Abstract
Rationale:
Frequent use and serious adverse effects related to nonsteroidal antiinflammatory drugs (NSAIDs) underscore the need to raise patient awareness about their potential risks. The partial success of patient- or provider-based interventions has recently led to renewed interest in combined approaches that focus on both the patient and physician. Therefore, this research tested a shared decision-making intervention for increasing patient-reported awareness of nonsteroidal antiinflammatory drug (NSAID) risk.
Methods:
A group randomized trial was performed in Alabama from 2005–2007. Intervention group physician practices received continuing medical education [CME] about NSAIDs and patient activation tools promoting risk assessment and communication during visits. Comparison group physician practices received only CME. Cross-sectional data were collected before and after the intervention. Generalized Linear Latent and Mixed Models with logistic link tested relationships among the intervention, study phase, intervention by study phase interaction, and patient-reported awareness of risks with either prescription or over-the-counter [OTC] NSAIDs.
Results:
347 patients at baseline and 355 patients at follow-up participated in this study. The intervention (Adjusted Odds Ratio [AOR]=0.74, p=0.248), follow-up study phase (AOR=1.31, p=0.300), and intervention by study phase interaction (AOR=0.98, p=0.942) were not significantly associated with patient-reported awareness of any prescription NSAID risk. The follow-up study phase was associated with increased odds of reporting any OTC NSAID risk awareness (AOR=2.99, p<0.001), but the patient activation intervention and intervention by study phase interaction were not significantly associated with patient-reported awareness of any OTC NSAID risk (AOR= 0.98, p=0.929; AOR=0.87, p=0.693, respectively). Black race and increasing age had significantly decreased odds of reporting any prescription or OTC NSAID risk awareness. Women and those with at least some college education had significantly increased odds of reporting awareness of any prescription or OTC NSAID risk.
Conclusions:
Our point of care intervention encouraging shared decision-making did not increase NSAID risk awareness.
Keywords: Non-Steroidal Anti-Inflammatory Agents; NSAIDs; decision making, shared; patient safety
Introduction
Nonsteroidal anti-inflammatory drugs (NSAIDs) have historically been one of the most frequently prescribed medication classes in adult outpatient medicine.1–3 The proportion of the US adult population who regularly used NSAIDs for at least 3 months increased from 9.1% to 12.8% during the period 2005 to 2010.4 Despite their common prescribing and use, chronic NSAIDs potentially expose patients to substantial toxicity and adverse effects, requiring careful balancing of benefits and risks.5 Historically, gastrointestinal complications of NSAIDs have received much publicity,6–8 but cardiovascular and renal risks may be equally as serious.9–16 Patients frequently fail to report concomitant use of over-the-counter (OTC) and prescription NSAID use,17–19 which may further exacerbate their risk. Serious adverse reactions also occur frequently when OTC NSAIDs are used alone or when combined with other medications (e.g., corticosteroids, anticoagulants, acetylsalicylic acid).17–23 Common prescribing and use of NSAIDs may lead to complacency among healthcare providers about discussing their risks and appropriate risk-prevention strategies. Collectively, these concerns underscore the need to raise patient awareness about NSAID risks.
Successful risk communication can be described in the context of the Health Belief Model.24 Within this theoretical framework, a patient must first understand a risk exists and that they are susceptible to it. They must then recognize potential adverse consequences from the risk. Lastly, they must believe their actions can minimize such risks. Interventions that can effectively raise patients’ risk awareness and perceived susceptibility are likely to enhance understanding of consequences and result in actions that limit high-risk behavior. Direct communication from the primary care physician, a credible information source, is fundamental to successful risk communication and increasing patient risk awareness.
Improving risk awareness remains a challenge. Isolated patient-based or provider-based interventions may produce modest change at best.25–28 The partial success of single-focus interventions has recently led to renewed interest in combined approaches that focus on both the patient and the physician.29 While the literature is replete with complex, multi-modal interventions aimed at changing patient behavior,30 there is little to guide researchers on the incremental impact of simple, direct interventions to improve patient risk awareness and safety. Although many studies have examined direct-to-patient approaches for improving medication adherence, few have focused directly on patient safety.31–37 Prior to the implementation of this research, an interpretive review of the literature related to shared decision-making and use of decision-aids suggested that such approaches were feasible, acceptable, and associated with increased knowledge of treatment decisions.38 Therefore, the objective of this research was to conduct a group-randomized trial testing whether a shared patient- and physician-based intervention contributed to increased patient-reported awareness of any prescription or OTC NSAID risk.
Methods
Design Overview.
A group randomized trial was designed to promote patient-physician NSAID risk communication. Community-based physician practices along with their patients were randomized to intervention or comparison groups. Intervention group physician practices received continuing medical education about NSAIDS and the patients within those practices were exposed to a patient-activation tool designed to promote personal risk assessment and communication during the clinical encounter. The patient activation tool used a checklist of “Yes” or “No” questions to assess risk factors for NSAID adverse events for prescription and OTC NSAID use (Appendix 1). The checklist required less than two minutes to complete. The patient activation tool also encouraged the patient to discuss NSAID risks identified on the checklist with their physician. The comparison group physician practices received only continuing medical education about NSAIDs and their patients did not use the activation tool. Study design was cross-sectional and serial, with independent groups of patients assessed at baseline and after the intervention. The study was approved by the University of Alabama at Birmingham Institutional Review Board (IRB).
Sampling and Recruitment
Physician Practice Recruitment.
