Abstract
Background
Arterial graft spasm is a severe complication after coronary artery bypass graft (CABG). Among numerous potential antispasmodic agents, systemic application of diltiazem and nitroglycerin had been investigated most frequently over the past three decades. However, it remains inconclusive if either or both agents could improve patient outcomes by preventing graft spasm when applied perioperatively, and, if so, which one would be a better choice. The current systematic review and network meta-analysis aims to summarize the data from all available randomized clinical trials of perioperative continuous intravenous infusion of diltiazem and/or nitroglycerin in patients undergoing on-pump CABG in order to define and compare their roles in graft spasm prevention and their impacts on perioperative outcomes.
Methods
We searched Ovid Medline, PubMed, CINAHL, Google Scholar and Cochrane Center for randomized controlled trials that reported outcome effects of perioperative continuous intravenous infusion of diltiazem and/or nitroglycerin in patients undergoing elective on-pump CABG. Conventional meta-analyses were conducted to evaluate the pairwise comparisons (diltiazem vs. placebo; nitroglycerin vs. placebo; diltiazem vs. nitroglycerin) on perioperative outcomes. Network meta-analyses were implemented to compare the three regimens through direct and indirect comparison.
Results
Twenty-seven studies involving 1,660 patients were included. Pairwise and network meta-analyses found no significant difference in mortality among the groups. There are four studies that reported blood flow measurements of internal mammary artery grafts intraoperatively after dissecting or immediately after distal anastomosis while patients were on continuous intravenous infusion of diltiazem and nitroglycerin. Although insufficient for data synthesis, the measured results from all four studies suggest that both diltiazem and nitroglycerin significantly increased blood flow of arterial grafts compared to placebo. For other perioperative outcomes, compared to diltiazem, patients that received nitroglycerin had higher odds of postoperative atrial fibrillation (OR = 2.67, 95% CI: 1.15 to 6.24) and higher peak serum cardiac enzymes. Patients that received placebo had higher odds of atrial fibrillation (OR = 3.00, 95% CI: 1.18 to 7.63) and lower odds of requiring inotrope support (OR = 0.19, 95% CI: 0.04 to 0.73) compared to diltiazem. Data from the network meta-analysis indicated that diltiazem had significantly lower odds of postoperative atrial fibrillation compared to nitroglycerin (OR = 0.39, 95% CI: 0.18 to 0.85). In fact, the rank from highest to lowest rates of postoperative atrial fibrillation was placebo>nitroglycerin>diltiazem. The rank from highest to lowest odds of requiring inotropic support is nitroglycerin> diltiazem>placebo. However, placebo had significantly higher odds of postoperative myocardial infarction than diltiazem (OR = 4.51, 95% CI: 1.34 to 15.25). The rank from highest to lowest odds of postoperative myocardial infarction, transient cardiac ischemic event and atrial fibrillation is placebo>nitroglycerin>diltiazem.
Conclusion
Compared to nitroglycerin and placebo, perioperative continuous intravenous infusion of diltiazem had stronger protective effects against postoperative ischemic cardiac injuries and atrial fibrillation although patients may need more inotropic support. The increased blood flow from diltiazem use in arterial grafts may potentially contribute to the drug’s outcome benefits.
Introduction
Coronary artery bypass graft (CABG) has been established as the standard procedure of revascularization for patients with multi-vessel coronary artery disease (CAD). The application of autologous grafts on arteries including internal mammary arteries (IMA), radial arteries (RA), gastroepiploic arteries and inferior epigastric arteries has greatly improved the short- and long-term outcomes of CABG. Compared with saphenous vein grafts (SVGs), arterial grafts have significantly higher graft patency over time. In fact, some authors proposed using total arterial grafts to replace SVGs, although, currently, there is insufficient clinical follow-up data to support this strategy[1–3].
One of the challenging issues with arterial grafts is graft spasm leading to intra- and/or post-operative myocardial ischemia and cardiac arrhythmia. While arterial graft spasm was first reported clinically in 1987[4,5], its mechanisms remain unclear. However, studies have indicated that it is likely multifactorial, including mechanical stimulation from graft manipulation, vasoactive molecules released from activated endothelial cells during cardiac reperfusion, the application of certain vasoactive drugs and so on. These insults cause increased extracellular influx and intracellular sarcoplasmic release of calcium ions through different signaling pathways. A previous meta-analysis indicated that perioperative application of different calcium channel blockers (CCBs) had significant outcome benefits for patients undergoing all types of cardiac surgeries requiring cardiopulmonary bypass (CPB)[5]. Topical application of CCBs on isolated IMAs and RAs consistently showed evidence of vasodilatation and increased blood flow[6,7].
As our understanding of its mechanisms improves, increasing effort has been devoted to developing effective pharmacological interventions for preventing graft spasm after CABG. The most investigated agents for this purpose are CCBs and nitrates. There are also clinical studies for alpha-1 blockers[8], phosphodiesterase III inhibitors, e.g. milrinone[9,10], calcium sensitizers, e.g. levosimendan [11], potassium channel openers, e.g. aprikalim[12], and prostacyclin analogues, e.g. iloprost[13]. These investigational drugs were applied in various ways: topically or intra-graft injection[6,14], systemically through bolus and/or intravenous (IV) infusion, or mixed in cardioplegia during CPB[15,16].
Diltiazem (DILT), a benzothiazepine-type CCB, is well known for relieving coronary spasm while uniquely being able to promote vasodilation without rebound tachycardia. Nitroglycerin (NTG) infusion is also one of the first line therapeutic interventions of unstable angina; injection of NTG into IMA grafts showed potent vasodilatation[17]. This systematic review and network meta-analysis therefore aims to summarize the available outcome data from clinical trials involving perioperative continuous IV infusion of DILT and/or NTG in patients undergoing on-pump CABG, and compare the drugs’ effects on graft blood flow, perioperative mortality, perioperative hemodynamic stability, ventricular functions, postoperative myocardial infarction (MI), new onset cardiac arrhythmias and requirement of inotropic support.
Materials and methods
Literature search strategy
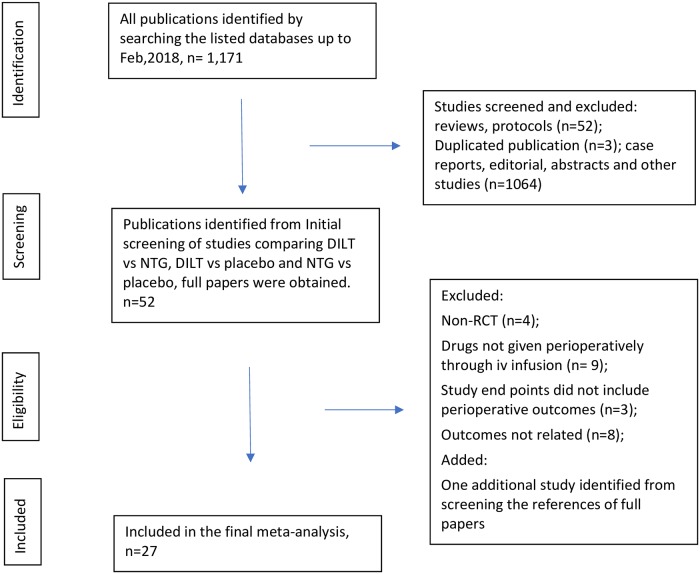
This systematic review and network meta-analysis was conducted based on the criteria of the Preferred Reporting Items for Systematic reviews and Meta-analysis (PRISMA) statement[18]. As shown in Fig 1, a comprehensive literature search was conducted using databases Ovid Medline, PubMed, EMBASE, CINAHL, Google Scholar and Cochrane Central Registry of Controlled Trials. These databases hosted papers published between 1946 and the end of February 2018. The search terms used were: “cardiac surgery”, “thoracic surgery”, “cardiac surgical procedures”, “coronary artery bypass”, “cardiopulmonary bypass”, “calcium channel blocker”, “diltiazem”, “nitrates”, “nitroglycerin”. The detailed search criteria applied in Ovid Medline are shown in S1 Table. The included studies should be randomized controlled trials (RCTs) or prospective cohort studies investigating effects of perioperative continuous IV infusion of DILT and/or NTG on arterial graft flow and perioperative outcomes in adult patients undergoing on-pump CABG. The seven reasons for a study to be excluded from final enrollment are: (1) the study is retrospective, (2) the study’s subjects were not limited to adult patients, (3) the studied drugs were administered topically, through intra-graft injection or mixed in cardioplegia, (4) the study was of non-cardiac surgeries, off-pump CABG or cardiac surgical procedures necessitating cardiac chamber opening, (5) the study was published as an abstract, a case report, case series, letter to the editor, editorial, narrative or systematic review, meta-analysis, or a study that did not report the investigated outcomes (6) the paper is a duplicated publication of an enrolled study, (7) the study was not approved by an institutional review board.
Fig 1. PRISMA flowchart.
The literature search and study enrollment. Flow chart for literature enrollment from identification to final synthesis according to the PRISMA protocol. DILT = diltiazem; NTG = nitroglycerin; RCT = randomized controlled trial.
The initially-identified studies were screened by one reviewer (XZ) for RCTs or prospective cohort studies. The screened publications were verified by the second reviewer (XY) before being enrolled into the final systematic review and network meta-analysis. We also manually searched through the references of the enrolled papers for potential studies not captured by the database searching strategy.
