Abstract
Aging, vascular function, and exercise are thought to have a common link in oxidative stress. Both antioxidant supplementation and exercise training have been identified as interventions that may reduce oxidative stress, but their interaction in older humans is not well understood.
Keywords: oxidative stress, vascular function, shear stress, blood flow, flow-mediated dilation
INTRODUCTION
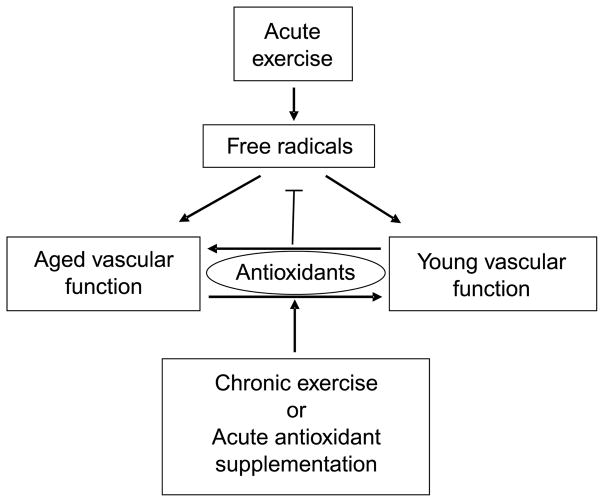
A reduction in nitric oxide (NO) bioavailability, perhaps secondary to free radical generation, has been implicated in the predominantly endothelium-mediated attenuation in vasodilatory capacity with age (32). The role of free radicals in this process is supported by the observation that plasma ascorbic acid (vitamin C), a powerful antioxidant (AO), administered by intra-arterial or venous infusion, transiently restores resting blood flow and endothelial function in aged subjects (14,16,33). Presumably by similar action, exercise training has been associated with a reduction in oxidative stress and improved vascular function (10). However, relatively little currently is known about the direct interaction between free radicals, AO, exercise, and vascular function in the elderly population (Fig. 1).
Figure 1.
A simplified schematic illustration of the complex and intriguing links between exercise, pro-oxidants and antioxidants, and vascular function with age. Specifically, our data support the concept that acute exercise increases the concentration of free radicals, which in turn, at least in terms of vascular function, seem to play a role in determining young or old vascular phenotypes. In contrast, chronic exercise or acute antioxidant supplementation, albeit by quite different pathways, lead to an increase in intravascular antioxidant capacity. As expected, this increased level of antioxidants inhibit free radical formation; however, the consequence of this elevated antioxidant capacity seems to have age-specific effects, with old vascular function being improved and young vascular function being attenuated. Our data suggest that chronic exercise training also can facilitate this improved vascular function in the elderly, but paradoxically, an acute increase in antioxidant capacity in this exercise trained state can have a negative impact, reversing the improved vascular function in the old.
Although both regular physical activity and AO supplementation have been identified as potential noninvasive means of controlling oxidative stress with advancing age, evidence for the efficacy of these interventions for disease prevention remains somewhat equivocal, apparently being highly dependent on intensity and dose (17). Central to the exercise training-induced improvement in vascular health is the regular increase in shear stress (at least at the conduit level), which raises production of endothelial-derived NO synthase and the subsequent release of NO, a potent vaso-dilator that is linked tightly to blood pressure control and endothelial health (40). Like exercise training, oral AO supplements are thought to evoke positive vascular effects, and some evidence exists for the ability of a pharmacological dose of vitamin C (ascorbate) to reduce vascular tone (16) and improve NO bioavailability (12). Although this reduction in free radical concentration seems beneficial for vascular health, it is noteworthy that the products of free radical generation, such as hydrogen peroxide (H2O2) and other free radicals, may act as both potent vasoconstrictors and vasodilators (22) and even increase NO bioavailability (34). As such, any AO intervention which reduces free radical levels will concomitantly protect NO levels but reduce vasodilation initiated by other free radicals, the balance of which will ultimately determine the impact of the intervention on systemic vascular tone and arterial blood pressure.
Despite the potentially negative impact of these age-associated changes, the practical consequence of reduced skeletal muscle blood flow in the elderly remains poorly understood. The paucity of information concerning effects of reduced perfusion on muscle function with healthy aging may be due in part to the difficulty of simultaneously assessing perfusion and metabolism within skeletal muscle. Using multiparametric nuclear magnetic resonance (NMR) techniques, we have begun to address this issue and have recently documented that the age-related decline in bulk limb blood flow also is evident when perfusion within the skeletal muscle is examined during and after lower limb exercise (5,13). However, in this study of a healthy, elderly cohort, muscle oxidative capacity, as assessed by phosphocreatine recovery, was not diminished, suggesting some maintenance of skeletal muscle functional capacity in a relatively hypoperfused state. This finding seems to support the concept that the elderly undergo beneficial adaptations within the skeletal muscle to overcome the age-related decline in perfusion and also raises the question of what effect an intervention capable of restoring perfusion toward that of the young might have on muscle function.
Therefore, with the recognition that exercise and age-related vascular dysfunction likely are associated with oxidative stress and armed with an ultrasound Doppler, an enteral AO cocktail (vitamins C and E and alpha lipoic acid) of known efficacy (25,38) (Fig. 2), and a novel NMR-based approach to simultaneously study muscle perfusion and metabolism (36), our group has set out to better elucidate these interactions (Fig. 1). It should be noted that this research, although having potentially clinically significant implications, was not designed as such. Rather, the goal of this work was to better understand the mechanistic links between exercise, age-related vascular dysfunction, and oxidative stress. For this reason, and the fact that this research was our group’s initial foray into this complex but intriguing area, the AO intervention used in these experiments was always acute in nature and therefore should not be interpreted to describe the pros/cons of chronic AO supplementation. This article reviews the findings of this work and puts it in context with the current literature.
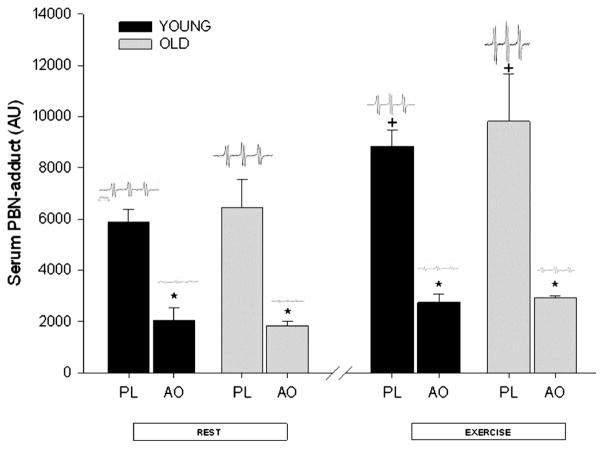
Figure 2.
