Abstract
Increasing levels of circulating estradiol during the follicular phase of the ovarian cycle act on the brain to trigger a sudden and massive release of gonadotropin-releasing hormone (GnRH) that evokes the pituitary luteinizing hormone surge responsible for ovulation in mammals. The mechanisms through which estrogen is able to exert this potent “positive feedback” influence upon the GnRH neurons are beginning to be unravelled. Recent studies utilizing mouse models with global and cell-specific deletions of the different estrogen receptors (ERs) have shown that estrogen positive feedback is likely to use an indirect pathway involving the modulation of ERα-expressing neurons that project to GnRH neurons. Conventional tract tracing studies in rats, and experiments involving conditional Pseudorabies virus tract tracing from GnRH neurons in the transgenic mouse, indicate that the dominant populations of ERα-expressing neuronal afferents to GnRH neurons reside in the anteroventral periventricular, median preoptic and periventricular preoptic nuclei. Together these estrogen-sensitive afferents to GnRH neurons form a periventricular continuum that can be referred to as rostral periventricular area of the third ventricle (RP3V) neurons. The neurochemical identity of some RP3V neurons has been determined and there is mounting evidence for important roles of glutamate, GABA, kisspeptin and neurotensin-expressing RP3V neurons in estrogen positive feedback. The definition of the key cluster of estrogen-sensitive neurons responsible for activating the GnRH neurons to evoke the GnRH surge (and ovulation) should be of substantial value to on-going efforts to understand the molecular and cellular basis of the estrogen positive feedback mechanism.
Keywords: AVPV, estrogen, GnRH, kisspeptin, LHRH, periventricular nucleus
The gonadotropin-releasing hormone (GnRH) neurons represent the final output pathway of the neuronal network controling fertility in all mammalian species. As a consequence of the pattern of GnRH neuron migration into the brain along the terminal and vomeronasal axons during embryogenesis (Wray, 2002), the GnRH neuron cell bodies exist as a scattered continuum throughout the basal forebrain of adult mammals. However, the majority of GnRH neurons extend axons to a highly circumscribed region within median eminence of the hypothalamus from where they release GnRH into the pituitary portal system to control gonadotrophin secretion (Herbison, 2006). This population of “neuroendocrine” GnRH neurons exhibits marked functional plasticity in the adult female brain; at mid-cycle, the GnRH neuron switches from delivering an episodic pattern of GnRH secretion to one of sustained high level output for several hours. This massive secretion of GnRH, termed the GnRH surge, is the primary trigger of the pituitary luteinizing hormone (LH) surge and, in turn, ovulation in all mammals (Caraty et al., 1995; Caraty et al., 1989; Ching, 1982; Clarke and Cummins, 1985; Levine et al., 1985; Levine et al., 1982; Moenter et al., 1992a; Moenter et al., 1992b; Moenter et al., 1990; Pau et al., 1993; Sarkar et al., 1976; Xia et al., 1992).
It is now recognized that changes in circulating estrogen levels are primarily responsible for driving the plasticity in GnRH neuron function that generates the GnRH surge in mammals. This paper intends to review what is known about (i) the changes in GnRH neuron activity at mid-cycle and (ii) our present understanding of how estrogen initiates these changes. In doing so, the case will be made for defining the rostral periventricular area of the third ventricle (RP3V) as a functional anatomical construct containing the key estrogen-regulated inputs to GnRH neurons necessary for estrogen positive feedback.
GnRH neuron excitability and secretion at mid-cycle
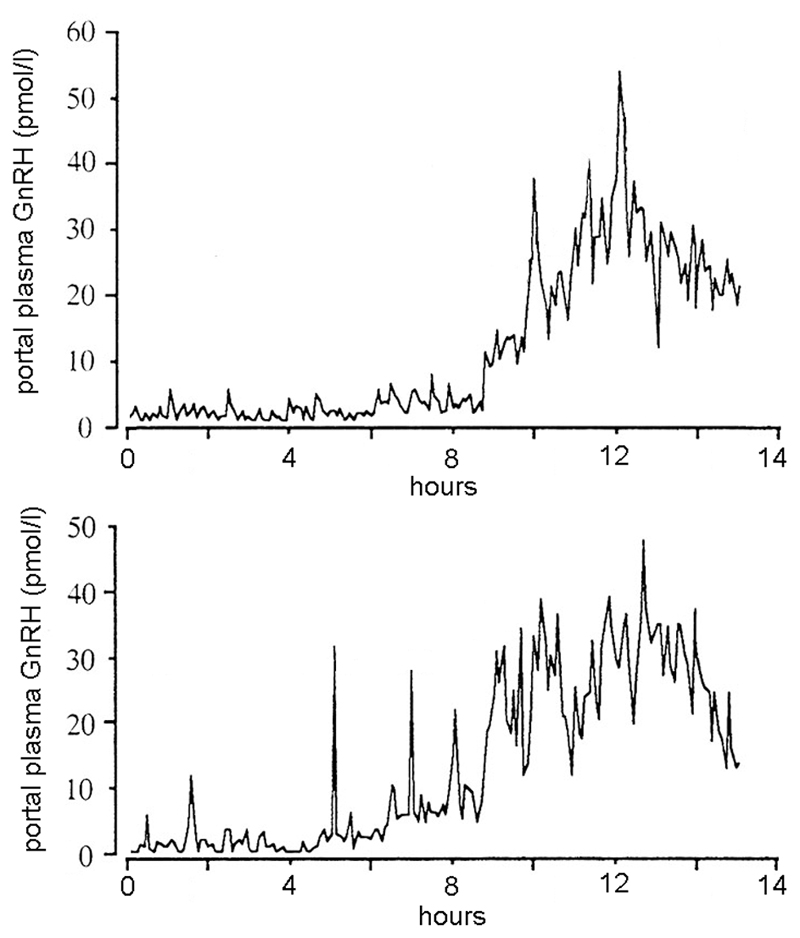
Studies in sheep have enabled the pattern of GnRH secretion into the portal vasculature to be studied in great detail. Analyses in the ewe have shown that the pattern of GnRH release leading up to the surge changes from a strictly pulsatile pattern to one of significant non-episodic GnRH release intermingled with high frequency pulsatile GnRH secretion through to a sudden “explosion” of extremely high GnRH release (Fig.1) (Caraty et al., 1989; Clarke, 1993; Evans et al., 1995; Moenter et al., 1992a). This massive increase in GnRH secretion continues for a period of >24h, considerably beyond the duration of the LH surge it induces, before returning to a strictly episodic pattern of release (Caraty et al., 1995; Moenter et al., 1990).
Figure 1.
Dynamics of GnRH surge in the portal vasculature of two ovariectomized, estrogen-treated ewes. Adapted from Caraty, A., Locatelli, A. and Martin, G. B., 1989, Journal of Endocrinology. 123, 375-382 © Society for Endocrinology (1989). Reproduced by permission.
The results of GnRH measurement studies in other species are similar to that of ewes but, because of technical constraints, less detailed information has been available. In the monkey, both median eminence and third ventricular monitoring of GnRH concentrations has shown that a marked increase in GnRH release occurs at the time of the LH surge (Pau et al., 1993; Xia et al., 1992). The precise profile of GnRH release over this time has not been studied, but seems likely to be comprised of pulsatile events superimposed upon a high basal outpouring of GnRH (Xia et al., 1992). Like the sheep, the GnRH surge in the monkey long outlasts the LH surge (Xia et al., 1992).
Secretion studies in the rat have used push-pull or microdialysis approaches to sample GnRH levels in the median eminence/arcuate nucleus region. Although hampered by a lack of good temporal resolution, these studies have, nevertheless, reported a clear increase in GnRH secretion correlated with the LH surge (Ching, 1982; Levine and Ramirez, 1982; Sarkar et al., 1976). The dynamics of GnRH surges in the rat appear to take the form of a marked increase in GnRH pulse amplitude with more minor variations in GnRH pulse frequency and inter-pulse secretion (Levine, 1997; Levine and Ramirez, 1982; Park and Ramirez, 1989; Sisk et al., 2001). Whether the proestrous increase in GnRH secretion persists for several hours after the end of the LH surge, as it does in the sheep and monkey, is not clear.
Together, these studies suggest that the GnRH neurons exhibit a fundamentally different pattern of secretory activity for several hours each cycle to evoke the GnRH/LH surge. Experiments using the expression of the immediate-early gene c-Fos, as a marker of electrical activation, have indicated that GnRH neurons are indeed activated co-incident with the occurrence of the GnRH surge (Hoffman et al., 1993). Furthermore, a recent extracellular electrophysiological study using a daily LH surge mouse model, has reported that the mean firing rate of GnRH neurons is elevated in the afternoon of the expected surge (Christian et al., 2005). Thus, as would be expected, the increase in GnRH secretion into the portal vasculature at the time of the surge results from a change in the electrical excitability of GnRH neurons, and this occurs, at least in part, at the level of their cell bodies.
