Abstract
Community-acquired carriage and infections due to extended-spectrum beta-lactamase producing Enterobacteriaceae (ESBL-E) are increasing worldwide, resulting in increased morbidity, mortality and healthcare costs. The origins of community-acquired ESBL-E carriage and infections remain unclear. Bean sprouts are a potential source of Enterobacteriaceae for the community, as illustrated by outbreaks of pathogenic Enterobacteriaceae in the past. The current study focuses on contamination of retail bean sprouts with ESBL-E in the Netherlands. Of 131 bean sprout samples purchased between 2013 and 2016, 25 (19%) were contaminated with ESBL-E. The detected isolates were almost exclusively Klebsiella spp. and co-resistance to other antibiotics was observed frequently. Over time there was substantial genetic diversity between isolates. On the other hand, isolates from samples closely matched in time were frequently clonally related, indicative of batch contamination. Remarkably, no Escherichia coli was found. In conclusion, bean sprouts frequently harbor ESBL-E, which is a potential source for consumers.
Introduction
Over the past 15 years human carriage and infections due to antimicrobial resistant Enterobacteriaceae have increased substantially, and concomitantly the impact on morbidity, mortality and healthcare costs are rising [1–4]. Infections caused by extended-spectrum beta-lactamase producing Enterobacteriaceae (ESBL-E) originally were a hospital related problem, however, this has shifted to a largely community-acquired problem [5]. The reservoirs and transmission routes of community acquired ESBL-E are poorly understood and seem to be a multifactorial problem.
Risk factors for ESBL-E carriage can be classified as intrinsic and extrinsic. Intrinsic risk factors decrease the natural barriers of the body, such as decreased gastric acid production caused by proton-pump inhibitors or decreased colonization resistance due to antibiotic use [6–8]. Extrinsic risk factors largely entail the frequency and intensity of contact with ESBL-E. Travel to high endemic areas and contact with ESBL-E positive family members and pets are typical examples [9–12]. Many food items have been shown to contain ESBL-E and as such are potential sources for acquisition of ESBL-E by humans. In recent years meat has gained much interest as a potential source, but a large variety of food items are contaminated with ESBL-E, including vegetables and drinking water [13–20]. One study from the Netherlands and one from South-Korea reported ESBL-E on bean sprouts, among other vegetables [19,21]. This is relevant as bean sprouts are often consumed raw, and as such have a higher risk of transmission than food items that are cooked before consumption [22]. It has been shown in the past that bean sprouts carry the potential to be the source of large-scale community outbreaks with pathogenic Enterobacteriaceae, as was the case with E. coli O104:H4 causing hemolytic-uremic syndrome in Germany in 2011 [23,24]. The aim of the current study is to investigate to what extend bean sprouts in the Netherlands are contaminated with ESBL-E.
Materials and methods
Study design
Bean sprout samples were purchased from supermarkets and grocery stores (including: ethnic markets and green grocers) in the Netherlands from December 2013 until January 2016. For the ESBL-E prevalence survey a maximum of one bean sprout sample per store per day was included. For all the samples, the following variables were noted: store of purchase, date of purchase and if available the expiration date. Besides the samples for the prevalence survey, extra samples per store per day were obtained in the first sampling period (2013–2014) to determine the presence of batch contamination. Isolates from the additional samples were used only to determine the presence of batch contamination and were excluded from the other analyses.
Microbiological methods
Per sample, twelve grams of bean sprouts were enriched in 15 mL tryptic soy broth (TSB). After overnight incubation, 100 μL of the TSB was transferred to a selective TSB, containing cefotaxime (0.25 mg/L) and vancomycin (8 mg/L) (TSB-VC). After overnight incubation, 10 μL of the TSB-VC was subcultured on an ESBL screening agar, EbSA (AlphaOmega, ‘s-Gravenhage, the Netherlands), consisting of a split McConkey agar plate containing cloxacillin (400 mg/L), vancomycin (64 mg/L) and either cefotaxime or ceftazidime (1 mg/L). Species identification (VITEK-MS, bioMérieux, Marcy l’Etoile, France) and antibiotic susceptibility testing (VITEK2, bioMérieux, Marcy l’Etoile, France) were performed for all oxidase-negative Gram-negative isolates that grew on the EbSA. Minimal inhibitory concentrations (MIC) are given in mg/L. The production of ESBL was phenotypically confirmed with the combination disk diffusion method for cefotaxime (30 μg), ceftazidime (30 μg) and cefepime (30 μg). All with and without clavulanic acid (10 μg) (Rosco, Taastrup, Denmark). Test results were considered positive if the diameter of the inhibition zone was ≥5 mm larger for the disk with clavulanic acid as compared to the disk without clavulanic acid [25,26]. For interpretation of the phenotypic susceptibility testing EUCAST clinical breakpoints–bacteria (v 7.1) was used [27].
Whole genome sequencing (WGS), genome assembly and quality control (QC)
Phenotypically confirmed ESBL-E isolates were sequenced on a MiSeq (Illumina, San Diego, United States) and assembled with CLC Genomics Workbench 9.0, 9.0.1 or 9.5.2 (Qiagen, Hilden, Germany) as was previously described in more detail [28]. As quality control parameters, the following criteria were used: coverage: ≥ 30; number of scaffolds: ≤1000; N50: ≥ 15,000 bases and maximum scaffold length: ≥ 50,000 bases.
Analyses of WGS data: species determination, resistance gene detection, Multi Locus Sequence Typing (MLST) and whole-genome MLST (wgMLST)
Assembled genomes were analyzed using an open access bioinformatics web tool (https://cge.cbs.dtu.dk/services/cge/, DTU, Copenhagen). This was done with ResFinder for analyses of resistance genes, PlasmidFinder for plasmid replicons and MLST 1.8 for MLST [29–31]. The services are combined in the bacterial analysis pipeline–batch upload mode [32]. This analysis pipeline also incorporates species determination with KmerFinder [33]. In case of conflicting results between the phenotypical MALDI-TOF and genetic KmerFinder 2.0, final species determination was based on the rpoB sequence [34].
wgMLST was performed using Ridom SeqSphere+, version 3.4.0 (Ridom, Münster, Germany). The species specific wgMLST typing schemes used in this study (K. pneumoniae and K. oxytoca) are described by Kluytmans–van den Bergh et al [28]. The pairwise genetic difference between isolates was calculated by dividing the number of allele differences by the total number of shared alleles from the typing scheme present in both sequences, using a pairwise ignoring missing values approach. Species-specific thresholds for relatedness were used [28]. Using pairwise comparisons, a distance matrix was built. The relatedness of the isolates was inferred using the Neighbor-Joining method [35]. The Neighbor-Joining trees were constructed using MEGA6 [36].
