Abstract
The relative importance of lipid rafts vs. specialized rafts termed caveolae to influence signal transduction is not known. Here we show that in cells lacking caveolae, the dually acylated protein, endothelial nitric oxide synthase (eNOS), localizes to cholesterol-rich lipid raft domains of the plasma membrane. In these cells, expression of caveolin-1 (cav-1) stimulates caveolae biogenesis, promotes the interaction of cav-1 with eNOS, and the inhibition of NO release from cells. Interestingly, in cells where cav-1 does not drive caveolae assembly, despite equal levels of cav-1 and eNOS and localization of both proteins to raft domains of the plasmalemma, the physical interaction of eNOS with cav-1 is dramatically less resulting in less inhibition of NO release. Thus, cav-1 concentrated in caveolae, not in rafts, is in closer proximity to eNOS and is necessary for negative regulation of eNOS function, thereby providing the first clear example of spatial regulation of signaling in this organelle that is distinct from raft domains.
The lipid raft hypothesis formulated more than 10 years ago (1), postulated the existence of lipid rafts as dynamic assemblies of cholesterol and sphingolipids in the plasma membrane. Caveolae are specialized lipid rafts because of the ability of caveolins to initiate caveolae biogenesis from raft-derived components. The proposed functions of rafts/caveolae are diverse and somewhat controversial, including cholesterol transport (2, 3), endocytosis (4), potocytosis (5), and signal transduction (6–9). In addition, there is much confusion in the literature where the distinction among lipid rafts, flattened caveolae, or caveolae proper is less than clear, thus making it difficult to ascertain which cellular functions might be attributable to rafts, caveolae, or caveolins, per se.
For example, because cholesterol-modifying drugs such as cyclodextrins remove cholesterol from plasmalemmal rafts and caveolae, these reagents cannot distinguish between signaling events occurring in these compartments. Moreover, from a biophysical perspective, it is unknown whether the curvature and structure of plasmalemma-attached caveolae with caveolin-1 (cav-1) as the coat protein or whether the presence of cav-1 protein in the plasma membrane, per se, is necessary for the effects of cav-1 on any target protein or function. This distinction is important because although cav-1 is the main structural protein of caveolae, it may also exist in lipid rafts without the formation of plasmalemma-attached caveolae (10), be found in the trans-Golgi network as originally described (9), or found in other organelles (11).
Endothelial nitric-oxide synthase (eNOS) is a dually acylated signaling protein responsible for the production of nitric oxide (NO) in the cardiovascular system. N-myristoylated and cysteine palmitoylation of eNOS targets eNOS to the cytoplasmic face of the Golgi and to plasmalemmal caveolae. eNOS in these domains can interface with several aspects of signal transduction systems through direct interactions with several protein partners, including cav-1, dynamin-2, the intracellular domain III of certain G protein-coupled receptors (12), calmodulin (13), heat shock protein 90 (hsp90; ref. 14), and serine/threonine protein kinase Akt (protein kinase B; ref. 15). These regulated interactions are associated with either inhibition or stimulation of the enzyme activity.
Several laboratories have shown that eNOS directly interacts in coimmunoprecipitation and domain-mapping studies with residues 82–101 of the caveolae coat protein, cav-1 (16, 17), and that cav-1 or the surrogate caveolin peptide negatively regulate eNOS function in vitro. Recently, evidence supporting the in vivo function of this interaction has been shown by delivery of the eNOS binding motif of cav-1 into living cells or tissues by using either permeabilization techniques or peptide chimeras that permit cellular internalization without endocytic degradation. In both instances, the caveolin peptide, but not a scrambled control peptide, attenuates NO release and blocks NO-dependent functions, thus supporting a potential in vivo role for the eNOS–caveolin interaction (18, 19).
Given the complexity of examining the importance of cav-1 in signaling and the fact that most cells contain dynamic assemblies of raft domains and caveolae, we sought to find a suitable mammalian cell system to clearly distinguish between signaling in raft domains vs. caveolae. Thus, here we examine the importance of cav-1 as a negative regulator of eNOS by using heterologous systems that permit a clear distinction between signaling in lipid rafts vs. caveolae.
Materials and Methods
Cells.
Fischer rat thyroid (FRT) cells were cultured in Ham's F-12/Coon's modified media (Sigma) containing penicillin (100 units/ml) and streptomycin (100 μg/ml) supplemented with 5% FBS (Life Technologies, Grand Island, NY). The human prostate cancer cell line (LNCaP; American Type Culture Collection) was cultured in RPMI medium 1640 containing penicillin, streptomycin, and 10% (vol/vol) FBS.
Construction of Adenoviral Vectors.
Recombinant adenoviruses (Ads) containing the cDNA encoding cav-1 (Adcav-1), eNOS fused with green fluorescent protein (AdeNOS-GFP), and β-galactosidase (Adβ-gal) were generated as follows. The early region 1 (E1) of a full-length serotype 5 wild Ad was deleted and replaced by a cDNA sequence encoding canine myc-tagged cav-1, bovine eNOS fused with GFP, or β-gal under control of cytomegalovirus (CMV) promoter. Recombination, amplification, and titering were performed by using standard methodology. Recombinant Ad containing the cDNA encoding eNOS was a gift of Z. Katusic (Mayo Clinic, Rochester, MN) and was generated as described (20).