The α-phenyl-tert-butylnitrone (PBN) spin adducts assessed by electron paramagnetic resonance (EPR) spectroscopy under the conditions of rest and after exercise with placebo (PL) and the acute effects of the oral antioxidant cocktail (AO) in young and old subjects (n = 12). Inlayed, in the upper panel are representative individual examples of PBN EPR spectra in each scenario. +Significantly different from resting levels; *Significantly different levels during AO trial than matched PL condition. [Adapted from Donato AJ, Uberoi A, Bailey DM, Wray DW, Richardson RS. Exercise-induced brachial artery vasodilation: effects of antioxidants and exercise training in elderly men. Am. J. Physiol. Heart Circ. Physiol. 2010; 298(2):H671–8. Copyright © 2010 The American Physiological Society. Used with permission.]
AGE, OXIDATIVE STRESS, EXERCISE, AND VASCULAR FUNCTION
Age-Related Decline in Arterial Vasodilation
One of the predominant vascular phenotypes with advancing sedentary aging is impaired endothelial function in conduit and small resistance arteries. The majority of data on aging and endothelial dysfunction support the theory that an elevated production of reactive oxygen species or other free radicals inactivate NO, thus attenuating arterial vasodilation (14,33). To study this phenomenon, we have implemented an acute handgrip exercise model, initially documented by Shoemaker et al. (31). This approach provides the opportunity to study conduit arterial function that is likely in a state of heightened oxidative stress as a consequence of elevated skeletal muscle metabolic activity and free radical outflow to interstitial fluid and vascular plasma (2,9).
Our research, using this forearm model, has revealed a severe age-related impairment in brachial artery vasodilation during rhythmic handgrip exercise (Fig. 3). Specifically, the highest workload produced approximately 7% brachial artery vasodilation in young subjects and approximately 3% brachial artery vasodilation in older subjects. To our knowledge, these data are the first to demonstrate that brachial artery vasodilation is markedly compromised with advanced age during submaximal exercise, findings that are comparable to data produced by our laboratory (23,39) and others (6,14) that have documented similar age-related reductions in brachial artery flow-mediated vasodilation (FMD). Although endothelial dysfunction may not be isolated in this more complex exercise model, with similar age-related vasodilator deficiencies, it is tempting to speculate that these findings using the exercise model may represent a similar endothelial dysfunction observed in other FMD studies of aging.
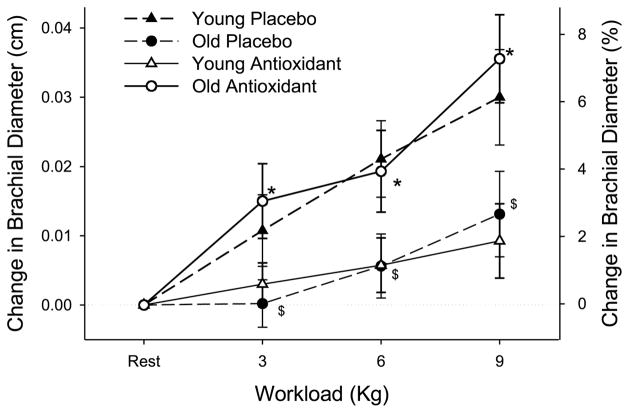
Figure 3.
The change in brachial artery diameter (upper panel) in young and old subjects at rest and at three levels of handgrip exercise either after the acute ingestion of the antioxidant cocktail (AO) or placebo. Values for percent change in brachial diameter are not exact and are displayed solely for reference purposes. $Diameter changes in the old subjects placebo significantly decreased from the young placebo trial. *Diameter changes during AO trial significantly increased from placebo in old subjects. [Adapted from Donato AJ, Uberoi A, Bailey DM, Wray DW, Richardson RS. Exercise-induced brachial artery vasodilation: effects of antioxidants and exercise training in elderly men. Am. J. Physiol. Heart Circ. Physiol. 2010; 298(2):H671–8. Copyright © 2010 The American Physiological Society. Used with permission.]
AO and Arterial Vasodilation in the Old
Chronic oral AO therapy has proven largely ineffective in reducing mortality associated with cardiovascular disease. Conversely, the acute supraphysiological infusion of vitamin C in aged subjects seems to transiently restore endothelial-dependent vasodilation and blood flow, suggesting that elevations in free radicals have a deleterious effect on endothelial function, most likely through a reduction in NO bioavailability (14,16,33). Again, using the forearm exercise model, our data demonstrate that, in a likely pro-oxidant state (i.e., exercise-induced elevation in circulating free radicals, Fig. 2), the acute ingestion of an oral AO effectively ameliorates the age-related reduction in brachial artery vasodilation whether expressed as a percentage change in vessel diameter (Fig. 3) or in absolute terms with initial age-related differences in baseline diameters (11). These data are in contrast to the findings of Eskurza et al. (14) who failed to see any improvement in brachial artery FMD of resting older subjects with chronic oral vitamin C supplementation. These disparate results may be explained by several important differences between these studies. First, our study used an acutely ingested AO cocktail composed of several potent AOs (vitamins C and E and α-lipoic acid), which was directly validated using EPR spectroscopy to reduce blood free radical concentrations (Fig. 2), rather than a vitamin C supplement alone. Second, our experimental design used acute small muscle mass exercise (handgrip), which, in addition to locally generating free radicals that may or may not recirculate in a great enough concentration to play a role, produced greater blood flow and therefore more shear stress-mediated oxidants in the vessel being studied than a FMD protocol (3). Thus, the potential role for AOs could be greater in this type of experimental paradigm than studies using FMD. Taken together, these data suggest that oral acute AO supplementation exerts a beneficial effect on exercise-induced arterial vasodilation in older subjects, in part because of the use of an experimental paradigm that exposes the vessel of interest to sustained elevations in shear stress. Additionally, it should be noted that, in these studies, there was no impact of the acute AO intervention on either shear rate or muscle blood flow. This lack of an effect on blood flow is in contrast to other studies that have improved muscle blood flow in the elderly during handgrip exercise by the intra-arterial infusion of ascorbic acid (8,19). This discrepancy in results could again be a consequence of experimental differences (e.g., the achievement of greater intravascular ascorbic acid levels by infusion rather than oral AO supplementation).