Data extraction
Data from enrolled studies were extracted into a spreadsheet by two reviewers (XZ, XY), independently. This data included sample size, geographic regions, age, sex, race/ethnicity and outcomes regarding cardiac functions. Disputations during the process of literature searching and data extraction were resolved upon reaching consensus through discussions with all the co-authors.
The complete texts of the enrolled studies were inspected by the authors independently and the following outcome parameters were extracted: patient characteristics, measurement of arterial graft blood flow, perioperative mortality, incidence of post-operative MI (post-MI), postoperative atrial fibrillation (A-fib), transient cardiac ischemic event (TIE), inotrope requirement, peak postoperative cardiac enzymes and hemodynamic parameters, such as heart rate (HR), cardiac index (CCI), mean blood pressure (mBP) and mean pulmonary arterial pressure (mPAP) and pulmonary artery wedge pressure (PAWP).
The primary outcomes are graft blood flow alteration and perioperative mortality. The remaining perioperative outcomes were further categorized into: (1) cardiac protection outcomes including post-MI, TIE, A-fib and postoperative peak cardiac enzymes. (2) cardiac function outcomes including HR, mBP, mPAP, PAWP and requirement of inotropic support.
Although the results from the individual studies were reported in different formats, the continuous variables were all converted in the data extraction spreadsheet prepared for future meta-analysis, if necessary. The values were converted to mean and standard deviation, the dichotomous variables for frequency of events.
Quality assessment
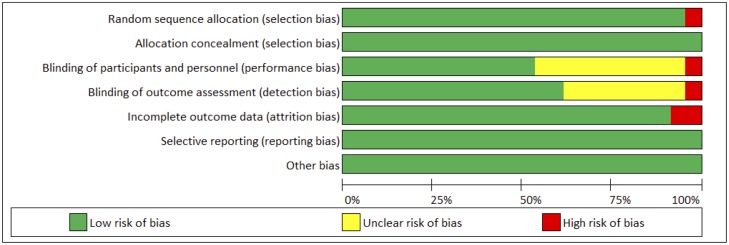
The RCTs were evaluated by two reviewers using the Cochrane risk of bias assessment tool[19], which evaluated 6 domains including random assignment, allocation concealment, blinding of participants, incomplete outcome data, selective outcome reporting and other sources of bias. The assessment of “yes,” “no,” or “unclear” was assigned to each domain for respective designation of a low, high, or unclear risk of bias. If “unclear” or “no” was assigned to one or less domains, the study was evaluated as having a low risk of bias. If over four domains were assigned “unclear” or “no”, the study was evaluated as having a moderate risk[20], see Fig 2.
Fig 2. Quality assessment of enrolled clinical trials.
Quality assessment was conducted using the Cochrane risk of bias assessment tool. Risk of bias assessment for included studies in meta-analysis was classified as “high”, “low” or “unclear”.
Statistical analyses
First, the pairwise meta-analyses were conducted for each included outcome using random-effects model. Odds ratios (ORs) and 95% confidence intervals (95% CIs) were estimated for binary outcomes. Mean differences (MDs) and 95% CIs were calculated for continuous outcomes. The pooled OR is considered statistically significant if 95% CI did not contain 1, and the pooled MD is considered statistically significant if 95% CI did not contain 0. Individual and pooled estimates were illustrated using forest plots.
Second, network meta-analysis (NMA) was performed to incorporate multiple comparisons for each available outcome using multivariate meta-analyses, where the within-network heterogeneity was assumed common and the heterogeneity variance was estimated using restricted maximum likelihood (REML). For all three pairwise comparisons (closed triangle loop), both direct and indirect comparisons were integrated to evaluate the effect sizes (ORs, MDs) and 95% CIs. For those outcomes with any two pairwise comparisons available (open triangle loop), indirect estimates for the third pairwise comparison were estimated (ORs, MDs and 95% CIs).
Global test for inconsistency was performed using the Wald test statistic, which follows a chi-squared distribution under the consistency assumption[21]. P-value greater than 0.05 indicates no evidence of inconsistency. The rank probability of three treatment effects were computed using the surface under the cumulative ranking curve (SUCRA)[22]. Publication bias was evaluated using funnel plots. Sensitivity analysis was conducted by excluding studies with extreme results, defined as larger than twice or smaller than half of the pooled results. All analyses were conducted using Stata 14 (Stata Corp, College Station, TX).
Results
Baseline characteristics for included studies
A total of 1,660 patients were recorded in the 27 included studies. The clinical characteristics are shown in Table 1. Twenty-three trials belonged to two-arm trials, three were in the category of three-arm trials and one was in the category of four-arm trials.
Table 1. Study characters of enrolled clinical trials.
| Authors, years, journals | Sample size | Arterial Grafts | Drug application | Drug dosage | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NMA study lists | Journal | Total | DILT | NTG | Placebo | IMA | Radial | Drug starting | Drug ending | Drug route | DILT dosage | NTG dosage |
| Donegani 1986[23] | Thorac cardiovas Surgeon | 40 | 20 | 20 | not specified | induction GA | 48 h after releasing aortic cross clamp | IV infusion | 0.5~3.0 mcg/kg/min | 0.5 ~1.5 mcg/kg/min | ||
| Hannes 1993[24] | Eur J Cardiothorac Surg | 91 | 44 | 47 | Yes | initiation of CPB | 24 h after releasing aortic cross clamp | IV infusion | 0.1 mg/kg/h | 1 mcg/kg/min | ||
| Seitelberger 1994[25] | J Thorac Cardiovasc Surg | 120 | 60 | 60 | Yes | initiation of CPB | 24h after releasing of aortic cross clamp | IV infusion | 0.1 mg/kg/h | 1 mcg/kg/min | ||
| Hannes 1995[26] | European Heart Journal | 66 | 31 | 33 | Yes | initiation of CPB | 24 h after releasing aortic cross clamp | IV infusion | 0.1 mg/kg/h | 1 mcg/kg/min | ||
| Keilich 1997[27] | International Journal of Angiology | 211 | 104 | 107 | Yes | initiating CPB | 24 h after releasing aortic cross clamp | IV infusion | 0.1 mg/kg/h | 1 mcg/kg/min | ||
| Malhotra 1997[28] | Eur J Cardiothorac Surg | 71 | 34 | 37 | Yes | initiation of CPB | 24 h after starting drug infusion | IV infusion | 0.1 mg/kg/h | 1 mcg/kg/min | ||
| Lischke 1997[29] | Anesthetist | 55 | 29 | 26 | not specified | before induction of GA | reach ICU postoperatively | IV bolus and infusion | 0.15 mg/kg, then 3mcg/kg/min | 1 mcg/kg/min | ||
| Shapira 2000[30] | Ann Thorac Surg | 161 | 77 | 84 | Yes | induction GA | 24h post operatively | IV infusion, oral | 0.1mg/kg/min | 0.1 mcg/kg/min | ||
| Lassnigg 2001[31] | Wien Klin Wochenschr | 49 | 24 | 25 | Yes | initiation of CPB | 24 h post op | IV infusion | 0.1 mg/kg/h | 1 mcg/kg/min | ||
| Hirnle 2000[32] | kardiol Pol | 49 | 24 | 25 | Yea | 48 h before CABG | 24 h post op | oral and IV infusion | 0.1mg/kg/min | 1mg/h | ||
| Zhang 2003[33] | Natl Med J China | 40 | 20 | 20 | Yes | initiation of CPB | 24 h after releasing of aortic cross clamp | IV infusion | 0.1 mg/kg/h | 1mcg/kg/min | ||
| Tabel 2004[34] | Eur J Cardiothorac Surg | 60 | 30 | 30 | Yes | Sternotomy | after second flow measurement | IV infusion | 0.05~0.1 mg/kg/h | 0.25~2.5 mcg/kg/min | ||
| Colson 1992[35] | J Cardiothorac Vasc Anesth | 29 | 15 | 14 | not specified | induction of GA | IV infusion | 2ug/kg/min | ||||
| Zanardo 1993[36] | J Cardiothorac Vasc Anesth | 24 | 12 | 12 | not specified | induction of GA | 24 h post op | IV infusion | 2mcg/kg/min | |||
| Amano 1995[37] | Chest | 23 | 13 | 10 | not specified | Sternotomy | not specified | IV bolus, infusion and oral | 0.1 mg/kg bolus, 2 mcg/kg/min until unclamp, then oral 30mg q8h | |||
| Babin-Ebell 1996[38] | Eur J Cardio-thoracic Surg | 70 | 33 | 37 | Yes | induction GA | 72h after releasing aortic cross clamp | IV infusion | 0.1 mg/kg/h | |||
| Yavuz 2002[39] | Med Sci Monit | 30 | 15 | 15 | Yes | 24 h pre-op | 48 h post op | IV infusion | 2 mcg kg/min | |||
| Fansa 2003[40] | Med Sci Monit | 30 | 15 | 15 | Yes | initiation of CPB | conclusion of CPB | IV infusion | ||||
| Erdem 2015[41] | Bra J Cardiovas Surg | 140 | 70 | 70 | Yes | yes | induction of GA | IV infusion | 2.5 mcg/kg/min | |||
| Thomson 1984[42] | Anesthesiology | 20 | 9 | 11 | not specified | before induction of GA | until opening of pericardium | IV infusion | 0.5 mcg/kg/min | |||
| Gallagher 1986[43] | Anesthesiology | 81 | 41 | 40 | not specified | initiating CPB | no specified | IV infusion | 1 mcg/kg/min | |||
| Withington 1988[44] | European Heart Journal | 14 | 7 | 7 | not specified | releasing cross clamp | not specified | IV infusion | 1 mcg/kg/min | |||
| Lell et al. 1993[45] | J Card Surg | 30 | 20 | 10 | Yes | Induction of GA | initiation of CPB | IV infusion | 1 or 2 mcg/kg/min | |||
| Knothe 1993[46] | Herz | 30 | 15 | 15 | Yes | induction of GA | after releasing aortic cross clamp | IV infusion | 1.5 mcg/kg/min | |||
| Apostolidou 1999[47] | Ann Thorac Surg | 47 | 30 | 17 | Yes | after releasing aortic cross clamp | 24 h post op | IV infusion | 0.5~1 mcg/kg/min | |||
| Chen 2000[48] | Chinese Journal of Surgery | 40 | 20 | 20 | not specified | 30 mins before induction of GA | 24 h post op | IV infusion | 10ug/kg/min | |||
| Zvara 2000[49] | J Cardiothorac Vasc Anesth | 39 | 20 | 20 | Yes | induction of GA | 6 h after extubation | IV infusion | 2 mcg/kg/min | |||
NMA, network meta-analysis; CABG, coronary artery bypass graft; CPB, cardiopulmonary bypass; GA, general anesthesia; IV, intravenous.