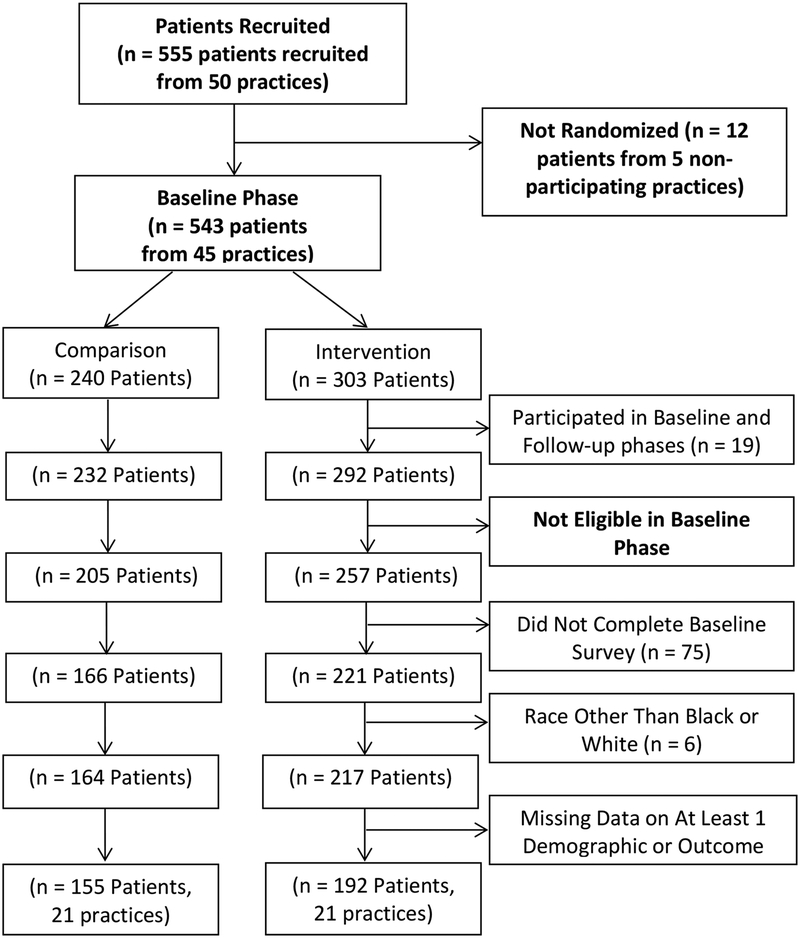
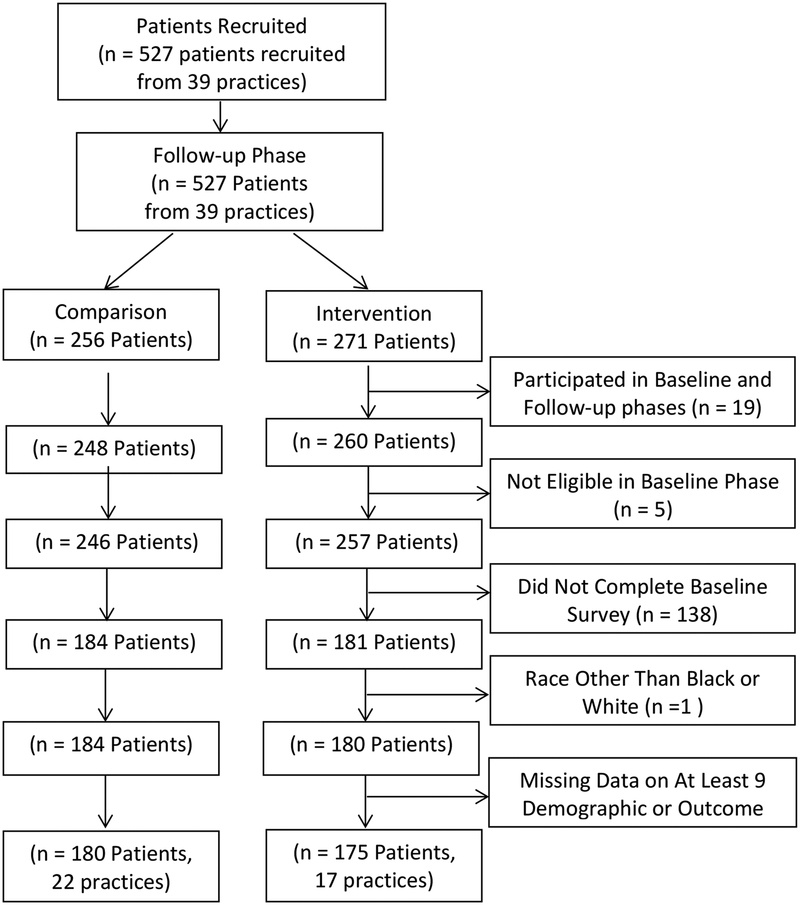
The physician recruitment pool was derived from the SK&A vendor physician list, Alabama Practice Based Network (APBRN) database, and American Medical Association (AMA) data base. The SK&A is a vendor that provides comprehensive lists and databases of healthcare professionals (http://www.skainfo-direct.com/index1.cfm). Family practitioners, general practitioners and general internal medicine physicians in private, community-based practices in Alabama were identified. Practices with 5 or more physicians were excluded to avoid large group or academic practices that may not be representative of general community practice. Recruitment involved weekly faxes, emails and letters depending on the physician’s preference for communication. If there was no preference specified, faxes were used for recruitment. Overall, 66 practices indicated initial interest in study participation. Individual practices received a personal call to confirm their interest in the project. Fifty (n=50) practices were enrolled (received study materials) for the study and recruited patients. Five practices, representing 12 potential participants, were withdrawn prior to randomization because they had few or no eligible patients and/or quit responding to communications. A total of 45 practices were randomized and 42 remained enrolled at completion of the baseline phase. For the follow-up phase, 39 practices were enrolled and remained until completion of the study.
Patient Recruitment and Interview Administration.
Patient eligibility criteria included the following: (1) an established patient (i.e., previously seen) from one of the participating primary care physician practices; (2) currently taking presciption NSAIDs; and (3) willingness to provide contact information, informed consent, and complete a 30 minute follow-up telephone interview. For the baseline phase, patients had to be at least 65 years of age. The age criterion was relaxed to 50 years of age in the follow-up phase to facilitate greater enrollment. Eligible patients who completed an office screening survey (Appendix 2) were subsequently contacted by telephone for a more in-depth interview. The survey was administered using computer assisted telephone interview (CATI) protocols. The computer software contained checks for logical consistency and out-of-range errors. Interviewers underwent formal training with certification of competency before beginning data collection. Patients completing the telephone survey were given a $20 gift card.