Interaction Between Exercise Training, AO, and Arterial Vasodilation
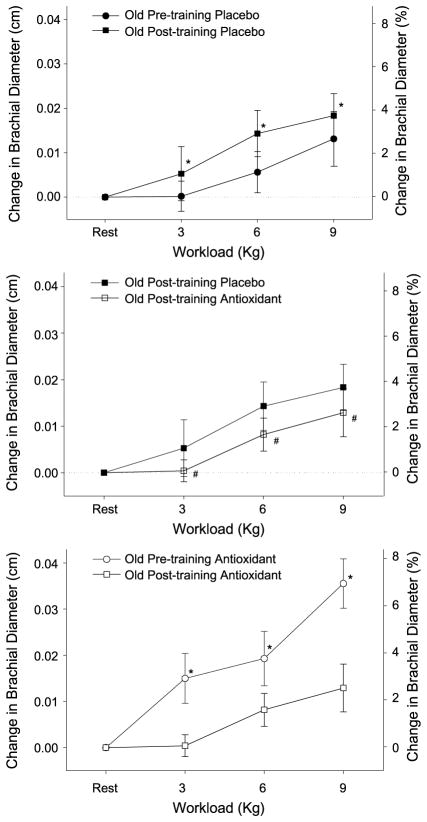
Exercise training has been documented to increase endogenous AOs in aged animals (18), and therefore it is possible that the acute administration of supplemental AOs in the trained state would have little or no effect. Our recent data not only support this contention but, in fact, reveal a negative effect of acute AO administration on brachial artery vasodilation in the trained state (Fig. 4, middle panel). Indeed, there was a very different response to the acutely ingested AO cocktail in the trained and untrained state (Fig. 4, lower panel), where the positive consequences of the AOs when untrained starkly contrast with the effects after exercise training. This is in agreement with our recent observation that in an untrained state, acute AO administration had little effect on endothelial function as measured by FMD and blood pressure, yet after exercise training, both were negatively affected by an acute AO-induced reduction in free radicals (Fig. 5) (38).
Figure 4.
The effect of 6 wk of single leg knee-extensor exercise training on the change in brachial artery diameter (upper panel), the acute impact of the antioxidant cocktail (AO) on this response after training (middle panel), and the acute effect of the oral antioxidant cocktail in the exercise trained and untrained state in old subjects at rest and at three levels of handgrip exercise (lower panel). Values for percent change in brachial diameter are not exact and are displayed solely for reference purposes. It should be noted that baseline resting diameters did not differ as a consequence of exercise training. *Diameter changes during post-training trial significantly increased from pretraining. #Diameter changes during posttraining trial with the AO significantly decreased from pre-training placebo trial. [Adapted from Donato AJ, Uberoi A, Bailey DM, Wray DW, Richardson RS. Exercise-induced brachial artery vasodilation: effects of antioxidants and exercise training in elderly men. Am. J. Physiol. Heart Circ. Physiol. 2010; 298(2):H671–8. Copyright © 2010 The American Physiological Society. Used with permission.]
Figure 5.
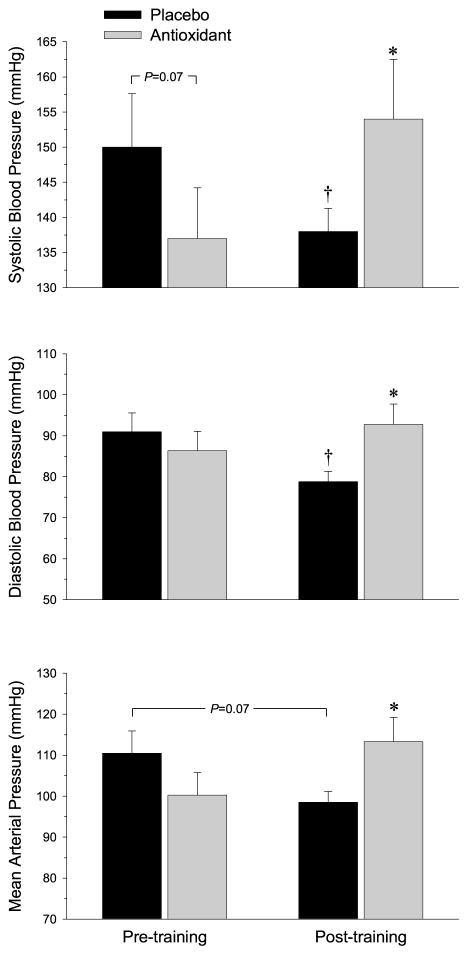
The acute effect of antioxidant administration on resting arterial blood pressure. Changes in resting arterial blood pressure after acute consumption of placebo (black bars) and the oral antioxidant (AO) cocktail (gray bars) before and after exercise training and acute oral AO supplementation. *Significantly different than placebo, P < 0.05; †Significantly different than pretraining, P < 0.05. (Reprinted from Wray DW, Uberoi A, Lawrenson L, Bailey DM, Richardson RS. Oral antioxidants and cardiovascular health in the exercise-trained and untrained elderly: a radically different outcome. Clin. Sci. (Lond.) 2009; 116(5): 433–41. Copyright © 2009 Portland Press Ltd. Used with permission.)
Our investigations do not address the mechanisms responsible for the deleterious combined effect of exercise training and acute AO administration on exercise-induced brachial vasodilation. However, we speculate that this is likely the result of an acute disturbance in the balance between pro- and AO forces. Oxidative stress is typically regarded as an unwanted byproduct of cellular oxidation and is seen as a negative risk factor for cellular and vascular health (4). However, as previously mentioned, the downstream consequences of free radicals, such as H2O2 and other free radicals, may act as both potent vasoconstrictors and vasodilators (22) and, as such, possess the capacity to alter vascular responsiveness. Thus, it is tempting to suggest that while exercise training alone evokes an appropriate adaptation to the increase in oxidative stress, the even greater reduction in free radical concentration after acute AO administration may have removed oxidative species that possesses some beneficial vaso-active properties. Thus, this possible vasodilatory role of free radicals (25) may explain the loss of exercise-induced brachial artery vasodilation in the posttraining acute AO condition (Fig. 4, lower panel). These somewhat paradoxical findings regarding the interaction between exercise training responses and AO, albeit acute AO supplementation, do not stand in complete isolation. Indeed, there is growing evidence that exercise training, so far only in young humans, in combination with chronic AO supplementation severely attenuates exercise-induced adaptations (28). These emerging findings reinforce the concept that oxidative stress seems to be an important, if not essential, signaling process in much the same way as we have documented in terms of vascular function in young healthy people (25).