Quality of enrolled studies
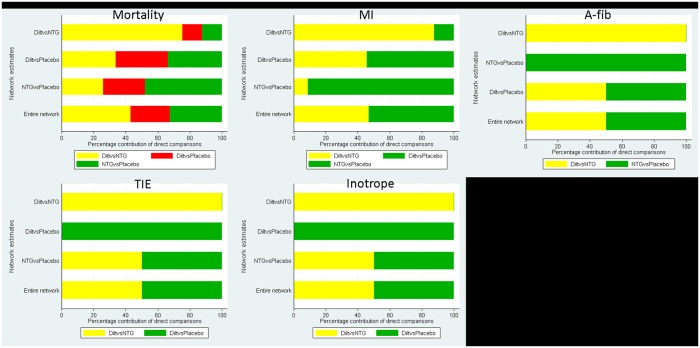
Two reviewers (XZ and XY) independently assessed concealment of allocation, blinding, and adequacy of analyses. Table 2 presented the quality assessment results using the Cochrane risk of bias assessment tool, with a score ranging from 5 to 7. Note that risk of bias can differ across different outcomes of interest, as each outcome draws from a different subset of studies. To ensure the relative contributions of different sources of direct evidence are accounted for appropriately, we presented risk of bias for each network estimate that integrated pairwise comparisons for primary outcomes. In Fig 3, the colors represent the risk of bias (green: low, yellow: moderate, red: high).
Table 2. Risk of bias for enrolled studies.
| Selection bias | Performance bias | Detection bias | Attrition bias | Reporting bias | Other bias | ||
|---|---|---|---|---|---|---|---|
| Study List | Random sequence generation | Allocation concealment | Blinding of participants and personnel | Blinding of outcome assessment | Incomplete outcome data | Selective reporting | Other sources of bias |
| Donegani 1986 | high risk, prospective non-randomized | unclear | low risk | low risk | low risk | low risk | low risk |
| Hannes1993 | low risk | unclear | low risk | low risk | low risk | low risk | low risk |
| Seitelberger 1994 | low risk | unclear | low risk | low risk | low risk | low risk | low risk |
| Hannes1995 | low risk | unclear | low risk | low risk | low risk | low risk | low risk |
| Keilich 1997 | low risk, "patients were randomly assingned to …" | unclear, did not specify | low risk, the outcome is unlikely to be affected by not complete blinding | low risk, not blind record review, however unlikely to be influenced | low risk | low risk | low risk |
| Malhotra 1997 | low risk "random assignement…done" | unclear, did not specify | low risk, the outcome is unlikely to be affected by not complete blinding | low risk, record review, unlikely to be influenced by not blinding | low risk | low risk | low risk |
| Lischk 1997 | low risk | low risk | low risk, double blinded | low risk, double blinded | low risk | low risk | low risk |
| Hirnle 2000 | low risk | unclear | low risk | low risk | low risk | low risk | low risk |
| Shapira 2000 | low risk, last digit of medical record number | unclear | low risk | low risk | low risk, missing data in 16/161, however, long term outcomes not included in meta- analysis | low risk | low risk |
| Lassnigg 2001 | low risk randomly assigned | unclear | low risk | low risk | unclear, one patient excluded after procedure | low risk | low risk |
| Zhang 2003 | unclear, randomization done with date of surgery | unclear | low risk | low risk | low risk | low risk | low risk |
| Tabel 2003 | low risk | unclear | low risk | low risk | low risk | low risk | low risk |
| Colso 1992 | low risk | low risk | low risk, double blinded | low risk | low risk | low risk | low risk |
| Zanardo 1993 | low risk | unclear | low risk | low risk | low risk | low risk | low risk |
| Armano 1995 | low risk | low risk | unclear | unclear | low risk | low risk | low risk |
| Babin-Ebell 1996 | low risk | unclear | low risk | low risk | low risk | low risk | low risk |
| Yavuz 2002 | low risk | unclear | low risk | low risk | low risk | low risk | low risk |
| Fansa 2003 | high risk, prospective non-randomized | unclear | low risk | low risk | low risk | low risk | low risk |
| Erdem 2015 | low risk | unclear | low risk | low risk | low risk | low risk | low risk |
| Thomson 1984 | low risk | unclear | low risk | low risk | low risk | low risk | low risk |
| Gallag 1986 | low risk | low risk | low risk, double blinded | low risk | low risk | low risk | low risk |
| Withington 1988 | low risk | unclear | low risk | low risk | low risk | low risk | low risk |
| Lell 1993 | low risk | unclear | low risk | low risk | low risk | low risk | low risk |
| Knothe 1993 | low risk | low risk | low risk | low risk | low risk | low risk | low risk |
| Apostolidou 1999 | low risk, computer randomization | low risk | low risk | low risk | low risk | low risk | low risk |
| Chen 2000 | unclear | unclear | low risk | low risk | low risk | low risk | low risk |
| Zvara 2000 | low risk | low risk | low risk, double blinded | low risk | low risk | low risk | low risk |
Fig 3. Network estimate for risk of bias for primary outcomes.
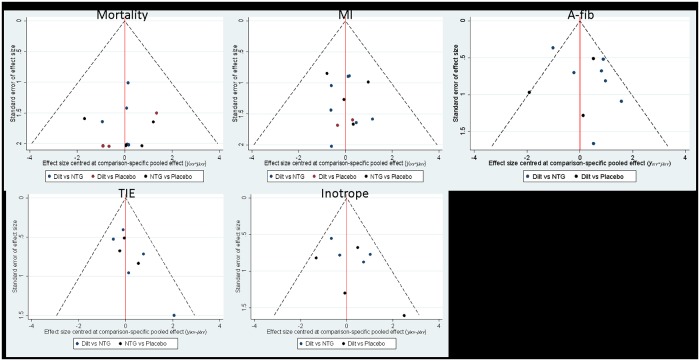
For primary outcomes of closed-loop or open-loop network estimates, we presented funnel plots comparing any active intervention with non-intervention from pairwise studies. In Fig 4, we observed there were no indications of asymmetry on funnel plots of the pooled estimates, where different colors represent different pairwise comparisons.
Fig 4. Funnel plots for primary outcomes.
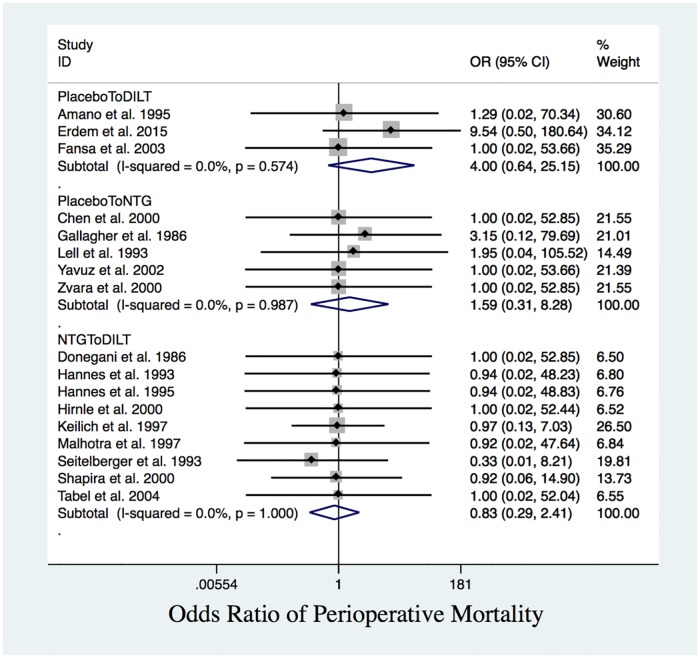
Perioperative mortality
Pairwise comparisons did not reveal significant differences in perioperative mortality between patients receiving DILT, NTG or placebo (Table 3 and Fig 5).