Figures 1a/1b provide the Consort Diagrams describing patient recruitment, enrollment, and study disposition for the baseline and follow-up phases, respectively. Data collection for the baseline phase of the study occurred from 6/23/2005 until 4/3/2006 and from 6/12/2006 until 2/20/2007 for the follow-up phase. During the baseline phase, 555 patients were recruited and 543 were assigned based on their physician practice to either the intervention (n=303) or comparison (n=240) group. Of those, 192 patients in the intervention group and 155 patients in the comparison group completed the study. Thus, 73.8% of eligible patients in the baseline phase were included in the analytical sample. In the follow-up phase, 527 patients were recruited and assigned based on their physician practice to either the patient activation intervention (n=271) or comparison (n=256) group. Of those, 175 patients in the intervention group and 180 patients in the comparison group completed the study. Thus, 70.6% of eligible patients in the follow-up phase were included in the analytical sample.
Figure Legend 1a.
Consort Diagram - Baseline
Figure Legend 1b.
Consort Diagram – Follow-up
Measurements.
All patient characteristics and endpoint measures were derived from patient self-report. Both study endpoints were ascertained at baseline and at follow-up with the following questions, “Do you know of any problems or risks connected with taking prescription [or over-the-counter] NSAIDs?” Both questions were the same with the exception of the word “prescription” or “over-the-counter”. The response set included “Yes”, “No”, “Not Sure”, and “Refused”. Participants responding in the affirmative were asked about what specific risks for which they were aware although no cafeteria-style list of risks was provided to prompt events from which to choose. For analytical purposes, only responses of “Yes” and “No” were used.
Patient characteristics collected included race, sex, age, education level, adequacy of income, and insurance status, many of which have been associated with health literacy disparities. Race was categorized as black or white given the limited representation of other racial/ethnic groups [n=7]. Sex was categorized as either female or male. Age in years was derived from date of birth and the interview date, and reported as a continuous variable. Education level was collapsed to high school or below or at least some college. A dichotomous variable indicating adequacy of income was determined using a “Yes” or “No” response to the question, “Currently, is your income enough to meet your basic needs for food, housing, clothing, and medical care?” Insurance status was collapsed into one of 2 categories described as Medicaid or uninsured as compared to some other type of insurance (e.g., Medicare, private).
As a surrogate measure assessing retention of the intervention, patients assigned to the intervention physician practice groups were asked if they remembered seeing the patient activation checklist. Those responding affirmatively were subsequently asked whether they discussed their answers from the intervention checklist with their doctor.
Analytical Approach.
Analysis began by examining distributions and univariate statistics for all variables. All patient characteristics and endpoint measures were described as proportions or means for the overall sample as well as by the intervention and comparison groups within the baseline and follow-up phases. Bivariable tests of proportions or t-tests were used to evaluate differences between the intervention and comparison groups within each study phase. Bivariable tests of proportion were also used to evaluate differences within the intervention and comparison groups, by study phase. Because this study employed a group randomized design with patients nested within physician practices, a multivariable Generalized Linear Latent and Mixed Model (GLLAMM) with logistic link was used to collectively test the relationship between the patient activation intervention, study phase, intervention by study phase interaction term, and the primary study endpoints while controlling for differences in patient characteristics in the baseline and follow-up phases. Clustering of observations within physician practices was accounted for as a random effect. For the models, the intervention variable represents the differences between intervention and comparison groups at baseline and follow-up. The study phase variable represents the differences between from baseline to follow-up. Finally, the interaction variable represents the differential over-time change for the intervention versus comparison group, isolating the effectiveness of the intervention. In addition to intervention and study phase variables, all multivariable models included race, sex, age, education, income adequacy, and insurance status as covariates to manage potential imbalances often observed in randomized group designs. Chi-square tests followed by multivariable GLLAMMs were used to perform post-hoc per-protocol analyses comparing patient-reported NSAID risk awareness between those who remembered the intervention and those who did not. All analyses were conducted using STATA 12.143 using an a-priori alpha level of 0.05.
Results
Sample Characteristics at Baseline and Follow-up
Patient characteristics by phase and intervention/comparison group are reported in Table 1. Overall during the baseline phase, the majority of the sample was female (74.35%) and reported adequate income (73.20%). The mean age and standard deviation was 74.09 ± 6.91 years. Approximately one-third of the baseline sample reported being of the black race (30.26%) and had at least some college education (33.14%). Whereas, 10.66% of the sample reported being either uninsured or enrolled in a Medicaid health insurance plan.
Table 1.
Demographic Characteristics of Patients, Randomized by Physician Practice, Participating in the NSAID1 Study At Baseline or Follow-up
| Baseline Phase | Follow-up Phase | |||||||
|---|---|---|---|---|---|---|---|---|
| Overall % n = 347 |
Comparison % n = 155 |
Intervention % n = 192 |
p | Overall % n = 355 |
Comparison % n = 180 |
Intervention % n = 175 |
p | |
| Black Race | 30.26 | 33.55 | 27.60 | 0.231 | 38.59 | 30.56 | 46.86 | 0.002 |
| Female Sex | 74.35 | 77.42 | 71.88 | 0.240 | 72.11 | 73.33 | 70.86 | 0.603 |
| Mean Age in Years (SD) | 74.09 (6.91) | 73.91 (7.37) | 74.24 (6.54) | 0.661 | 63.48 (9.67) | 63.55 (9.29) | 63.40 (10.06) | 0.877 |
| Some College | 33.14 | 28.39 | 36.98 | 0.091 | 43.66 | 47.78 | 39.43 | 0.113 |
| Adequate Income | 73.20 | 68.39 | 77.08 | 0.069 | 72.68 | 72.22 | 68.00 | 0.051 |
| Medicaid/Uninsured | 10.66 | 14.19 | 7.81 | 0.056 | 18.03 | 15.56 | 20.57 | 0.219 |
| Reported Awareness of Any Prescription NSAID1 Risk | 44.67 | 47.10 | 42.71 | 0.414 | 55.49 | 61.11 | 49.71 | 0.031 |
| Reported Awareness of Any Over-the-Counter NSAID1 Risk | 25.15 (n = 342) |
24.68 (n = 154) |
25.53 (n = 188) |
0.856 | 52.97 (n = 353) |
56.74 (n = 178) |
49.14 (n = 175) |
0.153 |
Nonsteroidal anti-inflammatory Drug (NSAID)
Overall during the follow-up phase, the majority of the sample was female (72.11%) and reported adequate income (72.68%). The mean age and standard deviation was 63.48 ± 9.67 years as a result of relaxing the age eligibility criterion to 50 years. More than one-third of the baseline sample reported being of the black race (38.59%), had at least some college education (43.66%), and 18.03% were either uninsured or enrolled in a Medicaid health insurance plan. The intervention and comparison group physician practices differed with respect to the proportion patients reporting their race as black (46.86% vs. 30.56%, p=0.002) and having an adequate income (68.00% vs. 72.22%, p=0.051).