An unresolved issue is that of precisely which populations of neuroendocrine GnRH neurons may be involved in the GnRH surge. There is ample evidence for quite marked heterogeneity within the GnRH neuronal population (Herbison, 2006) and the c-Fos studies mentioned above indicated that it is a sub-population of GnRH neurons located in the rostral preoptic area (rPOA) around the organum vasculosum of the lamina terminalis (OVLT) that is involved in the surge in rodents (Lee et al., 1990; Wintermantel et al., 2006). In addition, changes in GnRH biosynthesis also appear to exist preferentially in GnRH neurons located around the OVLT (Rubin and King, 1994). Thus, there is some evidence for a “surge population” of GnRH neurons in rodents. Similar c-Fos studies in sheep and monkeys have not highlighted an anatomically-delineated sub-population of GnRH neurons involved in the surge (Moenter et al., 1993; Witkin et al., 1994). Whether the GnRH neurons involved in the surge might only be “surge neurons” or also participate in generating pulsatile GnRH release or be involved in negative feedback remains an important unresolved issue. One hypothesis holds that the GnRH neurons responsible for the surge are a subset of the population that is independent of those involved in pulsatile release (Kimura and Funabashi, 1998).
Mechanism of estrogen positive feedback to GnRH neurons
The rising follicular-phase concentration of circulating estradiol is the key signal driving the GnRH surge in spontaneous ovulators. In rodents, a circadian mechanism is very often coupled to the surge mechanism to ensure that the time of onset of the surge (and ovulation) is co-ordinated with sexual behaviour. Thus, in rats and mice, the GnRH/LH surge only occurs when the rising estrogen and circadian clock signals co-incide (Chappell, 2005; Everett and Sawyer, 1950; Legan and Karsch, 1975). However, in sheep and primates, there is no clear requirement for a circadian input in generating the GnRH/LH surge (Karsch et al., 1997; Xia et al., 1992).
How does estrogen exert such a potent stimulatory effect on GnRH neuron secretion once every cycle to generate the surge? One clue lies in the dynamic of the estrogen signal that is interpreted by the GnRH neuron network. In primates, sheep and rodents, the LH/GnRH surge is only induced by an estrogen signal of sufficiently high or increasing levels that lasts for several hours (Bronson, 1981; Caraty et al., 1989; Legan et al., 1975; Moenter et al., 1990; Sarkar and Fink, 1980; Xia et al., 1992; Yamaji et al., 1971). Experiments examining the exact period of elevated estrogen exposure required in the ewe have suggested that a 7-14h period is the absolute minimum (Evans et al., 1997). Importantly, studies in both the rat (Legan et al., 1975) and sheep (Evans et al., 1997) have demonstrated that estrogen does not need to be present at the actual time of surge initiation for a normal GnRH surge to occur. This indicates that the GnRH neuronal network “reads” a prolonged, high concentration estrogen state as the appropriate signal to initiate positive feedback. The requirement for prolonged estrogen exposure would be compatible with the idea that estrogen acts, at least in part, in a classical genomic manner through estrogen receptors (ERs) to alter gene expression within the GnRH neuronal network. This concept has very recently been supported by a study showing that mice expressing a mutated ERα that is unable to bind to DNA estrogen response elements, is unable to generate positive feedback (Glidewell-Kenney et al., 2007).
Where is estrogen acting within the GnRH neuronal network? As it would appear that classical ERs are involved, one strategy has been to evaluate the roles and locations of the different ER isoforms (ERα and ERβ) involved in estrogen positive feedback. The generation of mutant mouse models with deletions of ERs has been especially useful in this regard. The global ERαKO mouse lines are infertile whereas ERβKO mutants show a range of reproductive phenotypes (Couse et al., 2003; Dupont et al., 2000; Krege et al., 1998; Lubahn et al., 1993). To evaluate estrogen positive feedback in these mouse lines, we used an ovariectomized, estrogen-only replacement paradigm to evaluate the LH surge and c-Fos expression in GnRH neurons. These studies revealed that estrogen positive feedback was normal in ERβKO mice, but absent in ERαKO mice (Wintermantel et al., 2006). Studies were then undertaken using ERα- and ERb-selective agonists (Hegele-Hartung et al., 2004) in wild-type mice to examine which receptor isoform was sufficient to generate positive feedback. These studies found that the ERα agonist generated normal positive feedback (Wintermantel et al., 2006) whereas the ERβ agonist was unable to initiate c-Fos expression in GnRH neurons or generate an LH surge (unpublished observations, Porteous, Wintermantel & Herbison).
Together, the above studies clearly indicated that ERα was both necessary and sufficient for estrogen positive feedback to occur in mice. As GnRH neurons do not express ERα (Herbison and Pape, 2001), this indicated that estrogen acted indirectly upon GnRH neurons to bring about their activation. Previous studies had suggested roles for ERα-expressing neurons, glia and endothelial cells in the positive feedback mechanism (Herbison, 1998; Petersen et al., 2003; Prevot, 2002; Rage et al., 1997). To refine further the cell types involved in this pathway, we used a Cre-LoxP approach to delete ERα specifically from neurons in the forebrain. Neuron-specific ERαKO mutants failed to exhibit estrogen positive feedback (Wintermantel et al., 2006) and thereby revealed that neurons expressing ERα are the critical cell type interacting with GnRH neurons to bring about estrogen positive feedback (Fig.2). This does not, of course, discount roles for ERα-expressing glia or endothelial cells in this mechanism, but does indicate that they are unable to bring about positive feedback by themselves.
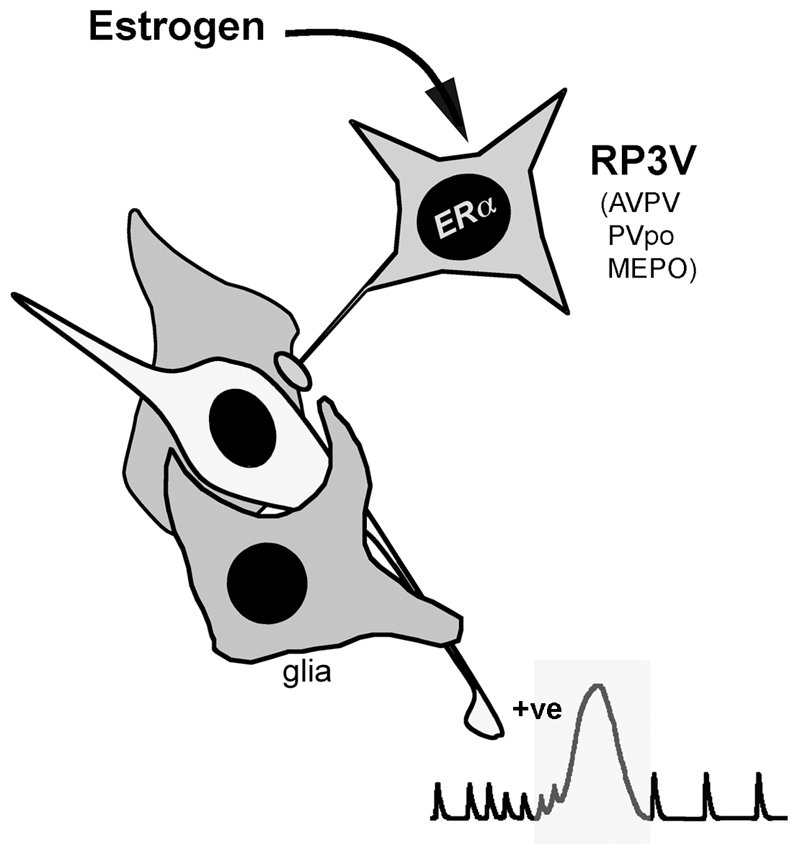
Figure 2.
Schematic representation of the key pathway underlying estrogen positive feedback in the rodent. A population of neurons scattered through the anteroventral periventricular (AVPV), periventricular preoptic (PVpo) and median preoptic (MEPO) nuclei project directly to GnRH neurons and express ERα. Together, they are referred to as the rostral periventricular area of the third ventricle (RP3V) population of neurons. The pale cell is a GnRH neuron.
The hypothesis that estrogen acted via ER-expressing interneurons to regulate GnRH neurons was generated initially in the early 1980s by Shivers and colleagues (Shivers et al., 1983) who reported that GnRH neurons were unable to bind radiolabeled estradiol. That experiment has now been repeated with more sensitive reagents that have shown that some GnRH neurons do indeed bind estradiol (Hrabovszky et al., 2000). Nevertheless, the new evidence from ER mutant mice clearly demonstrates that direct actions of estradiol on GnRH neurons through ERβ or other non-classical estrogen-binding molecules are not sufficient for positive feedback.