Table 3. Pairwise comparison of perioperative outcomes.
| Pairwise meta-analysis (mortality) | ||||
| Included Studies | Comparisons | OR (95% CI) | I2 | P |
| 7 studies | NTG vs. DILT | 0.83 (0.29, 2.41) | 0.00% | 1.000 |
| 5 studies | Placebo vs. NTG | 1.59 (0.31, 8.28) | 0.00% | 0.987 |
| 3 studies | Placebo vs. DILT | 4.00 (0.64, 25.15) | 0.00% | 0.574 |
| Pairwise meta-analysis (Cardiac protection) | ||||
| Included Studies | Comparisons | OR (95% CI) | I2 | P |
| post-MI | ||||
| 7 studies | NTG vs. DILT | 1.83 (0.77, 4.30) | 0.00% | 0.975 |
| 4 studies | Placebo vs. NTG | 2.24 (0.80, 6.28) | 0.00% | 0.596 |
| 1 studies | Placebo vs. DILT | 6.20 (0.27, 141.32) | - | - |
| TIE | ||||
| 5 studies | NTG vs. DILT | 1.67(0.99, 2.82) | 3.9% | 0.384 |
| 4 studies | Placebo vs. NTG | 1.42 (0.73, 2.75) | 0.0% | 0.707 |
| A-fib | ||||
| 7 studies | NTG vs. DILT | 2.67 (1.15, 6.24) | 62.20% | 0.014 |
| 2 studies | Placebo vs. DILT | 3.00 (1.18, 7.63) | 0.0% | 0.782 |
| Pairwise meta-analysis (Cardiac protection) | ||||
| Included Studies | Comparisons | MD (95% CI) | I2 | P |
| CK | ||||
| 1 study | NTG vs. Placebo | -36.00 (-232.18, 160.18) | NA | NA |
| 5 studies | DILT vs. NTG | -90.29 (-156.79, -23.79) | 0.00% | 0.691 |
| CKMB | ||||
| 6 studies | DILT vs. NTG | -12.47 (-18.33, -6.61) | 63.5% | 0.018 |
| 1 study | DILT vs. Placebo | -1.30 (-7.29,4.69) | NA | NA |
| Trop-T | ||||
| 4 Studies | DILT vs. NTG | -0.66 (-0.87, -0.44) | 0.0% | 0.42 |
| Pairwise meta-analysis (Cardiac function) | ||||
| Included Studies | Comparisons | OR (95% CI) | I2 | P |
| Inotrope | ||||
| 4 studies | NTG vs. DILT | 1.78 (0.78, 4.07) | 26.1% | 0.255 |
| 2 studies | Placebo vs. DILT | 0.19 (0.04, 0.73) | 0.0% | 0.419 |
| Pairwise meta-analysis (Cardiac function) | ||||
| Included Studies | Comparisons | MD (95% CI) | I2 | P |
| CCI | ||||
| 5 studies | DILT vs. NTG | -0.02 (-0.18, 0.13) | 21.3% | 0.279 |
| 2 studies | NTG vs. Placebo | 0.16 (-0.98, 0.42) | 69.2% | 0.072 |
| 1 study | DILT vs. Placebo | -0.10 (-0.62, 0.42) | NA | NA |
| HR | ||||
| 5 studies | NTG vs. Placebo | 2.54 (-6.22, 11.29) | 95.1% | 0.00 |
| 1 study | DILT vs. NTG | -13.00 (-23.56, -2.45) | NA | NA |
| 1 study | DILT vs. Placebo | -9.40 (-18.88, 0.08) | NA | NA |
| mBP (mmHg) | ||||
| 3 studies | NTG vs. Placebo | -2.61 (-11.70, 6.48) | 98.30% | 0.00 |
| 1 study | DILT vs. NTG | 2.00 (-7.92, 11.92) | NA | NA |
| 1 study | DILT vs. Placebo | 1.90 (-8.54, 12.34) | NA | NA |
| mPAP (mmHg) | ||||
| 2 studies | NTG vs. Placebo | -2.29 (-4.81, 0.23) | 29.40% | 0.23 |
| 1 study | DILT vs. NTG | -1.00 (-3.04, 1.04) | NA | NA |
| PAWP (mmHg) | ||||
| 4 studies | NTG vs. Placebo | -1.05 (-1.43, -0.70) | 0.0% | 0.64 |
| 1 study | DILT vs. NTG | -0.50 (-2.97, 1.97) | NA | NA |
| 1 study | DILT vs. Placebo | 0.80 (-1.84, 3.44) | NA | NA |
Note: OR greater than 1 favor the first treatment.
Fig 5. Perioperative mortality.
Forest plot of OR of perioperative mortality. The differences among the interventions are statistically insignificant.
The network meta-analysis for mortality between two treatment groups and one placebo group (Table 4) implied that DILT, NTG and placebo were comparable when integrating the direct and indirect comparison results, where no inconsistency was detected (global test for inconsistency indicated p = 0.39). However, the SUCRA values implied that NTG and DILT were comparable, but better than placebo in preventing perioperative mortality (Table 5).
Table 4. Network meta-analysis results for mortality, post MI, TIE, A-fib, and inotrope.
| Network meta-analysis (mortality), no inconsistency (p** = 0.39) | |||
| OR (95%CI) | Placebo | DILT | NTG |
| Placebo (vs.) | - | 1.42 (0.39, 5.22) | 1.37 (0.40, 4.64) |
| DILT (vs.) | 0.70 (0.19, 2.57) | - | 0.96 (0.35, 2.64) |
| NTG (vs.) | 0.73 (0.22, 2.47) | 1.04 (0.38, 2.81) | - |
| Network meta-analysis (post-MI), no inconsistency (p** = 0.99) | |||
| OR (95%CI) | Placebo | DILT | NTG |
| Placebo (vs.) | - | 4.51 (1.34, 15.25) | 2.26 (0.85, 5.99) |
| DILT (vs.) | 0.22 (0.07, 0.75) | - | 0.50 (0.20, 1.24) |
| NTG (vs.) | 0.44 (0.17, 1.18) | 2.00 (0.80, 4.96) | - |
| Network meta-analysis (TIE), no inconsistency (p** = 0.93) | |||
| OR (95%CI) | Placebo | DILT | NTG |
| Placebo (vs.) | - | 2.21 (0.94, 5.21) * | 1.43 (0.73, 2.77) |
| DILT (vs.) | 0.45 (0.19, 1.07) * | - | 0.64 (0.38, 1.11) |
| NTG (vs.) | 0.70 (0.36, 1.36) | 1.55 (0.90, 2.66) | - |
| Network meta-analysis (A-fib), no inconsistency (p** = 0.46) | |||
| OR (95%CI) | Placebo | DILT | NTG |
| Placebo (vs.) | - | 2.86 (0.65, 12.61) | 1.10 (0.20, 5.97) * |
| DILT (vs.) | 0.35 (0.08, 1.55) | - | 0.39 (0.18, 0.85) |
| NTG (vs.) | 0.91 (0.17, 4.88) * | 2.58 (1.18, 5.67) | - |
| Network meta-analysis (Inotrope), no inconsistency (p** = 0.14) | |||
| OR (95%CI) | Placebo | DILT | NTG |
| Placebo (vs.) | - | 0.51 (0.16, 1.61) | 0.28 (0.06, 1.21) * |
| DILT (vs.) | 1.95 (0.62, 6.11) | - | 0.55 (0.21, 1.41) |
| NTG (vs.) | 3.57 (0.82, 15.51) * | 1.83 (0.71, 4.72) | - |
vs.: row versus column. OR less than 1 favor the treatment specified in the row; OR greater than 1 favor the treatment specified in the column;
*: indirect comparison;
**: p-value from global test for inconsistency.
Table 5. SUCRA scores for network meta-analysis.
| SUCRA score (%) | DILT | NTG | Placebo |
|---|---|---|---|
| Mortality | 65.0 | 68.6 | 16.4 |
| Post-MI | 94.4 | 52.8 | 2.8 |
| TIE | 94.8 | 46.1 | 9.1 |
| A-fib | 95.2 | 27.5 | 27.2 |
| Inotrope | 45.5 | 5.8 | 98.6 |
| CCI | 51.3 | 27.3 | 71.4 |
| HR | 97.0 | 11.8 | 41.2 |
| mPAP | 76.9 | 54.5 | 18.5 |
| PAWP | 46.4 | 87.3 | 16.3 |
| CK | 93.4 | 31.8 | 24.7 |
| CKMB | 77.9 | 3.5 | 68.6 |
| Trop-T | 100.0 | 0.0 | NA |
| mBP | 35.6 | 75.0 | 39.4 |
Note: the scores are inversely related to the frequencies of complications or the values of continuous variables.
Cardiac protection outcomes
Four trials reported arterial graft flow measurements, but the data were insufficient for synthesis. One study revealed that, compared to placebo, continuous perioperative IV infusion of DILT significantly increased IMA blood flow[41]. Results from two other studies showed that patients receiving NTG had significantly higher blood flow in IMA or radial grafts[50,51], while a study comparing DILT to NTG showed that patients in the DILT group had significantly higher IMA blood flow[34].