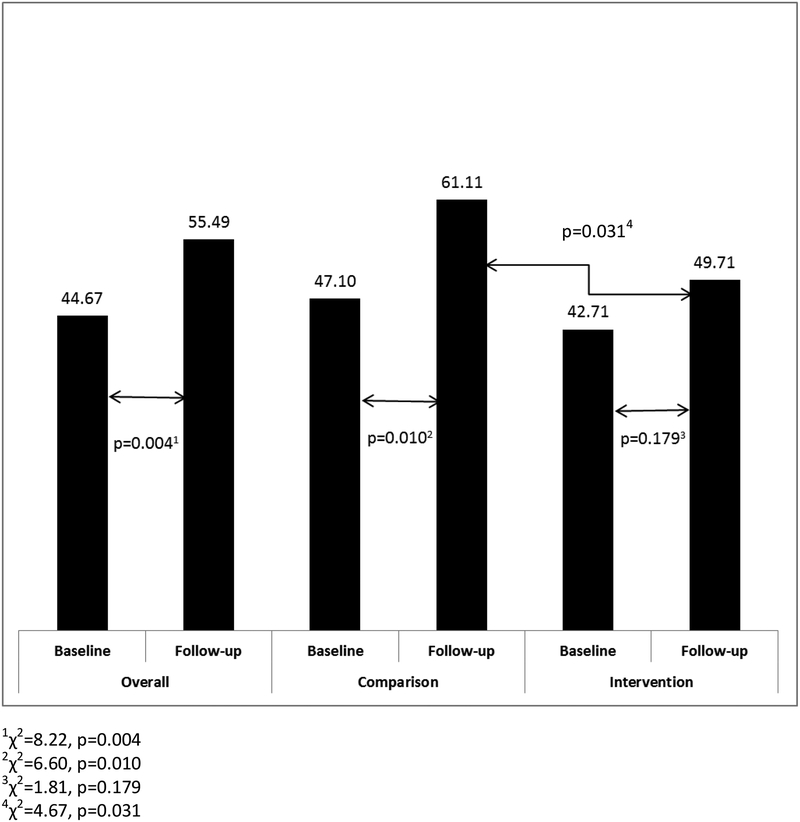
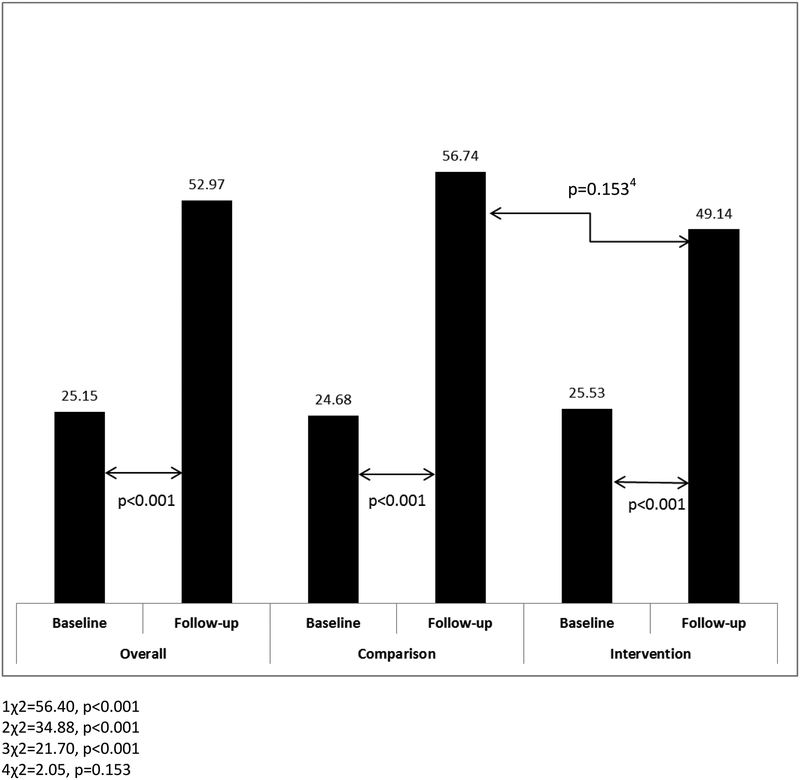
During the baseline phase, there were no significant differences between intervention and comparison physician group practices with respect to the proportion of patients reporting awareness of any prescription NSAID risk (42.71% vs. 47.10%, p=0.414) or OTC NSAID risk (25.53% vs. 24.68%, p=0.856). At follow-up, a significantly smaller proportion of patients reported awareness of any prescription NSAID risk for intervention compared to the comparison physician group practices (49.71% vs. 61.11%, p=0.031) [Figure 2]. However, there was no difference with respect to awareness of any OTC NSAID risk for the intervention and comparison groups (49.14% vs. 56.74%, p=0.153) [Figure 3].