Statistical analyses
Data were analyzed using Statistical Package for Social Science software (IBM SPSS Statistics 24.0, Armonk, NY). To test for differences in ESBL-E prevalence between supermarket chains and grocery stores and between the different supermarket chains, the Fischer exact test was used. As a measure of diversity between the isolates the Simpson Diversity Index (SID) was calculated based on MLST [37,38]. Confidence intervals of percentages were calculated with GraphPad QuickCalcs (GraphPad Software, La Jolla, California).
Accession number
Raw sequencing reads were submitted to the European Nucleotide Archive of the European Bioinformatics Institute and are available under the study accession number PRJEB25080.
Results
ESBL-E prevalence in bean sprouts
A total of 131 bean sprout samples were tested for the presence of ESBL-E of which 25 (19.1%) tested positive (Table 1). The ESBL-E prevalence varied depending on the store of purchase. Between supermarket chains the largest difference in ESBL-E prevalence was between chains three and four, with a prevalence of 45.0% and 4.8%, respectively. In general, samples from supermarkets were more frequently contaminated with ESBL-E than samples from grocery stores (ESBL-E prevalence of 25.3% and 5.0% respectively, p = 0.007).
Table 1. ESBL-E prevalence in bean sprout samples in the Netherlands, 2013–2016.
| No. samples | ESBL-E positive (%) | 95% CI | P | |
|---|---|---|---|---|
| Prevalence survey samples | 131 | 25 (19.1) | 13.2–26.7 | |
| Store of purchase (N = 131) | 0.013 | |||
| Supermarket chain 1 | 23 | 5 (21.7) | 9.2–42.3 | |
| Supermarket chain 2 | 23 | 7 (30.4) | 15.4–51.1 | |
| Supermarket chain 3 | 20 | 9 (45.0) | 25.8–65.8 | |
| Supermarket chain 4 | 21 | 1 (4.8) | <0.01–24.4 | |
| Supermarket chain 5 | 4 | 1 (25.0) | 3.4–71.1 | |
| Grocery store 1 | 10 | 0 (0) | 0.00–32.1 | |
| Grocery store 2 | 10 | 1 (10) | <0.01–42.6 | |
| Grocery store 3 | 10 | 1 (10) | <0.01–42.6 | |
| Grocery store 4 | 10 | 0 (0) | 0.00–32.1 | |
| Supermarket (N = 131) | 0.007 | |||
| Yes | 91 | 23 (25.3) | 17.4–35.1 | |
| No | 40 | 2 (5) | 0.5–17.4 |
CI confidence interval, P p-value of the Fischer exact test.
K. pneumoniae was the predominant species (n = 21, 80.8%), followed by K. oxytoca (n = 3, 11.5%) and K. variicola (n = 1, 3.8%). One sample contained an ESBL-producing E. cloacae (3.8%) besides an ESBL-producing K. pneumoniae. ESBL-producing E. coli was not found.
Results of antimicrobial-susceptibility testing are shown in Tables 2 and 3. Besides the ESBL phenotype, high rates of resistance were found against ciprofloxacin (69.2%), trimethoprim-sulfamethoxazole (80.8%) and tobramycin (84.6%). Combined resistance against these three antibiotics was present in 50.0% of the isolates. Resistance against piperacillin-tazobactam was found in two of 26 isolates (7.7%). All isolates were susceptible to meropenem and colistin.
Table 2. Susceptibility profiles to different beta-lactams, detected extended-spectrum beta-lactamase (ESBL) genes, plasmid replicons and multi-locus sequence types (MLST) of ESBL-producing Enterobacteriaceae isolates from bean sprouts in the Netherlands.
| Species ID |
AMC | TZP | CTX | CAZ | ESBL genes | Plasmid replicons | MLST |
|---|---|---|---|---|---|---|---|
| K. pneumoniae | |||||||
| 13 | 8 | ≤4 | 4 | ≤1 | blaCTX-M-14 | IncFII,IncFIB(K),ColRNAI | ST-1296 |
| 5 | 16 | 8 | 8 | 2 | blaCTX-M-3 | IncFII(K) | ST-1565 |
| 19 | 4 | ≤4 | ≤1 | ≤1 | blaSHV-2 | IncFIA(HI1),IncFIB(K), IncHI1B,ColRNAI | ST-2176 |
| 16 | 8 | ≤4 | 8 | ≤1 | blaCTX-M-14 | IncFII,IncFIB(K),ColRNAI | ST-2657 |
| 6 | 16 | 32 | ≥64 | 4 | blaSHV-2 | IncFIA(HI1),IncR | ST-2658 |
| 20 | 16 | 16 | 8 | 2 | blaCTX-M-3, blaSHV-99a |
IncFIA(HI1),IncFIB(pKPHS1),IncFIB(K),IncFII(K),IncR,IncQ1,ColRNAI | ST-2659 |
| 21 | 16 | 8 | 8 | 2 | blaCTX-M-3, blaSHV-99a |
IncFIA(HI1),IncFIB(pKPHS1),IncFIB(K),IncFII(K),IncR,IncQ1,ColRNAI | ST-2659 |
| 18 | 16 | 8 | 32 | ≤1 | blaSHV-2 | IncFIA(HI1),IncR | ST-280 |
| 14 | 8 | 8 | 16 | 16 | blaCTX-M-27 | IncFIA(HI1),IncFIB(K) | ST-37 |
| 15 | 8 | 8 | ≥64 | 16 | blaCTX-M-27 | IncFIA(HI1),IncFIB(K) | ST-37 |
| 17 | 16 | 8 | 8 | 2 | blaSHV-2 | IncFIA(HI1),IncFII, IncFIB(K) | ST-39 |
| 8 | 8 | ≤4 | ≤1 | ≤1 | blaSHV-2 | IncFiA(HI1),IncR,ColRNAI | ST-392 |
| 7,9 | 4 | ≤4 | ≤1 | ≤1 | blaSHV-2 | IncFiA(HI1),IncR,ColRNAI | ST-392 |