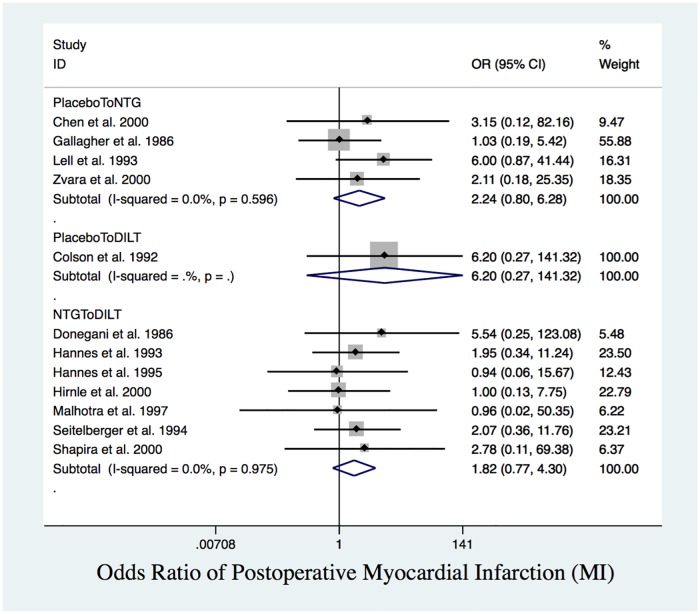
From the NMA, post-MI had a closed triangle loop with no inconsistency (global test for inconsistency indicated p = 0.99). Although pairwise meta-analysis results for post-MI were not significant (Fig 6), network meta-analysis results revealed that, compared to DILT, placebo had higher rates of post-MI (OR = 4.51, 95% CI: 1.34 to 15.25) (Table 4).
Fig 6. Postoperative MI.
Forest plot of OR of postoperative MI. There was no significant difference in pairwise comparison between placebo and NTG, placebo and DILT, NTG and DILT.
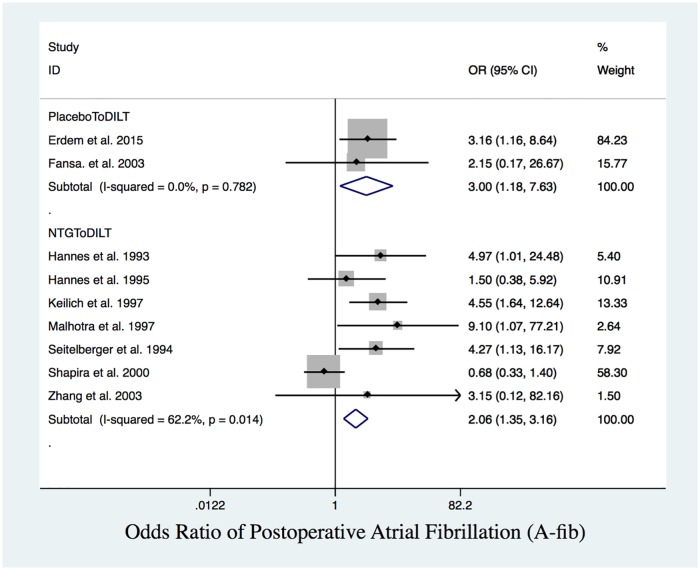
Pairwise meta-analysis results showed that patients who received NTG (OR = 2.67, 95% CI: 1.15 to 6.24) and placebo (OR = 3.00, 95% CI: 1.18 to 7.63) had higher rates of postoperative A-fib than those who received DILT (Table 2 and Fig 7).
Fig 7. Postoperative A-fib.
Forest plot of OR of postoperative A-fib. DILT had significantly lower odds than NTG and placebo.
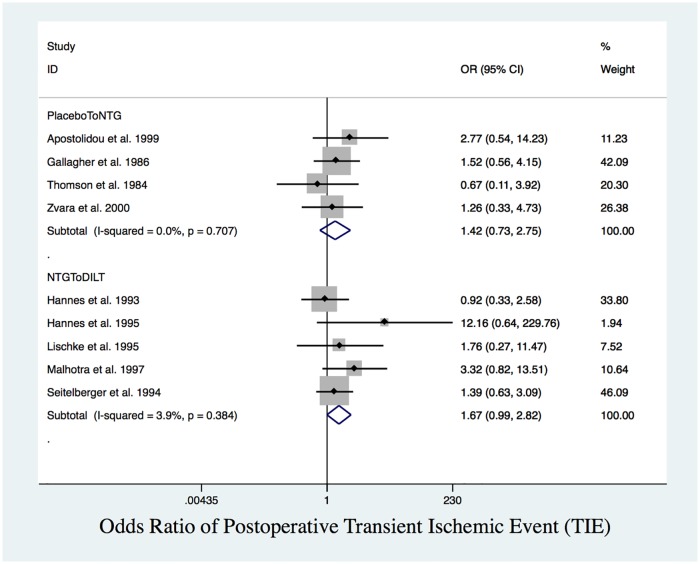
Since post-MI had a closed triangle loop with no inconsistency, we presented the network meta-analysis results by integrating direct and indirect evidence. For open triangle sloops (TIE, A-fib) with no inconsistency (global test for inconsistency indicated p = 0.93, p = 0.46, respectively), we presented both the direct estimates from conventional meta-analyses and the indirect estimates from network meta-analyses (Tables 4 and 5). From the NMA, TIE and A-fib had an open triangle loop with no inconsistency. Based exclusively on indirect comparisons (Table 4), placebo had a higher but statistically insignificant rate of TIE than DILT (Fig 8), while NTG had a significantly higher rate of A-fib (Fig 7) than DILT (OR = 2.58, 95% CI: 1.18 to 5.67).
Fig 8. Postoperative TIE.
Forest plot of OR of postoperative TIE. There was no statistically significant difference between placebo and NTG, NTG and DILT.
The SUCRA values indicated that DILT ranked the highest in terms of protecting the heart from post-MI, TIE and A-fib (94.4%, 94.8%, 95.2%, respectively) (Table 5). The network meta-analysis results were consistent with the pairwise comparisons. The ranking from highest to lowest odds of post-MI, TIE and A-fib is placebo>NTG>DILT.
In addition, we observed that patients who received NTG also had higher post-operative peak cardiac enzymes: CK (MD = 90.29, 95% CI: 23.79 to 156.79), CKMB (MD = 12.47, 95% CI: 6.61 to 18.33) and Troponin (MD = 0.66, 95% CI: 0.44 to 0.87) than DILT (Table 2).
Cardiac function outcomes
In pairwise meta-analyses, patients treated with DILT had significantly lower post-operative HR than those with NTG (MD = 13, 95% CI: -23.56 to -2.45). Patients who received placebo had lower PAWP compared with NTG (MD = -1.05, 95% CI: -1.43 to -0.70). Compared with DILT, patients who received placebo had lower odds of requiring postoperative inotrope support (OR = 0.19, 95% CI: 0.04 to 0.73). Among the interventions, there was no significant difference in CCI or mBP (Table 2 and Fig 9). Based exclusively on indirect comparisons with no inconsistency, we observed that NTG patients needed more inotropic support compared to placebo as well, although the result was not significant. The SUCRA score ranking from highest to lowest indicated rates of needing inotropic support is NTG> DILT>placebo (Table 5).
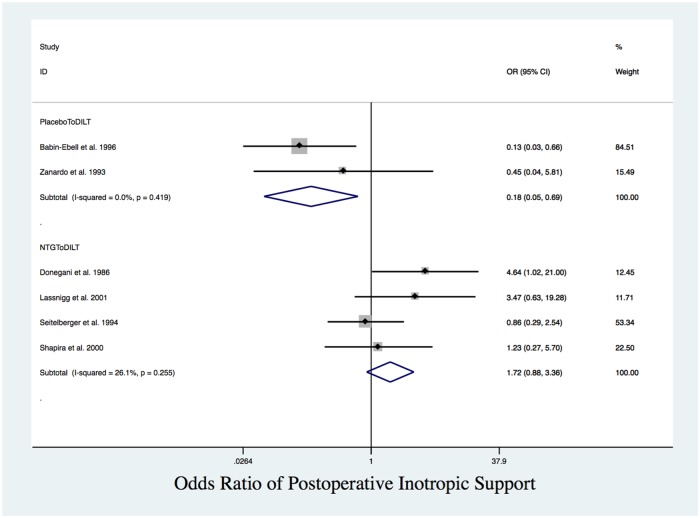
Fig 9. Requirement of postoperative inotropic support.
Forest plot of OR of postoperative inotropic support between placebo and DILT, NTG and DILT. Patients who received DILT had significantly higher odds of needing inotropic support compared to those who received placebo.
Discussion
To summarize the results of our current conventional and network meta-analyses, intraoperative measurements indicated that both DILT and NTG increased graft blood flow, which may help preventing graft spasm. However, the data are insufficient for synthesis. Compared with placebo, patients who received perioperative continuous IV infusion of DILT had significantly lower odds of postoperative cardiac ischemia and A-fib, but patients on DILT may need more inotropic support compared to placebo. Compared with NTG, the significantly lower HR and postoperative peak cardiac enzymes in patients on DILT indicates that DILT may be superior to NTG in preventing postoperative cardiac ischemic injuries.
