Abstract
The Nociceptin/Orphanin FQ (N/OFQ) peptide NOP receptor is coupled to pertussis toxin (PTX)-sensitive G proteins (Gi/o) whose activation leads to the inhibition of both cAMP production and calcium channel activity, and to the stimulation of potassium currents. The label free dynamic mass redistribution (DMR) approach has been demonstrated useful for investigating the pharmacological profile of G protein-coupled receptors. Herein, we employ DMR technology to systematically characterize the pharmacology of a large panel of NOP receptor ligands. These are of peptide and non-peptide nature and display varying degrees of receptor efficacy, ranging from full agonism to pure antagonism. Using Chinese hamster ovary (CHO) cells expressing the human NOP receptor we provide rank orders of potency for full and partial agonists as well as apparent affinities for selective antagonists. We find the pharmacological profile of NOP receptor ligands to be similar but not identical to values reported in the literature using canonical assays for Gi/o-coupled receptors. Our data demonstrate that holistic label-free DMR detection can be successfully used to investigate the pharmacology of the NOP receptor and to characterize the cellular effects of novel NOP receptor ligands.
Introduction
Nociceptin/Orphanin FQ (N/OFQ) is a 17 amino-acid (FGGFTGARKSARKLANQ) neuropeptide that binds with high affinity to the N/OFQ peptide (NOP) receptor [1, 2]. The NOP receptor mainly couples to pertussis toxin (PTX)-sensitive G proteins (Gi/o) whose activation leads to lowering of cAMP levels and inhibition of calcium channels, but also to the stimulation of potassium currents [3]. Its pharmacology has been classically studied in vitro with bioassays such as the electrically stimulated mouse vas deferens, and biochemical assays based on [35S]GTPγS binding and inhibition of forskolin-stimulated cAMP production. More recently, bioluminescence resonance energy transfer (BRET) based assays allowed the investigation of NOP/G protein and NOP/β-arrestin interactions demonstrating that several synthetic agonists are biased toward activation of G protein signaling over β-arrestin recruitment [4, 5]. Moreover, our knowledge about the binding pocket of the NOP receptor has been broadened substantially by the availability of the crystal structure of the NOP receptor in complex with different antagonists [6, 7]. The identification of several NOP receptor selective ligands [3, 8, 9] made it possible to test the in vivo consequences of selective stimulation or blockage of the NOP receptor. Complementary information has been collected using genetically modified animals such as mice [10] and rats [11] deficient in expression of the NOP receptor or the N/OFQ peptide precursor [12], and mice expressing a NOP-eGFP fusion protein from the native NOP receptor locus [13]. Pharmacological and genetic studies demonstrated the involvement of the N/OFQ-NOP receptor system in the control of different biological functions including pain, mood and anxiety, food intake, learning and memory, locomotion, drug abuse, cough and micturition reflexes, cardiovascular homeostasis, intestinal motility and immune responses [3, 14, 15].
NOP is a G protein-coupled receptor (GPCR), GPCRs are macromolecules belonging to the largest family of membrane proteins in the human genome. They are involved in the control of virtually all physiological processes and represent one of the main targets for prescribed medicines, in fact about 36% of all therapeutics mediate their effects through GPCRs [16]. The development of GPCR research in physiology and pharmacology led to a significant expansion of both available knowledge and methods for investigating these receptors [17–20]. The continuous acceleration in knowledge acquisition on GPCR conformational complexity (e.g. X-ray and CryoEM near atomic resolution structures) and how different ligands perturbate receptor signaling cascades (i.e. biased agonism), might increase the challenge in translating the effects elicited by receptor ligands from the medicinal chemistry to the biological level [21]. For this reason, the use of phenotypic biosensor technology platforms capable to measure whole cell integrated responses might provide a new angle towards detection and differentiation of promising GPCR ligands.
Such methods, rather than focusing at single readout assays (e.g. GTP/GDP exchange, second messengers’ levels modulation, protein-protein interaction, protein phosphorylation, etc.) make it possible to obtain a more global view of receptor-dependent cellular perturbations. The mostly used, are based on special biosensors (electron-conducting or light-diffracting plates) that allow translation of the receptor-dependent holistic cellular response to physical parameters such as variations in impedance or modulations of wavelength shift of an incident light in real time [22]. These assays are used in laboratories from both industry and academia and may be advantageous for identifying novel molecular entities with favorable in vitro profiles before translation to in vivo investigations. This is in part due to the possibility to test drug candidates non-invasively in several types of cellular backgrounds, including primary cell cultures.
The dynamic mass redistribution (DMR) assay is based on an optical biosensor technology, and was recently developed to monitor receptor signaling responses including those mediated by GPCRs (for details on the method see [23, 24]). It has already been applied to study the pharmacological properties of new ligands acting at various GPCRs such as the urotensin-II [25], β2 adrenergic [26, 27], muscarinic M3 [28], purinergic P2Y [29], formyl peptide [30], and protease activated [31, 32] receptors. Classical opioid receptors, the mu [33], kappa and delta receptors [34], were also evaluated with DMR. No data are yet available for the NOP receptor. Thus, in the present study we performed a systematic pharmacological characterization of the NOP receptor using a label-free optical DMR-based biosensor, cells expressing the human NOP receptor, and a large panel of NOP ligands with a wide spectrum of pharmacological activities.
Materials and methods
Drugs and reagents
The peptides N/OFQ, N/OFQ(1–13)-NH2, UFP-112, UFP-101, [F/G]N/OFQ(1–13)-NH2, [Nphe1]N/OFQ(1–13)-NH2, [Arg14,Lys15]N/OFQ, Ac-RYYRIK-NH2, and PWT2-N/OFQ were synthesized in house following previously described procedures [35, 36]. The non-peptide molecules Ro 65–6570, C-24, and J-113397 were synthesized in our laboratories. SB-612111 and naloxone were from Tocris bioscience (Bristol, UK). AT-090 and AT-127 were provided by N Zaveri (Astraea Therapeutics, Mountain View, USA). Pertussis toxin was from Sigma (Taufkirchen, DE). Hanks balanced salt solution (HBSS) was from Invitrogen (Darmstadt, DE), HEPES was from Applichem (Darmstadt, DE). All tissue culture media and supplements were from Invitrogen (Darmstadt, DE) and were of the highest purity available. Concentrated solutions of ligands were made in ultrapure water or dimethyl sulfoxide and kept at—20°C until use.
Cells
Chinese Hamster Ovary (CHO) cells stably expressing the human NOP receptor (CHONOP) were kindly provided by D.G. Lambert (University of Leicester, UK). CHOdelta were supplied by E Varga (The University of Arizona, USA), CHOmu and CHOkappa were both provided by L Toll (Torrey Pines Institute for Molecular Studies, Port St. Lucie, USA), CHO cells were used as control. Cells were cultured in Dulbecco's Modified Eagle Medium: Nutrient Mixture F-12 (DMEM/F12) supplemented with 10% (v/v) Fetal Calf Serum (FCS), 100 U/ml penicillin, 100 μg/ml streptomycin, 2 mM L-glutamine, 15 mM HEPES. The medium was supplemented with 400 μg/ml G418 to maintain expression.
Experimental protocol
For DMR measurements the label-free EnSight Multimode Plate Reader (Perkin Elmer, MA, US) was used. Cells were seeded at 12,000 cells/well onto fibronectin-coated 384 well DMR microplates and cultured for 20 h to obtain confluent monolayers. Cells were starved in assay buffer (Hank’s Balanced Salt Solution (HBSS) with 20 mM HEPES, 0.01% Bovine Serum Albumin (BSA) fraction V) for 1 hr prior the addition of compounds. Serial dilutions were made in the assay buffer. After reading baseline, compounds were added using a semiautomatic liquid handler Selma (Analytik Jena AG, Jena, DE). Online additions of 10 μL compounds were carried out in a volume of 30 μl/well. Antagonists were incubated 30 min before agonist injection, then DMR changes were recorded for 3000 sec. Agonists responses represented in traces were described as picometer (pm) shifts over time (sec) following baseline normalization. Maximum picometers (pm) modification (Peak) and area under the curve (AUC) were used to generate concentration response curves. All the experiments were carried out at 37°C. For a detailed description of the methods see [24] and [37]
Data analysis
All the data were elaborated using Graph Pad Prism 6.0 (La Jolla, CA, US). Concentration response curves were fitted by log logistic four parameter equation. Data are expressed as mean + or ± sem of n experiments and were analyzed statistically using one-way analysis of variance followed by Dunnett’s test for multiple comparisons. Agonist potencies are given as pEC50 i.e. the negative logarithm to base ten of the molar concentration of an agonist that produces half of the maximal effect. Agonist maximal effect, i.e. the maximal effect that an agonist can elicit in a given preparation under particular experimental conditions, has been also expressed as intrinsic activity (α) by dividing the Emax of the agonist under study by that elicited by the reference full agonist (N/OFQ) in parallel experiments. Antagonists were assayed at single concentrations against the concentration-response curve to the agonist and their potencies expressed as pKB according to the following equation: pKB = log(CR-1)-log[A], where CR is the ratio between agonist potency (expressed as EC50) in the presence and absence of antagonist and [A] is the molar concentration of antagonist. KB refers to the equilibrium dissociation constant of a ligand determined by means of a functional assay [38]. In a separate series of experiments SB-612111 was tested using the classical Schild protocol.