What is the location and identity of ERα-expressing neurons involved in the estrogen positive feedback mechanism? Lesion studies undertaken in rats have clearly demonstrated the key importance of cells within the anteroventral periventricular nucleus (AVPV), more than any other brain region, in the positive feedback mechanism. Lesions of the AVPV were shown to result in persistent estrus and the abolition of the estrogen- or estrogen plus progesterone-induced LH surges (Ronnekleiv and Kelly, 1988; Wiegand and Terasawa, 1982; Wiegand et al., 1980). Although not administered specifically into the AVPV, estradiol implants placed in the medial preoptic area (but not elsewhere) were found to be able to generate an LH surge in the rat (Goodman, 1978; Kalra and McCann, 1975) while similarly positioned implants of anti-estrogens inhibited the estrogen-induced LH surge (Petersen and Barraclough, 1989; Petersen et al., 1989). There is also evidence for abundant ERα and ERβ expression within neurons of the AVPV (Herbison and Theodosis, 1992b; Orikasa et al., 2002; Shughrue et al., 1997) and conventional tracing studies have indicated that ER-expressing AVPV neurons are likely to project directly to the GnRH neurons (Gu and Simerly, 1997; Simonian et al., 1999). Furthermore, cells in the AVPV express c-Fos at the time of the surge suggesting that they are also activated by estrogen positive feedback (Le et al., 1999). As ERα-containing neurons of the AVPV receive direct inputs from the suprachiasmatic nucleus in the rat (Watson et al., 1995), they may also represent a site of integration of circadian and estrogen inputs in the regulation of GnRH neurons in rodents (Herbison, 1998; Petersen et al., 2003; Simerly, 2002). Together, these experimental findings strongly suggest that estrogen regulates AVPV neurons to activate the GnRH neurons.
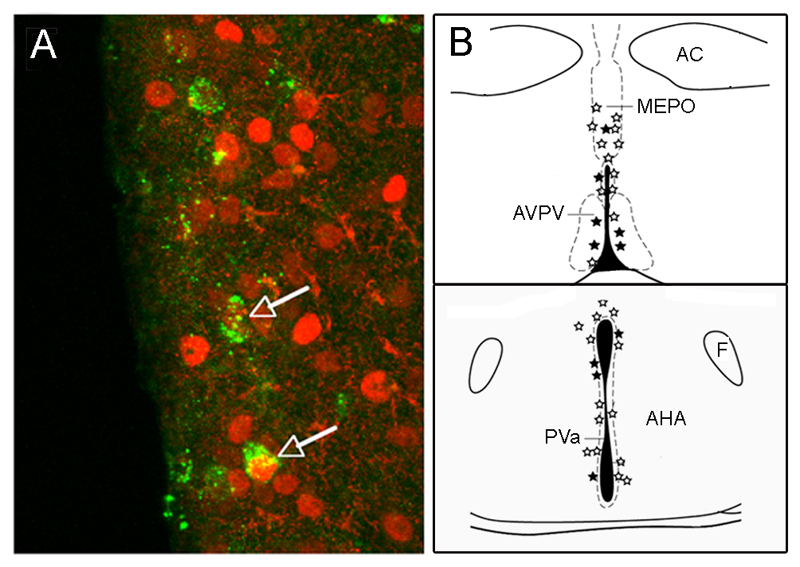
Using a conditional pseudorabies virus (PRV) tracing approach (DeFalco et al., 2001), we have been able to determine the locations of neurons that innervate the sub-population of GnRH neurons residing in the rPOA of the mouse (Campbell, 2007; Wintermantel et al., 2006). By combining this approach with ERα immunocytochemistry, it has been possible to establish the locations of ERα-expressing neurons that project to the sub-population of GnRH neurons likely involved in generating the GnRH/LH surge (Fig.3). We have found that virtually all ERα-afferents reside in the median preoptic nucleus (MEPO), AVPV, and preoptic and anterior hypothalamic divisions of the periventricular nucleus (PVpo/a), with a very few ERα-expressing primary afferents identified in the arcuate nucleus (Fig.3) (Wintermantel et al., 2006). While confirming the long-suspected arrangement between the AVPV and GnRH neurons, this study also highlighted that neurons adjacent to the AVPV in the PV and MEPO, were also direct ERα-expressing primary afferents.
Figure 3.
Definition of ERα-expressing neurons projecting to rostral preoptic area GnRH neurons using Cre-conditional Pseudorabies virus tract tracing. A. Neurons in the anteroventral periventricular nucleus (AVPV) projecting to GnRH neurons are defined by the expression of green fluorescent protein. Immunolabelling for ERα (red) reveals two of these neurons (arrows) to be ERα-expressing afferents to GnRH neurons. B. Schematic coronal representation of the location of neurons projecting to GnRH neurons (stars) at the level of the preoptic area (top) and anterior hypothalamic nuclei (AHA) (bottom). Filled stars represent primary afferents that express ERα. AC, anterior commissure; F, fornix; MEPO, median preoptic nucleus; PVa, periventricular nucleus, anterior hypothalamic area division. Adapted and reproduced from Neuron, Vol 52, Wintermantel TM, Campbell RE, Porteous R, Bock D, Grone HJ, Todman MG, Korach KS, Greiner E, Perez CA, Schutz G, Herbison AE, “Definition of estrogen receptor pathway critical for estrogen positive feedback to gonadotropin-releasing hormone neurons and fertility” 271-280, ©2006 with permission from Elsevier.
The case for the RP3V
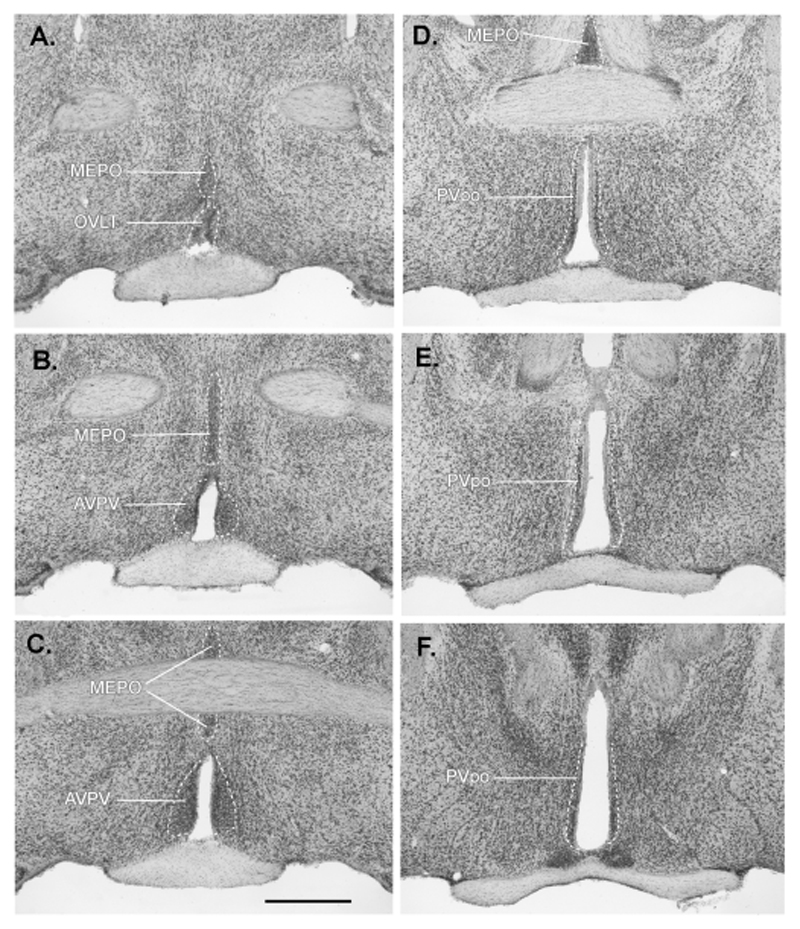
Whereas the role of AVPV cells in estrogen positive feedback is well documented (see above), neurons within the MEPO and PV have received relatively little attention in terms of the positive feedback mechanism. A series of Nissl-stained sections from an adult female C57BL/6J mouse through these areas is shown in Fig.4. Interestingly, in the landmark lesioning studies by Wiegand and Terasawa, lesions of the PVpo (termed ASR in that paper) blocked the estrogen-induced LH surge in rats (Wiegand and Terasawa, 1982). Furthermore, in c-Fos mapping studies undertaken by Hoffman and colleagues, it was clear that increased numbers of c-Fos-expressing neurons at the time of the surge were located within the AVPV as well as more caudally within the PVpo (Le et al., 1999). Similarly, following the identification of ERα-expressing afferents in the PVpo/a with the PRV approach, we re-evaluated c-Fos expression in the PV of mice and found that the numbers of cells positive for c-Fos was increased in the PVpo at the time of positive feedback. A re-evaluation of the MEPO in the same study failed, however, to find any evidence for increased c-Fos in this region in response to estrogen (Wintermantel et al., 2006). Evidence in support of the PVpo, in particular, as a brain region containing primary afferents to the GnRH neurons, including ERα-expressing afferents, also comes from conventional tracing approaches in the rat (Hahn and Coen, 2006; Le et al., 1999; Simonian et al., 1999).
Figure 4.
Nissl-stained coronal sections through the preoptic area of an adult female C57BL/6J mouse highlighting the three brain regions (anteroventral periventricular (AVPV), median preoptic (MEPO) and periventricular preoptic (PVpo) nuclei) in which ER-expressing neuronal afferents to GnRH neurons are found (RP3V neurons). The 40μm-thick sections are arranged in rostral to caudal order (A-F) with 40μm between sections. Scale bar = 500μm. OVLT, organum vasculosum of the lamina terminalis.
