+/-;ERβKO-/- mutant mice and compared with recordings from wild-type and GnRH-pericam+/-;ERβKO+/+ mice littermates. All genotypes demonstrated the exact same response to E2 (Fig.3A,C) evoking a significant (p<0.05) increase in [Ca2+]i transients in 50% and 48% of GnRH neurons from ERβ mutants and controls, respectively. This indicated that ERβ was not required for this response. To address directly the involvement of ERα in activating [Ca2+]i transients, GnRH neurons from GnRH-Pericam mice were tested with 10 or 100nM 16α-LE2, an ERα-selective ligand with equimolar potency to E2 (20). Application of 10nM 16α-LE2 induced [Ca2+]i transients in 2 of 10 cells (Fig.3B) while 100nM 16α-LE2 induced [Ca2+]i transients in 10 of 20 cells from 12 animals with a similar pattern to that evoked by 15 min 100nM E2 (Fig.3D). Together, these data indicated that E2 interacts with ERα within the cell to initiate calcium transients in GnRH neurons.
Figure 3. The stimulatory effect of estradiol is mediated by ER–α.
A. Representative trace from a GnRH neuron recorded in an ERβKO x GnRH-pericam mouse that shows the typical stimulatory response to E2. B. Recording from a GnRH neuron of a GnRH-pericam mouse that was stimulated by the application of the ERa-selective agonist 16α-LE2. C. Mean (+SEM) Ca2+ transient frequency in GnRH-pericam (Pericam) and GnRH-pericam x ERβKO mice following exposure to increasing doses of E2 (*** p<0.001 vs. ACSF). D. Mean (+SEM) Ca2+ transient frequency of “slow” GnRH neurons in response to a single dose of 100 nM E2 (grey bars) or the ER-α selective agonist 16α-LE2 (black bars) (*** p<0.001 vs. ACSF).
E2 acts through a non-transcriptional mechanism to generate [Ca2+]i transients dependent upon IP3 receptors
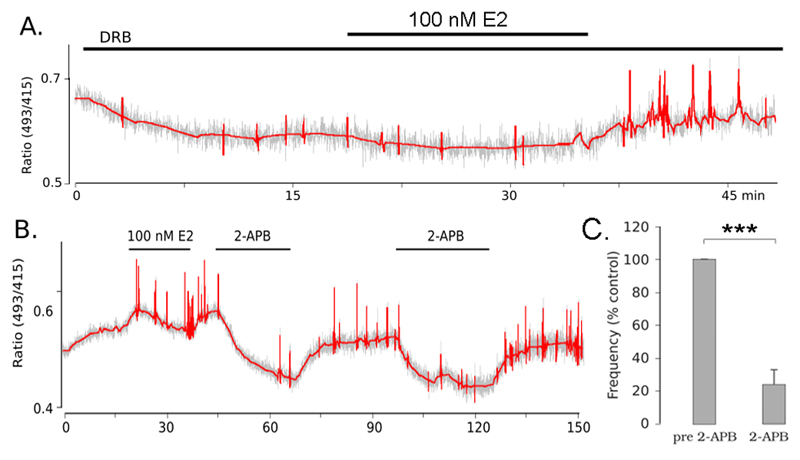
The 15min time delay is relatively long for “rapid” estrogen effects and could conceivably result from transcriptional actions. To evaluate this, experiments were performed using the RNA synthesis blocker DRB in a protocol similar to that shown to be effective for mouse olfactory placode GnRH neurons (11). Brain slices were treated with 150μM DRB 15 minutes prior to and during the E2 exposure. Three of 7 cells from 4 animals, treated with 100 nM E2 in the presence of DRB responded with an activation of [Ca2+]i transients (Fig. 4A), indicating that transcription is not necessary for the generation of this response.
Figure 4. Estradiol activates calcium transients in a transcription-independent mechanism requiring IP3 receptors.
A. Representative trace from a GnRH neuron showing a response to 100 nM E2 in the presence of 150 μM DRB. B. Representative trace from a GnRH neuron activated by 100 nM E2 in which calcium transients were inhibited by treatment with 100μM 2-APB. Calcium transients reappeared after removal of 2-APB and the inhibition was repeatable upon exposure to a second dose of 2-APB. C. Bar graph showing the average (± SEM) frequency of calcium transients in the 15 minutes preceding 2-APB and during the exposure to 2-APB expressed as percentage of the control period.