Adenoviral Transduction of FRT and LNCaP Cells with Cav-1 and eNOS.
Two days after plating, cells were infected with appropriate Ads in serum-free medium for 4 h. All Ads were used at multiplicities of infection of 20 and 5 for infecting FRT and LNCaP cells, respectively. The viruses were removed after 4 h, and cells were left to recover for 1–4 days (depending on experiment) in complete media. In preliminary experiments with the β-gal virus, these conditions were optimal for infecting nearly 100% of cells.
Stable Transfection.
We used the clone of FRT cells stably expressing cav-1 (T2). The procedure for stable transfection of these cells has been described (21).
NO Release.
For measurement of basal NO production, 48 h after infection with Adβ-gal, Adcav-1, and AdeNOS, media was replaced with fresh media containing 100 μM l-sepiapterin (Cayman Chemicals, Ann Arbor, MI) and left for another 48 h (4 days after the initial infection). For measurement of stimulated NO release, cells were washed with serum-free medium followed by stimulation with ionomycin (1 μM; Sigma) for 15 min. Media were processed for the measurement of nitrite (NO2−), the stable breakdown product of NO in aqueous solution, by NO-specific chemiluminescence as described (22). In addition, NO2− release from noninfected with eNOS control cells or cells infected with Adβ-gal was subtracted to control for background levels of NO2− found in samples.
Western Blotting.
Cell lysates were obtained and processed for Western blot as described (23).
Immunofluorescence.
Cells cultured on 35-mm glass-bottomed dishes were fixed, permeabilized, and stained with Abs as described (21). Slides were mounted with Slowfade (Molecular Probes), and cells were observed with an inverted Zeiss microscope fitted with a Bio-Rad MRC 600 confocal imaging system.
Treatment with β-Trimethyl Cyclodextrin (CD).
Cells were incubated with medium without serum for 18 h, medium was changed into the fresh one containing 10 mM β-trimethyl CD (Sigma). Cells were incubated for 1 h, washed with ice-cold Dulbecco's phosphate-buffered saline (DPBS), and processed for detergent-free sucrose fractionation as described below or incubated with 1 μM ionomycin in serum-free medium for 15 min and processed for NO release. To determine the reversibility of CD action, cells treated with CD were washed with serum-free medium and incubated for another 1 h in the presence of CD loaded with cholesterol (30 μg/ml; Sigma).
Sodium Carbonate Extraction Followed by Sucrose Gradient Fractionation.
To determine raft association of both eNOS and cav-1, we used a detergent-free method developed by Song et al. (24) with a modification for smaller volumes. Briefly, cells were homogenized and sonicated as described (24), lysates (0.25 ml) adjusted to 42.5% sucrose with 0.25 ml of 85% sucrose in Mes-buffered saline (MBS) (25 mM Mes, pH 6.5/150 mM NaCl). The 0.5 ml of lysate (in 42.5% sucrose and 0.25 M Na2CO3) was placed at the bottom of a 2.4-ml centrifuge tube and overlaid with 1.4 ml of 30% sucrose in 0.25 M Na2CO3 and 0.3 ml of 5% sucrose in 0.25 M Na2CO3. The gradients were centrifuged for 18–20 h at 48,000 rpm in a TL100 centrifuge by using a TLS55 rotor. Ten 0.22-ml fractions were collected from the top of the gradient and processed for Western blotting. In addition to eNOS, cav-1 and β-actin membranes were blotted with polyclonal Ab to the Golgi marker β-COP (1:1000; Affinity BioReagents, Golden, CO).
Immunoprecipitation.
Cav-1 was immunoprecipitated from cultured LNCaP and FRT cells with polyclonal Ab (Santa Cruz Biotechnology) as described (25). Immunocomplexes were isolated, electrophoresed, and Western blotted with anti-cav-1 and with anti-eNOS mAbs (Transduction Laboratories, Lexington, KY).
Electron Microscopy (EM)
Embedding of Cell Monolayers.
Cells were fixed for 1 h at room temperature in 1% glutaraldehyde (Electron Microscopy Sciences, Fort Washington, PA) in 0.1 M cacodylate buffer (pH 7.4) postfixed in 1% osmium tetroxide (Electron Microscopy Sciences) in the same buffer, stained with 2% aqueous uranyl acetate, and dehydrated in a graded series of ethanol. They were then detached from the plastic dish by using propylene oxide and embedded in EMbed 812 resin (Electron Microscopy Sciences). Sections (60 nm) were cut with a Reichert Ultracut E ultramicrotome, stained with 2% uranyl acetate and lead citrate, and examined in a Philips Electronic Instruments (Mahwah, NJ) 410 electron microscope.
Quantitative EM.