Figure Legend 2.
Proportion of Patients Reporting Awareness of Any Prescription NSAID Risk Overall and by Study Group at Baseline and Follow-up
Figure Legend 3.
Proportion of Patients Reporting Awareness of Any Over-the-Counter NSAID Risk Overall and by Study Group at Baseline and Follow-up
Patient-Reported Awareness of Any NSAID Risk by Study Phase
There was a significant increase in the proportion of patients reporting awareness of any prescription NSAID risk from baseline to follow-up phases for the overall sample (44.67% vs. 55.49%, p=0.004) as well as in the comparison group (47.10% vs. 61.11%, p=0.010) [Figure 2]. In contrast for the intervention group, the observed increase from baseline to follow-up trended toward a difference, but was not significant (42.71% vs. 49.71%, p=0.179) [Figure 2]. With respect to the proportion of patients reporting awareness of any OTC NSAID risk from baseline to follow-up, there were significant increases for the overall sample (25.15% vs. 52.97%, p<0.001), the comparison group (24.68% vs. 56.74%, p<0.001), and the intervention group (25.53% vs. 49.14%, p<0.001) [Figure 3].
Multivariable Analyses
In multivariable analyses, the patient activation intervention (Adjusted Odds Ratio [AOR]=0.74, p=0.248), follow-up study phase (AOR=1.31, p=0.300), intervention by study phase interaction (AOR=0.98, p=0.942) were not significantly associated with patients reporting awareness of any prescription NSAID risk [Table 2]. The follow-up study phase was associated with increased odds of reporting any OTC NSAID risk awareness (AOR=2.99, p<0.001), but the patient activation intervention and intervention by study phase interaction were not significantly associated with patient-reported awareness of any OTC NSAID risk (AOR= 0.98, p=0.929; AOR=0.87, p=0.693, respectively) [Table 3]. Patients reporting black race had significantly lower odds of reporting awareness of any prescription or OTC NSAID risk (AOR=0.59, p=0.009; AOR=0.52, p=0.001, respectively) as did each year of increasing age (AOR=0.97, p=0.009; AOR=0.97, p=0.007, respectively) [Tables 2 and 3]. Women and those with at least some college education had significantly higher odds of reporting awareness of any prescription NSAID risk (AOR=1.96, p<0.001; AOR=1.70, p=0.003, respectively) [Table 2] and awareness of any OTC NSAID risk (AOR=1.47, p=0.048; AOR=1.80, p=0.001, respectively) [Table 3].
Table 2.
| Adjusted Odds Ratio | 95% Confidence Interval | p | |
|---|---|---|---|
| Intervention Group | 0.74 | 0.45 – 1.23 | 0.248 |
| Follow-up Study Phase | 1.31 | 0.79 – 2.18 | 0.300 |
| Intervention Group by Study Phase Interaction3 | 0.98 | 0.50 – 1.89 | 0.942 |
| Black Race | 0.59 | 0.40 – 0.87 | 0.009 |
| Female Sex | 1.96 | 1.36 – 2.83 | <0.001 |
| Age in Years | 0.97 | 0.95 – 0.99 | 0.004 |
| Some College | 1.70 | 1.20 – 2.40 | 0.003 |
| Adequate Income | 1.08 | 0.73 – 1.60 | 0.710 |
| Medicaid/Uninsured | 0.72 | 0.43 – 1.18 | 0.193 |
Nonsteroidal anti-inflammatory Drug (NSAID)
Overall Sample (347 patients during baseline phase and 355 patients during follow-up phase)
The Intervention Group by Study Phase Interaction isolates the impact of the intervention
Table 3.
| Adjusted Odds Ratio | 95% Confidence Interval | p | |
|---|---|---|---|
| Intervention Group | 0.98 | 0.58 – 1.64 | 0.929 |
| Follow-up Study Phase | 2.99 | 1.77 – 5.07 | <0.001 |
| Intervention Group by Study Phase Interaction2 | 0.87 | 0.44 – 1.72 | 0.693 |
| Black Race | 0.52 | 0.35 – 0.77 | 0.001 |
| Female Sex | 1.47 | 1.00 – 2.15 | 0.048 |
| Age in Years | 0.97 | 0.95 – 0.99 | 0.007 |
| Some College | 1.80 | 1.27 – 2.57 | 0.001 |
| Adequate Income | 0.86 | 0.57 – 1.31 | 0.492 |
| Medicaid/Uninsured | 0.64 | 0.38 – 1.07 | 0.088 |
Nonsteroidal anti-inflammatory Drug (NSAID)
Overall Sample (342 patients during baseline phase and 353 patients during follow-up phase)
The Intervention Group by Study Phase Interaction isolates the impact of the intervention
Retention of Intervention
During follow-up, 45/165 (27.27%) of the respondents in the intervention group reported remembering the intervention. Less than half of those who reported remembering the intervention 17/42 (40.48%) discussed their answers from the checklist with their physician. In a post-hoc per protocol analysis, the proportion of patients who reported awareness of any prescription NSAID risk and remembered the intervention was not significantly higher than the proportion of those who did not remember the intervention (53.33% vs. 47.50%; p=0.504). As well, the proportion of patients who reported awareness of any OTC NSAID risk and remembered the intervention was not significantly higher than the proportion of those who did not remember the intervention (51.11% vs. 49.17%; p=0.824). These findings did not change with multivariable analyses.
Discussion
In our group randomized trial examining a strategy to promote patient-physician communication about safer NSAID use, there was an increase in patient-reported NSAID risk awareness over time in bivariable analyses for both intervention and comparison groups (Figures 2 and 3). The proportion of patients from enrolled physician practices with self-reported awareness of any prescription or OTC NSAID risk increased between the periods (6/2005–4/2006) and (6/2006 and 2/2007). Our patient activation intervention, when combined with continuing medical education about NSAIDs, did not have a significant effect on self-reported awareness of any prescription or OTC NSAID risk when compared to continuing medical education about NSAIDs alone in multivariable analyses. There are a number of historical and methodological factors that may explain these observed findings.