Previous studies from our laboratory have shown that spontaneous [Ca2+]i transients in GnRH neurons derive from release of calcium from IP3 receptors (IP3R). To test if the same mechanism is involved in the generation of E2-induced transients, 5 cells that were activated by E2 were treated with the IP3R blocker 2-APB. In 4 of 5 cells tested calcium transients were almost completely suppressed upon treatment with 2-APB (Fig. 4B,C).
E2 activates [Ca2+]i transients in GnRH neurons through a presynaptic mechanism involving GABA
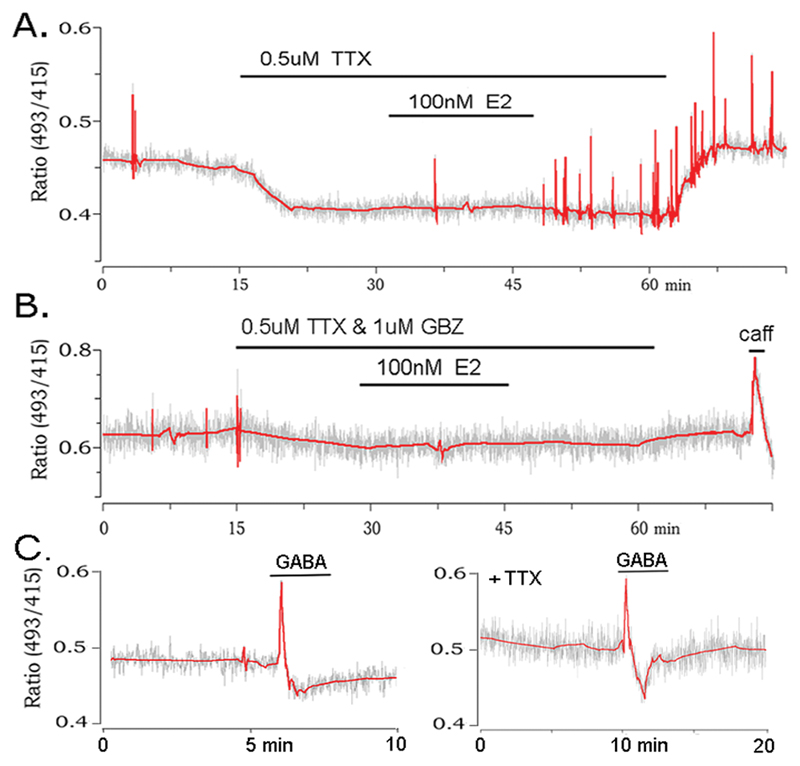
To examine the possibility that E2 acts directly upon GnRH neurons to generate [Ca2+]i transients, experiments were undertaken in the presence of tetrodotoxin (TTX) to block action potential-dependent transmission in the brain slice. Ten GnRH neurons from 7 animals were tested with 100nM E2 in the presence of TTX and three found to respond in the normal manner with an induction of [Ca2+]i transients (Fig.5A) and a latency of 15.3±2.1 min. This result suggested that E2 may act directly upon GnRH neurons to evoke [Ca2+]i transients but was inconsistent with the known absence of ERα in these cells (21). Alternatively, it was possible that E2 was influencing action potential-independent neurotransmitter release from nerve terminals synapsing with GnRH neurons. Electrophysiological studies had shown that these neurons are exposed to considerable action potential-independent GABA release (22, 23). Hence, experiments were repeated with the inclusion of 1mM gabazine, a GABAA receptor antagonist that (unlike bicuculline) does not fluoresce at the wavelengths used for Pericam recording, and blocks all GABAA receptor-mediated inhibitory postsynaptic currents (IPSCs) in GnRH neurons. In the presence of TTX and gabazine, E2 failed to induce [Ca2+]i transients in all 13 of 13 GnRH neurons from 4 animals (Fig.5B). This demonstrated that estrogen’s ability to evoke [Ca2+]i transients in GnRH neurons was critically dependent upon presynaptic, action potential-independent GABA release. This predicted that GABA itself would be able to regulate [Ca2+]i in GnRH neurons. Although it is not possible to simulate quantal GABA release we, nevertheless, tested GnRH neurons with brief (2min) applications of 50mM GABA. This was found to evoke a large transient increase in [Ca2+]i in 7 of 11 (64%) of GnRH neurons (Fig.5C). Application of 50mM GABA in the presence of 0.5mM TTX also induced a large calcium transient in 5 of 6 (83%) of GnRH neurons (Fig.5C).