| 11 | 16 | ≤4 | 8 | ≤1 | blaSHV-2 | IncFIA(HI1),IncR,ColRNAI | ST-45 |
| 12 | 8 | ≤4 | ≥64 | 8 | blaCTX-M-15 | IncFIB(K) | ST-45 |
| 10 | 8 | 8 | 2 | 2 | blaSHV-2 | IncFIA(HI1),Col(BS512),IncR, Col(MG828),ColRNAI | ST-485 |
| 4 | 16 | 8 | ≥64 | ≤1 | blaCTX-M-3, blaSHV-27 | IncFII(K),IncQ1 | ST-661 |
| 1,2,3 | 16 | ≤4 | 8 | ≤1 | blaCTX-M-3, blaSHV-27 | IncFII(K),IncQ1 | ST-661 |
| K. oxytoca | |||||||
| 29 | 8 | ≤4 | 8 | ≤1 | blaCTX-M-14 | IncFIA(HI1), IncFIB(pKPHS1),IncN | ST-195 |
| 33 | 8 | ≤4 | 4 | ≤1 | blaCTX-M-3 | IncN,IncU | ST-196 |
| 32 | 8 | ≤4 | ≥64 | 4 | blaCTX-M-27 | IncFIA(HI1),IncR | ST-2 |
| K. variicola | |||||||
| 34 | 8 | ≤4 | 8 | ≤1 | blaCTX-M-3 | IncN2,IncFIB(K),ColRNAI | ST-1142 |
| E. cloacae | |||||||
| 31 | ≥32 | 8 | ≥64 | 4 | blaCTX-M-15 | IncFII(pECLA),IncFIB(pENTE01), IncFIB(pECLA),ColRNAI | ST-144 |
aESBL genes called with a less than 100% identity and or length less than 100%. Minimum identity and length for call: 90.00% and 60% respectively. All plasmid replicons called by PlasmidFinder in default settings were reported. Shading within table indicates susceptibility interpretation according to EUCAST breakpoint table version 7.1, dark grey: resistant, light grey: intermediate and white: susceptible. ID isolate identification number, AMC amoxicillin-clavulanic acid, TZP piperacillin-tazobactam, CTX cefotaxim, CAZ ceftazidime, ESBL extended-spectrum beta-lactamase, MLST multilocus sequencing typing, ST sequence type.
Table 3. Detected genes associated with resistance to different classes of antibiotics and phenotypic susceptibility profiles to most of these classes of antibiotics.
| Species ID | TOB | CIP | TMP | SXT | Aminoglycoside† | Quinolone | TMP | SUL | TET | MAC |
|---|---|---|---|---|---|---|---|---|---|---|
| K. pneumoniae | ||||||||||
| 13 | 8 | 0.5 | ≥16 | ≥320 | aac(3)-IIda,strA,strB | oqxAa,oqxBa, QnrS1 | dfrA1 | sul1, sul2 | tet(A), tet(D) | |
| 5 | 8 | ≥4 | ≥16 | ≥320 | aadA16a,aac(6')Ib-cr | oqxAa,oqxBa, QnrB49a,QnrS1 | dfrA27 | sul1 | tet(A)a | mph(A) |
| 19 | 8 | 1 | ≤0.5 | ≤20 | aac(3)-IIda,strA,strB | oqxAa,oqxBa, QnrS1 | sul2 | tet(D) | ||
| 16 | 8 | 0.5 | ≥16 | ≤20 | aac(3)-IIda | oqxAa,oqxBa, QnrS1 | dfrA1 | sul1 | tet(A) | |
| 6 | 8 | ≥4 | ≥16 | ≥320 | aac(3)-IIda,aadA16a, strAa,strBa, aac(6')Ib-cr |
oqxAa,oqxBa, QnrB6 | dfrA27 | sul1, sul2 | tet(D) | |
| 20,21 | 8 | ≥4 | ≥16 | ≥320 | aac(3)-IIda,aadA16a, aph(3')-Iaa,strA,strB, aac(6')Ib-cr | oqxAa,oqxBa, QnrB49a,QnrS1 | dfrA27 | sul2 | tet(A)a | mph(A) |
| 18 | 8 | 0.5 | 8 | 40 | aac(3)-IIda,aadA16a, strAa,strBa,aacA4a, aac(6')Ib-cra | oqxAa,oqxBa, QnrB6 | dfrA27 | sul1, sul2 | tet(D) | |
| 14 | 2 | ≥4 | ≥16 | ≥320 | aadA16a,aac(6')Ib-cr | oqxA,oqxB | dfrA27 | sul1 | tet(D) | |
| 15 | 4 | ≥4 | ≥16 | ≥320 | aadA16a,aac(6')Ib-cr | oqxA,oqxB | dfrA27 | sul1 | tet(D) | |
| 17 | 8 | 0.5 | ≥16 | ≥320 | aac(3)-IIda, strAa,strBa | oqxAa,oqxBa, QnrS1 | dfrA14a | sul2 | tet(A)a | mph(A) |
| 7,8,9 | 8 | ≥4 | ≥16 | ≥320 | aac(3)-IIda,aadA16a, aac(6')Ib-cr | oqxAa,oqxBa | dfrA27 | sul1, sul2 | tet(A)a, tet(D) | |
| 11 | 8 | ≤0.25 | ≤0.5 | ≤20 | aac(3)-IIda, strAa,strBa | oqxAa,oqxBa, QnrB6 | sul1, sul2 | tet(D) | ||
| 12 | ≤1 | 1 | ≥16 | ≥320 | strA,strB | oqxAa,oqxBa, QnrS1 | dfrA14a | sul2 | ||
| 10 | 8 | ≥4 | ≥16 | ≥320 | aac(3)-IIda,aadA16a, aac(6')Ib-cr | oqxAa,oqxBa, QnrB49a | dfrA27 | sul1, sul2 | tet(A)a, tet(D) | |
| 4 | 8 | 2 | ≥16 | ≥320 | aac(3)-IIda,aadA16a, aph(3')-Iaa,strA,strB, aac(6')Ib-cr | oqxAa,oqxBa, QnrB49a,QnrS1 | dfrA27 | sul1, sul2 | tet(A)a | mph(A) |
| 1,2,3 | 8 | 2 | ≥16 | ≥320 | aac(3)-IIda,aadA16a, aph(3')-Iaa,strA,strB, aac(6')Ib-cr | oqxAa,oqxBa, QnrB49a,QnrS1 | dfrA27 | sul1, sul2 | tet(A)a | mph(A) |
| K. oxytoca | ||||||||||
| 29 | ≤1 | ≤0.25 | ≤0.5 | ≤20 | aph(3')-Iaa | |||||
| 33 | 8 | 0.5 | ≥16 | ≥320 | strAa,strBa,aacA4, aac(6')Ib-cra | QnrS1 | dfrA14a | sul2 | ||
| 32 | 8 | ≥4 | ≥16 | ≥320 | aac(3)-IIda,aadA16a, aac(6')Ib-cr | QnrB52 | dfrA27 | sul1 | tet(A)a | |
| K. variicola | ||||||||||
| 34 | ≤1 | ≤0.25 | ≤0.5 | ≤20 | oqxAa,oqxBa | |||||
| E. cloacae | ||||||||||
| 31 | 8 | 0.5 | ≥16 | ≥320 | aac(3)-IIaa, aadA1a,strA,strB, aac(6')Ib-cr | QnrB1a | dfrA14a | sul2 | tet(A)a | |
aGenes called with a less than 100% identity and or length less than 100%. Minimum identity and length for call: 90.00% and 60% respectively.