Results
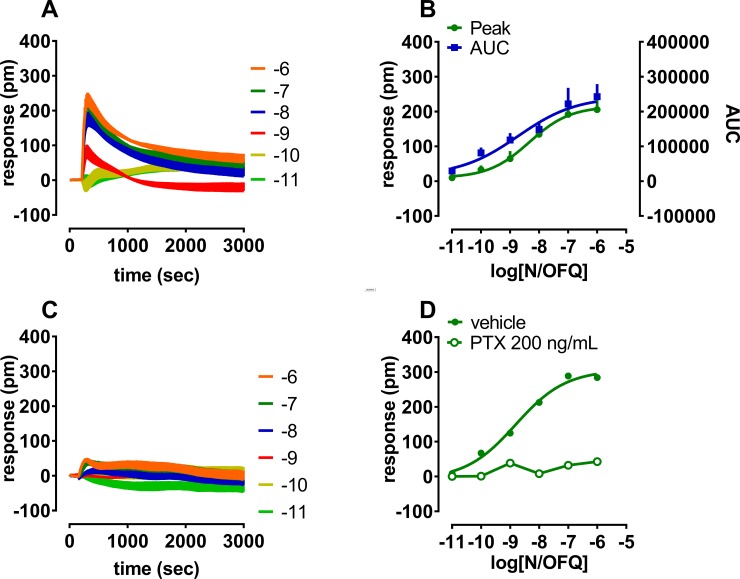
Cellular effects of the endogenous peptide N/OFQ were measured over time at increasing concentrations applying the DMR technology; at 1 μM the signal-to-noise ratio calculated was in the range 4–4.5. N/OFQ effects were thereafter computed in sigmoidal curves as peaks and areas under the curve (AUC); similar values of potency were obtained by fitting the two parameters (pEC50 8.33 and 8.73, respectively) (Fig 1A and 1B). For simplicity concentration response curves to NOP agonists are later presented as DMR peaks. To confirm the prevalent Gi/o nature of the N/OFQ-stimulated DMR responses, N/OFQ effects were measured after 20 h pretreatment with 200 ng/mL PTX. The Gi/o selective ADP-ribosylator largely diminished the N/OFQ DMR signal confirming the signaling preferences of the NOP receptor (Fig 1C and 1D). Importantly, N/OFQ was completely inactive when tested in wild type CHO cells (Table 1).
Fig 1.
Concentration response curve to N/OFQ (10 pM– 1 μM) in the absence (panels A and B) and presence of 200 ng/mL PTX (panels C and D). Baseline corrected DMR representative tracings are shown in panels A and C and concentration response curves in panels B and D. Sigmoidal curves to N/OFQ computed as peak and AUC are shown as mean + sem of at least 3 experiments performed in triplicate (panel B).
Table 1. DMR responses to high concentrations of ligands in CHO and CHONOP cells.
| CHO | CHONOP | |
|---|---|---|
| (pm ± sem) | (pm± sem) | |
| N/OFQ | 23±9 | 205±29* |
| N/OFQ(1–13)NH2 | 21±8 | 267±17* |
| [Arg14,Lys15]N/OFQ | 40±20 | 271±16* |
| UFP-112 | 25±7 | 248±38* |
| PWT2-N/OFQ 0.1 μM | 24±5 | 198±55* |
| PWT2-N/OFQ 1 μM | 467±23* | ~ 350* |
| [Nphe1]N/OFQ(1–13)NH2 | 20±6 | 180±21* |
| [F/G]N/OFQ(1–13)NH2 | 24±2 | 200±17* |
| UFP-101 | 25±6 | ~ 40 |
| Ac-RYYRIK-NH2 | 17±8 | 182±11* |
| Ro 65–6570 | 23±8 | 210±19* |
| AT-090 | 21±3 | 252±15* |
| AT-127 | 20±4 | 191±5* |
| SB-612111 | 26±3 | -10±9 |
| J-113397 | 17±8 | 5±16 |
| C-35 | 16±4 | -9±14 |
| C-24 | 21±5 | -13±15 |
| Dermorphin | 35±12 | 25±20 |
| DPDPE | 21±5 | 12±13 |
| Dynorphin A | 7±4 | 32±14 |
| Naloxone | 23±5 | 15±12 |
| FSK | -172±23* | -164±15* |
| ATP | 177±15* | 185±12* |
| Buffer | 18±10 | 12±22 |
PWT2-N/OFQ was tested at 0.1 and 1 μM, FSK and ATP at 100 μM, all the other compounds at 1 μM.
*p < 0.05 vs buffer according to one-way ANOVA followed by the Dunnett’s test for multiple comparisons.
DMR effects of NOP full and partial agonists
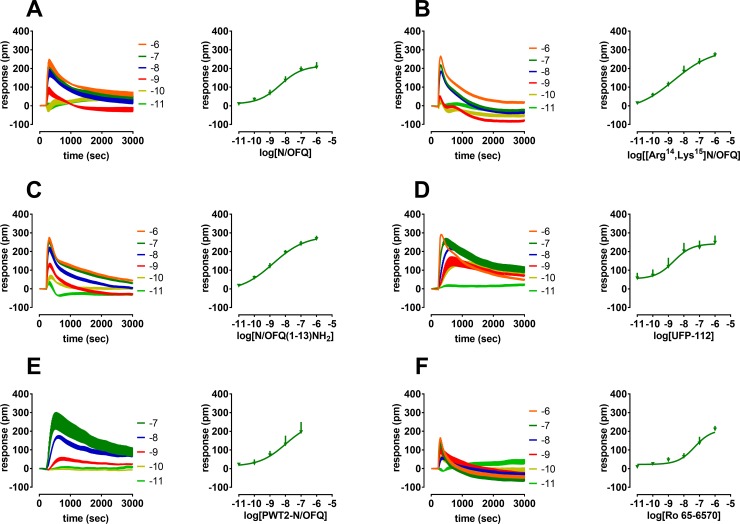
The rank order of potency of selective agonists was determined in the DMR assay by studying a panel of NOP ligands encompassing full and partial agonist activity together with the endogenous peptide N/OFQ (Fig 2A). N/OFQ(1–13)-NH2, a peptide constituted by the minimal sequence maintaining the same activity as N/OFQ, mimicked the stimulatory effects of the endogenous peptide with similar potency (pEC50 8.80) and efficacy (Emax 267) (Fig 2C). The N/OFQ derivatives [Arg14,Lys15]N/OFQ and UFP-112 also displayed similar effects as N/OFQ showing comparable high potency (pEC50 8.63 and 8.66) and maximal effects (Emax 271 and 248) (Fig 2B and 2D). The effects of the recently developed N/OFQ tetrabranched peptide PWT2-N/OFQ were tested up to 0.1 μM since at 1 μM this compound was active in wild type CHO cells (Table 1) and potency and maximal effects estimated were similar to that of N/OFQ (pEC50 ~ 8, Emax 198) (Fig 2E). The effects of the tetrabranched peptide appeared, in 3 out of 6 experiments, longer lasting than those elicited by N/OFQ. Of note, the Emax of PWT2-N/OFQ calculated as AUC were not significantly, yet higher than those of N/OFQ (Fig 3B). Ro 65–6570, one of the most commonly used non-peptide NOP agonists, produced a concentration-dependent increase in the DMR signal without reaching the stimulation plateau; the application of higher concentrations of compound was not possible since Ro 65–6570 exhibited a DMR response in wild type CHO cells when tested at 10 μM. The concentration response curve for Ro 65–6570 was constrained to the estimated maximal effects and a value of potency of ~ 7.3 was calculated (Fig 2F).
Fig 2.
Concentration response curve to N/OFQ (panel A), [Arg14,Lys15]N/OFQ (panel B), N/OFQ(1–13)-NH2 (panel C), UFP-112 (panel D), PWT2-N/OFQ (panel E), and Ro 65–6570 (panel F). Representative raw DMR tracings are represented on the left of each panel and average sigmoidal curves on the right. Data are mean + sem of at least 3 separate experiments performed in triplicate.