Immediately prior to and during the study period, there was significant scientific, regulatory, and media attention focused on NSAID safety in response to the findings of APPROVe trial16. Results from emerging studies and coverage in popular press likely contributed to the temporal trends for increased patient reporting of awareness of NSAID risks for both groups. In a study of community-based patients, 55 years or older, with disabling hip and knee osteoarthritis in Ontario, Canada, 94.8% of patients were aware of the Vioxx® (rofecoxib) recall within 20 weeks of its issuance.44 Most patients heard about the recall through television (94.7%) or newspapers (34.9%) or a close associate such as a family member, friend neighbor or colleague (24.9%). In that study, approximately 57% of respondents (548/968) reported that of all medications available in Canada have at least some probability of a “life-threatening” side effect. In the current study, 49% to 60% participants in the follow-up phase of the current study reported awareness of any prescription or OTC NSAID risk, in line with the results of the Canadian study. Collectively, this historical threat to internal validity, along with the use of an active comparison group, may have blunted the effect of the designed patient activation intervention. It is possible that the over-time change represents an effect of both the comparison and intervention components. Unfortunately, in the absence of a third, intervention-naïve comparison group, this research is unable to draw upon the power of randomization to determine if this overall increase in NSAID risk awareness was due to the continuing medical education component that both groups received or simply due to a temporal trend. Only a minority of patients (27.27%) allocated to the intervention remembered the intervention and less than half (40.48%) who remembered it, reported using it, calling into question the viability and sustainability of such a low-intensity intervention. As noted in the Canadian study, only 3.9% of the overall sample of respondents reported hearing about the Vioxx® recall from their primary care doctor and only 1.25% heard about it from their pharmacist.39 The proportion of respondents was significantly increased to 25.3% and 10.8% for primary care doctors and pharmacists, respectively, in the subgroup of active Vioxx users. In the current study, post-hoc analysis revealed that 51.44% of patients reported that they were counseled on at least one of the following NSAID-related risks: gastrointestinal, hypertension, myocardial infarction, or renal risks. Importantly, a significantly smaller proportion of patients in the intervention group was counseled compared to the comparison group physicians (45.35% vs. 57.39, p=0.025). Despite accounting for clustering of patients within physician practices as a random effect in multivariable modeling, this finding may suggest differences in physician practices with respect to counseling at the practice level and may bias the effect of the intervention toward the null.
Promoting an active approach by patients, as the intervention in this study does, without reciprocity from providers may set up an awkward encounter between patient and provider if a patient wants to engage in dialogue and the provider does not respond. The Ask Me 3 Program (http://www.npsf.org/for-healthcare-professionals/programs/ask-me-3/), endorsed by the National Patient Safety Foundation to improve active communication between patients and healthcare providers encourages patients to ask 3 key questions at every healthcare encounter, providers to be prepared to answer those questions, and for health-systems to promote such dialogue. To be effective, patients, providers, and health-systems must work in harmony to optimize patient safety. While intended to activate patients, the intervention studied herein may have not effectively created enough synergy among all essential partners.
Patient characteristics may have also been biased towards lower risk awareness in the follow-up intervention group. There was a significantly larger proportion of African American patients and a significantly smaller proportion of patients with at least some college education in the intervention group at follow up. Minorities and those with less education may be less likely to initiate dialogue with their provider. Apparent differences in intervention and comparison group physician practices’ awareness of any prescription or OTC risk at follow-up were likely related to patient imbalances. Patient imbalance when randomization occurs at the practice level is a common problem in group-randomized trials and adjustment for these observed imbalances were incorporated in multivariable analyses. At minimum, these results highlight the need to focus on risk communication among African Americans, those without a college education, males, and the elderly.
Finally, the primary endpoint measures, self-report of awareness of any prescription or OTC NSAID risk, may overestimate true knowledge of patient knowledge. Despite the risk profile of NSAIDs being highlighted in official product labeling and well documented in the peer-reviewed literature and lay media, the results reported herein likely represent the ceiling of NSAID risk awareness, which is suboptimal. We have observed previously that approximately 9% of patients do not have objectively tested risk knowledge despite reporting risk awareness (unpublished data available on request). Future work should include more objective measures of risk knowledge. It is also possible that not all NSAID risks are of equal concern or importance to patients and physicians; and the combination of these into a common endpoint measure may have created some noise in our signal, biasing the study toward the null.
Conclusion
Our point of care intervention encouraging patient activation and shared decision-making did not have a significant effect on the proportion of patients from physicians in private, community-based practices in Alabama self-reporting awareness of any prescription or OTC NSAID risk during the study period. There is an ongoing need for more “methodological science” about the how to build simple interventions to promote patient-doctor communication.
Acknowledgements
This research was supported in part by the Agency for Healthcare Research and Quality (AHRQ) Centers for Education and Research on Therapeutics cooperative agreement (U18-HS010389).
The research contained herein was presented, in part, at the AHRQ 2009 Annual Conference and 2013 Annual Research Meeting of Academy Health in Baltimore, MD on June 23–25, 2013.
Appendix 1.
Patient Intervention Checklist
Appendix 2.
Patient Screening survey
Footnotes
Competing Interests
The authors declare that they have no competing interests.
Contributor Information
Michael J. Miller, Department of Pharmacy: Clinical and Administrative Sciences – Tulsa, College of Pharmacy, The University of Oklahoma Health Sciences Center, 918-660-3586 (Telephone), 918-660-3009 (Fax), michael-miller@ouhsc.edu.
Jeroan J. Allison, Department of Quantitative Health Sciences, Associate Vice Provost for Health Disparities Research, University of Massachusetts Medical School, 55 Lake Avenue North, Worcester MA 01655, Jeroan.Allison@umassmed.edu.
Daniel J. Cobaugh, ASHP Research and Education Foundation, 7272 Wisconsin Avenue, Bethesda, MD 20814, DCobaugh@ashp.org.