†aac(6')Ib-cr confers resistance to aminoglycosides and quinolones.
Shading within table indicates susceptibility interpretation according to EUCAST breakpoint table version 7.1, dark grey: resistant, light grey: intermediate and white: susceptible. ID isolate identification number, TOB tobramycin, CIP ciproflacin, TMP trimethoprim, SXT trimethoprim-sulfamethoxazole, SUL sulphonamide, TET tetracyclin, MAC macrolide.
Genetic characteristics of ESBL-E isolated from bean sprouts
Quality control results and recoded file names to access files from ENA are displayed in S1 Table and S2 Table respectively. All of the phenotypically confirmed ESBL-E isolates contained at least one ESBL gene (Table 2). The following ESBL genes were detected: in 9 (34.6%) isolates the blaSHV-2 gene, in 5 (19.2%) the blaCTX-M-3 gene, in 4 (15.4%) both the blaSHV-27 gene and blaCTX-M-3 gene, in 3 (11.5%) the blaCTX-M-14 gene, in 3 (11.5%) the blaCTX-M-27 gene and in 2 (7.7%) the blaCTX-M-15 gene. In two isolates containing the blaCTX-M-3 gene, the blaSHV-99 gene was also detected, with one mismatching nucleotide. The most frequently detected plasmid replicons as reported by PlasmidFinder 1.2 were IncFIA, IncFIB, IncFII, Col and IncR (Table 2). Most of the plasmid replicons called by PlasmidFinder were variants on the genes in the database [31]. MLST results of the isolates are shown in Table 2. Unknown MLST types were submitted to the corresponding databases. Three new MLST types were added for K. pneumoniae (ST2657, ST2658 and ST2659) and two were added for K. oxytoca (ST195 and ST196). The Simpson index of diversity (1-D) based on MLST was 0.96, 95% CI 0.92–1.00, demonstrating a high diversity between the isolates. Genes associated with resistance to aminoglycosides, quinolones, trimethoprim, sulphonamides, tetracyclines and macrolides were frequently present (Table 3).
wgMLST
The genetic relatedness of the 21 K. pneumoniae isolates from the prevalence survey is shown in Fig 1. Isolates were either clonally related or had large genetic diversity. The median genetic distance of K. pneumoniae isolate-to-isolate comparisons was 0.0002; range, 0.0000–0.0016 for clonally related isolates (n = 11), and 0.8524; range 0.1082–0.8721 for non-clonally related isolates (n = 199). Four clusters were identified; one cluster consisted of four isolates, one cluster contained three isolates and two clusters contained two isolates. Isolates within clusters came from samples that had expiration dates closely matched in time. The longest time between expiration dates within a cluster was 18 days. Clustering isolates were detected in samples purchased from different supermarkets. wgMLST analysis of the three K. oxytoca isolates from the prevalence study revealed no clonal relatedness. The smallest genetic distance was between isolates 29 and 33, which was 0.71. The genetic distance between isolates 29 and 32 and between 32 and 33 were both 0.99.
Fig 1. Neighbor-joining tree based on the wgMLST analysis of K. pneumonia isolates from the prevalence survey of bean sprout samples (2013–2016), using a pairwise ignore missing values approach.
Scale shows genetic distance. wgMLST clusters are color coded (ID and MLST) as is the store of purchase. The date is the expiration date unless labeled with * in which case date of purchase of the sample is depicted. ID isolate identification number, MLST multilocus sequence typing, ST sequence type, S supermarket chain, R retailer.
Batch contamination of bean sprout samples
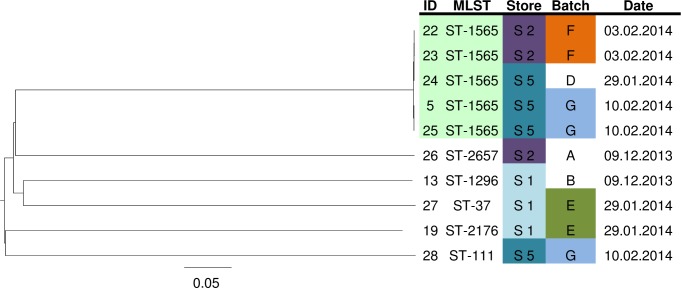
In the period from December 2013 until March 2014 additional samples were purchased from different supermarkets, to investigate the occurrence of batch contamination with ESBL-E. Twenty-seven samples, coming from seven batches; three batches consisted of two samples, three batches consisted of five samples and one batch consisted of six samples. Analyzing the samples in a batch-by-batch manner shows three separate batch contamination events: two batches with K. pneumoniae (batch F and G) and one with K. oxytoca (batch F; Table 4). When comparing the 10 K. pneumoniae isolates from the batch contamination experiment with each other using wgMLST, contamination of five samples with one clone was found (Fig 2). This cluster occurred over a time period of thirteen days and was spread over two supermarket chains.