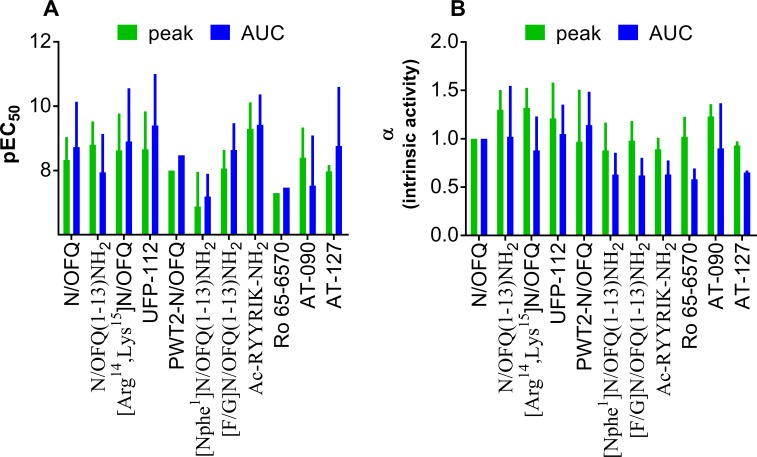
Fig 3.
Comparison of potencies (pEC50 + CL95%, panel A) and maximal effects (α+ SD, panel B) of NOP receptor agonists obtained by computing maximal DMR peaks or areas under the curve (AUC).
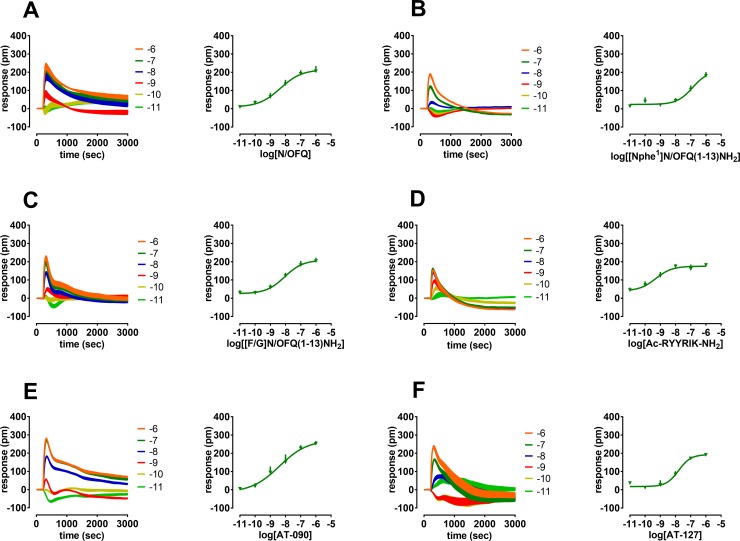
In Fig 4 DMR traces of NOP partial agonists are displayed and computed as concentration response curves in comparison with N/OFQ (Fig 4A). The peptide [Nphe1]N/OFQ(1–13)-NH2 stimulated the NOP receptor mimicking the effects of N/OFQ but with lower potency (~30-fold) and maximal effects (Emax 180) (Fig 4B). The first N/OFQ related peptide showing reduced efficacy, [F/G]N/OFQ(1–13)-NH2, concentration dependently stimulated DMR effects with comparable potency (pEC50 8.06) as the endogenous peptide (Fig 3C). The hexapeptide Ac-RYYRIK-NH2 evoked a concentration dependent stimulation of the NOP receptor with estimated potency approximately 10-fold higher than N/OFQ and similar maximal effects (Emax 182) (Fig 4D). The recently characterized non-peptide agonists AT-090 and AT-127 showed high potency (pEC50 8.40 and 7.97) and maximal effects (Emax 252 and 191) (Fig 4E and 4F).
Fig 4.
Concentration response curve to N/OFQ (panel A), [Nphe1]N/OFQ (panel B), [F/G]N/OFQ(1–13)-NH2 (panel C), Ac-RYYRIK-NH2 (panel D), AT-090 (panel E), and AT-127 (panel F). Representative raw DMR tracings are represented on the left of each panel and average sigmoidal curves on the right. Data are mean + sem of at least 3 separate experiments performed in triplicate.
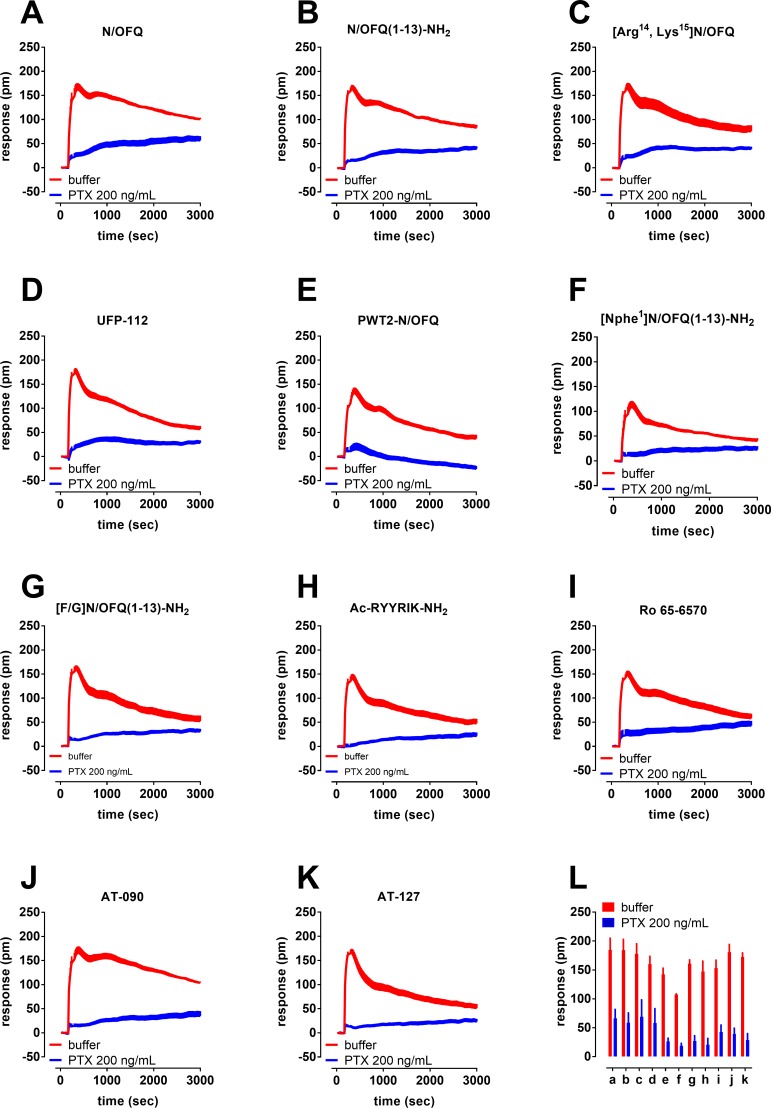
In a separate series of experiments, the nature of the NOP-DMR signal elicited by full and partial agonists was investigated by testing the ligands at the single concentration of 1 μM (with the exception of PWT2-N/OFQ that was tested at 0.1 μM) in cells treated with PTX (Fig 5). The effects of all compounds were largely blunted by toxin pretreatment with residual DMR signal ranging from 15 to 40% of the control response (Fig 5L).
Fig 5.
Representative DMR traces for NOP receptor agonists (panels A-K) tested at 1 μM or at 0.1 μM (PWT2-N/OFQ, panel E), in the absence and presence of 200 ng/mL PTX. The effects of the same compounds are reported as DMR peaks in the absence and in the presence of 200 ng/mL PTX in panel L. Data in panel A-K are mean + sem of a single experiment performed in triplicate. Data in panel L are mean + SD of 3 separate experiments performed in triplicate.
Comparison of the effects of high concentrations of ligands in wild type CHO and CHONOP cells is shown in Table 1.