For each sample, about 20 random pictures were taken at ×10,800 and printed at a final magnification of ×26,900. The basolateral membrane was identified by the presence of a thin plastic film resulting from the dissolution of the plastic cell culture dish by propylene oxide. Caveolae and coated pits were counted when a neck connecting them to the cell surface was clearly visible. Smooth and coated vesicles were also counted, but only when they were within 50 nm of the plasma membrane. The total length of plasma membrane was estimated by counting intersections with vertical and horizontal lines of a lattice grid with a 30-μm spacing between lines.
Statistics.
NO release data are expressed as mean ± SEM. Comparisons were made by using a two-tailed Student's t test. Differences were considered as significant at P < 0.05 (*) or P < 0.01 (**).
Results and Discussion
To address whether lipid rafts, caveolae, and/or caveolins can influence NO signaling, we initially used FRT cells that lack cav-1 and plasmalemmal caveolae, but express cav-2. In these cells, overexpression of cav-1 stimulates caveolae biogenesis (26) and the redistribution of cav-2 from the Golgi to Triton X-100 insoluble lipid-rich domains of plasma membrane (21). As seen in Table 1, parental FRT cells did not have a single plasmalemma attached caveolae but contained ample uncoated vesicles, clathrin-coated pits, and vesicles based on quantitative EM. FRT cells stably transfected with the cav-1 cDNA (T2 cells) or adenoviral expression of cav-1 into parental FRT cells induced the formation of caveolae but did not change the number of uncoated vesicles, clathrin-coated pits, and vesicles similar to previous findings.
Table 1.
Quantitative morphology of plasma membrane structures in FRT and LNCaP cells
| Caveolae | Uncoated vesicles | Coated pits | Coated vesicles | Length of membrane, mm | |
|---|---|---|---|---|---|
| FRT + Adβ-gal | 0 | 51 | 27 | 39 | 0.337 |
| T2 | 107 | 61 | 38 | 33 | 0.213 |
| FRT + Adcav-1 | 75 | 92 | 32 | 56 | 0.285 |
| LNCaP + Adβ-gal | 0 | 24 | 36 | 47 | 0.253 |
| LNCaP + Adcav-1 | 0 | 113 | 22 | 26 | 0.229 |
FRT and LNCaP cells were plated and infected with Adβ-gal or Adcav-1 as described in Materials and Methods. Two days after infection, cells were fixed, postfixed, and processed for EM. Numbers of plasma membrane structures, i.e., caveolae with a visible connection with the cell surface; as well as uncoated and coated vesicles and coated pits within 50 nm from the cell surface were assessed as described in Materials and Methods.
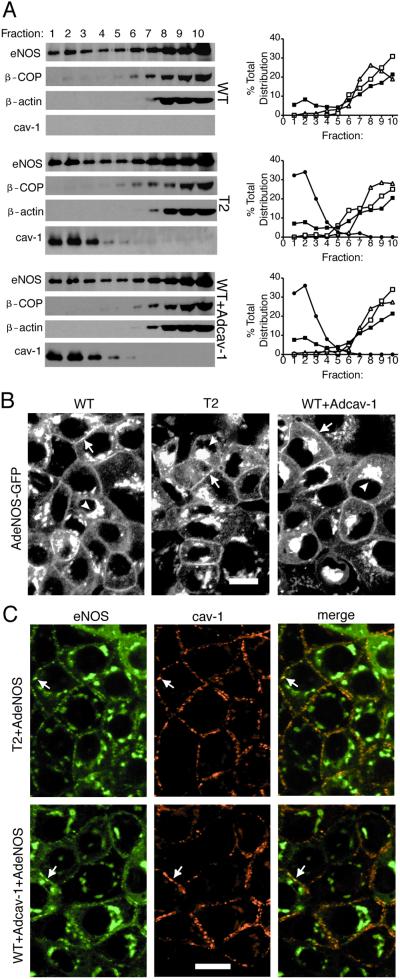
Because eNOS has been reported to be found in plasma membrane caveolae of endothelial cells based on immunofluorescence (25) and immunogold labeling (27), we infected FRT cells with an Ad expressing eNOS and compared the distribution of eNOS by using detergent-free microdomain fractionation and immunofluorescence microscopy. In parental FRT cells lacking cav-1, eNOS was distributed into both light buoyant membranes (fractions 1–3 containing raft and caveolae-enriched proteins) and heavy membranes (fractions 7–10) as compared with β-COP and β-actin, which were enriched in heavy membrane fractions (Fig. 1A Top, densitometry far Right). Stable expression (T2 cells, Fig. 1A Middle, densitometry far Right) and adenoviral transduction of FRT cells with cav-1 (Adcav-1, Fig. 1A Bottom, densitometry far Right) resulted in cav-1 distributing into light membrane fractions. Under these conditions, the presence of cav-1 did not influence eNOS trafficking into or out of light fractions of the gradient, suggesting that eNOS can target to light buoyant membrane fractions independently of cav-1 or caveolae.
Figure 1.