The goal of CABG is to restore blood supply to ischemic heart through arterial and venous grafts. Graft spasm during and early after the procedure has negative impacts on the cardiac function and hemodynamic stability. CCBs and nitrates are two major categories of coronary vasodilators that were investigated for preventing graft spasm. Among the CCBs, DILT, a benzothiazepine, and verapamil, a phenylalkylamine, were considered suitable for perioperative continuous IV infusion due to their negative chronotropic effect. Studies in vitro and in vivo have consistently demonstrated that CCBs, topically applied or directly injected into the grafts, can significantly increase blood flow in human IMA and the radial artery (RA) grafts. Data from studies by Erdem[41] and Tabel[34] showed that continuous IV infusion of DILT is superior to both placebo and NTG in improving blood flow in dissected left IMA and RA segments before graft anastomosis. Although there were no data of direct flow measurement postoperatively, the lower incidence of cardiac ischemia and cardiac arrhythmia from the current meta-analysis indicated that coronary blood flow was likely better maintained early postoperatively in patients who received perioperative DILT infusion.
Our meta-analysis showed that intraoperative infusion of DILT resulted in significantly lower odds of postoperative A-fib. This is particularly interesting as the most recent ACCF/AHA guideline for CABG in 2011 states that nondihydropyridine CCBs such as DILT can be useful to control the ventricular rate in the setting of A-fib but are not indicated for prophylaxis[52]. However, this recommendation was based on one meta-analysis published in 1991[53]. In that study, verapamil, a nondihydropyridine CCB, failed to show a protective effect against the development of supraventricular arrhythmias (SVAs) after CABG. The discrepancy between that meta-analysis and ours may be due to the differential efficacy of oral verapamil and intravenous DILT against SVAs and the significant improvements to surgical and perioperative managements over the years. The results from our meta-analysis suggest that DILT might be useful for prophylaxis against A-fib after CABG.
The mechanism of cardiac protection by DILT is likely multifactorial. DILT inactivates cell surface L-type calcium channels, preventing the extracellular influx and sarcoplasmic release of calcium ions, promoting smooth muscle relaxation and therefore dilation of native and grafted coronary vessels[54]. Additionally, experimental data suggest that DILT could be involved in regulating endothelial function. DILT reduces the blood concentration of endothelin-1, a potent vasoconstrictor released from vascular endothelium, which may promote vascular smooth muscle relaxation through nitric oxide (NO) related signaling pathways[55,56]. In fact, there is evidence suggesting DILT could up-regulate NO synthase gene expression in endothelial cells[57]. Besides increasing blood supply through dilating coronary vessels, DILT may also have significant anti-inflammatory effects by regulating pro- and anti-inflammatory cytokines[40,58]. Clinically, Haak et al.[59] found that endothelin-1 level in circulation was elevated during and immediately after CABG, and perioperative DILT IV infusion significantly reduced endothelin-1 release. Compared to those who received NTG, patients who received DILT had more favorable hemodynamic status early postoperatively.
Nitrate family molecules, including NTG, have been applied clinically to relieve acute and chronic angina pectoris since 1876. Its mechanism was found to be NO mediated vasodilation of coronary arteries to improve oxygen supply to ischemic cardiac muscle, and afterload reduction to decrease oxygen demanding from the heart[60–62]. Previous studies confirmed that topical application and intravascular injection of NTG had potent vasodilatory properties[63]. However, despite their effectiveness in relieving acute chest pain, the long-term outcome benefit of nitrates in patients with CAD remains questionable. It is well documented that patients continuously taking nitrates quickly develop significant tolerance to the drugs, which not only diminish the treatment effects, but also had the tendency of causing more cardiac ischemic events[64]. Nakamura et al.[65], found that chronic usage of nitrates in patients with CAD may be associated with increased mortality. A large-scale retrospective study showed that pre-operative IV infusion of NTG failed to provide short term outcome benefits in patients who underwent CABG for unstable angina, patients on preoperative NTG also required prolonged postoperative mechanical ventilation and had more acute cardiovascular events[66]. The results from the current meta-analysis also suggest that perioperative continuous IV infusion of NTG had less cardiac protective effects in patients undergoing CABG compared to DILT; patients receiving NTG are more likely to have cardiac arrhythmic events compared to placebo and DILT. Therefore, perioperative continuous IV infusion of NTG may not be beneficial for patients having CABG.
In this study, network meta-analysis was used to compare treatment effectiveness of three treatment groups. The results suggest that DILT has the best protective effects against cardiac ischemia and arrhythmia in patients undergoing on-pump CABG. The advantage of network meta-analysis, an extension of traditional pairwise meta-analysis, is that it has the advantage of comparing multiple treatments with few or no head-to-head comparison data available. It can also help to determine the best available treatment and provide clinical guidelines. By “recycling” the data from prior studies, network meta-analysis is a cost-efficient statistical tool for comparing multiple interventions.
The current systematic review and network meta-analysis has some limitations, however: Firstly, due to the heterogeneity in the designs of the original studies, many critical outcomes cannot be evaluated because of unavailable or insufficient published data. Secondly, the sample sizes in most of the enrolled studies are relatively small, as we did not enroll unpublished data. This limitation in the quantity and quality of data could affect the power of the pairwise and network meta-analyses. Thirdly, the studied population in the enrolled studies were elective patients whose clinical conditions were relatively stable. Clinically more complicated, unstable patients were mostly excluded before or during the studies. This may be a confounding factor for the analyses of perioperative mortality, as it suggests that the conclusions drawn from the current meta-analysis may not apply to patients with different severities of clinical conditions. Most importantly, the studies enrolled were from the last three decades, an extensive period that has seen significant improvements in surgical techniques, perioperative and long-term medical managements of the patients undergoing on- or off-pump CABG. Studies have shown significant reduction of mortality as well as improvement of short- and long-term outcomes[67,68]. Due to the unavailability of anatomical evidence supporting the relief of graft spasm after closing of sternum as well as insufficient long-term follow-up data of DILT and NTG applied perioperatively, the overall outcome benefits of perioperative continuous IV infusion of DILT and/or NTG in patients undergoing on-pump CABG remains uncertain.
In conclusion, the current systematic review and network meta-analysis suggests that, compared to NTG and placebo, perioperative continuous IV infusion of DILT had stronger protective effects against postoperative ischemic cardiac injuries and A-fib. Possibly, DILT’s outcome benefits may be due to increased blood flow in arterial grafts. However, compared to placebo, patients may need more inotropic support.
Supporting information
Comprehensive database search, including the components of “Epub Ahead of Print” and “In-Process & Other Non-Indexed Citations”, was conducted initially on 11/15/2016, the search had been continuously updated monthly until the date when the manuscript was submitted.
(DOCX)
The complete 27 checklist items pertain to the content of the current systematic review and network meta-analysis. Items s# 16, 23 and 27 are unfilled because there are no additional analyses conducted, there is no institutional funding support for this study.
(DOC)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The authors received no specific funding for this work.