Thus, on the basis of neuroanatomical tracing, c-Fos activity and lesioning studies, it would appear that the key estrogen-sensitive neurons projecting to GnRH neurons are located in a periventricular continuum that includes the AVPV, but also extends caudally into the PVpo and dorsally into the MEPO. Thus, it is important to recognize that neurons outside the AVPV are also likely to be involved in the estrogen positive feedback mechanism. As such, the definition of a functional anatomical construct (RP3V = MEPO + AVPV + PVpo) comprised of the neuronal afferents involved in estrogen feedback to GnRH neurons should be useful (Fig.2). This is a similar concept to the naming of the anteroventral third ventricle (AV3V) by Johnson to represent a functionally-related group of neurons residing in different rostral hypothalamic nuclei involved in controlling body fluid balance (Johnson, 1985). The RP3V concept also neatly expands the boundaries of the AVPV as the periventricular visceromotor pattern generator for neuroendocrine GnRH neurons (Thompson and Swanson, 2003).
Neurochemical phenotypes of RP3V neurons
The neurochemical identity of the RP3V ER-expressing neurons is not well established at present. A variety of classical neurotransmitters and neuropeptides are synthesized by rostral periventricular neurons and several of these populations are known to express ERα; these include glutamate (Eyigor et al., 2004; Ottem et al., 2004), GABA (Herbison, 1997; Ottem et al., 2004), dynorphin (Simerly, 1991), enkephalin (Simerly, 1991; Yuri and Kawata, 1994), galanin (Bloch et al., 1992), kisspeptin (Smith et al., 2005), substance P (Okamura et al., 1994), calcitonin gene-related peptide (Herbison and Theodosis, 1992a) and neurotensin (Herbison and Theodosis, 1992b) neurons. At present, a strong case can be made for the involvement of ERα-expressing glutamate, GABA, kisspeptin and neurotensin RP3V neurons in the estrogen positive feedback mechanism (for reviews see (Herbison, 2006; Petersen et al., 2003; Smith and Jennes, 2001)). A brief outline of the data available for each candidate is provided below.
a). Glutamate
Glutamate receptor antagonists administered into the ventricular system of rats blocked the occurrence of the LH surge (Brann and Mahesh, 1991; Brann et al., 1993; Lopez et al., 1990) and glutamate levels within the vicinity of the GnRH neuron cell bodies, but not their terminals, increased at the time of the surge (Jarry et al., 1995; Ping et al., 1994). Although such studies have not addressed the RP3V glutamatergic cells themselves, the data are consistent with the hypothesis that estrogen activates these cells to stimulate the GnRH neurons. Dual-label immunocytochemical studies have shown that the numbers of glutamatergic inputs to GnRH neurons are likely to increase at the time of the surge (Ottem et al., 2004). Intriguingly, these glutamatergic inputs may be dual-phenotype GABA-glutamate terminals originating from the AVPV in which the synthesis of glutamate goes up and that of GABA goes down prior to the surge (Ottem et al., 2004).
b). GABA
There is a consistent set of data that show GABA levels within the general vicinity of the GnRH cell bodies rise in the morning and then fall precipitously in the afternoon just before the onset of the LH surge (Jarry et al., 1995; Jarry et al., 1988; Mitsushima et al., 2002; Robinson et al., 1991; Tin Tin Win et al., 2004). Pharmacological studies with GABAA receptor antagonists have further indicated that this fall in GABA release is necessary for the GnRH surge to proceed (Herbison and Dyer, 1991; Kimura and Jinnai, 1994; Seltzer and Donoso, 1992). These data are in agreement with the idea that a reduction in net inhibitory GABAergic input to the GnRH neurons (dis-inhibition) must occur to enable GnRH neuron activation to generate the surge. However, it is unclear how this afternoon decline in GABA release occurs, particularly as estrogen has been shown to exert a predominant stimulatory effect upon preoptic GABAergic neurons (Herbison, 1998; Mitsushima et al., 2002).
c). Kisspeptin
The recent discovery of the key importance of kisspeptin-GPR54 signaling in puberty (de Roux et al., 2003; Seminara et al., 2003) has led to the evaluation of its role in the GnRH surge mechanism. To date, the results of these studies look very promising and suggest that kisspeptin is a “high-order” component of the surge generating mechanism. Kisspeptin neurons are (i) located within the AVPV and PVpo (Clarkson and Herbison, 2006; Smith et al., 2006), (ii) exhibit a female-dominant, sexually dimorphic distribution (Clarkson and Herbison, 2006; Kauffman et al., 2007), (iii) express ERα (Smith et al., 2005), and (iv) are activated to express c-Fos at the time of estrogen positive feedback in both the rat (Smith et al., 2006) and mouse (Clarkson & Herbison, unpublished). Together with unpublished evidence that RP3V kisspeptin neurons expressing ERα are primary afferents to rPOA GnRH neurons (Campbell, Clarkson & Herbison), these observations provide a strong neuroanatomical case for kisspeptin in estrogen feedback. In addition, however, there is functional evidence that kisspeptin is required for the GnRH surge to occur (Kinoshita et al., 2005), that GnRH neurons express GPR54 (Irwig et al., 2004), and that kisspeptin is the most potent activator of GnRH neuron firing yet discovered (Han et al., 2005). Hence, it seems very likely that the rising follicular phase concentrations of estrogen stimulate directly RP3V kisspeptin neurons that, in turn, activate GnRH neurons to evoke the GnRH surge.
d). Neurotensin
The infusion of neurotensin into the preoptic area of the rat increases the magnitude of the LH surge (Akema et al., 1987; Ferris et al., 1984) whereas the administration of neurotensin antisera reduces the size of the LH surge (Alexander et al., 1989). Neither treatment influences the timing of the LH surge, indicating that neurotensin neurons are not involved in the circadian component of surge generation in the rat. Neurotensin neurons in the AVPV express ERα (Herbison and Theodosis, 1992b) and estrogen increases their biosynthesis of neurotensin (Alexander and Leeman, 1994; Alexander et al., 1989). As GnRH neurons were shown recently to express neurotensin receptors (Smith and Wise, 2001), it is possible that RP3V neurotensin neurons are stimulated by estrogen to help activate the GnRH neurons prior to the surge.
Although progress has been made towards identifying the RP3V population of neurons and determining the phenotypes of neurons within it, little headway has been made in terms of understanding how estrogen modulates the excitability of RP3V neurons. What are the downstream gene targets for estrogen in RP3V neurons? The progesterone receptor (PR) is one possibility as estrogen clearly increases its expression in the AVPV and the receptor may then be modulated in a ligand-independent manner (Levine, 1997) or by astroglial-derived progesterone (Micevych et al., 2003; Micevych et al., 2007). Although the role of ovarian-derived progesterone in positive feedback is unclear, the antagonism or deletion of PRs interrupts the LH surge (Levine, 1997). For populations such as the neurotensin and kisspeptin neurons, there is evidence suggesting that the slowly increasing estradiol levels of the follicular phase act to gradually increase neuropeptide mRNA expression by these cells (Smith et al., 2006; Smith and Wise, 2001). However, the relationship of slowly increasing mRNA expression to an abrupt increase in neuropeptide secretion from terminals is not known. Whereas a circadian input might appear to be an attractive candidate underlying the sudden onset of the surge in rodents, it does not explain the equally abrupt onset of the GnRH surge in sheep and primates where circadian factors are of little or no consequence. These pieces of information illustrate the complexity of the molecular machinery that is likely to underlie the activation of RP3V neurons by estrogen. Indeed, it seems almost certain that estrogen will regulate multiple genes involved in regulating diverse cellular functions within individual RP3V neurons. The elucidation of these pathways and their interactions will represent a major step forward in our understanding of estrogen-regulated brain function and the generation of the GnRH/LH surge.
Summary
There is little doubt that the estrogen positive feedback signal responsible for driving rodent GnRH neurons into a surge mode of secretion results from the activation of ERα-expressing neuronal afferents to GnRH neurons (Fig.2). Evidence for a role of AVPV ERα-expressing neurons in this pathway is now overwhelming. However, new findings, considered alongside a re-evaluation of older data, indicate that adjacent ERα-positive neurons in the PVpo and MEPO are also likely to be involved in this mechanism. While the AVPV may indeed be the central nucleus in the pathway, it remains that there is a continuum of ERα-expressing GnRH neuron afferents running the length of the rostral periventricular nuclei. As such, it is proposed that the topographic description of “AVPV, PVpo and MEPO estrogen-receptive afferents to GnRH neurons” be replaced by the functional neuroanatomical construct of “RP3V neurons” (Fig.2).