Table 4. Presence of ESBL-E in bean sprout samples purchased from supermarkets in batches.
| Batch | No. samples in batch |
No. samples ESBL-E positive (%) in batch | Clones present in >1 sample in batch | No. samples with clone and species | MLST of clone | Date of purchase | Store | ID |
|---|---|---|---|---|---|---|---|---|
| A | 2 | 1 (50) | no | 1x K. pneumoniae | ST-2657 | 09/12/13 | 2 | 26 |
| B | 2 | 1 (50) | no | 1x K. pneumoniae | ST-1296 | 09/12/13 | 1 | 13 |
| C | 2 | 0 (0) | no | - | 17/12/13 | 5 | ||
| D | 5 | 1 (20) | no | 1x K. pneumoniae | ST-1565a | 29/01/14 | 5 | 24 |
| E | 5 | 2 (40) | no | 1x K. pneumoniae | ST-2176 | 29/01/14 | 1 | 19 |
| no | 1x K. pneumoniae | ST-37 | 27 | |||||
| F | 5 | 4 (80) | yes | 2x K. oxytoca | ST-195a | 03/02/14 | 2 | 29,30 |
| yes | 2x K. pneumoniae | ST-1565a | 22,23 | |||||
| G | 6 | 3 (50) | yes | 2x K. pneumoniae | ST-1565a | 10/02/14 | 5 | 5,25 |
| no | 1x K. pneumoniae | ST-111 | 28 |
a Isolates with the same ST are also clonally related on basis of wgMLST analyses. ESBL-E, extended-spectrum beta-lactamase producing Enterobacteriaceae, MLST multilocus sequence typing, ST sequence type, isolate identification number.
Fig 2. Neighbor-joining tree based on the wgMLST analysis of K. pneumonia isolates from multiple batches of bean sprouts, using a pairwise ignore missing values approach.
Scale shows genetic distance. A batch was defined as bean sprout samples purchased on the same day from the same supermarket chain. wgMLST clusters are color coded (ID and MLST) as are store and batch of purchase. The date is the date of purchase of the sample. ID isolate identification number, MLST multilocus sequence typing, ST sequence type, S supermarket chain, R retailer.
Discussion
To our knowledge this is the first study focusing specifically on the ESBL-E prevalence in retail bean sprouts to date. An ESBL-E prevalence of 19% was found, being almost exclusively ESBL-producing Klebsiella spp. No ESBL-producing E. coli were found. The ESBL-E isolates found over time were either genetically highly diverse or clearly within the thresholds of clonal relatedness between epidemiologically related isolates as described by Kluytmans–van den Bergh et al [28]. The clonally related isolates always came from samples that were purchased within weeks of each other, which is suggestive for batch contamination. These findings indicate that there is a continuous influx of unrelated ESBL-E isolates and no prolonged persistence of specific clones.
A remarkable finding is that 96.2% of the isolates are of the genus Klebsiella, 80.8% being K. pneumoniae. The complete absence of ESBL-producing E. coli is noteworthy. We are unaware of factors favoring the growth of K. pneumoniae or suppressing the growth of other pathogens in the bean sprout production process. Other studies that present data on ESBL-E from bean sprouts reported similar high percentages of K. pneumoniae, namely, 80% and 84% in the studies by Reuland et al. (the Netherlands) and Kim et al. (South Korea) respectively [19,21].
Besides the resistance to beta-lactams, we found high levels of co-resistance to important antimicrobial agents like ciprofloxacin, trimethoprim-sulfamethoxazole and tobramycin. Combined resistance to these three antibiotics and the ESBL phenotype was present in 50% of the isolates. This rate of co-resistance is higher than what is found in ESBL-E isolates from human carriage, namely 12% [39]. Combined resistance to the three antibiotics and the ESBL phenotype for K. pneumoniae from blood cultures in four peripheral hospitals in the South of the Netherlands was 18.3% (11 of 60 isolates, time period 1-1-2010–1-1-2018, unpublished data). None of the isolates from the current study showed resistance to carbapenems or colistin.
The ESBL-genes detected in bean sprouts have also been detected in the human population. For instance the blaCTX-M-15 and blaCTX-M-14 genes, which were detected in bean sprouts in the current study, are among the most frequently detected ESBL genes in human carriage and bloodstream infections in the Netherlands [40–42]. Also blaCTX-M-3 and blaCTX-M-27 have been reported to be present in bloodstream infections in the Netherlands [42]. The most commonly detected ESBL gene in the current study, blaSHV-2, has also been reported to be present in clinical samples [40]. However, the blaSHV genes are not always typed to the specific variants within the group, making comparison of the exact SHV types difficult [41,43]. Although similar ESBL genes are found in bean sprouts and humans, it is difficult to judge the impact of the ESBL-gene reservoir in bean sprouts on humans, as this study was not designed to make a direct comparison. However, bean sprouts have the potential to spread Enterobacteriaceae to humans, as has been shown by multiple outbreaks of pathogenic Enterobacteriaceae in humans in the past [23,24,44]. Therefore, our findings should be considered as a potential threat for humans but based on the current information we cannot quantify the size of the effect.
Our study has some strengths and limitations. Strengths of the study are the fact that samples were taken in different time periods, showing that ESBL-E contamination of bean sprouts is not an incidental finding. In addition, sensitive culture techniques were employed, using broth enrichment and a validated ESBL screening agar [45]. Furthermore, genetic confirmation of ESBL genes and precise typing and clustering methods using whole genome sequencing were used.
A limitation of the study is that we did not use a (semi-) quantitative culture method to quantify the load of the ESBL-E in bean sprouts. This information could have been important to estimate the possible impact for humans [22].
A further limitation of the study is the lack of information on the label of the bean sprouts. For instance, no information on place of production or production batches was given on the label and expiration dates were not always present. For the analyses, batches were defined as samples purchased from the same supermarket chain or store, with the same expiration date or date of purchase, depending on the availability of the information. No tracking codes or batch numbers from producers were present on the packages, which would have allowed for more precise analyses. Care was taken to achieve a representative sample of bean sprouts sold in the Netherlands and minimize the effect of batch contamination on the reported ESBL-E prevalence.