References
- 1.Le J, Baskett RJF, Buth KJ, Hirsch GM, Brydie A, Gayner R, et al. A pilot randomized controlled trial comparing CABG surgery performed with total arterial grafts or without. Journal Of Cardiothoracic Surgery. 2015;10: 1 10.1186/s13019-014-0203-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tatoulis J. Total arterial coronary revascularization. Multimedia Manual of Cardiothoracic Surgery. 2013;2013: mmt017. [DOI] [PubMed] [Google Scholar]
- 3.Erdil N, Nisanoglu V, Eroglu T, Fansa I, Cihan HB, Battaloglu B. Early outcomes of radial artery use in all-arterial grafting of the coronary arteries in patients 65 years and older. Texas Heart Institute Journal. 2010;37: 301–306. [PMC free article] [PubMed] [Google Scholar]
- 4.Sarabu MR, McClung JA, Fass A, Reed GE. Early postoperative spasm in left internal mammary artery bypass grafts. Ann Thorac Surg. 1987;44: 199–200. [DOI] [PubMed] [Google Scholar]
- 5.Wijeysundera DN, Beattie WS, Rao V, Karski J. Calcium antagonists reduce cardiovascular complications after cardiac surgery: a meta-analysis. J Am Coll Cardiol. 2003;41: 1496–1505. [DOI] [PubMed] [Google Scholar]
- 6.Battaloglu B, Nisanoglu V, Erdil N, Ozgur B, Eroglu T, Aydin N, et al. Effects of pretreatment with different topical vasodilators on blood flow in the internal mammary artery: a prospective randomized study. Heart Surg Forum. 2007;10: E136–40. [DOI] [PubMed] [Google Scholar]
- 7.Hou H, Wang J, Wang Z, Liu X, Marinko M, Novakovic A, et al. Effect of Benidipine in Human Internal Mammary Artery and Clinical Implications. Ann Thorac Surg. 2016;101: 1789–1795. 10.1016/j.athoracsur.2015.10.029 [DOI] [PubMed] [Google Scholar]
- 8.Locker C, Mohr R, Paz Y, Lev-Ran O, Herz I, Uretzky G, et al. Pretreatment with alpha-adrenergic blockers for prevention of radial artery spasm. Ann Thorac Surg. 2002;74: S1368–70. [DOI] [PubMed] [Google Scholar]
- 9.He GW, Yang CQ. Inhibition of vasoconstriction by phosphodiesterase III inhibitor milrinone in human conduit arteries used as coronary bypass grafts. J Cardiovasc Pharmacol. 1996;28: 208–214. [DOI] [PubMed] [Google Scholar]
- 10.Liu JJ, Doolan LA, Xie B, Chen JR, Buxton BF. Direct vasodilator effect of milrinone, an inotropic drug, on arterial coronary bypass grafts. FANZCA. Journal of Thoracic & Cardiovascular Surgery. 1997;113: 108–113. [DOI] [PubMed] [Google Scholar]
- 11.Ertuna E, Turkseven S, Amanvermez D, Ayik F, Yagdi T, Yasa M. Effects of levosimendan on isolated human internal mammary artery and saphenous vein: concurrent use with conventional vasodilators. Fundam Clin Pharmacol. 2016;30: 226–234. 10.1111/fcp.12185 [DOI] [PubMed] [Google Scholar]
- 12.He GW, Yang CQ. Inhibition of vasoconstriction by potassium channel opener aprikalim in human conduit arteries used as bypass grafts. Br J Clin Pharmacol. 1997;44: 353–359. 10.1046/j.1365-2125.1997.00640.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ege T, Gur O, Karadag CH, Duran E. Evaluation of iloprost to prevent vasospasm in coronary artery bypass grafts. J Int Med Res. 2010;38: 1759–1763. 10.1177/147323001003800521 [DOI] [PubMed] [Google Scholar]
- 14.Roubos N, Rosenfeldt FL, Richards SM, Conyers RA, Davis BB. Improved preservation of saphenous vein grafts by the use of glyceryl trinitrate-verapamil solution during harvesting. Circulation. 1995;92: II31–6. [DOI] [PubMed] [Google Scholar]
- 15.Yaliniz H, Tokcan A, Zeren H, Ulus T, Kisacikoglu B, Salih OK, et al. Effects on reperfusion injury of adding diltiazem to tepid blood cardioplegia. Heart Surg Forum. 2004;7: E434–9. [DOI] [PubMed] [Google Scholar]
- 16.Nomura F, Matsuda H, Nakano S, Ohtani M, Takami H, Hirose H, et al. Enhancement of cardiac prostacyclin release during reperfusion after diltiazem supplemented potassium cardioplegia. J Cardiovasc Surg (Torino). 1991;32: 26–30. [PubMed] [Google Scholar]
- 17.He GW, Buxton BF, Rosenfeldt FL, Angus JA, Tatoulis J. Pharmacologic dilatation of the internal mammary artery during coronary bypass grafting. J Thorac Cardiovasc Surg. 1994;107: 1440–1444. [PubMed] [Google Scholar]
- 18.Hutton B, Salanti G, Caldwell DM, Chaimani A, Schmid CH, Cameron C, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med. 2015;162: 777–784. 10.7326/M14-2385 [DOI] [PubMed] [Google Scholar]
- 19.Higgins JPT, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343: 5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chung JH, Lee SW. Assessing the quality of randomized controlled urological trials conducted by korean medical institutions. Korean J Urol. 2013;54: 289–296. 10.4111/kju.2013.54.5.289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.White IR, Barrett JK, Jackson D, Higgins JP. Consistency and inconsistency in network meta-analysis: model estimation using multivariate meta-regression. Res Synth Methods. 2012;3: 111–125. 10.1002/jrsm.1045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Trinquart L, Attiche N, Bafeta A, Porcher R, Ravaud P. Uncertainty in Treatment Rankings: Reanalysis of Network Meta-analyses of Randomized Trials. Ann Intern Med. 2016;164: 666–673. 10.7326/M15-2521 [DOI] [PubMed] [Google Scholar]
- 23.Donegani E, Costa P, De Paulis R, di Summa M, Poletti GA, Pignatelli MG, et al. Myocardial protection by perioperative diltiazem drip: a clinical evaluation. Thoracic & Cardiovascular Surgeon. 1986;34: 168–171. [DOI] [PubMed] [Google Scholar]
- 24.Hannes W, Fasol R, Zajonc H, Schindler M, Schumacher B, Schlosser V, et al. Diltiazem provides anti-ischemic and anti-arrhythmic protection in patients undergoing coronary bypass grafting. Eur J Cardiothorac Surg. 1993;7: 239–245. [DOI] [PubMed] [Google Scholar]
- 25.Seitelberger R, Hannes W, Gleichauf M, Keilich M, Christoph M, Fasol R. Effects of diltiazem on perioperative ischemia, arrhythmias, and myocardial function in patients undergoing elective coronary bypass grafting. J Thorac Cardiovasc Surg. 1994;107: 811–821. [PubMed] [Google Scholar]
- 26.Hannes W, Seitelberger R, Christoph M, Keilich M, Kulinna C, Holubarsch C, et al. Effect of peri-operative diltiazem on myocardial ischaemia and function in patients receiving mammary artery grafts. Eur Heart J. 1995;16: 87–93. [DOI] [PubMed] [Google Scholar]
- 27.Keilich M, Kulinna C, Seitelberger R, Fasol R. Postoperative Follow-up of Coronary Artery Bypass Patients REceiving Calcium Antagonist Diltiazem. INternational Journal of Angiology. 1997;6: 8–12. [Google Scholar]
- 28.Malhotra R, Mishra M, Kler TS, Kohli VM, Mehta Y, Trehan N. Cardioprotective effects of diltiazem infusion in the perioperative period. European Journal of Cardio-Thoracic Surgery. 1997;12: 420–427. [DOI] [PubMed] [Google Scholar]
- 29.Lischke V, Probst S, Behne M, Dieterich HA. [Prevention of myocardial ischemia. Study following aortocoronary bypass operation with the calcium antagonist diltiazem]. Anaesthesist. 1995;44: 92–100. [DOI] [PubMed] [Google Scholar]
- 30.Shapira OM, Alkon JD, Macron DS, Keaney JFJ, Vita JA, Aldea GS, et al. Nitroglycerin is preferable to diltiazem for prevention of coronary bypass conduit spasm. Ann Thorac Surg. 2000;70: 883–888. [DOI] [PubMed] [Google Scholar]
- 31.Lassnigg A, Wutte M, Grubhofer G, Chevtchik O, Podesser B, Simon-Kupilik N, et al. Diltiazem versus nitroglycerin for myocardial protection following coronary artery bypass grafting as assessed by dobutamine stress echocardiography. Wien Klin Wochenschr. 2001;113: 439–445. [PubMed] [Google Scholar]
- 32.Hirnle T., Stachurski A., Negrusz-Kawecka M., Halawa B., Bross T. Myocardial protection during coronary artery by-pass surgery with nitroglycerin or diltiazem. kardiologia polska. 2000;52: 277–284. [Google Scholar]
- 33.Zhang P, Chen G, Zhang P, Zheng K, Wang G. [Cardioprotective effects of diltiazem infusion in the perioperative period in patients undergoing coronary artery bypass grafting with extracorporeal circulation]. Chung-Hua i Hsueh Tsa Chih [Chinese Medical Journal]. 2003;83: 1387–1390. [PubMed] [Google Scholar]
- 34.Tabel Y, Hepaguslar H, Erdal C, Catalyurek H, Acikel U, Elar Z, et al. Diltiazem provides higher internal mammary artery flow than nitroglycerin during coronary artery bypass grafting surgery. European Journal of Cardio-Thoracic Surgery. 2004;25: 553–559. 10.1016/j.ejcts.2004.01.004 [DOI] [PubMed] [Google Scholar]
- 35.Colson P, Medioni P, Saussine M, Seguin JR, Cuchet D, Grolleau D, et al. Hemodynamic effect of calcium channel blockade during anesthesia for coronary artery surgery. Journal of Cardiothoracic & Vascular Anesthesia. 1992;6: 424–428. [DOI] [PubMed] [Google Scholar]
- 36.Zanardo G, Michielon P, Rosi P, Teodori T, Antonucci F, Caenaro G, et al. Effects of a continuous diltiazem infusion on renal function during cardiac surgery. Journal of Cardiothoracic & Vascular Anesthesia. 1993;7: 711–716. [DOI] [PubMed] [Google Scholar]