The studies identifying the RP3V have been undertaken in rodents. Whether a similar neuroanatomical grouping exists for estrogen positive feedback to GnRH neurons in other species is not known. Certainly, studies in the ewe indicate that the key site of estrogen action in evoking the GnRH/LH surge is not within the preoptic area but located in the mediobasal hypothalamus (Blache et al., 1991; Caraty et al., 1997). Equally, the limited studies that have been undertaken in primates suggest that a mediobasal hypothalamic network is sufficient for positive feedback to occur (Ferin et al., 1974). Thus, it is very likely that the locations of the relevant estrogen-sensitive neurons in sheep and primates are different from those of the rodent. Nevertheless, available evidence in other species suggests that the positive feedback pathway is organized in a similar indirect manner requiring ER-expressing afferents inputs to GnRH neurons (see (Herbison, 2006)). Thus, it seems likely that RP3V-equivalent populations exist in other mammals and these await elucidation.
Acknowledgements
The author is supported by the Wellcome Trust (UK), Health Research Council of New Zealand and Royal Society Marsden Fund of New Zealand. Drs Rebecca Campbell and Christine Jasoni are thanked for commenting on an earlier draft of this manuscript.
Abbreviations
- AV3V
anteroventral third ventricle
- AVPV
anteroventral periventricular nucleus
- GnRH
gonadotropin-releasing hormone
- MEPO
median preoptic nucleus
- PR
progesterone receptor
- RP3V
rostral periventricular area of the third ventricle
- rPOA
rostral preoptic area
- PRV
Pseudorabies virus
- PV
periventricular nucleus
References
- Akema T, Praputpittaya C, Kimura F. Effects of preoptic microinjection of neurotensin on luteinizing hormone secretion in unanesthetized ovariectomized rats with or without estrogen priming. Neuroendocrinology. 1987;46:345–349. doi: 10.1159/000124843. [DOI] [PubMed] [Google Scholar]
- Alexander MJ, Leeman SE. Estrogen-inducible neurotensin immunoreactivity in the preoptic area of the female rat. Journal of Comparative Neurology. 1994;345:496–509. doi: 10.1002/cne.903450403. [DOI] [PubMed] [Google Scholar]
- Alexander MJ, Mahoney PD, Ferris CG, Carraway RE, Leeman SE. Evidence that neurotensin participates in the central regulation of the preovulatory surge of luteinizing hormone in the rat. Endocrinology. 1989;124:783–788. doi: 10.1210/endo-124-2-783. [DOI] [PubMed] [Google Scholar]
- Blache D, Fabre-Nys CJ, Venier G. Ventromedial hypothalamus as a target for oestradiol action on proceptivity, receptivity and luteinizing hormone surge of the ewe. Brain Research. 1991;546:241–249. doi: 10.1016/0006-8993(91)91488-m. [DOI] [PubMed] [Google Scholar]
- Bloch GJ, Kurth SM, Akesson TR, Micevych PE. Estrogen-concentrating cells within cell groups of the medial preoptic area: sex differences and co-localization with galanin- immunoreactive cells. Brain Research. 1992;595:301–308. doi: 10.1016/0006-8993(92)91064-l. [DOI] [PubMed] [Google Scholar]
- Brann DW, Mahesh VB. Endogenous excitatory amino acid involvement in the preovulatory and steroid-induced surge of gonadotropins in the female rat. Endocrinology. 1991;128:541–1547. doi: 10.1210/endo-128-3-1541. [DOI] [PubMed] [Google Scholar]
- Brann DW, Ping L, Mahesh VB. Role of non-NMDA receptor neurotransmission in steroid and preovulatory gonadotropin surge expression in the female rat. Molecular and Cellular Neuroscience. 1993;4:92–297. doi: 10.1006/mcne.1993.1037. [DOI] [PubMed] [Google Scholar]
- Bronson FH. The regulation of luteinizing hormone secretion by estrogen: relationships among negative feedback, surge potential, and male stimulation in juvenile, peripubertal, and adult female mice. Endocrinology. 1981;108:506–16. doi: 10.1210/endo-108-2-506. [DOI] [PubMed] [Google Scholar]
- Campbell RE. Defining the gonadotropin-releasing hormone (GnRH) neuronal network: Transgenic approaches to understand neurocircuitry. J Neuroendocrinol. 2007 doi: 10.1111/j.1365-2826.2007.01561.x. in press. [DOI] [PubMed] [Google Scholar]
- Caraty A, Antoine C, Delaleu B, Locatelli A, Bouchard P, Gautron JP, Evans NP, Karsch FJ, Padmanabhan V. Nature and bioactivity of gonadotropin-releasing hormone (GnRH) secreted during the GnRH surge. Endocrinology. 1995;136:3452–60. doi: 10.1210/endo.136.8.7628381. [DOI] [PubMed] [Google Scholar]
- Caraty A, Fabre-Nys C, Delaleu B, Locatelli A, Bruneau G, Karsch FJ, Herbison A. Evidence that the mediobasal hypothalamus is the primary site of action of estradiol in inducing the preovulatory GnRH surge in the ewe. Endocrinology. 1997;139:1752–1760. doi: 10.1210/endo.139.4.5904. [DOI] [PubMed] [Google Scholar]
- Caraty A, Locatelli A, Martin GB. Biphasic response in the secretion of gonadotrophin-releasing hormone in ovariectomized ewes injected with oestradiol. Journal of Endocrinology. 1989;123:375–382. doi: 10.1677/joe.0.1230375. [DOI] [PubMed] [Google Scholar]
- Chappell PE. Clocks and the black box: circadian influences on gonadotropin-releasing hormone secretion. J Neuroendocrinol. 2005;17:119–30. doi: 10.1111/j.1365-2826.2005.01270.x. [DOI] [PubMed] [Google Scholar]
- Ching M. Correlative surges of LHRH, LH and FSH in pituitary stalk plasma and systemic plasma of rat during proestrus. Effect of anaesthetics. Neuroendocrinology. 1982;34:279–285. doi: 10.1159/000123313. [DOI] [PubMed] [Google Scholar]
- Christian CA, Mobley JL, Moenter SM. Diurnal and estradiol-dependent changes in gonadotropin-releasing hormone neuron firing activity. Proc Natl Acad Sci U S A. 2005;102:15682–7. doi: 10.1073/pnas.0504270102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke IJ. Variable patterns of gonadotropin-releasing hormone secretion during the estrogen-induced luteinizing hormone surge in ovariectomized ewes. Endocrinology. 1993;133:1624–1632. doi: 10.1210/endo.133.4.8404603. [DOI] [PubMed] [Google Scholar]
- Clarke IJ, Cummins JT. Increased gonadotropin-releasing hormone pulse frequency associated with estrogen-induced luteinizing hormone surges in ovariectomized ewes. Endocrinology. 1985;116:2376–83. doi: 10.1210/endo-116-6-2376. [DOI] [PubMed] [Google Scholar]
- Clarkson J, Herbison AE. Postnatal Development of Kisspeptin Neurons in Mouse Hypothalamus; Sexual Dimorphism and Projections to Gonadotropin-Releasing Hormone Neurons. Endocrinology. 2006;147:5817–5825. doi: 10.1210/en.2006-0787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couse JF, Yates MM, Walker VR, Korach KS. Characterization of the hypothalamic-pituitary-gonadal (HPG) axis in estrogen receptor null mice reveals hypergonadism and endocrine sex-reversal in females lacking ERα but not ERβ. Mol Endocrinol. 2003;17:1039–1053. doi: 10.1210/me.2002-0398. [DOI] [PubMed] [Google Scholar]
- de Roux N, Genin E, Carel JC, Matsuda F, Chaussain JL, Milgrom E. Hypogonadotropic hypogonadism due to loss of function of the KiSS1-derived peptide receptor GPR54. Proc Natl Acad Sci U S A. 2003;100:10972–6. doi: 10.1073/pnas.1834399100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFalco J, Tomishima M, Liu H, Zhao C, Cai X, Marth JD, Enquist L, Friedman JM. Virus-assisted mapping of neural inputs to a feeding center in the hypothalamus. Science. 2001;291:2608–13. doi: 10.1126/science.1056602. [DOI] [PubMed] [Google Scholar]
- Dupont S, Krust A, Gansmuller A, Dierich A, Chambon P, Mark M. Effect of single and compound knockouts of estrogen receptors alpha (ERalpha) and beta (ERbeta) on mouse reproductive phenotypes. Development. 2000;127:4277–91. doi: 10.1242/dev.127.19.4277. [DOI] [PubMed] [Google Scholar]