AGE, AO, AND EXERCISE
Small Muscle Mass Exercise Training, Vascular Function, and Arterial Blood Pressure
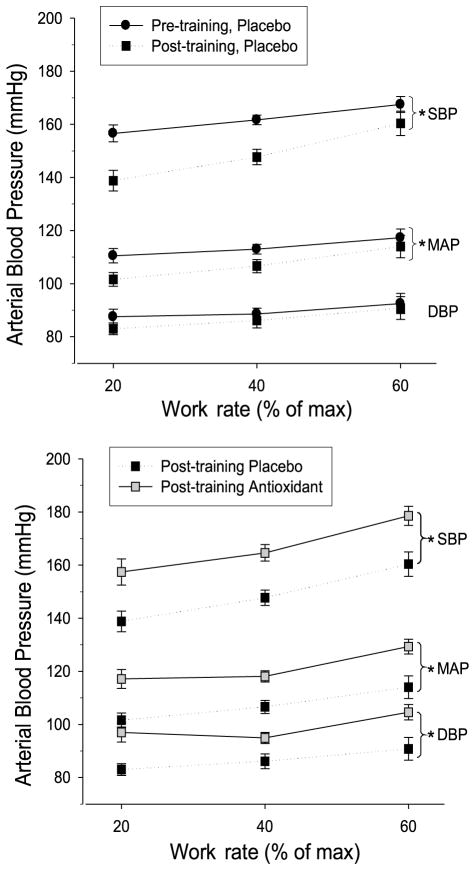
Cardiovascular adaptations to habitual, whole-body exercise training have been well described and include a significant reduction in resting and exercising arterial blood pressure in both young and old subjects (24). However, few studies have evaluated adaptations to exercise training of a small muscle mass, which allows training of muscle groups in the leg without central cardiovascular limitations (1,27). By using the single leg knee-extensor exercise training paradigm, we have demonstrated an increase in vascular function (Fig. 4, upper panel) as well as a clinically significant reduction in both resting and exercising arterial blood pressure after 6 wk of small muscle mass training (Figs. 5 and 6, upper panel). In terms of arterial blood pressure, these findings not only emphasize the importance of peripheral vascular adaptations as a result of habitual exercise but also reveal that even a relatively low-stress, small muscle mass exercise regime can provide a significant improvement in mildly hypertensive individuals. Clinically, these findings are of importance in terms of exercise adherence for the general population, which classically is poor when whole-body, high stress exercise is prescribed. Additionally, these observed cardiovascular benefits of a small muscle mass exercise modality may be of great benefit to patients with central cardiopulmonary limitations, such as chronic obstructive pulmonary disease and congestive heart failure, where whole-body exercise prescription is limited by both disease symptoms and patient compliance (26,30).
Figure 6.
The effect of exercise training and the combined impact of acute antioxidant administration and exercise training on arterial blood pressure. Arterial blood pressure during three intensities of acute knee-extensor exercise is shown before (black circles) and after (black squares) 6-wk of knee-extensor exercise training. SBP, systolic blood pressure; MAP, mean arterial pressure; DBP, diastolic blood pressure. *Significantly different from pretraining or placebo, P < 0.05. [Adapted from Wray DW, Uberoi A, Lawrenson L, Bailey DM, Richarson RS. Oral antioxidants and cardiovascular health in the exercise-trained and untrained elderly: a radically different outcome. Clin. Sci. (Lond.) 2009; 116(5):433–41. Copyright © 2009 Portland Press Ltd. Used with permission.]
Oxidative Stress, Arterial Blood Pressure, and Endothelial Function
Little consensus exists in the literature with regard to the efficacy of oral AOs on arterial blood pressure in humans. Recent work from our group (25) has verified the efficacy of an acutely ingested oral AO cocktail to increase plasma total AO capacity and reduce plasma alkoxy free radical concentration in young subjects by documenting a reduction in the electron paramagnetic resonance (EPR) signal intensity of alpha-phenyl-tert-butylnitrone adducts. Our most recent EPR data extend these observations to an elderly cohort (38), further verifying the ability of this acutely ingested AO cocktail to reduce oxidative stress both at rest and at the end of maximal exercise in older individuals (Fig. 2). To our knowledge, this was the first study in humans to evaluate changes in arterial blood pressure and endothelial function with an acute pharmacological AO dose directly proven to reduce plasma free radical content. Despite the marked reduction in oxidative stress, we observed only a tendency toward a reduction in resting arterial blood pressure (Fig. 5) and no effect during acute exercise after acute oral AO administration before exercise training.
Age, Exercise Training, and Oxidative Stress — Tipping the Balance
Healthy aging is associated with an increase in free radical production (33) and progressively increasing arterial blood pressure (7). Others have identified exercise training and AO supplementation as efficacious, noninvasive interventions to improve vascular health in older subjects (17,20). Our findings of a tendency for the acutely ingested AO cocktail to reduce mean arterial blood pressure at rest (Fig. 5) and the observed reduction in arterial blood pressure (Fig. 6, upper panel) and improved exercising FMD (39) after exercise training support these studies. However, contrary to our expectations, the combination of exercise training and acute oral AO administration did not interact to produce an additive beneficial outcome but, in fact, reversed the training-induced improvements in both resting and exercising arterial blood pressure (Figs. 5 and 6, lower panel) and endothelial function assessed by FMD (38).
By design, these studies focused on the clinical outcome of the interventions and, as such, do not address the mechanisms responsible for the deleterious combined effect of exercise training and acute AO administration on arterial blood pressure and endothelial function. Oxidative stress is typically regarded as an unwanted byproduct of cellular oxidation and is seen as a negative risk factor for cellular and vascular health (4). However, as already noted, the downstream consequences of free radicals, such as H2O2 and other reactive oxygen species, may act as both potent vasoconstrictors and vasodilators (22), dependent on the paradigm, and as such, possess the capacity to affect vascular tone. Therefore, multiple interventions (exercise training and acute AO supplementation) that elevate AO levels may ultimately tip the pro- and AO balance, resulting in detrimental changes in arterial blood pressure regulation and endothelial function.