Figure 5. The stimulatory effect of estradiol is mediated by action potential-independent GABA release and can be activated by exogenous GABA.
A. Representative example of a GnRH neuron treated with TTX but continuing to respond to 100 nM E2 in the normal manner. B. GnRH neuron treated with 100 nM E2 in the presence of both TTX and the GABAA receptor antagonist gabazine (GBZ) showing the failure of the cell to be activated by E2. C. Two examples of GnRH neurons (one in the presence of TTX) showing the ability of GABA to evoke a Ca2+ transient. Note difference in time scale from prior recordings.
E2 initiates bursts of miniature IPSCs in GnRH neurons
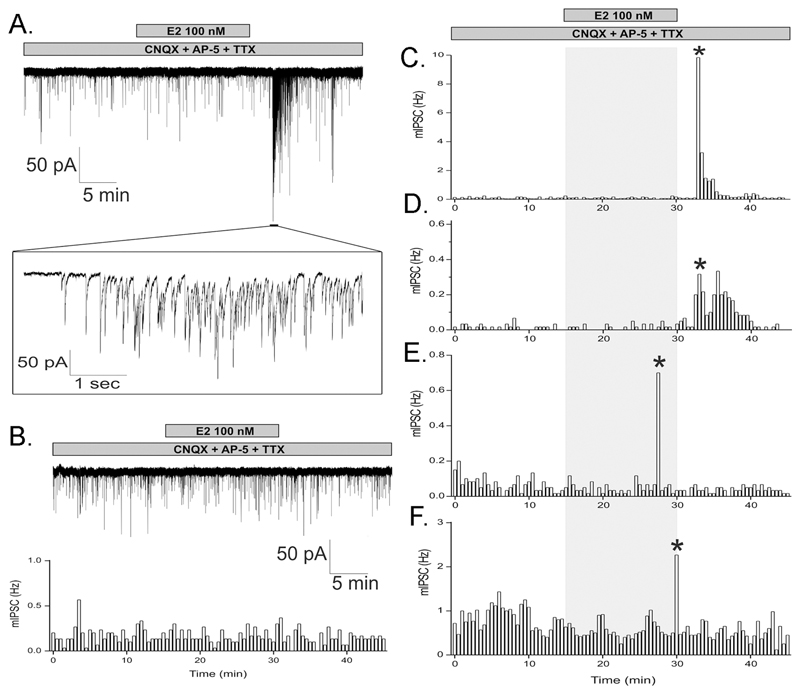
Together, the previous results indicated that E2 enhances action potential-independent GABA release upon GnRH neurons. To evaluate this hypothesis directly, GABAA receptor currents were recorded in GnRH neurons using whole-cell, voltage-clamp electrophysiology in brain slices of adult female GnRH-GFP transgenic mice. The exact same TTX-containing experimental protocol as that used in the calcium experiments was employed with the exception of the addition of glutamate receptor antagonists CNQX, 20 µM and AP5, 20 µM to ensure that only inhibitory post-synaptic currents (IPSCs) were measured. Miniature IPSCs (mean (+SEM) frequency 0.75+0.20 Hz; amplitude 29.5±3.3 pA; decay 15.5±0.7 ms) were blocked completely by the GABAA-receptor antagonists bicuculline (10mM) or gabazine (1mM) demonstrating that they reflect action potential-independent GABA activation of GABAA receptors on GnRH neurons. Remarkably, E2 (100nM) applied for 15 min resulted in an abrupt burst of IPSCs (frequency 7.3±4.3 Hz; amplitude 41.7±7.5 pA; duration 145±89 s; Fig.6A) with a mean latency of onset of 15.8±1.3 min (range 13-18 min) (Fig.5A) in four of 17 GnRH neurons from 13 animals (24%; Fig.6C-F). The IPSC frequency and characteristics did not change in the remaining GnRH neurons (Fig.6B).