Ectopic expression of cav-1 in FRT cells results in caveolae assembly but has no effect on eNOS trafficking. (A) Cav-1-negative parental (WT) FRT cells, T2, or parental FRT cells infected with Adcav-1 (WT + Adcav-1) were infected with AdeNOS, and isolation of raft domains was performed on bottom-loaded sucrose gradients as described. The relative enrichments in eNOS, β-COP, β-actin, and caveolin in equal volumes of each fraction were assessed. Right is quantitative densitometry reflecting the distribution of eNOS (filled box), β-COP (open box), β-actin (open triangle), or cav-1 (filled circle) throughout the gradient. (B) WT, T2, or parental FRT + Adcav-1 were infected with AdeNOS-GFP, and the localization of eNOS-GFP was examined in living cells. Arrows depict eNOS-GFP in the plasma membrane, and arrowheads show eNOS-GFP in perinuclear structures. (C) The localization of eNOS (Left) and cav-1 (Center) were performed by immunofluorescence microscopy in T2 and FRT cells infected with AdeNOS virus. Far Right shows the merged images. Arrows denote examples of colocalization of eNOS and cav-1 in plasma membrane. Calibration bars = 20 μm for B and C. Please refer to Table 1 for morphometric evaluation of caveolae and other vesicle populations.
Next we infected FRT cells (parental, T2 clone, and Adcav-1-infected) with adenoviral eNOS-GFP and examined eNOS trafficking in living cells. eNOS-GFP targeted to the plasma membrane (Fig. 1 B and C, arrows) and perinuclear region (Fig. 1 B and C, arrowheads) in both cav-1-negative and -positive FRT cells. Thus, cav-1-dependent caveolae formation did not change eNOS subcellular localization, which was consistent with the data in Fig. 1A. To determine whether both eNOS and cav-1 colocalize, we used dual immunofluorescence labeling of eNOS and cav-1 in fixed FRT cells (Fig. 1C). Merged images of eNOS and cav-1 labeling show that eNOS colocalized with cav-1 in plasma membrane (Fig. 1 B and C, denoted by arrows) of the cells (Fig. 1C Upper and Lower Right). Thus, imaging of both living and fixed cells expressing eNOS shows that although a large portion of eNOS colocalizes with cav-1 both in perinuclear and plasma membrane regions, eNOS targets to both domains independently of cav-1/caveolae.
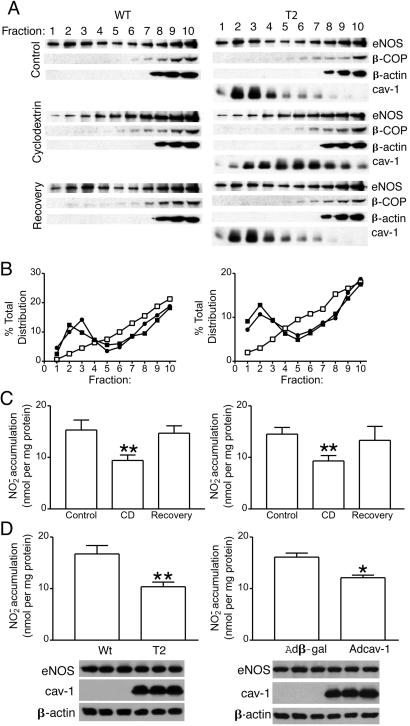
Next we examined the importance of cholesterol to eNOS trafficking and NO release in cells that either have only rafts or caveolae. Treatment of parental [wild type (WT), Fig. 1B Left] and stably expressing cav-1 FRT cells (T2, Fig. 1B Right) with β-trimethyl CD, which removes plasma membrane cholesterol from rafts/caveolae (28), caused a significant reduction of eNOS in the light membrane fractions (fractions 1–3) coinciding with an increase in intermediate buoyant density fractions 5–8 (Fig. 2A, compare Top and Middle in WT vs. T2). Similarly to eNOS, in T2 cells, the amount of cav-1 in light membranes decreased, and the relative enrichment of this protein as compared with control could be seen in intermediate and heavy density fractions (Fig. 2A Right, compare Top and Middle). Distribution of both β-COP and β-actin were unaffected by CD treatment. The effect of CD on the redistribution of both eNOS and cav-1 could be fully reversed to that seen in control cells (Fig. 2A Top) by removing CD followed by 2-h incubation with cholesterol (Fig. 2A Bottom and densitometric quantification of eNOS in control, CD, and CD + cholesterol treatment in B). Next, we examined the effects of cav-1 expression and CD treatment on calcium ionophore-induced NO release from cells. As seen in Fig. 2C (Left and Right), calcium ionophore-induced NO release was similar in cells lacking or expressing cav-1. Moreover, CD treatment blocked calcium ionophore-induced NO release from both cells to the same extent, an effect reversed by cholesterol reloading. Thus, the inability of cav-1 to influence eNOS trafficking and NO release in both cav-1-negative and -positive FRT cells along with the reversible redistribution of both eNOS and cav-1 by CD suggests that cav-1/caveolae presence does not influence eNOS targeting to rafts or the stimulated release of NO from cells. Moreover, mislocalization of eNOS from rafts/caveolae with CD blunts NO release, which is consistent with the paradox that localization of eNOS to the plasma membrane is necessary for agonist-induced NO release, whereas the complex of eNOS with cav-1 in resting cells likely reduces basal NO release.