AGE, AO, EXERCISE, AND MUSCLE PERFUSION AND ENERGETICS
Novel NMR-Based Approach to the Study of Muscle Function
We have used a unique interleaved pulse sequence to acquire perfusion and 31P data every 3 s, resulting in a highly resolved set of data collected in vivo (5,13). Measurements of perfusion within the skeletal muscle tissue are made using the arterial spin labeling (ASL) method (Fig. 7A). Skeletal muscle energetics are assessed through 31P NMR spectroscopy (Fig. 7B inlay), and from these spectra, phosphocreatine (PCr) depletion and recovery kinetics are evaluated (Fig. 7B and 7D) as an index of muscle oxidative demand and capacity, respectively. Both these measurements are unique in that they allow probing of perfusion and metabolism in a noninvasive manner with very high spatial and temporal resolution and do so in a focused region of interest within the exercising muscle tissue. The simultaneous acquisition of ASL and PCr provides the truly unique opportunity to examine the real-time interplay between perfusion and metabolism in the active skeletal muscle, providing new information concerning the “matching” of these parameters in the human leg. We have recently used this NMR-based approach to examine age-related changes in perfusion and metabolism during lower leg exercise (36), which was then extended to interrogate the role of oxidative stress using a similar experimental paradigm (37).
Figure 7.
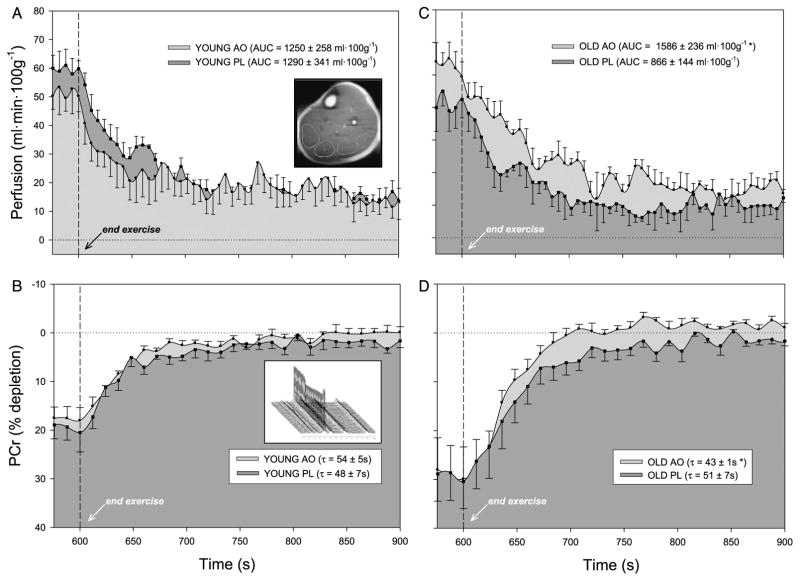
Lower leg perfusion kinetics in young (A) and old (C) subjects and phosphocreatine (PCr) resynthesis in young (B) and old (D) subjects at the end of 5-min plantar flexion exercise after consumption of either antioxidant (AO) cocktail (light gray shade) or placebo (PL; dark gray shade). AO consumption did not significantly alter perfusion or metabolism in the young subjects, whereas AO consumption significantly improved both perfusion and metabolism in the old. Dashed line indicates the cessation of exercise. Values are mean ± SE. AUC, area under the curve; τ, time constant. Inlays: Example 1H image of the lower leg with four regions of interest (ROI) which encompass volumes of both the soleus and gastrocnemius (A) and a stack plot of 31P spectra revealing changes in the phosphocreatine (PCr) peak (B) during plantar flexion exercise. [Adapted from Wray DW, Nishiyama SK, Monnet A, et al. Antioxidants and aging: NMR-based evidence of improved skeletal muscle perfusion and energetics. Am. J. Physiol. Heart Circ. Physiol. 2009; 297(5):H1870–5. Copyright © 2009 The American Physiological Society. Used with permission.]
Oxidative Stress and Skeletal Muscle Perfusion
The use of ASL has provided striking new evidence of the capacity of an acute AO intervention to improve skeletal muscle perfusion in the elderly. In the placebo condition, perfusion within the gastrocnemius and soleus muscle groups during plantar flexion exercise was attenuated by approximately 20% in older subjects (Fig. 7C) compared with young (Fig. 7A). After acute AO administration, exercise-induced perfusion and postexercise hyperemia were 30% to 40% greater in the elderly (Fig. 7C), whereas these hemodynamic parameters were unaffected by acute AO consumption in younger individuals (Fig. 7A). Viewed together, it seems that acute AO consumption effectively restores muscle perfusion to that of the younger group, ablating the peripheral hypoperfusion in the lower leg of elderly subjects.
In a similar cohort, Jablonski et al. (16) recently documented the ability of a high-dose (2 g over 20 min) intravenous infusion of ascorbic acid to improve resting leg blood flow in the elderly. Our NMR-based work extends these findings to exercise, demonstrating a similar magnitude of effect on hemodynamics but with a much lower dose, acutely ingested, over-the-counter AO cocktail. To our knowledge, this was the first study to identify the capacity of acute AO administration to restore the age-related decline in skeletal muscle perfusion during exercise. This new finding not only provided convincing evidence that oxidative stress plays a significant role in the well-documented decline of skeletal muscle blood flow with advancing age but also demonstrated an encouraging plasticity for this age-related adaptation. Whether this acute lack of vasodilation could be maintained with longer-term AO supplementation cannot be ascertained from the present findings, but this is an intriguing possibility that awaits further study.
Interestingly, acute AO administration did not improve hemodynamics in the young, which is most likely attributable to better endogenous AO capacity and therefore a lesser degree of oxidative stress in these subjects (33). In fact, despite evidence for the ability of the acutely ingested AO cocktail to reduce plasma free radical concentration both at rest and during exercise (25), end-exercise perfusion tended to be lower after AO consumption in the young group (Fig. 7A). This is in agreement with other recent work from our group identifying a negative effect of acute AO administration on exercise-induced vasodilation in young subjects (11), again adding credence to the concept that some level of free radical concentration is essential for normal function during exercise in young, healthy individuals. Again, these findings are somewhat parallel to the finding that exercise training in combination with chronic AO supplementation severely attenuates exercise-induced adaptations (28), suggesting a positive regulatory role for free radicals.