Figure 6. Estradiol initiates bursts of mIPSCs.
A. Whole-cell, voltage-clamp recordings in the presence of TTX and glutamate receptor antagonists CNQX and AP-5 from adult female GnRH neurons over a 45 min period during which 100nM E2 was administered to the bath for 15 min (bar). An abrupt increase in the frequency and amplitude of mIPSCs occurs 18 min after application of E2, part of which is expanded in the inset. B. Representative example of a GnRH neuron that did not respond to E2, using the same protocol as in panel A. Top shows actual trace and bottom shows 30s bin histogram of mIPSC frequency. C - F. mIPSC frequency histogram plots from four GnRH neurons that responded to E2 with a delayed burst in mIPSC (*).
Discussion
This study identifies a new mechanism of rapid, non-classical E2 action in the brain (Fig.7). Estrogens can exert multiple non-classical actions upon neurons and, where established, the underlying mechanisms have involved the direct modulation of signaling cascades within the neuron (1, 3, 24). We now show that the presynaptic terminal is also a direct site of rapid E2 action in the brain and that, using the classical ER, it is able to modulate GABA release dynamics that, in turn, controls [Ca2+]i transients in postsynaptic cells.
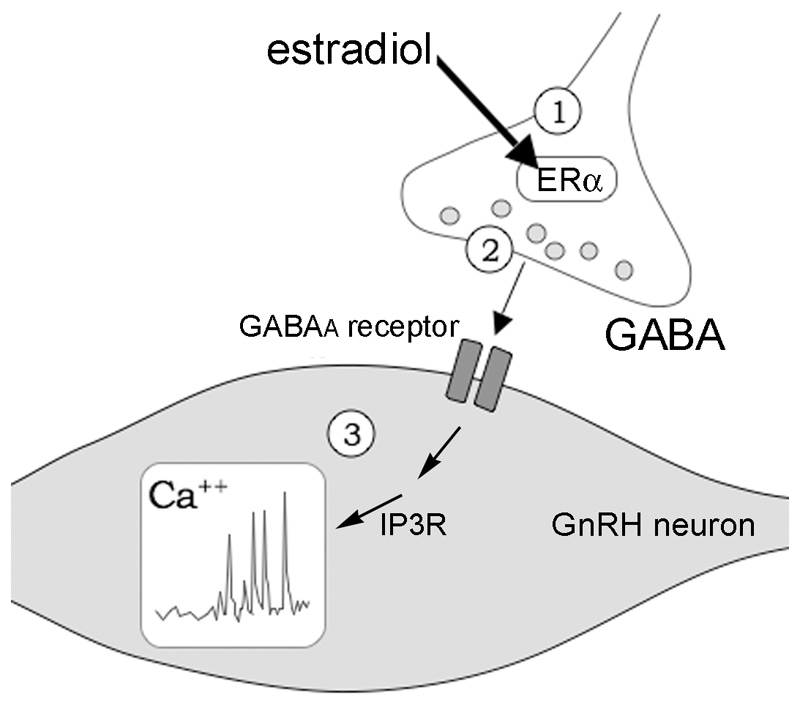
Figure 7. Proposed mechanism of presynpatic estrogen action on GnRH neurons.
Schematic diagram showing postulated mechanism of non-classical estradiol modulation of presynaptic GABAergic terminals to control calcium homeostasis in GnRH neurons. Estradiol (E2) binds ERα located in the presynaptic GABAergic nerve terminals ① to facilitate action potential-independent GABA release ② that acts through GABAA receptors to initiate intracellular calcium transients in an IP3 receptor-dependentt manner ③.