Figure 2.
Cav-1 does not influence the cholesterol sensitivity of eNOS trafficking or stimulated NO production but attenuates basal NO release. (A) Isolation of raft domains was performed in WT and T2 FRT cells that were treated with CD (10 mM for 1 h at 37°C) or after removal of cholesterol with CD-loaded cholesterol. Fractions were probed for eNOS, β-COP, β-actin, and cav-1 as mentioned previously. B shows relative densitometry of eNOS levels in control (filled square), CD treatment (open square), and recovery (filled circle). (C) The production of NO (assayed as NO2−) in response to calcium ionophore (1 μM) was examined in FRT (Left) and T2 cells (Right) expressing AdeNOS. Cells were treated with CD or vehicle and CD plus cholesterol as mentioned previously, and calcium ionophore was added for 15 min. NO2− was quantified in the media. The accumulation of NO2− from FRT cells not expressing eNOS was subtracted as a background. (D) The basal accumulation of NO2− in WT and T2 cells (Left) or WT cells infected with β-gal or Adcav-1 (Right), and AdeNOS was assessed. The graph shows accumulated NO2− normalized per mg of protein (mean ± SEM, n = 3 experiments in triplicate; **, P < 0.01; *, P < 0.05). The Inset below shows the level of expression of eNOS and cav-1 determined by Western blot in cells from which NO release was determined in one representative experiment performed in triplicate. Equal protein loading is confirmed by β-actin.
Because the interaction of eNOS with cav-1 is proposed to inhibit basal NO production, and on stimulation of cells the binding of CaM and hsp90 to eNOS relieves the negative regulation by cav-1 (29, 30), we examined basal accumulation of NO over 48 h in cells that have rafts and/or caveolae. Interestingly, the expression of cav-1 reduced basal NO release by ≈40% (**P < 0.01) and 28% (*P < 0.05) in T2 cells and Adcav-1-transduced cells compared with parental FRT cells, respectively (Fig. 2D, with inset Western blots documenting equal expression of eNOS, cav-1, and actin in triplicate). Thus, the presence of cav-1 does not influence calcium ionophore-stimulated NO production (Fig. 2C) but inhibits basal NO release (Fig. 2D), suggesting that cav-1 and/or cav-1-dependent generation of caveolae is responsible for inhibition of NO production.
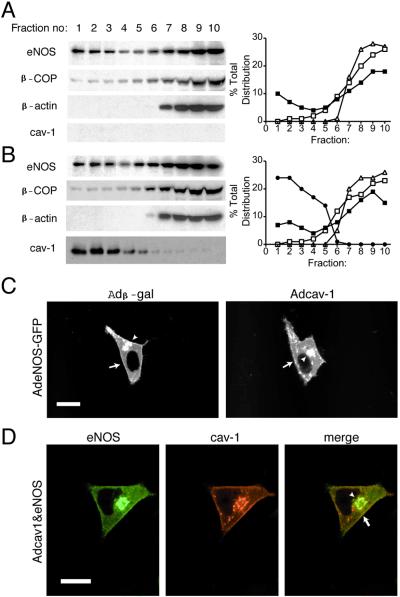
To distinguish between these two possibilities, whether cav-1, per se, or cav-1 in the context of caveolae could influence eNOS function, we used LNCaP cells. As seen in Table 1, LNCaP cells do not have a single plasmalemmal-attached caveolae based on quantitative EM, nor do they express cav-1 by Western blotting (Fig. 3A Left). Interestingly, adenoviral expression of cav-1 (Adcav-1) in LNCaP does not result in caveolae formation in these cells compared with FRT cells (see Fig. 3B and Table 1) but increases the number of uncoated vesicles that do not fuse with the plasma membrane. As seen in Fig. 3 A and B, in parental LNCaP and LNCaP infected with Adcav-1, eNOS distributes into light and heavy membrane domains, and eNOS-GFP targeting to the perinuclear region (Fig. 3C, arrowhead) and plasma membrane (Fig. 3C, arrow) in these cells is not affected by cav-1 expression. Moreover, eNOS and cav-1 colocalize in the perinuclear region (Fig. 3D, arrowhead) and plasma membrane (Fig. 3D, arrow) in fixed cells.
Figure 3.
Ectopic expression of cav-1 in LNCaP does not influence eNOS targeting. In A and B, the distribution of eNOS and other marker proteins was examined in parental LNCaP cells infected with β-gal (A) or Adcav-1 (B) and eNOS as mentioned previously (Left). The relative distribution of eNOS (filled square), β-COP (open square), β-actin (open triangle), and cav-1 (filled circle) is shown Right. In C, LNCaP cells infected with β-gal or Adcav-1 were infected with AdeNOS-GFP, and the localization of eNOS-GFP was examined in living cells. Arrows depict eNOS-GFP in the plasma membrane and arrowheads show eNOS-GFP in perinuclear structures. In D, the localization of eNOS (Left) and cav-1 (Center) were performed by immunofluorescence microscopy in LNCaP cells infected with AdeNOS virus. Right shows the merged images. Calibration bars = 20 μm for C and D.