- Evans NP, Dahl GE, Mauger DT, Padmanabhan V, Thrun LA, Karsch FJ. Does estradiol induce the preovulatory gonadotropin-releasing hormone (GnRH) surge in the ewe by inducing a progressive change in the mode of operation of the GnRH neurosecretory system. Endocrinology. 1995;136:5511–5519. doi: 10.1210/endo.136.12.7588302. [DOI] [PubMed] [Google Scholar]
- Evans NP, Dahl GE, Padmanabhan V, Thrun LA, Karsch FJ. Estradiol requirements for induction and maintenance of the gonadotropin-releasing hormone surge: implications for neuroendocrine processing of the estradiol signal. Endocrinology. 1997;138:5408–14. doi: 10.1210/endo.138.12.5558. [DOI] [PubMed] [Google Scholar]
- Everett J, Sawyer CH. A 24h periodicity in the "LH release apparatus" of female rats, disclosed by barbiturate sedation. Endocrinology. 1950;46:198–216. doi: 10.1210/endo-47-3-198. [DOI] [PubMed] [Google Scholar]
- Eyigor O, Lin W, Jennes L. Identification of neurones in the female rat hypothalamus that express oestrogen receptor-alpha and vesicular glutamate transporter-2. J Neuroendocrinol. 2004;16:26–31. doi: 10.1111/j.1365-2826.2004.01109.x. [DOI] [PubMed] [Google Scholar]
- Ferin M, Carmel PW, Zimmerman EA, Warren M, Perez R, Vande Wiele RL. Location of intrahypothalamic estrogen-responsive sites influencing LH secretion in the female Rhesus monkey. Endocrinology. 1974;95:1059–68. doi: 10.1210/endo-95-4-1059. [DOI] [PubMed] [Google Scholar]
- Ferris CF, Pan JX, Singer EA, Boyd ND, Carraway RE, Leeman SE. Stimulation of luteinizing hormone release after stereotaxic microinjection of neurotensin into the medial preoptic area of rats. Neuroendocrinology. 1984;38:144–151. doi: 10.1159/000123882. [DOI] [PubMed] [Google Scholar]
- Glidewell-Kenney C, Hurley LA, Pfaff L, Weiss J, Levine JE, Jameson JL. Nonclassical estrogen receptor alpha signaling mediates negative feedback in the female mouse reproductive axis. Proc Natl Acad Sci U S A. 2007;104:8173–7. doi: 10.1073/pnas.0611514104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman RL. The site of the positive feedback action of estradiol in the rat. Endocrinology. 1978;102:151–159. doi: 10.1210/endo-102-1-151. [DOI] [PubMed] [Google Scholar]
- Gu GB, Simerly RB. Projections of the sexually dimorphic anteroventral periventricular nucleus in the female rat. J Comp Neurol. 1997;384:142–164. [PubMed] [Google Scholar]
- Hahn JD, Coen CW. Comparative study of the sources of neuronal projections to the site of gonadotrophin-releasing hormone perikarya and to the anteroventral periventricular nucleus in female rats. J Comp Neurol. 2006;494:190–214. doi: 10.1002/cne.20803. [DOI] [PubMed] [Google Scholar]
- Han SK, Gottsch ML, Lee KJ, Popa SM, Smith JT, Jakawich SK, Clifton DK, Steiner RA, Herbison AE. Activation of gonadotropin-releasing hormone (GnRH) neurons by kisspeptin as a neuroendocrine switch for the onset of puberty. J Neurosci. 2005;25:11349–56. doi: 10.1523/JNEUROSCI.3328-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegele-Hartung C, Siebel P, Peters O, Kosemund D, Muller G, Hillisch A, Walter A, Kraetzschmar J, Fritzemeier KH. Impact of isotype-selective estrogen receptor agonists on ovarian function. Proc Natl Acad Sci U S A. 2004;101:5129–34. doi: 10.1073/pnas.0306720101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbison AE. Estrogen regulation of GABA transmission in rat preoptic area. Brain Res Bull. 1997;44:321–326. doi: 10.1016/s0361-9230(97)00210-4. [DOI] [PubMed] [Google Scholar]
- Herbison AE. Multimodal influence of estrogen upon gonadotropin-releasing hormone neurons. Endocr Rev. 1998;19:302–330. doi: 10.1210/edrv.19.3.0332. [DOI] [PubMed] [Google Scholar]
- Herbison AE. Physiology of the GnRH neuronal network. In: Neill JD, editor. Knobil and Neill's Physiology of Reproduction. Academic Press; San Diego: 2006. pp. 1415–1482. [Google Scholar]
- Herbison AE, Dyer RG. Effect on luteinizing hormone secretion of GABA receptor modulation in the medial preoptic area at the time of proestrous luteinizing hormone surge. Neuroendocrinology. 1991;53:317–320. doi: 10.1159/000125735. [DOI] [PubMed] [Google Scholar]
- Herbison AE, Pape JR. New evidence for estrogen receptors in gonadotropin-releasing hormone neurons. Front Neuroendocrinol. 2001;22:292–308. doi: 10.1006/frne.2001.0219. [DOI] [PubMed] [Google Scholar]
- Herbison AE, Theodosis DT. Immunocytochemical identification of oestrogen receptors in preoptic neurones containing calcitonin gene-related peptide in the male and female rat. Neuroendocrinology. 1992a;56:761–764. doi: 10.1159/000126304. [DOI] [PubMed] [Google Scholar]
- Herbison AE, Theodosis DT. Localisation of oestrogen receptors in preoptic neurons containing neurotensin but not tyrosine hydroxylase, cholecystokinin or luteinizing hormone-releasing hormone in the male and female rat. Neuroscience. 1992b;50:283–298. doi: 10.1016/0306-4522(92)90423-y. [DOI] [PubMed] [Google Scholar]
- Hoffman GE, Smith MS, Verbalis JG. c-Fos and related immediate early gene products as markers of activity in neuroendocrine systems. Front Neuroendocrinol. 1993;14:173–213. doi: 10.1006/frne.1993.1006. [DOI] [PubMed] [Google Scholar]
- Hrabovszky E, Shughrue PJ, Merchenthaler I, Hajszan T, Carpenter CD, Liposits Z, Petersen SL. Detection of estrogen receptor-β messenger ribonucleic acid and 125I-estrogen binding sites in luteinizing hormone-releasing hormone neurons of the rat brain. Endocrinology. 2000;141:3506–3509. doi: 10.1210/endo.141.9.7788. [DOI] [PubMed] [Google Scholar]
- Irwig MS, Fraley GS, Smith JT, Acohido BV, Popa SM, Cunningham MJ, Gottsch ML, Clifton DK, Steiner RA. Kisspeptin activation of gonadotropin releasing hormone neurons and regulation of KiSS-1 mRNA in the male rat. Neuroendocrinology. 2004;80:264–72. doi: 10.1159/000083140. [DOI] [PubMed] [Google Scholar]
- Jarry H, Leonhardt S, Schwarze T, Wuttke W. Preoptic rather than mediobasal hypothalamic amino acid neurotransmitter release regulates GnRH secretion during the estrogen-induced LH surge in the ovariectomized rat. Neuroendocrinology. 1995;62:479–486. doi: 10.1159/000127037. [DOI] [PubMed] [Google Scholar]
- Jarry H, Perschl A, Wuttke W. Further evidence that preoptic anterior hypothalamic GABAergic neurons are part of the GnRH pulse and surge generator. Acta Endocrinologica. 1988;118:573–579. doi: 10.1530/acta.0.1180573. [DOI] [PubMed] [Google Scholar]
- Johnson AK. The periventricular anteroventral third ventricle (AV3V): its relationship with the subfornical organ and neural systems involved in maintaining body fluid homeostasis. Brain Res Bull. 1985;15:595–601. doi: 10.1016/0361-9230(85)90209-6. [DOI] [PubMed] [Google Scholar]
- Kalra PS, McCann SM. The stimulatory effect on gonadotropin release of implants of estradiol or progesterone in certain sites in the central nervous system. Neuroendocrinology. 1975;19:289–302. doi: 10.1159/000122450. [DOI] [PubMed] [Google Scholar]
- Karsch FJ, Bowen JM, Caraty A, Evans NP, Moenter SM. Gonadotropin-releasing hormone requirements for ovulation. Biol Reprod. 1997;56:303–309. doi: 10.1095/biolreprod56.2.303. [DOI] [PubMed] [Google Scholar]
- Kauffman AS, Gottsch ML, Roa J, Byquist AC, Crown A, Clifton DK, Hoffman GE, Steiner RA, Tena-Sempere M. Sexual Differentiation of Kiss1 Gene Expression in the Brain of the Rat. Endocrinology. 2007 doi: 10.1210/en.2006-1540. [DOI] [PubMed] [Google Scholar]
- Kimura F, Funabashi T. Two Subgroups of Gonadotropin Releasing Hormone Neurons Control Gonadotropin Secretion in Rats. News Physiol Sci. 1998;13:225–231. doi: 10.1152/physiologyonline.1998.13.5.225. [DOI] [PubMed] [Google Scholar]
- Kimura F, Jinnai K. Bicuculline infusions advance the timing of luteinizing hormone surge in progestrous rats: comparisons with naloxone effects. Hormones and Behaviour. 1994;28:424–430. doi: 10.1006/hbeh.1994.1039. [DOI] [PubMed] [Google Scholar]