This acute positive outcome on the peripheral circulation after an acute AO-mediated reduction in vascular oxidative stress in the elderly is in contrast to the largely disappointing results from interventional trials concerning the effects of AO supplementation on many indicators of cardiovascular health (35). Such equivocal findings may be due, at least in part, to a tendency for clinical trials to focus on specific patient populations and also to a general lack of knowledge concerning in vivo AO status and efficacy. Although acute in nature, our laboratory-based studies have attempted to address both of these potentially confounding influences. Specifically, recruiting a focused cohort of subjects in good overall health with no diagnosed disease or use of prescription medication provided the opportunity to examine the impact of oxidative stress on muscle function in a “pre-clinical” context. Regarding efficacy, the acute AO intervention that we have used was selected based on previous studies from our group using EPR spectroscopy that have quantified the capacity of this AO cocktail to reduce plasma O2-centered free radical levels in both young (25) and older (38) subjects (Fig. 2). This previous work thus confirms the ability of the acute AO intervention to significantly attenuate vascular oxidative stress in an acute manner and lends confidence to the apparent link between reduced oxidative stress and improved muscle perfusion as measured by ASL.
Oxidative Stress and Skeletal Muscle Energetics
In the placebo condition, PCr depletion and the ratio of inorganic phosphate to PCr (Pi/PCr) were found to be 30%–40% greater in older compared with young subjects at a similar work rate, indicative of a greater “metabolic stress” during exercise in the elderly (Figs. 7B and D). In contrast to the pronounced effect of acute AO administration on perfusion in the elderly, this intervention did not alter PCr depletion or Pi/PCr, suggesting that age-related differences in muscle energetics during exercise was due to inherited differences in skeletal muscle metabolism or simply greater relative stress due to a diminished exercise capacity in the older group. To our knowledge, this was the first study to specifically examine the impact of oxidative stress on these parameters in younger and older individuals.
In contrast to the negligible effect of the acute AO supplementation on PCr depletion, PCr τ upon cessation of exercise was significantly faster in the elderly after AO consumption, suggestive of improved skeletal muscle energetics in this group (Fig. 7D). This remarkable and effect of the acute AO ingestion on skeletal muscle energetics in the elderly could be the result of either an AO-mediated change in muscle metabolism or a secondary consequence of the AO-mediated improvement in perfusion and thus O2 availability. Regarding the previous, although there is some indication that chronic AO administration may influence oxidative metabolism, the time course for the observed effect (2–3 h after consumption) in the present study makes this an unlikely mechanism for the observed improvement in PCr recovery. Alternately, we have identified the potential influence of perfusion on muscle energetics from NMR-based studies, with a clear association between O2 availability and the rate of PCr recovery at the end of exercise in healthy subjects (15). With this previous work as our basis, we speculate that the acute AO-induced increase in muscle perfusion observed in the older group augmented O2 delivery to the muscle tissue (Fig. 7C), which may be viewed as effectively improving O2 availability for oxidative metabolism within the skeletal muscle.
Evidence of Hemodynamic and Metabolic Reserve in Aging Skeletal Muscle
The observed improvement in perfusion and subsequent beneficial effect of muscle oxidative capacity after acute AO administration (Figs. 7C and D) raises the question of whether age-related changes in these parameters should be viewed as detrimental or adaptive. Theoretically, Fick’s equation dictates that any decline in perfusion will decrease O2 delivery and may jeopardize O2 availability to the exercising muscle tissue. However, we have previously reported an age-related decline in limb blood flow that is accompanied by improved O2 extraction, apparently compensating for the reduction in O2 delivery and thereby maintaining O2 consumption (21). In terms of metabolism, there is little consensus regarding the impact of age on muscle energetics (29). In this NMR-based work, we did not observe an age-related difference in PCr repletion after moderate-intensity exercise in the placebo condition (Fig. 7), supporting the body of evidence against a decline in metabolic capacity in leg skeletal muscle with healthy aging. Together, these previous and current findings suggest that healthy aging is associated with a decline in exercising peripheral blood flow but that this adaptation does not result in skeletal muscle dysfunction in this cohort.
With these previous studies demonstrating an apparent maintenance of skeletal muscle function with advancing age, it is noteworthy that acute AO administration unmasked such a marked improvement in both hemodynamic and metabolic parameters (Fig. 7), which may suggest a “reserve capacity” in the elderly. In pathophysiological states, such as peripheral vascular disease characterized by chronic ischemia, the detrimental effects of reduced perfusion on muscle function and energetics are well known. In this population, peripheral blood flow improves after an acute AO intervention, which may presumably also improve muscle energetics through improved O2 supply. Although less severe, perfusion and metabolic data from our studies in healthy older subjects parallel these clinical findings (Fig. 7), suggesting that the aging skeletal muscle may operate in a functional but somewhat depressed state because of elevated oxidative stress that may be acutely restored in the presence of a more favorable, AO environment. As these NMR-based assessments of muscle metabolism were of relatively short duration (5 min), it would be interesting to examine this concept of the muscle of the elderly, working in a somewhat depressed state in the face of a more prolonged exercise challenge and subsequently greater accumulation of oxidative stress.
CONCLUSIONS
We have revealed an age-related pro- and AO imbalance that affects vascular function, and exercise training is capable of restoring this equilibrium. Indeed, exercise training improves vascular function such that the AO-mediated reduction in free radicals negatively affects exercise-induced brachial artery vasodilation, as previously seen in young untrained subjects. Additionally, acute AO administration after exercise training negates exercise-induced improvements in blood pressure and FMD, returning subjects to a hypertensive state and blunting FMD. Finally, measurements using NMR revealed that acute AO supplementation significantly improved end-exercise and postexercise perfusion in older subjects but did not do so in the young. Concomitantly, muscle oxidative capacity (PCr recovery) was improved with acute AO ingestion in the old, again with no effect in the young. In combination, these age-specific findings suggest an important role for oxidative stress in the differing vascular responses between young and old. However, the paradoxical effects of acute AO supplementation, when combined with exercise training, reveal an intriguing, but complex, relationship between aging, oxidative stress, and vascular function (Fig. 1).