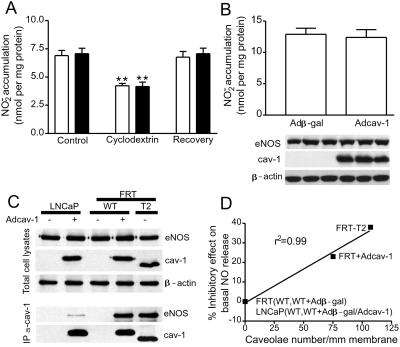
Next we examined calcium ionophore-stimulated NO production in the absence and presence of CD, as well as basal NO accumulation. As seen in Fig. 4A, calcium ionophore stimulates NO accumulation to the same extent in LNCaP vs. LNCaP expressing cav-1. In addition, CD attenuates ionophore-stimulated NO, in a cholesterol-reversible manner, showing that eNOS targets to rafts in parental LNCaP and that the presence of cav-1 does not influence the CD sensitivity. However, the expression of cav-1 in LNCaP, in the absence of caveolae formation, does not result in inhibition of basal NO release (Fig. 4B). Next, we examined whether the documented protein–protein interaction of eNOS with cav-1 is influenced by the absence or presence of caveolae as a potential mechanism to explain the effect of cav-1 on NO release. As seen in Fig. 4C, the expressions of eNOS, cav-1, and actin were similar in all lines examined. However, immunoprecipitation of cav-1 from LNCaP cells results in low level detection of coassociated eNOS (see lanes 1 and 2). The low level coassociation of cav-1 and eNOS in LNCaP is in contrast to FRT cells expressing cav-1 (WT + Adcav-1) and T2 cells stably expressing cav-1 where ample amounts of eNOS were coprecipitated with cav-1. In the absence of cav-1 in the cells, no eNOS was precipitated with the anti-cav-1 Ab (Fig. 4C, lanes 1 and 3). These results demonstrate that the presence of cav-1 in caveolae but not cav-1 in light buoyant density membranes or plasmalemmal rafts, enhances the interaction of eNOS with cav-1 and is responsible for the negative regulation of eNOS function. After plotting the percent inhibition of basal NO release against caveolae number in FRT and LNCaP cells, there is a striking inverse correlation between these two phenomena (r2 = 0.99), namely inhibition of NO release occurs as caveolae number increases (Fig. 4C). In contrast, there is no correlation (r2 = 0.49) between the levels of inhibition of NO release and the levels of cav-1 expression. Thus, our data strongly imply that inhibition of basal NO release in FRT cells by cav-1 depends on the abundance of caveolae and cav-1-associated eNOS within the organelle.
Figure 4.
Cav-1 per se does not influence NO release, but cav-1 in closer proximity to eNOS in caveolae interacts with eNOS and inhibits NO release. In A, the production of NO (assayed as NO2−) in response to calcium ionophore (1 μM) was examined in LNCaP cells expressing β-gal (open bars) or Adcav-1 (filled bars). Cells were treated or not with CD and CD plus cholesterol as mentioned previously, and calcium ionophore was added for 15 min. NO2− was quantified in the media. The accumulation of NO2− from LNCaP cells not expressing eNOS was subtracted as a background. In B, the basal accumulation of NO2− in LNCaP cells infected with Adβ-gal or Adcav-1 and AdeNOS was assessed. The graph shows accumulated NO2− normalized per mg of protein (mean ± SEM, n = 3 experiments in triplicate; **, P < 0.01). Inset below shows the level of expression of eNOS and cav-1 determined by Western blot in cells from which NO release was determined in one representative experiment performed in triplicate. Equal protein loading is confirmed by β-actin. In C, cav-1 and eNOS were expressed in LNCaP or FRT cells (Top reflects expression of protein in lysates), and cav-1 was immunoprecipitated from cells and the relative amount of associated eNOS determined by Western blotting (Bottom). Note that equal amounts of eNOS and cav-1 were recovered from cell lysates; however, the amount of cav-1-associated eNOS was markedly less in cells that cannot form caveolae. The different molecular mass of cav-1 introduced by Ad is the result of an myc tag on the carboxyl tail. In D, the percent inhibition of NO2− accumulation from parental FRT and LNCaP cells, T2 cells, and Adcav-1-transduced cells is plotted against the number of caveolae in these cells (see Table 1 for details). Data were correlated (r2 = 0.99) by first-order linear regression with GraphPad (San Diego) PRISM software.