- Kinoshita M, Tsukamura H, Adachi S, Matsui H, Uenoyama Y, Iwata K, Yamada S, Inoue K, Ohtaki T, Matsumoto H, Maeda K. Involvement of central metastin in the regulation of preovulatory luteinizing hormone surge and estrous cyclicity in female rats. Endocrinology. 2005;146:4431–6. doi: 10.1210/en.2005-0195. [DOI] [PubMed] [Google Scholar]
- Krege JH, Hodgin JB, Couse JF, Enmark E, Warner M, Magler JF, Sar M, Korach KS, Gustafsson J-A, Smithies O. Generation and reproductive phenotypes of mice lacking estrogen receptor β. Proc Natl Acad Sci U S A. 1998;95:15677–15682. doi: 10.1073/pnas.95.26.15677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le WW, Berghorn KA, Rassnick S, Hoffman GE. Periventricular preoptic area neurons coactivated with luteinizing hormone (LH)-releasing hormone (LHRH) neurons at the time of the LH surge are LHRH afferents. Endocrinology. 1999;140:510–9. doi: 10.1210/endo.140.1.6403. [DOI] [PubMed] [Google Scholar]
- Lee WS, Smith MS, Hoffman GE. Luteinizing hormone-releasing hormone neurons express Fos protein during the proestrous surge of luteinizing hormone. Proc Natl Acad Sci U S A. 1990;87:5163–7. doi: 10.1073/pnas.87.13.5163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legan SJ, Coon GA, Karsch FJ. Role of estrogen as initiator of daily LH surges in the ovariectomized rat. Endocrinology. 1975;96:50–56. doi: 10.1210/endo-96-1-50. [DOI] [PubMed] [Google Scholar]
- Legan SJ, Karsch FJ. A daily signal for the LH surge in the rat. Endocrinology. 1975;96:57–62. doi: 10.1210/endo-96-1-57. [DOI] [PubMed] [Google Scholar]
- Levine JE. New concepts of the neuroendocrine regulation of gonadotropin surges in rats. Biology of Reproduction. 1997;56:293–302. doi: 10.1095/biolreprod56.2.293. [DOI] [PubMed] [Google Scholar]
- Levine JE, Norman RL, Gliessman PM, Oyama TT, Bangsberg DR, Spies HG. In vivo gonadotropin-releasing hormone release and serum luteinizing hormone measurements in ovariectomized, estrogen-treated rhesus macaques. Endocrinology. 1985;117:711–21. doi: 10.1210/endo-117-2-711. [DOI] [PubMed] [Google Scholar]
- Levine JE, Pau KY, Ramirez VD, Jackson GL. Simultaneous measurement of luteinizing hormone-releasing hormone and luteinizing hormone release in unanesthetized, ovariectomized sheep. Endocrinology. 1982;111:1449–55. doi: 10.1210/endo-111-5-1449. [DOI] [PubMed] [Google Scholar]
- Levine JE, Ramirez VD. Luteinizing hormone-releasing hormone release during the rat estrous cycle and after ovariectomy, as estimated with push-pull cannulae. Endocrinology. 1982;111:1439–1446. doi: 10.1210/endo-111-5-1439. [DOI] [PubMed] [Google Scholar]
- Lopez F, Donoso A, Negro-Vilar A. Endogenous excitatory amino regulates the estradiol-induced LH surge in ovariectomized rats. Endocrinology. 1990;126:1771–1773. doi: 10.1210/endo-126-3-1771. [DOI] [PubMed] [Google Scholar]
- Lubahn DB, Moyer JS, Golding TS, Couse JF, Korach KS, Smithies O. Alteration of reproductive function but not prenatal sexual development after insertional disruption of the mouse estrogen receptor gene. Proc Natl Acad Sci U S A. 1993;90:11162–6. doi: 10.1073/pnas.90.23.11162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micevych P, Sinchak K, Mills RH, Tao L, LaPolt P, Lu JK. The luteinizing hormone surge is preceded by an estrogen-induced increase of hypothalamic progesterone in ovariectomized and adrenalectomized rats. Neuroendocrinology. 2003;78:29–35. doi: 10.1159/000071703. [DOI] [PubMed] [Google Scholar]
- Micevych PE, Chaban V, Ogi J, Dewing P, Lu JK, Sinchak K. Estradiol stimulates progesterone synthesis in hypothalamic astrocyte cultures. Endocrinology. 2007;148:782–9. doi: 10.1210/en.2006-0774. [DOI] [PubMed] [Google Scholar]
- Mitsushima D, Shwe TT, Funabashi T, Shinohara K, Kimura F. GABA release in the medial preoptic area of cyclic female rats. Neuroscience. 2002;113:109–14. doi: 10.1016/s0306-4522(02)00160-4. [DOI] [PubMed] [Google Scholar]
- Moenter SM, Brand RC, Karsch FJ. Dynamics of gonadotropin-releasing hormone (GnRH) secretion during the GnRH surge: insights into the mechanism of GnRH surge induction. Endocrinology. 1992a;130:2978–84. doi: 10.1210/endo.130.5.1572305. [DOI] [PubMed] [Google Scholar]
- Moenter SM, Brand RM, Midgley AR, Karsch FJ. Dynamics of gonadotropin-releasing hormone release during a pulse. Endocrinology. 1992b;130:503–10. doi: 10.1210/endo.130.1.1727719. [DOI] [PubMed] [Google Scholar]
- Moenter SM, Caraty A, Karsch FJ. The estradiol-induced surge of gonadotropin-releasing hormone in the ewe. Endocrinology. 1990;127:1375–1384. doi: 10.1210/endo-127-3-1375. [DOI] [PubMed] [Google Scholar]
- Moenter SM, Karsch FJ, Lehman MN. Fos expression during the estradiol-induced gonadotropin-releasing hormone (GnRH) surge of the ewe: induction in GnRH and other neurons. Endocrinology. 1993;133:896–903. doi: 10.1210/endo.133.2.8344224. [DOI] [PubMed] [Google Scholar]
- Okamura H, Yokosuka M, Hayashi S. Induction of substance P-immunoreactivity by estrogen in neurons containing estrogen receptors in the anteroventral periventricular nucleus of female but not male rats. Journal of Neuroendocrinology. 1994;6:609–615. doi: 10.1111/j.1365-2826.1994.tb00626.x. [DOI] [PubMed] [Google Scholar]
- Orikasa C, Kondo Y, Hayashi S, McEwen BS, Sakuma Y. Sexually dimorphic expression of estrogen receptor beta in the anteroventral periventricular nucleus of the rat preoptic area: implication in luteinizing hormone surge. Proc Natl Acad Sci U S A. 2002;99:3306–11. doi: 10.1073/pnas.052707299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottem EN, Godwin JG, Krishnan S, Petersen SL. Dual-phenotype GABA/glutamate neurons in adult preoptic area: sexual dimorphism and function. J Neurosci. 2004;24:8097–105. doi: 10.1523/JNEUROSCI.2267-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park OK, Ramirez VD. Spontaneous changes in LHRH release during the rat estrous cycle, as measured with repetitive push-pull perfusions of the pituitary gland in the same female rats. Neuroendocrinology. 1989;50:66–72. doi: 10.1159/000125203. [DOI] [PubMed] [Google Scholar]
- Pau KF, Berria M, Hess DL, Spies HG. Preovulatory gonadotropin-releasing hormone surge in ovarian-intact rhesus macaques. Endocrinology. 1993;133:1650–1656. doi: 10.1210/endo.133.4.8404606. [DOI] [PubMed] [Google Scholar]
- Petersen SL, Barraclough CA. Suppression of spontaneous LH surges in estrogen-treated ovariectomized rats by microimplants of antiestrogens into the preoptic brain. Brain Research. 1989;484:279–289. doi: 10.1016/0006-8993(89)90371-5. [DOI] [PubMed] [Google Scholar]
- Petersen SL, Cheuk C, Hartman RD, Barraclough CA. Medial preoptic microimplants of the antiestrogen, keoxifene, affect luteinizing hormone-releasing hormone mRNA levels, median eminence luteinizing hormone-releasing hormone concentrations and luteinizing hormone release in ovariectomized, estrogen-treated rats. J Neuroendocrinol. 1989;1:279–283. doi: 10.1111/j.1365-2826.1989.tb00116.x. [DOI] [PubMed] [Google Scholar]
- Petersen SL, Ottem EN, Carpenter CD. Direct and indirect regulation of gonadotropin-releasing hormone neurons by estradiol. Biol Reprod. 2003;69:1771–8. doi: 10.1095/biolreprod.103.019745. [DOI] [PubMed] [Google Scholar]
- Ping L, Mahesh VB, Wiedmeier VT, Brann DW. Release of glutamate and aspartate from the preoptic area during the progesterone-induced LH surge: in vivo microdialysis studies. Neuroendocrinology. 1994;59:318–324. doi: 10.1159/000126673. [DOI] [PubMed] [Google Scholar]
- Prevot V. Glial-neuronal-endothelial interactions are involved in the control of GnRH secretion. J Neuroendocrinol. 2002;14:247–55. doi: 10.1046/j.0007-1331.2001.00772.x. [DOI] [PubMed] [Google Scholar]