Our initial genetic and pharmacological analyses indicated that ERα rather than ERβ was essential for the stimulation of [Ca2+]i transients as the ERα selective ligand 16α-LE2 (20) was effective while the E2 response was maintained in GnRH-pericam+/-;ERβKO-/- mice. In confirmation of the involvement of a classical ER, we found that equimolar 17-α-estradiol did not stimulate [Ca2+]i transients. Furthermore, this effect was unlikely to occur through a plasma membrane receptor, as E2-6-BSA was unable to stimulate [Ca2+]i transients. The E2-6-BSA and 17-α-estradiol results are not technical false negatives as both compounds at these concentrations evoke effects in other assays within the laboratory (unpublished observations). GPR30 has recently been proposed to be a new E2-binding molecule (18) although it is important to note that considerable controversy surrounds the location of GPR30 within cells (25) and even its ability to bind E2 (26). Application of the GPR30 receptor agonist G-1 did not evoke [Ca2+]i transients suggesting that this receptor is not involved in regulating calcium dynamics in GnRH neurons. Together, these findings reinforce further the concept that classical ERs are often necessary for the non-classical rapid effects of E2 in the brain (3, 27) and demonstrate that E2 requires intracellular ERα to stimulate [Ca2+]i transients in GnRH neurons.
As GnRH neurons do not express ERα (21), it seemed unlikely that E2 was acting directly on GnRH neurons to modulate [Ca2+]i dynamics. As such, we were surprised to find that E2 induced [Ca2+]i transients in the absence of action potential-dependent transmission. However, GnRH neurons are known to receive substantial action potential-independent GABA transmission (22, 23) and so we speculated that E2 may be acting at the level of presynaptic GABAergic terminals to modulate GABA release that, in turn, controls the frequency of [Ca2+]i transients in GnRH neurons (Fig.7). This hypothesis has been supported by our calcium imaging and electrophysiological observations. Firstly, we show that the ability of E2 to activate [Ca2+]i transients in TTX-treated GnRH neurons is abolished completely by pre-treatment with a GABAA receptor antagonist, thereby identifying the necessity of action potential-independent GABA. The role of action potential-independent GABA release in brain function is not yet well understood (28) but clearly shown here to be involved in regulating postsynaptic calcium dynamics. Secondly, we show that E2 treatment enhances miniature IPSCs in GnRH neurons demonstrating that E2 facilitates action potential-independent GABA release directed at these cells. Finally, we show that treatment with exogenous GABA can itself initiate [Ca2+]i transients in GnRH neurons. It is important to note that, as with many characteristics reported for GnRH neurons (5), only a sub-population of GnRH neurons respond to E2 in this manner. The biological significance and nature of this heterogeneity remains unknown at present.
Precisely how E2-evoked GABA initiates [Ca2+]i transients in GnRH neurons is not yet established. We show that the mechanism is indeed non-classical as it is not dependent upon gene transcription. We also report that, like spontaneous [Ca2+]i transients (14), E2-GABA-evoked transients are dependent upon IP3Rs. Current studies in the laboratory are investigating this novel mechanism.
There is now good evidence that ERα is located within dendrites and nerve terminals (29–31) and that GABA release is modulated by E2 in multiple brain regions, including putative ERα-expressing GABAergic afferents to the GnRH neurons (32–34). Most compelling of all, however, is the recent work by Woolley and colleagues (35), who found that sub-populations of synaptic vesicles in hippocampal GABAergic neurons are associated with ERα and that 24h E2 treatment moved these vesicles towards the synapse. Although using a different E2 protocol, that ultrastructural study appears to have remarkable parallels with our own findings. Brought together, we suggest that E2 acts on ERα-associated GABA vesicles in terminals presynaptic to GnRH neurons to modulate action potential-independent GABA release (Fig.7). Whereas many non-classical effects of E2 occur within seconds or a few minutes (3), we note here that the activation of GABA release and subsequent [Ca2+]i transients takes ~ 15 min. The sub-cellular mechanisms within the GABAergic terminal responsible for this delay are not known but would be compatible with the several minute delay that might be required for mobilization and movement of vesicles to the synaptic cleft.