Our results have several implications for the biogenesis, trafficking, and regulation of acylated protein function in rafts and/or caveolae. Our data show that myristoylated and palmitoylated eNOS targets to cholesterol-rich raft domains independent of caveolae in both parental FRT and LNCaP cells. These finding argue against the idea that cav-1 may act as a trafficking scaffold to cargo acylated proteins from the trans-Golgi network to the plasma membrane. In addition, our data supports the idea of a two-step model for the trafficking of eNOS and other acylated proteins into the plasma membrane: (i) initial targeting of the lipid-modified protein into a raft domain, a function that occurs independently of cav-1, and (ii) the interaction of eNOS and perhaps other proteins with cav-1 in the context of a caveolae. If caveolae are absent, but rafts are still present as in parental FRT cells, parental LNCaP, or LNCaP cells expressing cav-1, eNOS behaves as if it is in a raft, weakly interacts with cav-1, and is not subject to tonic negative regulation by cav-1 in the plasma membrane. Conversely, if caveolae are present, the total amount of eNOS in an isolated raft/caveolae domain does not change, but the level of the protein–protein interaction is greater and inhibition of NO release occurs.
These findings, using eNOS as a downstream target of cav-1, imply a different mode of action for caveolin in signaling pathways as compared with its role in H-Ras signaling. Previous work by Roy et al. (31) has shown that caveolin-dependent trafficking of cholesterol can influence H-Ras-mediated activation of Raf activity in the plasma membrane. In this model, overexpression of dominant negative caveolin or cholesterol removal (with CD) attenuates H-Ras- but not K-Ras-stimulated Raf activity without influencing Raf recruitment, implying that caveolin-mediated delivery of cholesterol was important for raft/caveolae assembly and efficient signaling from H-Ras to Raf. Our work supports the importance of cholesterol for eNOS trafficking and activity because the appearance of eNOS in buoyant membrane fractions and stimulated NO release is perturbed by CD treatment. However, the presence of cholesterol, per se, either in rafts or in domains where cav-1 resides, cannot explain the negative regulation of basal NO production in cells when caveolae biogenesis occurs. We favor the hypothesis that certain caveolae-enriched proteins (i.e., eNOS) may be regulated by cav-1 in the context of a plasma membrane-attached caveola or perhaps by the recruitment of other regulatory molecules in the caveolae, but not to a raft. Thus, our work underscores the importance of plasmalemmal microdomains in regulating signaling and provides a unique model for the distinction between lipid rafts and caveolae.
Acknowledgments
We thank Dr. Rodriquez-Boulon (Cornell Medical School, New York) for helpful comments with the manuscript and for FRT cells and Dr. Katusic for the eNOS Ad. This work is supported by National Institute of Health Grants RO1 HL57665, HL61371, and HL64793 (to W.C.S.).
Abbreviations
- eNOS
endothelial nitric oxide synthase
- cav-1
caveolin-1
- Ad
adenovirus
- β-gal
β-galactosidase
- FRT
Fischer rat thyroid
- T2
clone of FRT cells stably expressing cav-1
- LNCaP
human prostate cancer cell line
- GFP
green fluorescence protein
- CD
cyclodextrin
- WT
wild type
- EM
electron microscopy
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Simons K, van Meer G. Biochemistry. 1988;27:6197–6202. doi: 10.1021/bi00417a001. [DOI] [PubMed] [Google Scholar]
- 2.Fielding P E, Fielding C J. Biochemistry. 1995;34:14288–14292. doi: 10.1021/bi00044a004. [DOI] [PubMed] [Google Scholar]
- 3.Smart E J, Ying Y, Donzell W C, Anderson R G. J Biol Chem. 1996;271:29427–29435. doi: 10.1074/jbc.271.46.29427. [DOI] [PubMed] [Google Scholar]
- 4.Schnitzer J E, Oh P, McIntosh D P. Science. 1996;274:239–242. doi: 10.1126/science.274.5285.239. [DOI] [PubMed] [Google Scholar]
- 5.Anderson R G, Kamen B A, Rothberg K G, Lacey S W. Science. 1992;255:410–411. doi: 10.1126/science.1310359. [DOI] [PubMed] [Google Scholar]