- Rage F, Lee BJ, Ma YJ, Ojeda SR. Estradiol enhances prostaglandin E2 receptor gene expression in luteinizing hormone-releasing hormone (LHRH) neurons and facilitates the LHRH response to PGE2 by activating a glia-to-neuron signaling pathway. J Neurosci. 1997;17:9145–9156. doi: 10.1523/JNEUROSCI.17-23-09145.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson JE, Kendrick KM, Lambart CE. Changes in the release of gamma-aminobutyric acid and catecholamines in the preoptic/septal area prior to and during the preovulatory surge of luteinizing hormone in the ewe. Journal of Neuroendocrinology. 1991;3:393–399. doi: 10.1111/j.1365-2826.1991.tb00293.x. [DOI] [PubMed] [Google Scholar]
- Ronnekleiv OK, Kelly MJ. Plasma prolactin and luteinizing hormone profiles during the estrous cycle of the female rat: effects of surgically induced persistent estrus. Neuroendocrinology. 1988;47:133–141. doi: 10.1159/000124903. [DOI] [PubMed] [Google Scholar]
- Rubin BS, King JC. The number and distribution of detectable luteinizing hormone (LH)-releasing hormone cell bodies changes in association with the preovulatory LH surge in the brains of young but not middle-aged female rats. Endocrinology. 1994;134:467–474. doi: 10.1210/endo.134.1.8275960. [DOI] [PubMed] [Google Scholar]
- Sarkar DK, Chiappa SA, Fink G, Sherwood NM. Gonadotropin-releasing hormone surge in pro-oestrous rats. Nature. 1976;264:461–463. doi: 10.1038/264461a0. [DOI] [PubMed] [Google Scholar]
- Sarkar DK, Fink G. Luteinizing hormone releasing factor in pituitary stalk plasma from long-term ovariectomized rats: effects of steroids. J Endocrinol. 1980;86:511–524. doi: 10.1677/joe.0.0860511. [DOI] [PubMed] [Google Scholar]
- Seltzer AM, Donoso AO. Restraining action of GABA on estradiol-induced LH surge in the rat: GABA activity in brain nuclei and effects of GABA mimetics in the medial preoptic nucleus. Neuroendocrinology. 1992;55:28–34. doi: 10.1159/000126093. [DOI] [PubMed] [Google Scholar]
- Seminara SB, Messager S, Chatzidaki EE, Thresher RR, Acierno JS, Jr, Shagoury JK, Bo-Abbas Y, Kuohung W, Schwinof KM, Hendrick AG, Zahn D, et al. The GPR54 gene as a regulator of puberty. N Engl J Med. 2003;349:1614–27. doi: 10.1056/NEJMoa035322. [DOI] [PubMed] [Google Scholar]
- Shivers BD, Harlan RE, Morrell JI, Pfaff DW. Absence of oestradiol concentration in cell nuclei of LHRH-immunoreactive neurones. Nature. 1983;304:345–7. doi: 10.1038/304345a0. [DOI] [PubMed] [Google Scholar]
- Shughrue PJ, Lane MV, Merchenthaler I. Comparative distribution of estrogen receptor-α and -β mRNA in the rat central nervous system. Journal of Comparative Neurology. 1997;388:507–525. doi: 10.1002/(sici)1096-9861(19971201)388:4<507::aid-cne1>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Simerly RB. Prodynorphin and proenkephalin gene expression in the anteroventral periventricular nucleus of the rat: sexual differentiation and hormonal regulation. Molecular and Cellular Neurosciences. 1991;2:473–484. doi: 10.1016/1044-7431(91)90014-f. [DOI] [PubMed] [Google Scholar]
- Simerly RB. Wired for reproduction: organization and development of sexually dimorphic circuits in the mammalian forebrain. Annu Rev Neurosci. 2002;25:507–36. doi: 10.1146/annurev.neuro.25.112701.142745. [DOI] [PubMed] [Google Scholar]
- Simonian SX, Spratt DP, Herbison AE. Identification and characterization of estrogen receptor a-containing neurons projecting to the vicinity of the gonadotropin-releasing hormone perikarya in the rostral preoptic area of the rat. J Comp Neurol. 1999;411:346–358. doi: 10.1002/(sici)1096-9861(19990823)411:2<346::aid-cne13>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Sisk CL, Richardson HN, Chappell PE, Levine JE. In vivo gonadotropin-releasing hormone secretion in female rats during peripubertal development and on proestrus. Endocrinology. 2001;142:2929–36. doi: 10.1210/endo.142.7.8239. [DOI] [PubMed] [Google Scholar]
- Smith JT, Cunningham MJ, Rissman EF, Clifton DK, Steiner RA. Regulation of Kiss1 Gene Expression in the Brain of the Female Mouse. Endocrinology. 2005 doi: 10.1210/en.2005-0488. [DOI] [PubMed] [Google Scholar]
- Smith JT, Popa SM, Clifton DK, Hoffman GE, Steiner RA. Kiss1 neurons in the forebrain as central processors for generating the preovulatory luteinizing hormone surge. J Neurosci. 2006;26:6687–94. doi: 10.1523/JNEUROSCI.1618-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MJ, Jennes L. Neural signals that regulate GnRH neurones directly during the oestrous cycle. Reproduction. 2001;122:1–10. doi: 10.1530/rep.0.1220001. [DOI] [PubMed] [Google Scholar]
- Smith MJ, Wise PM. Neurotensin gene expression increases during proestrus in the rostral medial preoptic nucleus: potential for direct communication with gonadotropin-releasing hormone neurons. Endocrinology. 2001;142:3006–13. doi: 10.1210/endo.142.7.8256. [DOI] [PubMed] [Google Scholar]
- Thompson RH, Swanson LW. Structural characterization of a hypothalamic visceromotor pattern generator network. Brain Res Brain Res Rev. 2003;41:153–202. doi: 10.1016/s0165-0173(02)00232-1. [DOI] [PubMed] [Google Scholar]
- Tin Tin Win S, Mitsushima D, Shinohara K, Kimura F. Sexual dimorphism of GABA release in the medial preoptic area and luteinizing hormone release in gonadectomized estrogen-primed rats. Neuroscience. 2004;127:243–50. doi: 10.1016/j.neuroscience.2004.04.056. [DOI] [PubMed] [Google Scholar]
- Watson RE, Langub MC, Engle MG, Maley BE. Estrogen-receptive neurons in the anteroventral periventricular nucleus are synaptic targets of the suprachiasmatic nucleus and peri-suprachiasmatic region. Brain Research. 1995;689:254–264. doi: 10.1016/0006-8993(95)00548-5. [DOI] [PubMed] [Google Scholar]
- Wiegand SJ, Terasawa E. Discrete lesions reveal functional heterogeneity of suprachiasmatic structures in regulation of gonadotropin secretion in the female rat. Neuroendocrinology. 1982;34:395–404. doi: 10.1159/000123335. [DOI] [PubMed] [Google Scholar]
- Wiegand SJ, Terasawa E, Bridson WE, Goy RW. Effects of discrete lesions of preoptic and suprachiasmatic structures in the female rat. Neuroendocrinology. 1980;31:147–157. doi: 10.1159/000123066. [DOI] [PubMed] [Google Scholar]
- Wintermantel TM, Campbell RE, Porteous R, Bock D, Grone HJ, Todman MG, Korach KS, Greiner E, Perez CA, Schutz G, Herbison AE. Definition of estrogen receptor pathway critical for estrogen positive feedback to gonadotropin-releasing hormone neurons and fertility. Neuron. 2006;52:271–80. doi: 10.1016/j.neuron.2006.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkin JW, Xiao E, Popilskis S, Ferin M, Silverman A. Fos expression in the gonadotropin-releasing hormone (GnRH) neuron does not increase during the ovarian steroid-induced GnRH surge in the rhesus monkey. Endocrinology. 1994;135:956–961. doi: 10.1210/endo.135.3.8070392. [DOI] [PubMed] [Google Scholar]
- Wray S. Development of gonadotropin-releasing hormone-1 neurons. Front Neuroendocrinol. 2002;23:292–316. doi: 10.1016/s0091-3022(02)00001-8. [DOI] [PubMed] [Google Scholar]
- Xia L, Van Vugt D, Alston EJ, Luckhaus J, Ferin M. A surge of gonadotropin-releasing hormone accompanies the estradiol-induced gonadotropin surge in the rhesus monkey. Endocrinology. 1992;131:2812–2820. doi: 10.1210/endo.131.6.1446619. [DOI] [PubMed] [Google Scholar]
- Yamaji T, Dierschke DJ, Hotchkiss J, Bhattacharya AN, Surve AH, Knobil E. Estrogen induction of LH release in the rhesus monkey. Endocrinology. 1971;89:1034–1041. doi: 10.1210/endo-89-4-1034. [DOI] [PubMed] [Google Scholar]
- Yuri K, Kawata M. Estrogen receptor-immunoreactive neurons contain calcitonin gene-related peptide, methionine-enkephalin or tyrosine hydroxylase in the female rat preoptic area. Neuroscience Research. 1994;21:135–141. doi: 10.1016/0168-0102(94)90155-4. [DOI] [PubMed] [Google Scholar]