A final limitation of the study was the analysis of the plasmid content of the detected isolates. PlasmidFinder results are reported which enable comparisons to other studies, but further analyses into the epidemiology of the plasmids would have been a valuable addition. However, unraveling plasmid DNA from chromosomal DNA from the available short-read sequencing data is still a conundrum and beyond the scope of this study.
Despite these caveats several conclusions can be made on the ESBL-E prevalence of the different stores. First, there are differences in ESBL-E prevalence between supermarket chains. Supermarket chain one, two and three have an ESBL-E prevalence of more than 20%, whereas supermarket chain four has a prevalence of 4.8%. The bean sprouts sold by supermarket chain two and three are likely to have an overlapping origin, as clonality in isolates from these supermarkets is common. Supermarket chain five was discontinued during the study period explaining the small number of samples. Second, a higher ESBL-E prevalence was found in samples from supermarkets compared to those from smaller retailers. The underlying causes of the differences in ESBL-E prevalence in the different stores are unknown. This may be caused by differences in the production process, network of transportation and differences in production scales with intrinsic possibilities of cross-contamination. Further studies are warranted to reveal the causes of contamination to decrease the overall ESBL-E prevalence in bean sprouts.
A final point on the ESBL-E contamination of bean sprout samples is the presence of batch contamination. High genetic variability of the ESBL-E isolates was seen in the bean sprout samples over time. In contrast, from samples purchased within three weeks of each other clonally related isolates were frequently cultured. Furthermore, in the experiment focusing on batch contamination, a single wgMLST clone was found in five different packages purchased from two different supermarket chains (Figs 1 and 2). These two observations support the hypothesis of batch contamination, however, not all samples from these batches were ESBL-E positive. This may indicate a varying load of ESBL-E or overgrowth with abundantly present non-fermenting bacteria. Also, the previously mentioned limitation of the definition of a batch may play a role.
In conclusion, 19.1% of bean sprout samples are contaminated with ESBL-E, with a remarkable high percentage of Klebsiella isolates in the absence of E. coli. The isolates are resistant to several other classes of antibiotics and are genetically highly diverse over time. Therefore, bean sprouts are a possible community source of ESBL-producing Klebsiella spp. Further investigations to the points of entry of ESBL-E into the production process and countermeasures against these entry points are warranted. Unfortunately, research looking into food as a vehicle for the spread of antimicrobial resistance in humans is greatly hampered by the lack of transparency in the food production process. In the current article the factory or even country of origin of the samples was not traceable for the researchers. We strongly suggest working towards a situation where basic information such as which country or countries the products were produced are clearly marked on the food items.
Supporting information
(DOCX)
(DOCX)
Acknowledgments
We thank the team of curators of the Institut Pasteur MLST and whole genome MLST databases for curating the data and making them publicly available at http://bigsdb.pasteur.fr. This publication also made use of the PubMLST website (https://pubmlst.org/) developed by Keith Jolley (Jolley & Maiden 2010, BMC Bioinformatics, 11:595) and sited at the University of Oxford. The development of that website was funded by the Wellcome Trust.
Data Availability
All raw sequencing reads are available from the ENA database (accession number PRJEB25080). Stores of purchase have been anonymized.
Funding Statement
The authors received no specific funding for this work.
References
- 1.Woerther PL, Burdet C, Chachaty E, Andremont A. Trends in human fecal carriage of extended-spectrum beta-lactamases in the community: Toward the globalization of CTX-M. Clin Microbiol Rev. 2013;26: 744–758. 10.1128/CMR.00023-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Karanika S, Karantanos T, Arvanitis M, Grigoras C, Mylonakis E. Fecal Colonization with Extended-spectrum Beta-lactamase-Producing Enterobacteriaceae and Risk Factors among Healthy Individuals: A Systematic Review and Metaanalysis. Clin Infect Dis. 2016; 10.1093/cid/ciw283 [DOI] [PubMed] [Google Scholar]
- 3.European Centre for Disease Prevention and Control (ECDC). Antimicrobial resistance surveillance in Europe 2014 Annual Report of the European Antimicrobial Resistance Surveillance Network (EARS-Net). Stockholm: ECDC; 2015. [Internet]. 2015. 10.2900/23549 [DOI] [Google Scholar]
- 4.Tumbarello M, Spanu T, Di Bidino R, Marchetti M, Ruggeri M, Trecarichi EM, et al. Costs of bloodstream infections caused by Escherichia coli and influence of extended-spectrum-beta-lactamase production and inadequate initial antibiotic therapy. Antimicrob Agents Chemother. 2010;54: 4085–4091. 10.1128/AAC.00143-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Livermore DM, Canton R, Gniadkowski M, Nordmann P, Rossolini GM, Arlet G, et al. CTX-M: Changing the face of ESBLs in Europe [Internet]. Journal of Antimicrobial Chemotherapy. 2007. pp. 165–174. [DOI] [PubMed] [Google Scholar]
- 6.Huizinga P, van den Bergh MK, van Rijen M, Willemsen I, van ‘t Veer N, Kluytmans J. Proton Pump Inhibitor Use Is Associated With Extended-Spectrum β-Lactamase–Producing Enterobacteriaceae Rectal Carriage at Hospital Admission: A Cross-Sectional Study. Clin Infect Dis. 2016;702: ciw743. [DOI] [PubMed] [Google Scholar]