- 6.Lisanti M P, Scherer P E, Vidugiriene J, Tang Z, Hermanowski-Vosatka A, Tu Y H, Cook R F, Sargiacomo M. J Cell Biol. 1994;126:111–126. doi: 10.1083/jcb.126.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Okamoto C T. Adv Drug Delivery Rev. 1998;29:215–228. doi: 10.1016/s0169-409x(97)00080-x. [DOI] [PubMed] [Google Scholar]
- 8.Shaul P W, Anderson R G. Am J Physiol. 1998;275:L843–L851. doi: 10.1152/ajplung.1998.275.5.L843. [DOI] [PubMed] [Google Scholar]
- 9.Kurzchalia T V, Dupree P, Parton R G, Kellner R, Virta H, Lehnert M, Simons K. J Cell Biol. 1992;118:1003–1014. doi: 10.1083/jcb.118.5.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Scheiffele P, Verkade P, Fra A M, Virta H, Simons K, Ikonen E. J Cell Biol. 1998;140:795–806. doi: 10.1083/jcb.140.4.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li W P, Liu P, Pilcher B K, Anderson R G. J Cell Sci. 2001;114:1397–1408. doi: 10.1242/jcs.114.7.1397. [DOI] [PubMed] [Google Scholar]
- 12.Marrero M B, Venema V J, Ju H, He H, Liang H, Caldwell R B, Venema R C. Biochem J. 1999;343:335–340. [PMC free article] [PubMed] [Google Scholar]
- 13.Forstermann U, Pollock J S, Schmidt H H, Heller M, Murad F. Proc Natl Acad Sci USA. 1991;88:1788–1792. doi: 10.1073/pnas.88.5.1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garcia-Cardena G, Fan R, Shah V, Sorrentino R, Cirino G, Papapetropoulos A, Sessa W C. Nature (London) 1998;392:821–824. doi: 10.1038/33934. [DOI] [PubMed] [Google Scholar]
- 15.Fulton D, Gratton J P, McCabe T J, Fontana J, Fujio Y, Walsh K, Franke T F, Papapetropoulos A, Sessa W C. Nature (London) 1999;399:597–601. doi: 10.1038/21218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ju H, Zou R, Venema V J, Venema R C. J Biol Chem. 1997;272:18522–18525. doi: 10.1074/jbc.272.30.18522. [DOI] [PubMed] [Google Scholar]
- 17.Garcia-Cardena G, Martasek P, Masters B S, Skidd P M, Couet J, Li S, Lisanti M P, Sessa W C. J Biol Chem. 1997;272:25437–25440. doi: 10.1074/jbc.272.41.25437. [DOI] [PubMed] [Google Scholar]
- 18.Feron O, Belhassen L, Kobzik L, Smith T W, Kelly R A, Michel T. J Biol Chem. 1996;271:22810–22814. doi: 10.1074/jbc.271.37.22810. [DOI] [PubMed] [Google Scholar]
- 19.Bucci M, Gratton J P, Rudic R D, Acevedo L, Roviezzo F, Cirino G, Sessa W C. Nat Med. 2000;6:1362–1367. doi: 10.1038/82176. [DOI] [PubMed] [Google Scholar]
- 20.Chen A F, O'Brien T, Tsutsui M, Kinoshita H, Pompili V J, Crotty T B, Spector D J, Katusic Z S. Circ Res. 1997;80:327–335. doi: 10.1161/01.res.80.3.327. [DOI] [PubMed] [Google Scholar]
- 21.Mora R, Bonilha V L, Marmorstein A, Scherer P E, Brown D, Lisanti M P, Rodriguez-Boulan E. J Biol Chem. 1999;274:25708–25717. doi: 10.1074/jbc.274.36.25708. [DOI] [PubMed] [Google Scholar]
- 22.Sessa W C, Garcia-Cardena G, Liu J, Keh A, Pollock J S, Bradley J, Thiru S, Braverman I M, Desai K M. J Biol Chem. 1995;270:17641–17644. doi: 10.1074/jbc.270.30.17641. [DOI] [PubMed] [Google Scholar]
- 23.Sowa G, Liu J, Papapetropoulos A, Rex-Haffner M, Hughes T E, Sessa W C. J Biol Chem. 1999;274:22524–22531. doi: 10.1074/jbc.274.32.22524. [DOI] [PubMed] [Google Scholar]
- 24.Song K S, Li S, Okamoto T, Quilliam L A, Sargiacomo M, Lisanti M P. J Biol Chem. 1996;271:9690–9697. doi: 10.1074/jbc.271.16.9690. [DOI] [PubMed] [Google Scholar]
- 25.Garcia-Cardena G, Oh P, Liu J, Schnitzer J E, Sessa W C. Proc Natl Acad Sci USA. 1996;93:6448–6453. doi: 10.1073/pnas.93.13.6448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lipardi C, Mora R, Colomer V, Paladino S, Nitsch L, Rodriguez-Boulan E, Zurzolo C. J Cell Biol. 1998;140:617–626. doi: 10.1083/jcb.140.3.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rizzo V, McIntosh D P, Oh P, Schnitzer J E. J Biol Chem. 1998;273:34724–34729. doi: 10.1074/jbc.273.52.34724. [DOI] [PubMed] [Google Scholar]
- 28.Rothblat G H, de la Llera-Moya M, Atger V, Kellner-Weibel G, Williams D L, Phillips M C. J Lipid Res. 1999;40:781–796. [PubMed] [Google Scholar]
- 29.Michel J B, Feron O, Sacks D, Michel T. J Biol Chem. 1997;272:15583–15586. doi: 10.1074/jbc.272.25.15583. [DOI] [PubMed] [Google Scholar]
- 30.Gratton J P, Fontana J, O'Connor D S, Garcia-Cardena G, McCabe T J, Sessa W C. J Biol Chem. 2000;275:22268–22272. doi: 10.1074/jbc.M001644200. [DOI] [PubMed] [Google Scholar]
- 31.Roy S, Luetterforst R, Harding A, Apolloni A, Etheridge M, Stang E, Rolls B, Hancock J F, Parton R G. Nat Cell Biol. 1999;1:98–105. doi: 10.1038/10067. [DOI] [PubMed] [Google Scholar]