Acknowledgments
This research was supported by the National Heart, Lung, and Blood Institute grants HL-17731 and HL-09183, the Tobacco Related Disease Research Program grant 15RT-0100, and an American Heart Association Scientist Development Grant 0835209N.
References
- 1.Andersen P, Saltin B. Maximal perfusion of skeletal muscle in man. J Physiol. 1985;366:233–49. doi: 10.1113/jphysiol.1985.sp015794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bailey DM, Ainslie PN, Jackson SK, Richardson RS, Ghatei M. Evidence against redox regulation of energy homoeostasis in humans at high altitude. Clin Sci (Lond ) 2004;107(6):589–600. doi: 10.1042/CS20040085. [DOI] [PubMed] [Google Scholar]
- 3.Bailey DM, Davies B, Young IS, et al. EPR spectroscopic evidence of free radical outflow from an isolated muscle bed in exercising humans: functional significance of decreasing intracellular PO2 vs. increasing O2 flux. Adv Exp Med Biol. 2003;540:297–303. doi: 10.1007/978-1-4757-6125-2_42. [DOI] [PubMed] [Google Scholar]
- 4.Beckman JS, Koppenol WH. Nitric oxide, superoxide, and peroxynitrite: the good, the bad, and ugly. Am J Physiol. 1996;271(5 Pt 1):C1424–37. doi: 10.1152/ajpcell.1996.271.5.C1424. [DOI] [PubMed] [Google Scholar]
- 5.Carlier PG, Brillault-Salvat C, Giacomini E, Wary C, Bloch G. How to investigate oxygen supply, uptake, and utilization simultaneously by interleaved NMR imaging and spectroscopy of the skeletal muscle. Magn Reson Med. 2005;54(4):1010–3. doi: 10.1002/mrm.20649. [DOI] [PubMed] [Google Scholar]
- 6.Celermajer DS, Sorensen KE, Spiegelhalter DJ, Georgakopoulos D, Robinson J, Deanfield JE. Aging is associated with endothelial dysfunction in healthy men years before the age-related decline in women. J Am Coll Cardiol. 1994;24(2):471–6. doi: 10.1016/0735-1097(94)90305-0. [DOI] [PubMed] [Google Scholar]
- 7.Cheitlin MD. Cardiovascular physiology-changes with aging. Am J Geriatr Cardiol. 2003;12(1):9–13. doi: 10.1111/j.1076-7460.2003.01751.x. [DOI] [PubMed] [Google Scholar]
- 8.Crecelius AR, Kirby BS, Voyles WF, Dinenno FA. Nitric oxide but not vasodilating prostaglandins contributes to the improvement of exercise hyperemia via ascorbic acid in healthy older adults. Am J Physiol Heart Circ Physiol. 2010;299(5):H1633–41. doi: 10.1152/ajpheart.00614.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davies KJ, Quintanilha AT, Brooks GA, Packer L. Free radicals and tissue damage produced by exercise. Biochem Biophys Res Commun. 1982;107(4):1198–205. doi: 10.1016/s0006-291x(82)80124-1. [DOI] [PubMed] [Google Scholar]
- 10.DeSouza CA, Shapiro LF, Clevenger CM, et al. Regular aerobic exercise prevents and restores age-related declines in endothelium-dependent vasodilation in healthy men. Circulation. 2000;102(12):1351–7. doi: 10.1161/01.cir.102.12.1351. [DOI] [PubMed] [Google Scholar]
- 11.Donato AJ, Uberoi A, Bailey DM, Wray DW, Richardson RS. Exercise-induced brachial artery vasodilation: effects of antioxidants and exercise training in elderly men. Am J Physiol Heart Circ Physiol. 2010;298(2):H671–8. doi: 10.1152/ajpheart.00761.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dudgeon S, Benson DP, MacKenzie A, Paisley-Zyszkiewicz K, Martin W. Recovery by ascorbate of impaired nitric oxide-dependent relaxation resulting from oxidant stress in rat aorta. Br J Pharmacol. 1998;125(4):782–6. doi: 10.1038/sj.bjp.0702120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Duteil S, Bourrilhon C, Raynaud JS, et al. Metabolic and vascular support for the role of myoglobin in humans: a multiparametric NMR study. Am J Physiol Regul Integr Comp Physiol. 2004;287(6):R1441–9. doi: 10.1152/ajpregu.00242.2004. [DOI] [PubMed] [Google Scholar]
- 14.Eskurza I, Monahan KD, Robinson JA, Seals DR. Effect of acute and chronic ascorbic acid on flow-mediated dilatation with sedentary and physically active human ageing. J Physiol. 2004;556(Pt 1):315–24. doi: 10.1113/jphysiol.2003.057042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haseler LJ, Lin AP, Richardson RS. Skeletal muscle oxidative metabolism in sedentary humans: 31P-MRS assessment of O2 supply and demand limitations. J Appl Physiol. 2004;97(3):1077–81. doi: 10.1152/japplphysiol.01321.2003. [DOI] [PubMed] [Google Scholar]
- 16.Jablonski KL, Seals DR, Eskurza I, Monahan KD, Donato AJ. High-dose ascorbic acid infusion abolishes chronic vasoconstriction and restores resting leg blood flow in healthy older men. J Appl Physiol. 2007;103(5):1715–21. doi: 10.1152/japplphysiol.00533.2007. [DOI] [PubMed] [Google Scholar]
- 17.Ji LL. Exercise at old age: does it increase or alleviate oxidative stress? Ann N Y Acad Sci. 2001;928:236–47. doi: 10.1111/j.1749-6632.2001.tb05653.x. [DOI] [PubMed] [Google Scholar]
- 18.Ji LL, Leeuwenburgh C, Leichtweis S, et al. Oxidative stress and aging. Role of exercise and its influences on antioxidant systems. Ann N Y Acad Sci. 1998;854:102–17. doi: 10.1111/j.1749-6632.1998.tb09896.x. [DOI] [PubMed] [Google Scholar]