- 7.Reuland EA, Naiemi N, Kaiser AM, Heck M, Kluytmans JAJW, Savelkoul PHM, et al. Prevalence and risk factors for carriage of ESBL-producing Enterobacteriaceae in Amsterdam. J Antimicrob Chemother. 2016;71: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shitrit P, Reisfeld S, Paitan Y, Gottesman BS, Katzir M, Paul M, et al. Extended-spectrum beta-lactamase-producing Enterobacteriaceae carriage upon hospital admission: Prevalence and risk factors. J Hosp Infect. Elsevier Ltd; 2013;85: 230–232. 10.1016/j.jhin.2013.07.014 [DOI] [PubMed] [Google Scholar]
- 9.Arcilla MS, Hattem JM van, Haverkate MR, Bootsma MCJ, Genderen PJJ van, Goorhuis A, et al. Import and spread of extended-spectrum β-lactamase-producing Enterobacteriaceae by international travellers (COMBAT study): a prospective, multicentre cohort study. Lancet Infect Dis. 2017;17: 78–85. 10.1016/S1473-3099(16)30319-X [DOI] [PubMed] [Google Scholar]
- 10.Barreto Miranda IDM, Ignatius RPDM, Pfuller RDM, Friedrich-Janicke BDM, Steiner FDM, Paland MDM, et al. High carriage rate of ESBL-producing Enterobacteriaceae at presentation and follow-up among travellers with gastrointestinal complaints returning from India and Southeast Asia. J Travel Med. 2016;23: 1–7. 10.1093/jtm/tav024 [DOI] [PubMed] [Google Scholar]
- 11.Valverde A, Grill F, Coque TM, Pintado V, Baquero F, Cantón R, et al. High rate of intestinal colonization with extended-spectrum-β- lactamase-producing organisms in household contacts of infected community patients. J Clin Microbiol. 2008;46: 2796–2799. 10.1128/JCM.01008-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meyer E, Gastmeier P, Kola A, Schwab F. Pet animals and foreign travel are risk factors for colonisation with extended-spectrum beta-lactamase-producing Escherichia coli. Infection. 2012;40: 685–687. 10.1007/s15010-012-0324-8 [DOI] [PubMed] [Google Scholar]
- 13.Overdevest I, Willemsen I, Rijnsburger M, Eustace A, Xu L, Hawkey P, et al. Extended-spectrum β-lactamase genes of Escherichia coli in chicken meat and humans, the Netherlands. Emerg Infect Dis. 2011;17: 1216–1222. 10.3201/eid1707.110209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kluytmans JAJW, Overdevest ITMA, Willemsen I, Kluytmans-Van Den Bergh MFQ, Van Der Zwaluw K, Heck M, et al. Extended-spectrum beta-lactamase-producing escherichia coli from retail chicken meat and humans: Comparison of strains, plasmids, resistance genes, and virulence factors. Clin Infect Dis. 2013;56: 478–487. 10.1093/cid/cis929 [DOI] [PubMed] [Google Scholar]
- 15.Leverstein-van Hall MA, Dierikx CM, Cohen Stuart J, Voets GM, van den Munckhof MP, van Essen-Zandbergen A, et al. Dutch patients, retail chicken meat and poultry share the same ESBL genes, plasmids and strains. Clin Microbiol Infect. 2011;17: 873–880. 10.1111/j.1469-0691.2011.03497.x [DOI] [PubMed] [Google Scholar]
- 16.de Boeck H, Miwanda B, Lunguya-Metila O, Muyembe-Tamfum JJ, Stobberingh E, Glupczynski Y, et al. ESBL-positive enterobacteria isolates in drinking water. Emerg Infect Dis. 2012;18: 1019–1020. 10.3201/eid1806.111214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Egervärn M, Englund S, Ljunge M, Wiberg C, Finn M, Lindblad M, et al. Unexpected common occurrence of transferable extended spectrum cephalosporinase-producing Escherichia coli in Swedish surface waters used for drinking water supply. Sci Total Environ. 2017;587–588: 466–472. 10.1016/j.scitotenv.2017.02.157 [DOI] [PubMed] [Google Scholar]
- 18.van Hoek AHAM, Veenman C, van Overbeek WM, Lynch G, de Roda Husman AM, Blaak H. Prevalence and characterization of ESBL- and AmpC-producing Enterobacteriaceae on retail vegetables. Int J Food Microbiol. 2015;204: 1–8. 10.1016/j.ijfoodmicro.2015.03.014 [DOI] [PubMed] [Google Scholar]
- 19.Reuland EA, al Naiemi N, Raadsen SA, Savelkoul PHM, Kluytmans JAJW, Vandenbroucke-Grauls CMJE. Prevalence of ESBL-producing Enterobacteriaceae in raw vegetables. Eur J Clin Microbiol Infect Dis. 2014;33: 1843–1846. 10.1007/s10096-014-2142-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Blaak H, van Hoek AHAM, Veenman C, Docters van Leeuwen AE, Lynch G, van Overbeek WM, et al. Extended spectrum beta-lactamase- and constitutively AmpC-producing Enterobacteriaceae on fresh produce and in the agricultural environment. Int J Food Microbiol. 2014;168–169: 8–16. 10.1016/j.ijfoodmicro.2013.10.006 [DOI] [PubMed] [Google Scholar]
- 21.Kim H-S, Chon J-W, Kim Y-J, Kim D-H, Kim M, Seo K-H. Prevalence and characterization of extended-spectrum-β-lactamase-producing Escherichia coli and Klebsiella pneumoniae in ready-to-eat vegetables. Int J Food Microbiol. 2015;207: 83–6. 10.1016/j.ijfoodmicro.2015.04.049 [DOI] [PubMed] [Google Scholar]
- 22.Evers EG, Pielaat A, Smid JH, Van Duijkeren E, Vennemann FBC, Wijnands LM, et al. Comparative exposure assessment of esblproducing Escherichia coli through meat consumption. PLoS One. 2017;12 10.1371/journal.pone.0169589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bielaszewska M, Mellmann A, Zhang W, Köck R, Fruth A, Bauwens A, et al. Characterisation of the Escherichia coli strain associated with an outbreak of haemolytic uraemic syndrome in Germany, 2011: A microbiological study. Lancet Infect Dis. 2011;11: 671–676. 10.1016/S1473-3099(11)70165-7 [DOI] [PubMed] [Google Scholar]
- 24.Buchholz U, Bernard H, Werber D, Böhmer MM, Remschmidt C, Wilking H, et al. German Outbreak of Escherichia coli O104:H4 Associated with Sprouts. N Engl J Med. 2011;365: 1763–1770. 10.1056/NEJMoa1106482 [DOI] [PubMed] [Google Scholar]
- 25.Netherlands Society for Medical Microbiology. NVMM Guideline Laboratory detection of highly resistant microorganisms, version 2.0, 2012. Leeuwarden, Netherlands. 2012; Available: http://www.nvmm.nl/system/files/2012.11.15richtlijnBRMO%2528version2.0%2529-RICHTLIJN.pdf