Midge N. Ray, Department of Health Services Administration, School of Health Professions, University of Alabama at Birmingham, 1705 University Blvd, Birmingham, Alabama 35294-1212, midgeray@uab.edu.
Kenneth G. Saag, Director, Center for Education and Research on Therapeutics (CERTs) of Musculoskeletal, Disorders and Center for Outcomes Effectiveness Research and Education (COERE), Division of Clinical Immunology and Rheumatology, University of Alabama at Birmingham, ksaag@uab.edu.
References
- 1.Baum C, Kennedy DL, Forbes MB. (1985) Utilization of nonsteroidal antiinflammatory drugs. Arthritis & Rheumatism, 28(6), 686–692. [DOI] [PubMed] [Google Scholar]
- 2.Cherry DK, Hing E, Woodwell DA, Rechtsteiner EA. (2008) National Ambulatory Medical Care Survey: 2006 summary. National Health Statistics Reports, 3, 1–39. [PubMed] [Google Scholar]
- 3.National Center for Health Statistics. (2004) Health, United States, 2004 With Chartbook on Trends in the Health of Americans. Hyattsville, MD. [PubMed] [Google Scholar]
- 4.Zhou Y, Boudreau DM, Freedman AN. (2014) Trends in the use of aspirin and nonsteroidal anti-inflammatory drugs in the general U.S. population. Pharmacoepidemiology and Drug Safety, 23(1), 43–50. [DOI] [PubMed] [Google Scholar]
- 5.Saag KG, Cowdery JS. (1994) Nonsteroidal, anti-inflammatory drugs: balancing benefits and risks. Spine, 19, 1530–1534. [DOI] [PubMed] [Google Scholar]
- 6.Singh G (1998) Recent considerations in nonsteroidal anti-inflammatory drug gastropathy. The American Journal of Medicine, 105(1B), 31S–38S. [DOI] [PubMed] [Google Scholar]
- 7.Singh G, Triadafilopoulos G. (1999) Epidemiology of NSAID induced gastrointestinal complications. The Journal of Rheumatology Supplement, 26(Suppl 56), 18–24. [PubMed] [Google Scholar]
- 8.Straus WL, Ofman JJ. (2001) Gastrointestinal toxicity associated with nonsteroidal anti-inflammatory drugs. Epidemiologic and economic issues. Gastroenterology Clinics of North America, 30(4), 895–920. [DOI] [PubMed] [Google Scholar]
- 9.Delzell E, Shapiro S. (1996) Commentary on the National Kidney Foundation position paper on analgesics and the kidney. American Journal Kidney Diseases, 28(5), 783–785. [DOI] [PubMed] [Google Scholar]
- 10.Delzell E, Shapiro S. (1998) A review of epidemiologic studies of nonnarcotic analgesics and chronic renal disease. Medicine, 77(2), 102–121. [DOI] [PubMed] [Google Scholar]
- 11.Bach PH, Berndt WO, Delzell E, et al. (1998) A safety assessment of fixed combinations of acetaminophen and acetylsalicylic acid, coformulated with caffeine. Renal Failure, 20(6), 749–762. [DOI] [PubMed] [Google Scholar]
- 12.Feinstein AR, Heinemann LAJ, Curhan GC, et al. (2000) Relationship between nonphenacetin combined analgesics and nephropathy: a review. Ad Hoc Committee of the International Study Group on Analgesics and Nephropathy. Kidney International, 58(6), 2259–2264. [DOI] [PubMed] [Google Scholar]
- 13.Bleumink GS, Feenstra J, Sturkenboom MC, Stricker BH. (2003) Nonsteroidal anti-inflammatory drugs and heart failure. Drugs, 63(6), 525–534. [DOI] [PubMed] [Google Scholar]
- 14.Garcia Rodriguez LA, Hernandez-Diaz S. (2003) Nonsteroidal antiinflammatory drugs as a trigger of clinical heart failure. Epidemiology, 14(2), 240–246. [DOI] [PubMed] [Google Scholar]
- 15.Weaver A, Alderman M, Sperling R. (2003) Blood pressure control and rates of edema following the administration of the cyclooxygenase-2 specific inhibitors celecoxib versus rofecoxib in patients with systemic hypertension and osteoarthritis. The American Journal of Cardiology, 91(10), 1291–1292. [DOI] [PubMed] [Google Scholar]
- 16.Bresalier RS, Sandler RS, Quan H, et al. (2005) Cardiovascular events associated with rofecoxib in a colorectal adenoma chemoprevention trial. New England Journal of Medicine, 352(11), 1092–102. [DOI] [PubMed] [Google Scholar]
- 17.Pincus T, Swearingen C, Cummins P, Callahan LF. (2000) Preference for nonsteroidal antiinflammatory drugs versus acetaminophen and concomitant use of both types of drugs in patients with osteoarthritis. The Journal of Rheumatology, 27(4), 1020–1027. [PubMed] [Google Scholar]
- 18.Johnson RE, Ried LD. (1996) OTC drug use in an HMO: comparing the elderly and younger adults. Journal of Aging and Health, 8(1), 114–135. [DOI] [PubMed] [Google Scholar]
- 19.Scheiman JM, Fendrick AM. (2002) NSAIDs without a prescription: over-the-counter access, under-counted risks. The American Journal of Gastroenterology, 97(9), 2159–2161. [DOI] [PubMed] [Google Scholar]
- 20.Thomas J, Straus WL, Bloom BS. (2002) Over-the-counter nonsteroidal anti-inflammatory drugs and risk of gastrointestinal symptoms. The American Journal of Gastroenterology, 97(9), 2215–2219. [DOI] [PubMed] [Google Scholar]
- 21.Piper JM, Ray WA, Daugherty JR, Griffin MR. (1991) Corticosteroid use and peptic ulcer disease: role of nonsteroidal anti-inflammatory drugs. Annals of Internal Medicine, 114(9), 735–740. [DOI] [PubMed] [Google Scholar]
- 22.Shorr RI, Ray WA, Daugherty JR, Griffin MR. (1993) Concurrent use of nonsteroidal anti-inflammatory drugs and oral anticoagulants places elderly persons at high risk for hemorrhagic peptic ulcer disease. Archives of Internal Medicine, 153(14), 1665–1670. [PubMed] [Google Scholar]