- 19.Kirby BS, Voyles WF, Simpson CB, Carlson RE, Schrage WG, Dinenno FA. Endothelium-dependent vasodilatation and exercise hyperaemia in ageing humans: impact of acute ascorbic acid administration. J Physiol. 2009;587(Pt 9):1989–2003. doi: 10.1113/jphysiol.2008.167320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kojda G, Hambrecht R. Molecular mechanisms of vascular adaptations to exercise. Physical activity as an effective antioxidant therapy? Cardiovasc Res. 2005;67:187–97. doi: 10.1016/j.cardiores.2005.04.032. [DOI] [PubMed] [Google Scholar]
- 21.Lawrenson L, Poole JG, Kim J, Brown CF, Patel PM, Richardson RS. Vascular and metabolic response to isolated small muscle mass exercise: the effect of age. Am J Physiol Heart Circ Physiol. 2003;285:H1023–31. doi: 10.1152/ajpheart.00135.2003. [DOI] [PubMed] [Google Scholar]
- 22.Lucchesi PA, Belmadani S, Matrougui K. Hydrogen peroxide acts as both vasodilator and vasoconstrictor in the control of perfused mouse mesenteric resistance arteries. J Hypertens. 2005;23(3):571–9. doi: 10.1097/01.hjh.0000160214.40855.79. [DOI] [PubMed] [Google Scholar]
- 23.Nishiyama SK, Wray DW, Richardson RS. Aging affects vascular structure and function in a limb-specific manner. J Appl Physiol. 2008;105(5):1661–70. doi: 10.1152/japplphysiol.90612.2008. [DOI] [PubMed] [Google Scholar]
- 24.Pescatello LS, Franklin BA, Fagard R, Farquhar WB, Kelley GA, Ray CA American College of Sports Medicine position stand. Exercise and hypertension. Med Sci Sports Exerc. 2004;36(3):533–53. doi: 10.1249/01.mss.0000115224.88514.3a. [DOI] [PubMed] [Google Scholar]
- 25.Richardson RS, Donato AJ, Uberoi A, et al. Exercise-induced brachial artery vasodilation: role of free radicals. Am J Physiol Heart Circ Physiol. 2007;292(3):H1516–22. doi: 10.1152/ajpheart.01045.2006. [DOI] [PubMed] [Google Scholar]
- 26.Richardson RS, Leek BT, Gavin TP, et al. Reduced mechanical efficiency in chronic obstructive pulmonary disease but normal peak VO2 with small muscle mass exercise. Am J Respir Crit Care Med. 2004;169(1):89–96. doi: 10.1164/rccm.200305-627OC. [DOI] [PubMed] [Google Scholar]
- 27.Richardson RS, Saltin B. Human muscle blood flow and metabolism studied in the isolated quadriceps muscles. Med Sci Sports Exerc. 1998;30(1):28–33. doi: 10.1097/00005768-199801000-00005. [DOI] [PubMed] [Google Scholar]
- 28.Ristow M, Zarse K, Oberbach A, et al. Antioxidants prevent health-promoting effects of physical exercise in humans. Proc Natl Acad Sci U S A. 2009;106(21):8665–70. doi: 10.1073/pnas.0903485106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Russ DW, Kent-Braun JA. Is skeletal muscle oxidative capacity decreased in old age? Sports Med. 2004;34(4):221–9. doi: 10.2165/00007256-200434040-00002. [DOI] [PubMed] [Google Scholar]
- 30.Shephard RJ. Exercise for patients with congestive heart failure. Sports Med. 1997;23(2):75–92. doi: 10.2165/00007256-199723020-00002. [DOI] [PubMed] [Google Scholar]
- 31.Shoemaker JK, Halliwill JR, Hughson RL, Joyner MJ. Contributions of acetylcholine and nitric oxide to forearm blood flow at exercise onset and recovery. Am J Physiol. 1997;273(5 Pt 2):H2388–95. doi: 10.1152/ajpheart.1997.273.5.H2388. [DOI] [PubMed] [Google Scholar]
- 32.Taddei S, Galetta F, Virdis A, et al. Physical activity prevents age-related impairment in nitric oxide availability in elderly athletes. Circulation. 2000;101(25):2896–901. doi: 10.1161/01.cir.101.25.2896. [DOI] [PubMed] [Google Scholar]
- 33.Taddei S, Virdis A, Ghiadoni L, et al. Age-related reduction of NO availability and oxidative stress in humans. Hypertension. 2001;38(2):274–9. doi: 10.1161/01.hyp.38.2.274. [DOI] [PubMed] [Google Scholar]
- 34.Uppu RM, Nossaman BD, Greco AJ, et al. Cardiovascular effects of peroxynitrite. Clin Exp Pharmacol Physiol. 2007;34(9):933–7. doi: 10.1111/j.1440-1681.2007.04641.x. [DOI] [PubMed] [Google Scholar]
- 35.Willcox BJ, Curb JD, Rodriguez BL. Antioxidants in cardiovascular health and disease: key lessons from epidemiologic studies. Am J Cardiol. 2008;101(10A):75D–86D. doi: 10.1016/j.amjcard.2008.02.012. [DOI] [PubMed] [Google Scholar]
- 36.Wray DW, Nishiyama SK, Monnet A, et al. Multiparametric NMR-based assessment of skeletal muscle perfusion and metabolism during exercise in elderly persons: preliminary findings. J Gerontol. 2009;64(9):968–74. doi: 10.1093/gerona/glp044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wray DW, Nishiyama SK, Monnet A, et al. Antioxidants and aging: NMR-based evidence of improved skeletal muscle perfusion and energetics. Am J Physiol Heart Circ Physiol. 2009;297(5):H1870–5. doi: 10.1152/ajpheart.00709.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wray DW, Uberoi A, Lawrenson L, Bailey DM, Richardson RS. Oral antioxidants and cardiovascular health in the exercise-trained and untrained elderly: a radically different outcome. Clin Sci (Lond) 2009;116(5):433–41. doi: 10.1042/CS20080337. [DOI] [PubMed] [Google Scholar]
- 39.Wray DW, Uberoi A, Lawrenson L, Richardson RS. Evidence of preserved endothelial function and vascular plasticity with age. Am J Physiol Heart Circ Physiol. 2006;290(3):H1271–7. doi: 10.1152/ajpheart.00883.2005. [DOI] [PubMed] [Google Scholar]
- 40.Yetik-Anacak G, Catravas JD. Nitric oxide and the endothelium: history and impact on cardiovascular disease. Vascul Pharmacol. 2006;45(5):268–76. doi: 10.1016/j.vph.2006.08.002. [DOI] [PubMed] [Google Scholar]