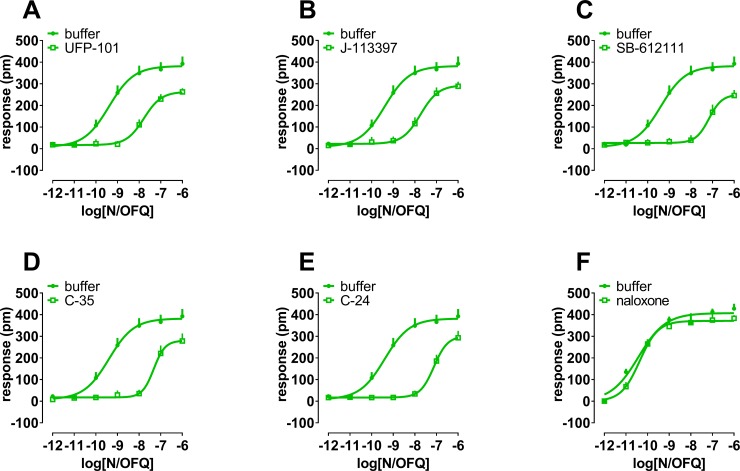
DMR effects of antagonists at the NOP and classical opioid receptors
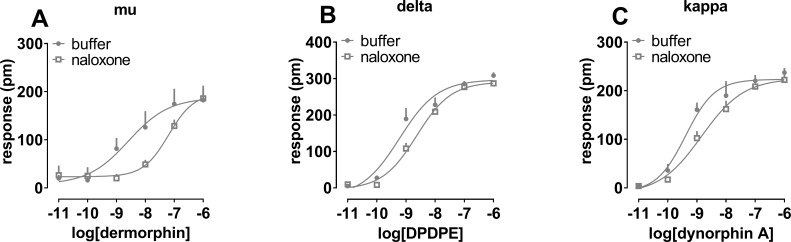
Finally, the effects of the classical opioid receptor antagonist naloxone, and the NOP receptor antagonists UFP-101, J-113397, SB-612111, C-35, and C-24 were tested (at fixed concentrations of 1 μM) against the concentration response curve to N/OFQ. These compounds did not produce any effect per se in CHONOP cells with the exception of UFP-101 which elicited a stimulatory effect approximately corresponding to 20% of the maximal effects of N/OFQ (S1 Fig). All the compounds elicited a rightward shift of the concentration response curve to N/OFQ (Fig 6) with estimated pKB values of 7.60, 7.65, 8.25, 8.07, and 8.30 for UFP-101, J-113397, SB-612111, C-35, and C-24, respectively. The antagonists caused a slight depression of the N/OFQ maximal effects at the concentrations tested. On the contrary naloxone did not modify the concentration response curve to N/OFQ. The opioid antagonist was also tested in cells expressing the classical opioid receptors against the standard agonists dermorphin (pEC50 8.59), DPDPE (pEC50 9.22), and dynorphin A (pEC50 9.39) for mu, delta, and kappa receptors, respectively. Naloxone shifted to the right the concentration response curves to opioid agonists without affecting their maximal effects with estimated pKB values of 8.37 at mu, 7.53, at delta, and 7.35 at kappa opioid receptor (Fig 7).
Fig 6.
Concentration response curve to N/OFQ in the absence and presence of 1 μM UFP-101 (panel A), J-113397 (panel B), SB-612111 (panel C), C-35 (panel D), C-24 (panel E), and naloxone (panel F). Data are mean + sem of at least 3 separate experiments performed in triplicate.
Fig 7.
Concentration response curve to dermorphin (panel A), DPDPE (panel B), and dynorphin A (panel C) in the absence and presence of 100 nM naloxone. Data are mean + sem of 3 separate experiments performed in triplicate.
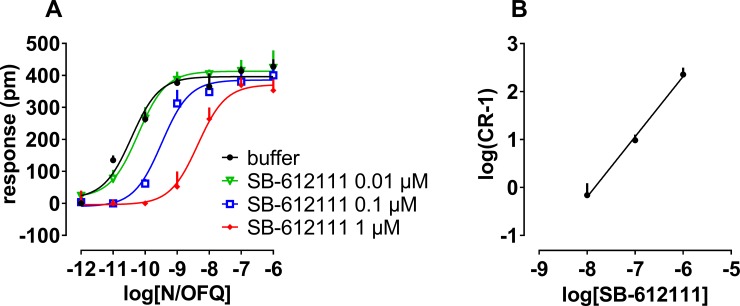
The antagonist nature of SB-612111 was further characterized by the classical Schild protocol by challenging the concentration-response curve to N/OFQ with increasing concentrations of the antagonist. SB-612111 rightward shifted the agonist curve without significantly affecting its maximal effects; a pA2 of 7.84 and a slope value close to 1 were obtained from the relative Schild plot (Fig 8).
Fig 8.
Concentration-response curves to N/OFQ in absence and presence of increasing concentrations (10 nM– 1 μM) of SB-612111 (panel A). The corresponding Schild plot is shown in panel B. Data are mean + sem of 3 separate experiments performed in triplicate.
In a separate series of experiments, the effects of increasing concentrations of N/OFQ and Ac-RYYRIK-NH2 were studied in the absence and presence of SB-612111 (1 μM), The antagonist produced a similar dextral displacement of the concentration response curve to N/OFQ and Ac-RYYRIK-NH2 and the calculated pKB values were 7.53 and 7.21, respectively (Fig 9).
Fig 9.
Concentration response curve to N/OFQ (panel A) and Ac-RYYRIK-NH2 (panel B) in the absence and presence of 1 μM SB-612111. Data are mean + sem of at least 3 separate experiments performed in triplicate.
Pharmacological parameters of the NOP ligands investigated in the present study have been schematically summarized in Table 2.
Table 2. Agonist potencies (pEC50) and intrinsic activity (α), and antagonist potencies (pKB) of the compounds tested in the CHONOP cell DMR assay.
| pEC50 | α | pKB | |
|---|---|---|---|
| N/OFQ | 8.33 (7.63–9.04) |
1.00 | |
| N/OFQ(1–13)NH2 | 8.80 (8.07–9.53) |
1.30 | |
| [Arg14,Lys15]N/OFQ | 8.63 (7.48–9.77) |
1.32 | |
| UFP-112 | 8.66 (7.47–9.84) |
1.21 | |
| PWT2-N/OFQ | ~8.00 | 0.97 | |
| [Nphe1]N/OFQ(1–13)NH2 | 6.88 (5.80–7.96) |
0.88 | |
| [F/G]N/OFQ(1–13)NH2 | 8.06 (7.48–8.64) |
0.98 | |
| Ac-RYYRIK-NH2 | 9.30 (8.49–10.12) |
0.89 | |
| Ro 65–6570 | ~7.30 | 1.02 | |
| AT-090 | 8.40 (7.46–9.34) |
1.23 | |
| AT-127 | 7.97 (7.42–8.17) |
0.93 | |
| UFP-101 | crc incomplete | 7.60 (7.44–7.76) |
|
| SB-612111 | inactive | 8.25 (7.98–8.53) |
|
| J-113397 | inactive | 7.65 (7.45–7.85) |
|
| C-35 | inactive | 8.07 (7.90–8.24) |
|
| C-24 | inactive | 8.30 (8.04–8.57) |
|
Inactive means that up to 1 μM the compound did not promote any DMR response.
Discussion
In the present study we have used the DMR technique that allows an integrated non-invasive measurement of cellular function, to investigate the pharmacological profile of the human NOP receptor in recombinant cells. A panel of peptide and non-peptide selective NOP ligands with a wide range of potency and efficacy, from full agonism to pure antagonism were studied. PTX experiments revealed that NOP signaling in CHO cells is largely yet not exclusively due to Gi/o coupling. The DMR pharmacological profile of the NOP receptor in terms of rank order of potency of full and partial agonists and apparent affinity of selective antagonists is similar although not identical to that reported in the literature using standard assays for Gi/o coupled receptors.
N/OFQ stimulated the DMR response in CHONOP cells but not in CHO cells demonstrating that this signal exclusively derives from the interaction of N/OFQ with the NOP receptor protein. The same is true for all the agonists evaluated since we selected their concentration range based on lack of DMR signal in CHO cells. Regarding the transduction pathway involved in the NOP dependent DMR signal, pretreating the cells with PTX largely inhibited the DMR signal elicited by N/OFQ. Consistently, the DMR response to all NOP agonists were depleted by toxin treatment, to a larger extent for partial than full agonists. This result demonstrated that in CHO cells the DMR signal is mainly, albeit not completely, due to NOP coupling with G proteins of the Gi/o family. PTX treatment is known to block most of the inhibitory Gα proteins through ADP-ribosylation of a Cys351 residue [39]. Importantly, previous reports described that the NOP receptor is able to couple to PTX-insensitive G proteins such as Gz and G16 [40], but also to G12 and G14 [41]. However the PTX resistant DMR signal elicited by N/OFQ as well as NOP agonists in CHO cells is too small to investigate further. In the future we will look for cells (possibly expressing the native NOP receptor) in which the Gi/o independent component of the DMR signal in response to N/OFQ is large enough to be investigated in deconvolution studies. Moreover, despite mechanistic details of arrestin catalytic activation are now being described [42], the lack of functional G proteins does not allow for arrestin-mediated signaling. [43]. Therefore these deconvolution studies will be validated by employing CRISPR/Cas9-edited cells lacking in turn G proteins or arrestins.