- 26.European Committee on Antimicrobial Susceptibility Testing. EUCAST guidelines for detection of resistance mechanisms and specific resistances of clinical and / or epidemiological importance. European Society of Clinical Microbiology and Infectious Diseases, Basel, Switzerland. 2013; Available: http://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Resistance_mechanisms/EUCAST_detection_of_resistance_mechanisms_v1.0_20131211.pdf
- 27.The European Committee on Antimicrobial Susceptibility Testing. Breakpoint tables for interpretation of MICs and zone diameters, version 7.1 [Internet]. 2017. Available: http://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Breakpoint_tables/v_7.1_Breakpoint_Tables.pdf
- 28.Kluytmans-van den Bergh MFQ, Rossen JWA, Bruijning-Verhagen PCJ, Bonten MJM, Friedrich AW, Vandenbroucke-Grauls CMJE, et al. Whole genome multilocus sequence typing of extended-spectrum beta-lactamase-producing Enterobacteriaceae. J Clin Microbiol. 2016;54: 2919–2927. 10.1128/JCM.01648-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zankari E, Hasman H, Cosentino S, Vestergaard M, Rasmussen S, Lund O, et al. Identification of acquired antimicrobial resistance genes. J Antimicrob Chemother. 2012;67: 2640–2644. 10.1093/jac/dks261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Larsen M V., Cosentino S, Rasmussen S, Friis C, Hasman H, Marvig RL, et al. Multilocus sequence typing of total-genome-sequenced bacteria. J Clin Microbiol. 2012;50: 1355–1361. 10.1128/JCM.06094-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Carattoli A, Zankari E, García-Fernández A, Voldby Larsen M, Lund O, Villa L, et al. In silico detection and typing of plasmids using PlasmidFinder and plasmid multilocus sequence typing. Antimicrob Agents Chemother. 2014;58: 3895–903. 10.1128/AAC.02412-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thomsen MCF, Ahrenfeldt J, Cisneros JLB, Jurtz V, Larsen MV, Hasman H, et al. A bacterial analysis platform: An integrated system for analysing bacterial whole genome sequencing data for clinical diagnostics and surveillance. PLoS One. 2016;11: 1–14. 10.1371/journal.pone.0157718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Larsen M V., Cosentino S, Lukjancenko O, Saputra D, Rasmussen S, Hasman H, et al. Benchmarking of methods for genomic taxonomy. J Clin Microbiol. 2014;52: 1529–1539. 10.1128/JCM.02981-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Martínez-Romero E, Rodríguez-Medina N, Beltrán-Rojel M, Silva-Sánchez J, Barrios-Camacho H, Pérez-Rueda E, et al. Genome misclassification of Klebsiella variicola and Klebsiella quasipneumoniae isolated from plants, animals and humans. Salud Publica Mex. 2018;60: 56–62. doi: 10.21149/8149 [DOI] [PubMed] [Google Scholar]
- 35.Saitou N, Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol. 1987;4: 406–25. Available: http://www.ncbi.nlm.nih.gov/pubmed/3447015 10.1093/oxfordjournals.molbev.a040454 [DOI] [PubMed] [Google Scholar]
- 36.Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. MEGA6: Molecular evolutionary genetics analysis version 6.0. Mol Biol Evol. 2013;30: 2725–2729. 10.1093/molbev/mst197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Simpson EH. Measurement of Diversity. Nature. 1949;163: 688–688. 10.1038/163688a0 [DOI] [Google Scholar]
- 38.Grundmann H, Hori S, Tanner G. Determining Confidence Intervals When Measuring Genetic Diversity and the Discriminatory Abilities of Typing Methods for Microorganisms. J Clin Microbiol. 2001;39: 4190–4192. 10.1128/JCM.39.11.4190-4192.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Reuland EA, Overdevest ITMA, al Naiemi N, Kalpoe JS, Rijnsburger MC, Raadsen SA, et al. High prevalence of ESBL-producing Enterobacteriaceae carriage in Dutch community patients with gastrointestinal complaints. Clin Microbiol Infect. 2013;19: 542–549. 10.1111/j.1469-0691.2012.03947.x [DOI] [PubMed] [Google Scholar]
- 40.Voets GM, Platteel TN, Fluit AC, Scharringa J, Schapendonk CM, Stuart JC, et al. Population distribution of Beta-lactamase conferring resistance to third-generation cephalosporins in human clinical Enterobacteriaceae in the Netherlands. PLoS One. 2012;7: e52102 10.1371/journal.pone.0052102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Willemsen I, Oome S, Verhulst C, Pettersson A, Verduin K, Kluytmans J. Trends in Extended Spectrum Beta-Lactamase (ESBL) producing enterobacteriaceae and ESBL genes in a Dutch teaching hospital, measured in 5 yearly point prevalence surveys (2010–2014). PLoS One. 2015;10: 1–10. 10.1371/journal.pone.0141765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Van Der Bij AK, Peirano G, Goessens WHF, Van Der Vorm ER, Van Westreenen M, Pitout JDD. Clinical and molecular characteristics of extended-spectrum-beta-lactamase-producing Escherichia coli causing bacteremia in the Rotterdam Area, Netherlands. Antimicrob Agents Chemother. 2011;55: 3576–3578. 10.1128/AAC.00074-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stuart JC, Dierikx C, Naiemi N Al, Karczmarek A, Van Hoek AHAM, Vos P, et al. Rapid detection of TEM, SHV and CTX-M extended-spectrum beta-lactamases in Enterobacteriaceae using ligation-mediated amplification with microarray analysis. J Antimicrob Chemother. 2010;65: 1377–1381. 10.1093/jac/dkq146 [DOI] [PubMed] [Google Scholar]
- 44.German institut for risk Assesment (BfR). Bundesinstitut für Risikobewertung. EHEC outbreak: BfR confirms contamination of sprouts with O104:H4 [Internet]. 2011 [cited 30 Dec 2017]. Available: http://www.bfr.bund.de/de/presseinformation/2011/17/ehec_ausbruch__bfr_bestaetigt_kontamination_von_sprossen_mit_o104_h4-70934.html
- 45.Overdevest ITMA, Willemsen I, Elberts S, Verhulst C, Kluytmans JAJW. Laboratory detection of extended-spectrum-beta-lactamase-producing Enterobacteriaceae: evaluation of two screening agar plates and two confirmation techniques. J Clin Microbiol. 2011;49: 519–22. 10.1128/JCM.01953-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
Data Availability Statement
All raw sequencing reads are available from the ENA database (accession number PRJEB25080). Stores of purchase have been anonymized.