It is important to note that this is now the third paper to show that estrogen rapidly modulates [Ca2+]i transients in GnRH neurons. The two previous studies have reported on effects of E2 on [Ca2+]i transients in GnRH neurons from embryonic olfactory placode cultures (11, 36). Whereas all three papers show that E2 can stimulate [Ca2+]i transients in GnRH neurons in the presence of TTX, the apparent underlying mechanism is completely different for each preparation. In embryonic monkey GnRH neurons this involves an E2-binding membrane receptor (36), while in early-cultured mouse embryonic GnRH neurons it requires an intracellular ERβ-mediated transcriptional event (11), and here, in adult mouse GnRH neurons, we report an indirect GABA-mediated mechanism. Whereas differences might be expected in the way E2 modulates embryonic and adult GnRH neurons, it is perhaps surprising to have such a divergence in mechanism of E2 action between embryonic GnRH neurons in the mouse and monkey.
In summary, the present study has identified a mechanism of non-classical E2 action that targets classical ERs within the presynaptic terminal to modulate action potential-independent GABA release. Changes in action potential-independent GABA release are further shown to have a key role in controlling [Ca2+]i dynamics in GnRH neurons. A similar scenario in which E2 targets glutamatergic nerve terminals to rapidly modulate postsynaptic neurons has been reported recently in the developing brain (37). This expands considerably the horizons of non-classical E2 regulation of neural function that have, until now, been focussed principally upon post-synaptic sites of action.
Acknowledgements
Bayer-Schering AG is thanked for the kind gift of 16-α-LE2 and ChemDiv for the gift of G-1.
Funding
This research was supported by the Wellcome Trust, Royal Society of New Zealand Marsden Fund and Health Research Council of New Zealand. IA was supported by IBRO, an Öveges Scholarship and OTKA grant 047217.
Footnotes
Disclosure Statement: The authors have nothing to disclose
References
- 1.McEwen BS, Alves SE. Estrogen actions in the central nervous system. Endocr Revs. 1999;20:279–307. doi: 10.1210/edrv.20.3.0365. [DOI] [PubMed] [Google Scholar]
- 2.Ronnekleiv OK, Kelly MJ. Diversity of ovarian steroid signaling in the hypothalamus. Front Neuroendocrinol. 2005;26:65–84. doi: 10.1016/j.yfrne.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 3.Kelly MJ, Levin ER. Rapid actions of plasma membrane estrogen receptors. Trends Endocrinol Metab. 2001;12:152–156. doi: 10.1016/s1043-2760(01)00377-0. [DOI] [PubMed] [Google Scholar]
- 4.Levine JE. New concepts of the neuroendocrine regulation of gonadotropin surges in rats. Biology of Reproduction. 1997;56:293–302. doi: 10.1095/biolreprod56.2.293. [DOI] [PubMed] [Google Scholar]
- 5.Herbison AE. Physiology of the GnRH neuronal network. In: Neill JD, editor. Knobil and Neill's Physiology of Reproduction. 3rd ed. San Diego: Academic Press; 2006. pp. 1415–1482. [Google Scholar]
- 6.Herbison AE. Multimodal influence of estrogen upon gonadotropin-releasing hormone neurons. Endocr Rev. 1998;19:302–330. doi: 10.1210/edrv.19.3.0332. [DOI] [PubMed] [Google Scholar]
- 7.Petersen SL, Ottem EN, Carpenter CD. Direct and indirect regulation of gonadotropin-releasing hormone neurons by estradiol. Biol Reprod. 2003;69:1771–1778. doi: 10.1095/biolreprod.103.019745. [DOI] [PubMed] [Google Scholar]