The DMR response to NOP activation by a series of NOP full agonists including the peptides N/OFQ(1–13)-NH2 [36], [Arg14, Lys15]N/OFQ [44], and UFP-112 [45], the N/OFQ tetrabranched derivative PWT2-N/OFQ [46], and the non-peptide molecule Ro 65–6570 [47] was investigated in the first series of experiments. These compounds mimicked the stimulatory effects of N/OFQ with similar maximal effects. Thus, in line with the original findings these molecules behave as full agonists at the NOP receptor. As far as agonist potency is concerned the following rank order has been measured:
This is in general in line with literature reports (see Table 2 of [3] that summarizes the action of these compounds in various assays/preparations at human recombinant and rodent native NOP receptors). However, there are some aspects that deserve attention. PWT2-N/OFQ has been reported to be more potent than N/OFQ in receptor binding, stimulated GTPγS binding, bioassay experiments [46] and more recently in a BRET based assay measuring NOP/G protein interaction [5]. In the present study the potency of PWT2-N/OFQ could not be precisely estimated since the compound produced off target effects at micromolar concentrations. This observation implies a certain loss of selectivity due to application of the PWT chemical modification to the N/OFQ peptide sequence and this is in line with bioassay studies in NOP knockout tissues where off target effects were observed with PWT2-N/OFQ but not N/OFQ [46]. PWT2-N/OFQ behavior in cells expressing the NOP receptor is interesting. In fact, in three out of six experiments, the tetrapeptide displayed a DMR response over time more sustained than N/OFQ. This is reminiscent of the behavior of this ligand in bioassay experiments where PWT2-N/OFQ elicited slow developing, long lasting and wash resistant effects [35]. Similar findings were obtained with different PWT peptides [48]. This feature, i.e. longer-lasting binding to the receptor, has been interpreted considering the mechanisms proposed to explain the mode of action of multivalent ligands that include receptor clustering, cooperative binding, rebinding and subsite binding [49]. This aspect of PWT2-N/OFQ action can be important since long lasting receptor binding contributes to prolongation of the duration of action (and eventually an increase in effect) in vivo [50]. As a matter of fact, in vivo PWT2-N/OFQ mimicked the spinal antinociceptive effects of the N/OFQ in models of nociceptive and neuropathic pain in mice and in non-human primates displaying approximately 40-fold higher potency and a remarkably prolonged duration of action [51]. Moreover when injected supraspinally in mice PWT2-N/OFQ stimulated food intake being 40 fold more potent than N/OFQ and eliciting larger effects [35].
A more detailed comparison of the present data with the literature shows that highly potent peptide agonists such as [Arg14, Lys15]N/OFQ and particularly UFP-112 were 10 to 30 fold more potent than N/OFQ in stimulated GTPγS binding and NOP/G protein interaction experiments while in the DMR assay this difference in potency is limited to 2 fold. Possibly differences in signal amplification, receptor desensitization and internalization between the assays may account for these differences.
In the second set of experiments a series of compounds with known partial agonist activity at the NOP receptor, the peptides [F/G]N/OFQ(1–13)-NH2 [52] and Ac-RYYRIK-NH2 [53] and the non peptides AT-090 and AT-127 [54], were evaluated. In the DMR assay all these compounds produced maximal effects that were not statistically different to those of N/OFQ. Similar results were obtained in calcium mobilization studies performed in cells co-expressing the NOP receptor and chimeric G proteins [54, 55]. On the contrary these same compounds consistently displayed significantly lower efficacy than N/OFQ in GTPγS binding and NOP/G protein interaction experiments [3, 5, 54]. As discussed in [56], this apparent discrepancy is probably due to the fact that the estimated efficacy of partial agonists strongly depends on the efficiency of the stimulus–response coupling which is different in the different assays. When the signal amplification phenomena make the efficiency of the stimulus–response coupling high, as in the case of DMR and calcium mobilization, ligand efficacy is overestimated. On the other hand, when there is little or no amplification and the efficiency of the stimulus-response coupling is low, as in the case of GTPγS binding and NOP/G protein interaction, ligand efficacy is underestimated. This phenomenon has been investigated in detail using a NOP-inducible expression system where the efficacy partial agonists could be manipulated to encompass full and partial agonism along with pure antagonism by changing the number of membrane receptors [57]. Importantly this does not happen only in recombinant systems but also when the receptor is investigated in a physiologically relevant environment. In fact [F/G]N/OFQ(1–13)-NH2 has been reported to act as a NOP antagonist in the electrically stimulated mouse vas deferent [52] and as a NOP full agonist in the mouse colon [58]. Interestingly, in vivo the compound acted as full agonist in the tail withdrawal assay [59], as partial agonist when measuring locomotor activity [60] and as a pure antagonist in the cardiovascular system, blocking N/OFQ-induced bradycardia and hypotension [61] in mice.
As far as potency of partial agonist is concerned the following rank order has been measured:
that perfectly matches previous results reported in the literature [3, 54].
In addition, evidence of negative DMR traces has been observed for some of the agonists tested in some but not all of the experiments carried out, e.g. Ac-RYYRIK-NH2 displayed, in some but not all of the experiments carried, a concentration dependent negative signal after 1000 sec with potency values determined at negative peaks being superimposable to those at positive peaks. The reasons for this action of Ac-RYYRIK-NH2 are unknown.
It is worthy of mention that previous photo-affinity labelling experiments demonstrated that the NOP binding pocket for Ac-RYYRIK-NH2 [62] and for N/OFQ [63] are distinct although overlapping, and this may favor the selection of different conformations and eventually coupling of the NOP receptor in response to these ligands. However, DMR responses to N/OFQ and Ac-RYYRIK-NH2 were equally sensitive to PTX and to the NOP selective antagonist SB-612111. These results exclude, at least under the present conditions, major differences in the way Ac-RYYRIK-NH2 activates the NOP receptor in comparison to the natural ligand N/OFQ.
Finally a panel of NOP antagonists including [Nphe1]N/OFQ(1–13)-NH2 [64], UFP-101 [65], J-113397 [66], SB-612111 [67], C-24 [68], and C-35 [69] were tested in DMR experiments per se and against the stimulatory effects elicited by N/OFQ. All non-peptide compounds did not modify per se the DMR baseline, while [Nphe1]N/OFQ(1–13)-NH2 and UFP-101 elicited a stimulatory action with maximal effects of 0.88 and 0.20 (N/OFQ = 1.00). A substantial body of evidence reviewed in [70] demonstrated the in vitro and in vivo NOP antagonist features of [Nphe1]N/OFQ(1–13)-NH2 and UFP-101. However there are also some limited results that suggest the elimination of ligand efficacy by the Phe1 / Nphe1 substitution might not be complete. In fact, sodium and GTP concentrations affected the potency of [Nphe1]N/OFQ(1–13)-NH2 in a manner similar to that of agonists (N/OFQ) but not of pure antagonists (J-113397). In electrophysiological experiments, C-24 or Trap-101 behaved as pure antagonists in control neurons and as inverse agonists in neurons microinjected with a NOP receptor coding plasmid. In contrast, UFP-101 acted as an antagonist in control cells while it displayed partial agonist behavior in transfected neurons [71]. Finally, it has been recently reported that both [Nphe1]N/OFQ(1–13)-NH2 and UFP-101 displayed some residual agonists activity (0.55 and 0.14, respectively) in a BRET NOP/G protein interaction assay [5]. The amount of agonist activity of [Nphe1]N/OFQ(1–13)-NH2 did not allow antagonist experiments to be performed with this compound while UFP-101, together with non peptide molecules, was further investigated for its ability to counteract N/OFQ stimulated DMR responses. All compounds produced a dextral displacement of the concentration response curve to N/OFQ with the following rank order of antagonist potency:
which is in agreement with data in the literature [3]. Importantly, in line with a large body of evidence the action of N/OFQ at the NOP receptor was not antagonized by naloxone. The antagonist nature of SB-612111 has also been investigated using the classical Schild analysis confirming the competitive nature of this NOP receptor selective antagonist [5, 67, 72, 73]. Moreover, the same panel of NOP antagonists has been recently evaluated in parallel experiments performed with a BRET NOP/G protein interaction assay obtaining superimposable results. Interestingly, antagonist potency correlated with ability to induce receptor stability and crystallogenesis. Using this screening strategy, two structures of NOP in complex with candidate ligands SB-612111 and C-35 were solved [7] and compared to that previously obtained using C-24 [6].
Collectively the results obtained in this study demonstrated that the DMR assay can be successfully used to investigate the pharmacology of the NOP receptor, to characterize the effects of novel NOP receptor ligands, and to explore their signaling profile. In general this study confirms and extends previous findings (see studies quoted in the introduction section) demonstrating the usefulness of the DMR as an “integrative pharmacology” approach to be used to complement reductionist signaling pathway based approaches [74]. The potential value of the DMR assay goes far beyond its utility for basic pharmacology and drug screening investigations. In fact, the DMR assay can be used for investigating cell sensitivity to endogenous signals and drugs in cell lines expressing the native receptor or in primary cultured cells obtained from normal animals and models of pathology. In the longer-term, DMR studies can be performed comparing cellular responses in primary culture cells from normal subjects and from patients, from patients at different stages of disease and from patients treated with different drugs. These kinds of studies will contribute to the translational of medicine knowledge thus reducing the gap between discoveries in biomedical science and their safe and effective clinical application.