- 23.Wilcox CM, Shalek KA, Cotsonis G. (1994) Striking prevalence of over-the-counter nonsteroidal anti-inflammatory drug use in patients with upper gastrointestinal hemorrhage. Archives of Internal Medicine, 154(1), 42–46. [PubMed] [Google Scholar]
- 24.Becker MH, Maiman LA. (1975) Sociobehavioral determinants of compliance with health and medical care recommendations. Medical Care, 13(1), 10–24. [DOI] [PubMed] [Google Scholar]
- 25.NHS Centre for Reviews and Dissemination. (1999) Getting Evidence into Practice. Effective Health Care Bulletin, 5(1), 1–16. [Google Scholar]
- 26.Kok G, van den Borne B, Mullen PD. (1997) Effectiveness of health education and health promotion: meta-analyses of effect studies and determinants of effectiveness. Patient Education and Counseling, 30(1), 19–27. [DOI] [PubMed] [Google Scholar]
- 27.Thomson O’Brien MA, Oxman AD, Davis DA, Haynes RB Freemantle N, Harvey EL. (2003) Educational outreach visits: effects on professional practice and health care outcomes (Cochrane Review). The Cochrane Library, Issue 2. [DOI] [PubMed] [Google Scholar]
- 28.Thomson O’Brien MA, Oxman AD, Davis DA, Haynes RB, Freemantle N, Harvey EL. (2003) Audit and feedback: effects on professional practice and health care outcomes (Cochrane Review). The Cochrane Library, Issue 2. [DOI] [PubMed] [Google Scholar]
- 29.Stroebel R, Scheitel SM, Fitz JS, Herman RA, Naessens JM, Scott CG, Zill DA, Muller L. (2002) A randomized trial of three diabetes registry implementation strategies in a community internal medicine practice. The Joint Commission Journal on Quality Improvement, 28(8), 441–450. [DOI] [PubMed] [Google Scholar]
- 30.Greenfield S, Kaplan SH, Ware JE, Yano EM, Frank HJ. (1988) Patients’ participation in medical care: effects on blood sugar control and quality of life in diabetes. Journal of General Internal Medicine, 3(5), 448–457. [DOI] [PubMed] [Google Scholar]
- 31.Weingarten SR, Henning JM, Badamgarav E, Knight K, Hasselblad V, Gano A Jr, Ofman JJ. (2002) Interventions used in disease management programmes for patients with chronic illness-which ones work? Meta-analysis of published reports. British Medical Journal, 325(7370), 925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guthrie RM. (2001) The effects of postal and telephone reminders on compliance with pravastatin therapy in a national registry: results of the first myocardial infarction risk reduction program. Clinical Therapeutics, 23(6), 970–980. [DOI] [PubMed] [Google Scholar]
- 33.Noel F, Berringer R, Patel U. (2002) Effect of early telephone and postal reminders on medication compliance with pravastatin therapy. Clinical Therapeutics, 24(1), 205–208. [DOI] [PubMed] [Google Scholar]
- 34.Simkins CV, Wenzloff NJ. (1986) Evaluation of a computerized reminder system in the enhancement of patient medication refill compliance. Drug Intelligence & Clinical Pharmacy, 20(10), 799–802. [DOI] [PubMed] [Google Scholar]
- 35.Ascione FJ, Brown GH, Kirking DM. (1985) Evaluation of a medication refill reminder system for a community pharmacy. Patient Education and Counseling, 7(2), 157–165. [DOI] [PubMed] [Google Scholar]
- 36.Feder G, Griffiths C, Eldridge S, Spence M. (1999) Effect of postal prompts to patients and general practitioners on the quality of primary care after a coronary event (POST): randomised controlled trial. British Medical Journal, 318(7197), 1522–1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Edwards R, Murphy P. (1999) Are postal prompts really ineffective? British Medical Journal, 319(7211), 711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Molenaar S Sprangers MA Postma-Schuit FC Rutgers EJ Noorlander J Hendriks J de Haes HC (2000) Interpretive review: feasibility and effects of decision aids. Medical Decision Making, 20(1), 112–27. [DOI] [PubMed] [Google Scholar]
- 39.Williams MV, Parker RM, Baker DW, Parikh NS, Pitkin K, Coates WC, Nurss JR. (1995) Inadequate functional health literacy among patients at two public hospitals. JAMA, 274(21), 1677–82. [PubMed] [Google Scholar]
- 40.Gazmararian JA, Baker DW, Williams MV, Parker RM, Scott TL, Green DC, Fehrenbach SN, Ren J, Koplan JP. (1999) Health literacy among Medicare enrollees in a managed care organization. 1999, 281(6), 545–51. [DOI] [PubMed] [Google Scholar]
- 41.Miller MJ, Degenholtz HB, Gazmararian JA, Lin CJ, Ricci EM, Sereika SM. (2007) Identifying elderly at greatest risk of inadequate health literacy: a predictive model for population-health decision makers. Research in Social and Administrative Pharmacy, 3(1), 70–85. [DOI] [PubMed] [Google Scholar]
- 42.Hanchate AD, Ash AS, Gazmararian JA, Wolf MS, Paasche-Orlow MK. (2008) The Demographic Assessment for Health Literacy (DAHL): a new tool for estimating associations between health literacy and outcomes in national surveys. Journal of General Internal Medicine, 23(10), 1561–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stata. (2013) Stata Statistical Software: Release 12.1. College Station, TX, StataCorp LP. [Google Scholar]
- 44.Hawker GA, Katz JN, Solomon DH. (2006) The patient’s perspective on the recall of Vioxx. The Journal of Rheumatology. 33, 1082–1088. [PubMed] [Google Scholar]