- 8.Wintermantel TM, Campbell RE, Porteous R, Bock D, Grone HJ, Todman MG, Korach KS, Greiner E, Perez CA, Schutz G, Herbison AE. Definition of estrogen receptor pathway critical for estrogen positive feedback to gonadotropin-releasing hormone neurons and fertility. Neuron. 2006;52:271–280. doi: 10.1016/j.neuron.2006.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Glidewell-Kenney C, Hurley LA, Pfaff L, Weiss J, Levine JE, Jameson JL. Nonclassical estrogen receptor alpha signaling mediates negative feedback in the female mouse reproductive axis. Proc Natl Acad Sci U S A. 2007;104:8173–8177. doi: 10.1073/pnas.0611514104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lagrange AH, Rønnekleiv OK, Kelly MJ. Estradiol-17β and μ-opioid peptides rapidly hyperpolarize GnRH neurons: a cellular mechanism of negative feedback. Endocrinology. 1995;136:2341–2344. doi: 10.1210/endo.136.5.7720682. [DOI] [PubMed] [Google Scholar]
- 11.Temple JL, Laing E, Sunder A, Wray S. Direct action of estradiol on gonadotropin-releasing hormone-1 neuronal activity via a transcription-dependent mechanism. J Neurosci. 2004;24:6326–6333. doi: 10.1523/JNEUROSCI.1006-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abe H, Terasawa E. Firing pattern and rapid modulation of activity by estrogen in primate luteinizing hormone releasing hormone-1 neurons. Endocrinology. 2005;146:4312–4320. doi: 10.1210/en.2005-0435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abraham IM, Han K, Todman MG, Korach KS, Herbison AE. Estrogen receptor β mediates rapid estrogen actions on gonadotropin-releasing hormone neurons in vivo. J Neurosci. 2003;23:5771–5777. doi: 10.1523/JNEUROSCI.23-13-05771.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jasoni CL, Todman MG, Strumia MM, Herbison AE. Cell type-specific expression of a genetically encoded calcium indicator reveals intrinsic calcium oscillations in adult gonadotropin-releasing hormone neurons. J Neurosci. 2007;27:860–867. doi: 10.1523/JNEUROSCI.3579-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Krege JH, Hodgin JB, Couse JF, Enmark E, Warner M, Magler JF, Sar M, Korach KS, Gustafsson J-A, Smithies O. Generation and reproductive phenotypes of mice lacking estrogen receptor β. Proc Natl Acad Sci U S A. 1998;95:15677–15682. doi: 10.1073/pnas.95.26.15677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Han SK, Todman MG, Herbison AE. Endogenous GABA release inhibits the firing of adult gonadotropin-releasing hormone neurons. Endocrinology. 2004;145:495–499. doi: 10.1210/en.2003-1333. [DOI] [PubMed] [Google Scholar]
- 17.Vasudevan N, Kow LM, Pfaff DW. Early membrane estrogenic effects required for full expression of slower genomic actions in a nerve cell line. Proc Natl Acad Sci U S A. 2001;98:12267–12271. doi: 10.1073/pnas.221449798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Revankar CM, Cimino DF, Sklar LA, Arterburn JB, Prossnitz ER. A transmembrane intracellular estrogen receptor mediates rapid cell signaling. Science. 2005;307:1625–1630. doi: 10.1126/science.1106943. [DOI] [PubMed] [Google Scholar]
- 19.Bologa CG, Revankar CM, Young SM, Edwards BS, Arterburn JB, Kiselyov AS, Parker MA, Tkachenko SE, Savchuck NP, Sklar LA, Oprea TI, et al. Virtual and biomolecular screening converge on a selective agonist for GPR30. Nature chemical biology. 2006;2:207–212. doi: 10.1038/nchembio775. [DOI] [PubMed] [Google Scholar]
- 20.Hegele-Hartung C, Siebel P, Peters O, Kosemund D, Muller G, Hillisch A, Walter A, Kraetzschmar J, Fritzemeier KH. Impact of isotype-selective estrogen receptor agonists on ovarian function. Proc Natl Acad Sci U S A. 2004;101:5129–5134. doi: 10.1073/pnas.0306720101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Herbison AE, Pape JR. New evidence for estrogen receptors in gonadotropin-releasing hormone neurons. Front Neuroendocrinol. 2001;22:292–308. doi: 10.1006/frne.2001.0219. [DOI] [PubMed] [Google Scholar]
- 22.Sim JA, Skynner MJ, Pape J-R, Herbison AE. Late postnatal reorganization of GABAA receptor signalling in native GnRH neurons. Eur J Neurosci. 2000;12:3497–3504. doi: 10.1046/j.1460-9568.2000.00261.x. [DOI] [PubMed] [Google Scholar]