Supporting information
Concentration response curves to UFP-101 (panel A), J-113397 (panel B), SB-612111 (panel C), C-35 (panel D), C-24 (panel E), and naloxone (panel F). Representative traces were obtained from a representative experiment performed in triplicate.
(DOCX)
Acknowledgments
We would like to thank Prof DG Lambert (University of Leicester, UK) for proofreading this article.
Data Availability
All relevant data are within the paper.
Funding Statement
This work was supported by 5x1000 Giovani Ricercatori (University of Ferrara, IT) and German Academic Exchange Service (DAAD) Short-Term Grants, 2015 to DM. Both funders provided support in the form of salary for DM. This work was also supported by a FAR grant to GC (University of Ferrara, IT). All funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Meunier JC, Mollereau C, Toll L, Suaudeau C, Moisand C, Alvinerie P, et al. Isolation and structure of the endogenous agonist of opioid receptor-like ORL1 receptor. Nature. 1995;377:532–5. 10.1038/377532a0 [DOI] [PubMed] [Google Scholar]
- 2.Reinscheid RK, Nothacker HP, Bourson A, Ardati A, Henningsen RA, Bunzow JR, et al. Orphanin FQ: a neuropeptide that activates an opioidlike G protein-coupled receptor. Science. 1995;270(5237):792–4. . [DOI] [PubMed] [Google Scholar]
- 3.Toll L, Bruchas MR, Calo G, Cox BM, Zaveri NT. Nociceptin/Orphanin FQ Receptor Structure, Signaling, Ligands, Functions, and Interactions with Opioid Systems. Pharmacological reviews. 2016;68(2):419–57. 10.1124/pr.114.009209 ; PubMed Central PMCID: PMC4813427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chang SD, Mascarella SW, Spangler SM, Gurevich VV, Navarro HA, Carroll FI, et al. Quantitative Signaling and Structure-Activity Analyses Demonstrate Functional Selectivity at the Nociceptin/Orphanin FQ Opioid Receptor. Molecular pharmacology. 2015;88(3):502–11. 10.1124/mol.115.099150 ; PubMed Central PMCID: PMC4551045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Malfacini D, Ambrosio C, Gro MC, Sbraccia M, Trapella C, Guerrini R, et al. Pharmacological Profile of Nociceptin/Orphanin FQ Receptors Interacting with G-Proteins and beta-Arrestins 2. PloS one. 2015;10(8):e0132865 10.1371/journal.pone.0132865 ; PubMed Central PMCID: PMC4527783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thompson AA, Liu W, Chun E, Katritch V, Wu H, Vardy E, et al. Structure of the nociceptin/orphanin FQ receptor in complex with a peptide mimetic. Nature. 2012;485(7398):395–9. Epub 2012/05/19. doi: nature11085 [pii] 10.1038/nature11085 ; PubMed Central PMCID: PMC3356928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miller RL, Thompson AA, Trapella C, Guerrini R, Malfacini D, Patel N, et al. The Importance of Ligand-Receptor Conformational Pairs in Stabilization: Spotlight on the N/OFQ G Protein-Coupled Receptor. Structure. 2015;23(12):2291–9. 10.1016/j.str.2015.07.024 ; PubMed Central PMCID: PMC4670589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mustazza C, Bastanzio G. Development of nociceptin receptor (NOP) agonists and antagonists. Medicinal research reviews. 2011;31(4):605–48. 10.1002/med.20197 . [DOI] [PubMed] [Google Scholar]
- 9.Zaveri NT. Nociceptin Opioid Receptor (NOP) as a Therapeutic Target: Progress in Translation from Preclinical Research to Clinical Utility. Journal of medicinal chemistry. 2016;59(15):7011–28. 10.1021/acs.jmedchem.5b01499 ; PubMed Central PMCID: PMC5001850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nishi M, Houtani T, Noda Y, Mamiya T, Sato K, Doi T, et al. Unrestrained nociceptive response and disregulation of hearing ability in mice lacking the nociceptin/orphaninFQ receptor. Embo J. 1997;16(8):1858–64. 10.1093/emboj/16.8.1858 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Homberg JR, Mul JD, de Wit E, Cuppen E. Complete knockout of the nociceptin/orphanin FQ receptor in the rat does not induce compensatory changes in mu, delta and kappa opioid receptors. Neuroscience. 2009;163(1):308–15. 10.1016/j.neuroscience.2009.06.021 . [DOI] [PubMed] [Google Scholar]
- 12.Koster A, Montkowski A, Schulz S, Stube EM, Knaudt K, Jenck F, et al. Targeted disruption of the orphanin FQ/nociceptin gene increases stress susceptibility and impairs stress adaptation in mice. Proc Natl Acad Sci U S A. 1999;96(18):10444–9. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ozawa A, Brunori G, Mercatelli D, Wu J, Cippitelli A, Zou B, et al. Knock-In Mice with NOP-eGFP Receptors Identify Receptor Cellular and Regional Localization. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2015;35(33):11682–93. 10.1523/JNEUROSCI.5122-14.2015 ; PubMed Central PMCID: PMC4540802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Witkin JM, Statnick MA, Rorick-Kehn LM, Pintar JE, Ansonoff M, Chen Y, et al. The biology of Nociceptin/Orphanin FQ (N/OFQ) related to obesity, stress, anxiety, mood, and drug dependence. Pharmacology & therapeutics. 2014;141(3):283–99. 10.1016/j.pharmthera.2013.10.011 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lambert DG. The nociceptin/orphanin FQ receptor: a target with broad therapeutic potential. Nature reviews Drug discovery. 2008;7(8):694–710. 10.1038/nrd2572 . [DOI] [PubMed] [Google Scholar]
- 16.Rask-Andersen M, Almen MS, Schioth HB. Trends in the exploitation of novel drug targets. Nature reviews Drug discovery. 2011;10(8):579–90. 10.1038/nrd3478 . [DOI] [PubMed] [Google Scholar]
- 17.Kenakin T, Christopoulos A. Signalling bias in new drug discovery: detection, quantification and therapeutic impact. Nature reviews Drug discovery. 2013;12(3):205–16. 10.1038/nrd3954 . [DOI] [PubMed] [Google Scholar]
- 18.Staus DP, Strachan RT, Manglik A, Pani B, Kahsai AW, Kim TH, et al. Allosteric nanobodies reveal the dynamic range and diverse mechanisms of G-protein-coupled receptor activation. Nature. 2016;535(7612):448–52. 10.1038/nature18636 ; PubMed Central PMCID: PMC4961583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thomsen AR, Plouffe B, Cahill TJ 3rd, Shukla AK, Tarrasch JT, Dosey AM, et al. GPCR-G Protein-beta-Arrestin Super-Complex Mediates Sustained G Protein Signaling. Cell. 2016;166(4):907–19. 10.1016/j.cell.2016.07.004 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lefkowitz RJ. A brief history of G-protein coupled receptors (Nobel Lecture). Angewandte Chemie. 2013;52(25):6366–78. 10.1002/anie.201301924 . [DOI] [PubMed] [Google Scholar]
- 21.Beaulieu JM. In vivo veritas, the next frontier for functionally selective GPCR ligands. Methods. 2016;92:64–71. 10.1016/j.ymeth.2015.08.018 . [DOI] [PubMed] [Google Scholar]
- 22.Lieb S, Michaelis S, Plank N, Bernhardt G, Buschauer A, Wegener J. Label-free analysis of GPCR-stimulation: The critical impact of cell adhesion. Pharmacological research. 2016;108:65–74. 10.1016/j.phrs.2016.04.026 . [DOI] [PubMed] [Google Scholar]
- 23.Grundmann M, Kostenis E. Label-free biosensor assays in GPCR screening. Methods in molecular biology. 2015;1272:199–213. 10.1007/978-1-4939-2336-6_14 . [DOI] [PubMed] [Google Scholar]
- 24.Schroder R, Schmidt J, Blattermann S, Peters L, Janssen N, Grundmann M, et al. Applying label-free dynamic mass redistribution technology to frame signaling of G protein-coupled receptors noninvasively in living cells. Nature protocols. 2011;6(11):1748–60. 10.1038/nprot.2011.386 . [DOI] [PubMed] [Google Scholar]