- 37.Amano J, Suzuki A, Sunamori M, Tofukuji M. Effect of calcium antagonist diltiazem on renal function in open heart surgery. Chest. 1995;107: 1260–1265. [DOI] [PubMed] [Google Scholar]
- 38.Babin-Ebell J, Keith PR, Elert O. Efficacy and safety of low-dose propranolol versus diltiazem in the prophylaxis of supraventricular tachyarrhythmia after coronary artery bypass grafting. European Journal of Cardio-Thoracic Surgery. 1996;10: 412–416. [DOI] [PubMed] [Google Scholar]
- 39.Yavuz S, Ayabakan N, Goncu MT, Ozdemir IA. Effect of combined dopamine and diltiazem on renal function after cardiac surgery. Medical Science Monitor. 2002;8: P45–50. [PubMed] [Google Scholar]
- 40.Fansa I, Gol M, Nisanoglu V, Yavas S, Iscan Z, Tasdemir O. Does diltiazem inhibit the inflammatory response in cardiopulmonary bypass?. Medical Science Monitor. 2003;9: P30–6. [PubMed] [Google Scholar]
- 41.Erdem O, Memetoglu ME, Tekin AI, Arslan U, Akkaya O, Kutlu R, et al. Effects of intraoperative diltiazem infusion on flow changes in arterial and venous grafts in coronary artery bypass graft surgery. Revista Brasileira de Cirurgia Cardiovascular: Orgao Oficial da Sociedade Brasileira de Cirurgia Cardiovascular. 2015;30: 459–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thomson IR, Mutch WA, Culligan JD. Failure of intravenous nitroglycerin to prevent intraoperative myocardial ischemia during fentanyl-pancuronium anesthesia. Anesthesiology. 1984;61: 385–393. [DOI] [PubMed] [Google Scholar]
- 43.Gallagher JD, Moore RA, Jose AB, Botros SB, Clark DL. Prophylactic nitroglycerin infusions during coronary artery bypass surgery. Anesthesiology. 1986;64: 785–789. [DOI] [PubMed] [Google Scholar]
- 44.Withington PS, Durcan JJ, Weir I, Innis R, Savage T. Haemodynamic and metabolic effects of prophylactic nitroglycerin infusion in the immediate period following coronary artery bypass grafting. Eur Heart J. 1988;9: 187–193. [DOI] [PubMed] [Google Scholar]
- 45.Lell W, Johnson P, Plagenhoef J, Samuelson P, Athanasuleas C, Hughes W, et al. The effect of prophylactic nitroglycerin infusion on the incidence of regional wall-motion abnormalities and ST segment changes in patients undergoing coronary artery bypass surgery. J Card Surg. 1993;8: 228–231. [DOI] [PubMed] [Google Scholar]
- 46.Knothe C, Boldt J, Zickmann B, Ballesteros M, Haufler G, Bruns F, et al. [Cardiac protection in heart surgery interventions by preventive drug administration before extracorporeal circulation. Studies with troponin T as a parameter for perioperative myocardial damage]. Herz. 1993;18: 379–386. [PubMed] [Google Scholar]
- 47.Apostolidou IA, Despotis GJ, Hogue CWJ, Skubas NJ, McCawley CA, Hauptmann EL, et al. Antiischemic effects of nicardipine and nitroglycerin after coronary artery bypass grafting. Ann Thorac Surg. 1999;67: 417–422. [DOI] [PubMed] [Google Scholar]
- 48.Chen X, Jiang Y, Xu M, Bao H, Mu X, Xiao L, et al. [Perioperative changes of plasma ET-1 in patients undergoing coronary artery bypass grafting and the effect of nitroglycerin]. Chung-Hua Wai Ko Tsa Chih [Chinese Journal of Surgery]. 2000;38: 669–671. [PubMed] [Google Scholar]
- 49.Zvara DA, Groban L, Rogers AT, Prielipp RC, Murphy B, Hines M, et al. Prophylactic nitroglycerin did not reduce myocardial ischemia during accelerated recovery management of coronary artery bypass graft surgery patients. Journal of Cardiothoracic & Vascular Anesthesia. 2000;14: 571–575. [DOI] [PubMed] [Google Scholar]
- 50.Zabeeda D, Medalion B, Jackobshvilli S, Ezra S, Schachner A, Cohen AJ. Comparison of systemic vasodilators: effects on flow in internal mammary and radial arteries. Ann Thorac Surg. 2001;71: 138–141. [DOI] [PubMed] [Google Scholar]
- 51.Arnaudov D, Cohen AJ, Zabeeda D, Hauptman E, Sasson L, Schachner A, et al. Effect of systemic vasodilators on internal mammary flow during coronary bypass grafting. Ann Thorac Surg. 1996;62: 1816–1819. [DOI] [PubMed] [Google Scholar]
- 52.Hillis LD, Smith PK, Anderson JL, Bittl JA, Bridges CR, Byrne JG, et al. 2011 ACCF/AHA Guideline for Coronary Artery Bypass Graft Surgery. A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Developed in collaboration with the American Association for Thoracic Surgery, Society of Cardiovascular Anesthesiologists, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2011;58: e123–210. 10.1016/j.jacc.2011.08.009 [DOI] [PubMed] [Google Scholar]
- 53.Andrews TC, Reimold SC, Berlin JA, Antman EM. Prevention of supraventricular arrhythmias after coronary artery bypass surgery. A meta-analysis of randomized control trials. Circulation. 1991;84: III236–44. [PubMed] [Google Scholar]
- 54.Garaliene V, Barsys V, Giedraitis S, Benetis R, Krauze A. The role of external Ca2+ in the action of Ca2+-channel agonists and antagonists on isolated human thoracic arteries. Journal of Physiology & Pharmacology. 2014;65: 35–31. [PubMed] [Google Scholar]
- 55.Wenzel RR, Duthiers N, Noll G, Bucher J, Kaufmann U, Luscher TF. Endothelin and calcium antagonists in the skin microcirculation of patients with coronary artery disease. Circulation. 1996;94: 316–322. [DOI] [PubMed] [Google Scholar]
- 56.Serebruany VL, Schlossberg ML, Edenbaum LR, Herzog WR, Gurbel PA. Serial changes of soluble endothelin-1 levels during myocardial ischaemia-reperfusion. Effects of magnesium, diltiazem and a novel MAC-1 inhibitor. Pharmacological Research. 1998;38: 165–172. 10.1006/phrs.1998.0349 [DOI] [PubMed] [Google Scholar]
- 57.Ding Y, Vaziri ND. Nifedipine and diltiazem but not verapamil up-regulate endothelial nitric-oxide synthase expression. Journal of Pharmacology & Experimental Therapeutics. 2000;292: 606–609. [PubMed] [Google Scholar]
- 58.Szabo C, Hasko G, Nemeth ZH, Vizi ES. Calcium entry blockers increase interleukin-10 production in endotoxemia. Shock. 1997;7: 304–307. [DOI] [PubMed] [Google Scholar]
- 59.Haak T, Matheis G, Kohleisen M, Ngo H, Beyersdorf F, Usadel KH. Endothelin during cardiovascular surgery: the effect of diltiazem and nitroglycerin. J Cardiovasc Pharmacol. 1995;26: S494–6. [PubMed] [Google Scholar]
- 60.Steinhorn BS, Loscalzo J, Michel T. Nitroglycerin and Nitric Oxide—A Rondo of Themes in Cardiovascular Therapeutics. N Engl J Med. 2015;373: 277–280. 10.1056/NEJMsr1503311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Asrress KN, Williams R, Lockie T, Khawaja MZ, De Silva K, Lumley M, et al. Physiology of Angina and Its Alleviation With Nitroglycerin: Insights From Invasive Catheter Laboratory Measurements During Exercise. Circulation. 2017;136: 24–34. 10.1161/CIRCULATIONAHA.116.025856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sievert H, Selzer G, Schneider W, Kober G, Kaltenbach M, Bussmann WD. Coronary stenosis dilation by low dose intravenous nitroglycerin. Eur Heart J. 1989;10: 134–136. [DOI] [PubMed] [Google Scholar]
- 63.Chanda J, Canver CC. Reversal of preexisting vasospasm in coronary artery conduits. Ann Thorac Surg. 2001;72: 476–480. [DOI] [PubMed] [Google Scholar]
- 64.Ignarro LJ. After 130 years, the molecular mechanism of action of nitroglycerin is revealed. Proc Natl Acad Sci U S A. 2002;99: 7816–7817. 10.1073/pnas.132271799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nakamura Y, Moss AJ, Brown MW, Kinoshita M, Kawai C. Long-term nitrate use may be deleterious in ischemic heart disease: A study using the databases from two large-scale postinfarction studies. Multicenter Myocardial Ischemia Research Group. Am Heart J. 1999;138: 577–585. [DOI] [PubMed] [Google Scholar]
- 66.Ali IS, Buth KJ, Maitland A. Impact of preoperative intravenous nitroglycerin on in-hospital outcomes after coronary artery bypass grafting for unstable angina. Am Heart J. 2004;148: 727–732. 10.1016/j.ahj.2004.04.003 [DOI] [PubMed] [Google Scholar]
- 67.Cornwell LD, Omer S, Rosengart T, Holman WL, Bakaeen FG. Changes over time in risk profiles of patients who undergo coronary artery bypass graft surgery: the Veterans Affairs Surgical Quality Improvement Program (VASQIP). JAMA Surg. 2015;150: 308–315. 10.1001/jamasurg.2014.1700 [DOI] [PubMed] [Google Scholar]
- 68.Abramov D, Tamariz MG, Fremes SE, Guru V, Borger MA, Christakis GT, et al. Trends in coronary artery bypass surgery results: a recent, 9-year study. Ann Thorac Surg. 2000;70: 84–90. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Comprehensive database search, including the components of “Epub Ahead of Print” and “In-Process & Other Non-Indexed Citations”, was conducted initially on 11/15/2016, the search had been continuously updated monthly until the date when the manuscript was submitted.
(DOCX)
The complete 27 checklist items pertain to the content of the current systematic review and network meta-analysis. Items s# 16, 23 and 27 are unfilled because there are no additional analyses conducted, there is no institutional funding support for this study.
(DOC)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.