- 23.Christian CA, Moenter SM. Estradiol induces diurnal shifts in GABA transmission to gonadotropin-releasing hormone neurons to provide a neural signal for ovulation. J Neurosci. 2007;27:1913–1921. doi: 10.1523/JNEUROSCI.4738-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kelly MJ, Lagrange AH, Wagner EJ, Rønnekleiv OK. Rapid effects of estrogen to modulate G protein-coupled receptors via activation of protein kinase A and protein kinase C pathways. Steroids. 1999;64:64–75. doi: 10.1016/s0039-128x(98)00095-6. [DOI] [PubMed] [Google Scholar]
- 25.Funakoshi T, Yanai A, Shinoda K, Kawano MM, Mizukami Y. G protein-coupled receptor 30 is an estrogen receptor in the plasma membrane. Biochem Biophys Res Commun. 2006;346:904–910. doi: 10.1016/j.bbrc.2006.05.191. [DOI] [PubMed] [Google Scholar]
- 26.Otto C, Rohde-Schulz B, Schwarz G, Fuchs I, Klewer M, Brittain D, Langer G, Bader B, Prelle K, Nubbemeyer R, Fritzemeier KH. GPR30 localizes to the endoplasmic reticulum and is not activated by estradiol. Endocrinology. 2008 doi: 10.1210/en.2008-0269. [DOI] [PubMed] [Google Scholar]
- 27.Abraham IM, Todman MG, Korach KS, Herbison AE. Critical in vivo roles for classical estrogen receptors in rapid estrogen actions on intracellular signaling in mouse brain. Endocrinology. 2004;145:3055–3061. doi: 10.1210/en.2003-1676. [DOI] [PubMed] [Google Scholar]
- 28.Staley KJ. Quantal GABA release: noise or not? Nat Neurosci. 1999;2:494–495. doi: 10.1038/9139. [DOI] [PubMed] [Google Scholar]
- 29.Adams MM, Fink SE, Shah RA, Janssen WG, Hayashi S, Milner TA, McEwen BS, Morrison JH. Estrogen and aging affect the subcellular distribution of estrogen receptor-alpha in the hippocampus of female rats. J Neurosci. 2002;22:3608–3614. doi: 10.1523/JNEUROSCI.22-09-03608.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Blaustein JD, Lehman MN, Turcotte JC, Greene G. Estrogen receptors in dendrites and axon terminals in the guinea pig hypothalamus. Endocrinology. 1992;131:281–290. doi: 10.1210/endo.131.1.1612006. [DOI] [PubMed] [Google Scholar]
- 31.Milner TA, McEwen BS, Hayashi S, Li CJ, Reagan LP, Alves SE. Ultrastructural evidence that hippocampal alpha estrogen receptors are located at extranuclear sites. J Comp Neurol. 2001;429:355–371. [PubMed] [Google Scholar]
- 32.Herbison AE. Estrogen regulation of GABA transmission in rat preoptic area. Brain Res Bull. 1997;44:321–326. doi: 10.1016/s0361-9230(97)00210-4. [DOI] [PubMed] [Google Scholar]
- 33.Flugge G, Oertel WH, Wuttke W. Evidence for estrogen-receptive GABAergic neurons in the preoptic/anterior hypothalamic area of the rat brain. Neuroendocrinology. 1986;43:1–5. doi: 10.1159/000124500. [DOI] [PubMed] [Google Scholar]
- 34.Ottem EN, Godwin JG, Krishnan S, Petersen SL. Dual-phenotype GABA/glutamate neurons in adult preoptic area: sexual dimorphism and function. J Neurosci. 2004;24:8097–8105. doi: 10.1523/JNEUROSCI.2267-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hart SA, Snyder MA, Smejkalova T, Woolley CS. Estrogen mobilizes a subset of estrogen receptor-alpha-immunoreactive vesicles in inhibitory presynaptic boutons in hippocampal CA1. J Neurosci. 2007;27:2102–2111. doi: 10.1523/JNEUROSCI.5436-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Abe H, Keen KL, Terasawa E. Rapid action of estrogens on intracellular calcium oscillations in primate luteinizing hormone-releasing hormone-1 neurons. Endocrinology. 2008;149:1155–1162. doi: 10.1210/en.2007-0942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schwarz JM, Liang SL, Thompson SM, McCarthy MM. Estradiol induces hypothalamic dendritic spines by enhancing glutamate release: a mechanism for organizational sex differences. Neuron. 2008;58:584–598. doi: 10.1016/j.neuron.2008.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]