- 25.Lee MY, Mun J, Lee JH, Lee S, Lee BH, Oh KS. A comparison of assay performance between the calcium mobilization and the dynamic mass redistribution technologies for the human urotensin receptor. Assay and drug development technologies. 2014;12(6):361–8. 10.1089/adt.2014.590 ; PubMed Central PMCID: PMC4142844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ferrie AM, Sun H, Fang Y. Label-free integrative pharmacology on-target of drugs at the beta(2)-adrenergic receptor. Scientific reports. 2011;1:33 10.1038/srep00033 ; PubMed Central PMCID: PMC3216520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carter RL, Grisanti LA, Yu JE, Repas AA, Woodall M, Ibetti J, et al. Dynamic mass redistribution analysis of endogenous beta-adrenergic receptor signaling in neonatal rat cardiac fibroblasts. Pharmacology research & perspectives. 2014;2(1). 10.1002/prp2.24 ; PubMed Central PMCID: PMC3968527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Deng H, Sun H, Fang Y. Label-free cell phenotypic assessment of the biased agonism and efficacy of agonists at the endogenous muscarinic M3 receptors. Journal of pharmacological and toxicological methods. 2013;68(3):323–33. 10.1016/j.vascn.2013.07.005 ; PubMed Central PMCID: PMC3858480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tran E, Sun H, Fang Y. Dynamic mass redistribution assays decode surface influence on signaling of endogenous purinergic P2Y receptors. Assay and drug development technologies. 2012;10(1):37–45. 10.1089/adt.2011.0392 ; PubMed Central PMCID: PMC3277731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Christensen HB, Gloriam DE, Pedersen DS, Cowland JB, Borregaard N, Brauner-Osborne H. Applying label-free dynamic mass redistribution assay for studying endogenous FPR1 receptor signalling in human neutrophils. Journal of pharmacological and toxicological methods. 2017;88(Pt 1):72–8. 10.1016/j.vascn.2017.07.003 . [DOI] [PubMed] [Google Scholar]
- 31.Fang Y, Ferrie AM. Optical biosensor differentiates signaling of endogenous PAR1 and PAR2 in A431 cells. BMC cell biology. 2007;8:24 10.1186/1471-2121-8-24 ; PubMed Central PMCID: PMC1925066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tang W, Huang B, Wang J, An L, Zhong H, Yang H, et al. A label-free screening approach targeted protease-activated receptor 1 based on dynamic mass redistribution in living cells. RSC Advances. 2017;7(68):43005–13. 10.1039/C7RA07927C [DOI] [Google Scholar]
- 33.Morse M, Tran E, Sun H, Levenson R, Fang Y. Ligand-directed functional selectivity at the mu opioid receptor revealed by label-free integrative pharmacology on-target. PloS one. 2011;6(10):e25643 10.1371/journal.pone.0025643 ; PubMed Central PMCID: PMC3189208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Morse M, Sun H, Tran E, Levenson R, Fang Y. Label-free integrative pharmacology on-target of opioid ligands at the opioid receptor family. BMC pharmacology & toxicology. 2013;14:17 10.1186/2050-6511-14-17 ; PubMed Central PMCID: PMC3602246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guerrini R, Marzola E, Trapella C, Pela M, Molinari S, Cerlesi MC, et al. A novel and facile synthesis of tetra branched derivatives of nociceptin/orphanin FQ. Bioorganic & medicinal chemistry. 2014;22(14):3703–12. 10.1016/j.bmc.2014.05.005 . [DOI] [PubMed] [Google Scholar]
- 36.Guerrini R, Calo G, Rizzi A, Bianchi C, Lazarus LH, Salvadori S, et al. Address and message sequences for the nociceptin receptor: a structure-activity study of nociceptin-(1–13)-peptide amide. Journal of medicinal chemistry. 1997;40(12):1789–93. 10.1021/jm970011b . [DOI] [PubMed] [Google Scholar]
- 37.Schroder R, Janssen N, Schmidt J, Kebig A, Merten N, Hennen S, et al. Deconvolution of complex G protein-coupled receptor signaling in live cells using dynamic mass redistribution measurements. Nature biotechnology. 2010;28(9):943–9. 10.1038/nbt.1671 . [DOI] [PubMed] [Google Scholar]
- 38.Neubig RR, Spedding M, Kenakin T, Christopoulos A, International Union of Pharmacology Committee on Receptor N, Drug C. International Union of Pharmacology Committee on Receptor Nomenclature and Drug Classification. XXXVIII. Update on terms and symbols in quantitative pharmacology. Pharmacological reviews. 2003;55(4):597–606. 10.1124/pr.55.4.4 . [DOI] [PubMed] [Google Scholar]
- 39.Mangmool S, Kurose H. G(i/o) protein-dependent and -independent actions of Pertussis Toxin (PTX). Toxins. 2011;3(7):884–99. 10.3390/toxins3070884 ; PubMed Central PMCID: PMC3202852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chan JS, Yung LY, Lee JW, Wu YL, Pei G, Wong YH. Pertussis toxin-insensitive signaling of the ORL1 receptor: coupling to Gz and G16 proteins. Journal of neurochemistry. 1998;71(5):2203–10. . [DOI] [PubMed] [Google Scholar]
- 41.Yung LY, Joshi SA, Chan RY, Chan JS, Pei G, Wong YH. GalphaL1 (Galpha14) couples the opioid receptor-like1 receptor to stimulation of phospholipase C. The Journal of pharmacology and experimental therapeutics. 1999;288(1):232–8. . [PubMed] [Google Scholar]
- 42.Eichel K, Jullie D, Barsi-Rhyne B, Latorraca NR, Masureel M, Sibarita JB, et al. Catalytic activation of beta-arrestin by GPCRs. Nature. 2018;557(7705):381–6. 10.1038/s41586-018-0079-1 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Grundmann M, Merten N, Malfacini D, Inoue A, Preis P, Simon K, et al. Lack of beta-arrestin signaling in the absence of active G proteins. Nature communications. 2018;9(1):341 10.1038/s41467-017-02661-3 ; PubMed Central PMCID: PMC5780443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Okada K, Sujaku T, Chuman Y, Nakashima R, Nose T, Costa T, et al. Highly potent nociceptin analog containing the Arg-Lys triple repeat. Biochemical and biophysical research communications. 2000;278(2):493–8. 10.1006/bbrc.2000.3822 . [DOI] [PubMed] [Google Scholar]
- 45.Rizzi A, Spagnolo B, Wainford RD, Fischetti C, Guerrini R, Marzola G, et al. In vitro and in vivo studies on UFP-112, a novel potent and long lasting agonist selective for the nociceptin/orphanin FQ receptor. Peptides. 2007;28(6):1240–51. 10.1016/j.peptides.2007.04.020 ; PubMed Central PMCID: PMC1975813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rizzi A, Malfacini D, Cerlesi MC, Ruzza C, Marzola E, Bird MF, et al. In vitro and in vivo pharmacological characterization of nociceptin/orphanin FQ tetrabranched derivatives. British journal of pharmacology. 2014;171(17):4138–53. 10.1111/bph.12799 ; PubMed Central PMCID: PMC4243985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rover S, Adam G, Cesura AM, Galley G, Jenck F, Monsma FJ Jr., et al. High-affinity, non-peptide agonists for the ORL1 (orphanin FQ/nociceptin) receptor. Journal of medicinal chemistry. 2000;43(7):1329–38. . [DOI] [PubMed] [Google Scholar]
- 48.Calo G, Rizzi A, Ruzza C, Ferrari F, Pacifico S, Gavioli EC, et al. Peptide welding technology—A simple strategy for generating innovative ligands for G protein coupled receptors. Peptides. 2018;99:195–204. 10.1016/j.peptides.2017.10.004 . [DOI] [PubMed] [Google Scholar]
- 49.Gestwicki JE, Cairo CW, Strong LE, Oetjen KA, Kiessling LL. Influencing receptor-ligand binding mechanisms with multivalent ligand architecture. Journal of the American Chemical Society. 2002;124(50):14922–33. . [DOI] [PubMed] [Google Scholar]
- 50.Vauquelin G, Charlton SJ. Long-lasting target binding and rebinding as mechanisms to prolong in vivo drug action. British journal of pharmacology. 2010;161(3):488–508. 10.1111/j.1476-5381.2010.00936.x ; PubMed Central PMCID: PMC2990149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rizzi A, Sukhtankar DD, Ding H, Hayashida K, Ruzza C, Guerrini R, et al. Spinal antinociceptive effects of the novel NOP receptor agonist PWT2-nociceptin/orphanin FQ in mice and monkeys. British journal of pharmacology. 2015;172(14):3661–70. 10.1111/bph.13150 ; PubMed Central PMCID: PMC4507167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Guerrini R, Calo G, Rizzi A, Bigoni R, Bianchi C, Salvadori S, et al. A new selective antagonist of the nociceptin receptor. British journal of pharmacology. 1998;123(2):163–5. 10.1038/sj.bjp.0701640 ; PubMed Central PMCID: PMC1565170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dooley CT, Spaeth CG, Berzetei-Gurske IP, Craymer K, Adapa ID, Brandt SR, et al. Binding and in vitro activities of peptides with high affinity for the nociceptin/orphanin FQ receptor, ORL1. The Journal of pharmacology and experimental therapeutics. 1997;283(2):735–41. . [PubMed] [Google Scholar]
- 54.Ferrari F, Cerlesi MC, Malfacini D, Asth L, Gavioli EC, Journigan BV, et al. In vitro functional characterization of novel nociceptin/orphanin FQ receptor agonists in recombinant and native preparations. European journal of pharmacology. 2016. 10.1016/j.ejphar.2016.10.025 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Camarda V, Fischetti C, Anzellotti N, Molinari P, Ambrosio C, Kostenis E, et al. Pharmacological profile of NOP receptors coupled with calcium signaling via the chimeric protein G alpha qi5. Naunyn-Schmiedeberg's archives of pharmacology. 2009;379(6):599–607. 10.1007/s00210-009-0396-x . [DOI] [PubMed] [Google Scholar]
- 56.Kenakin TP. Chapter 5—Agonists: The Measurement of Affinity and Efficacy in Functional Assays. A Pharmacology Primer (Third Edition). New York: Academic Press; 2009. p. 81–100. [Google Scholar]
- 57.McDonald J, Barnes TA, Okawa H, Williams J, Calo G, Rowbotham DJ, et al. Partial agonist behaviour depends upon the level of nociceptin/orphanin FQ receptor expression: studies using the ecdysone-inducible mammalian expression system. British journal of pharmacology. 2003;140(1):61–70. 10.1038/sj.bjp.0705401 ; PubMed Central PMCID: PMC1573999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rizzi A, Bigoni R, Calo G, Guerrini R, Salvadori S, Regoli D. [Nphe(1)]nociceptin-(1–13)-NH(2) antagonizes nociceptin effects in the mouse colon. European journal of pharmacology. 1999;385(2–3):R3–5. . [DOI] [PubMed] [Google Scholar]
- 59.Calo G, Rizzi A, Marzola G, Guerrini R, Salvadori S, Beani L, et al. Pharmacological characterization of the nociceptin receptor mediating hyperalgesia in the mouse tail withdrawal assay. British journal of pharmacology. 1998;125(2):373–8. 10.1038/sj.bjp.0702087 ; PubMed Central PMCID: PMC1565633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rizzi A, Bigoni R, Marzola G, Guerrini R, Salvadori S, Regoli D, et al. Characterization of the locomotor activity-inhibiting effect of nociceptin/orphanin FQ in mice. Naunyn-Schmiedeberg's archives of pharmacology. 2001;363(2):161–5. . [DOI] [PubMed] [Google Scholar]
- 61.Madeddu P, Salis MB, Milia AF, Emanueli C, Guerrini R, Regoli D, et al. Cardiovascular effects of nociceptin in unanesthetized mice. Hypertension. 1999;33(3):914–9. . [DOI] [PubMed] [Google Scholar]
- 62.Bes B, Meunier JC. Identification of a hexapeptide binding region in the nociceptin (ORL1) receptor by photo-affinity labelling with Ac-Arg-Bpa-Tyr-Arg-Trp-Arg-NH2. Biochemical and biophysical research communications. 2003;310(3):992–1001. . [DOI] [PubMed] [Google Scholar]
- 63.Mouledous L, Topham CM, Mazarguil H, Meunier JC. Direct identification of a peptide binding region in the opioid receptor-like 1 receptor by photoaffinity labeling with [Bpa(10),Tyr(14)]nociceptin. The Journal of biological chemistry. 2000;275(38):29268–74. 10.1074/jbc.M004971200 . [DOI] [PubMed] [Google Scholar]
- 64.Calo G, Guerrini R, Bigoni R, Rizzi A, Marzola G, Okawa H, et al. Characterization of [Nphe(1)]nociceptin(1–13)NH(2), a new selective nociceptin receptor antagonist. British journal of pharmacology. 2000;129(6):1183–93. 10.1038/sj.bjp.0703169 ; PubMed Central PMCID: PMC1571948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Calo G, Rizzi A, Rizzi D, Bigoni R, Guerrini R, Marzola G, et al. [Nphe1,Arg14,Lys15]nociceptin-NH2, a novel potent and selective antagonist of the nociceptin/orphanin FQ receptor. British journal of pharmacology. 2002;136(2):303–11. 10.1038/sj.bjp.0704706 ; PubMed Central PMCID: PMC1573345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ozaki S, Kawamoto H, Itoh Y, Miyaji M, Azuma T, Ichikawa D, et al. In vitro and in vivo pharmacological characterization of J-113397, a potent and selective non-peptidyl ORL1 receptor antagonist. European journal of pharmacology. 2000;402(1–2):45–53. . [DOI] [PubMed] [Google Scholar]
- 67.Zaratin PF, Petrone G, Sbacchi M, Garnier M, Fossati C, Petrillo P, et al. Modification of nociception and morphine tolerance by the selective opiate receptor-like orphan receptor antagonist (-)-cis-1-methyl-7-[[4-(2,6-dichlorophenyl)piperidin-1-yl]methyl]-6,7,8,9-tetrahy dro-5H-benzocyclohepten-5-ol (SB-612111). The Journal of pharmacology and experimental therapeutics. 2004;308(2):454–61. 10.1124/jpet.103.055848 . [DOI] [PubMed] [Google Scholar]
- 68.Goto Y, Arai-Otsuki S, Tachibana Y, Ichikawa D, Ozaki S, Takahashi H, et al. Identification of a novel spiropiperidine opioid receptor-like 1 antagonist class by a focused library approach featuring 3D-pharmacophore similarity. Journal of medicinal chemistry. 2006;49(3):847–9. 10.1021/jm0509851 . [DOI] [PubMed] [Google Scholar]
- 69.Trapella C, Fischetti C, Pela M, Lazzari I, Guerrini R, Calo G, et al. Structure-activity studies on the nociceptin/orphanin FQ receptor antagonist 1-benzyl-N-{3-[spiroisobenzofuran-1(3H),4'-piperidin-1-yl]propyl} pyrrolidine-2-carboxamide. Bioorganic & medicinal chemistry. 2009;17(14):5080–95. 10.1016/j.bmc.2009.05.068 . [DOI] [PubMed] [Google Scholar]
- 70.Calo’ G, Guerrini R. Medicinal Chemistry, Pharmacology, and Biological Actions of Peptide Ligands Selective for the Nociceptin/Orphanin FQ Receptor. Research and Development of Opioid-Related Ligands. ACS Symposium Series. 1131: American Chemical Society; 2013. p. 275–325.
- 71.Mahmoud S, Margas W, Trapella C, Calo G, Ruiz-Velasco V. Modulation of silent and constitutively active nociceptin/orphanin FQ receptors by potent receptor antagonists and Na+ ions in rat sympathetic neurons. Molecular pharmacology. 2010;77(5):804–17. 10.1124/mol.109.062208 ; PubMed Central PMCID: PMC2872970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Marti M, Mela F, Budri M, Volta M, Malfacini D, Molinari S, et al. Acute and chronic antiparkinsonian effects of the novel nociceptin/orphanin FQ receptor antagonist NiK-21273 in comparison with SB-612111. British journal of pharmacology. 2013;168(4):863–79. 10.1111/j.1476-5381.2012.02219.x ; PubMed Central PMCID: PMC3631376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Spagnolo B, Carra G, Fantin M, Fischetti C, Hebbes C, McDonald J, et al. Pharmacological characterization of the nociceptin/orphanin FQ receptor antagonist SB-612111 [(-)-cis-1-methyl-7-[[4-(2,6-dichlorophenyl)piperidin-1-yl]methyl]-6,7,8,9-tetrah ydro-5H-benzocyclohepten-5-ol]: in vitro studies. The Journal of pharmacology and experimental therapeutics. 2007;321(3):961–7. 10.1124/jpet.106.116764 . [DOI] [PubMed] [Google Scholar]
- 74.Lieb S, Littmann T, Plank N, Felixberger J, Tanaka M, Schafer T, et al. Label-free versus conventional cellular assays: Functional investigations on the human histamine H1 receptor. Pharmacological research. 2016;114:13–26. 10.1016/j.phrs.2016.10.010 . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Concentration response curves to UFP-101 (panel A), J-113397 (panel B), SB-612111 (panel C), C-35 (panel D), C-24 (panel E), and naloxone (panel F). Representative traces were obtained from a representative experiment performed in triplicate.
(DOCX)
Data Availability Statement
All relevant data are within the paper.