Key Points
Question
Could the favorable results from clinical experiences using a noninvasive, outpatient stereotactic ablative radiotherapy for medically frail patients with early-stage lung cancer be applicable for treating healthier patients traditionally managed exclusively with surgery?
Findings
In this analysis of data from a clinical trial of stereotactic body radiation therapy in 26 patients, targeted tumor control was high such that surgery for salvage was not generally needed. Median survival was higher in these patients with operable disease compared with the historical use in medically frail patients unable to tolerate surgery.
Meaning
These results support formal comparisons of surgery and stereotactic radiotherapy for early-stage lung cancer, and several trials are under way.
This analysis of data from a single-arm clinical trial investigates whether stereotactic body radiation therapy delivered on an outpatient basis for patients with operable early-stage lung cancer is associated with primary tumor control, survival, adverse events, and the need for surgical resection.
Abstract
Importance
Stereotactic body radiation therapy (SBRT) has become a standard treatment for patients with medically inoperable early-stage lung cancer. However, its effectiveness in patients medically suitable for surgery is unclear.
Objective
To evaluate whether noninvasive SBRT delivered on an outpatient basis can safely eradicate lung cancer and cure selected patients with operable lung cancer, obviating the need for surgical resection.
Design, Setting, and Participants
Single-arm phase 2 NRG Oncology Radiation Therapy Oncology Group 0618 study enrolled patients from December 2007 to May 2010 with median follow-up of 48.1 months (range, 15.4-73.7 months). The setting was a multicenter North American academic and community practice cancer center consortium. Patients had operable biopsy-proven peripheral T1 to T2, N0, M0 non–small cell tumors no more than 5 cm in diameter, forced expiratory volume in 1 second (FEV1) and diffusing capacity greater than 35% predicted, arterial oxygen tension greater than 60 mm Hg, arterial carbon dioxide tension less than 50 mm Hg, and no severe medical problems. The data analysis was performed in October 2014.
Interventions
The SBRT prescription dose was 54 Gy delivered in 3 18-Gy fractions over 1.5 to 2.0 weeks.
Main Outcomes and Measures
Primary end point was primary tumor control, with survival, adverse events, and the incidence and outcome of surgical salvage as secondary end points.
Results
Of 33 patients accrued, 26 were evaluable (23 T1 and 3 T2 tumors; 15 [58%] male; median age, 72.5 [range, 54-88] years). Median FEV1 and diffusing capacity of the lung for carbon monoxide at enrollment were 72.5% (range, 38%-136%) and 68% (range, 22%-96%) of predicted, respectively. Only 1 patient had a primary tumor recurrence. Involved lobe failure, the other component defining local failure, did not occur in any patient, so the estimated 4-year primary tumor control and local control rate were both 96% (95% CI, 83%-100%). As per protocol guidelines, the single patient with local recurrence underwent salvage lobectomy 1.2 years after SBRT, complicated by a grade 4 cardiac arrhythmia. The 4-year estimates of disease-free and overall survival were 57% (95% CI, 36%-74%) and 56% (95% CI, 35%-73%), respectively. Median overall survival was 55.2 months (95% CI, 37.7 months to not reached). Protocol-specified treatment-related grade 3, 4, and 5 adverse events were reported in 2 (8%; 95% CI, 0.1%-25%), 0, and 0 patients, respectively.
Conclusions and Relevance
As given, SBRT appears to be associated with a high rate of primary tumor control, low treatment-related morbidity, and infrequent need for surgical salvage in patients with operable early-stage lung cancer.
Trial Registration
ClinicalTrials.gov Identifier: NCT00551369
Introduction
Stereotactic body radiation therapy (SBRT) is a noninvasive cancer treatment in which numerous small and accurate radiation beams are used to deliver potent doses in 1 to 5 treatments to tumors in extracranial sites.1,2 The NRG Oncology Radiation Therapy Oncology Group (RTOG) 0236 trial,3 as well as several other international cooperative group trials,4,5 has shown that SBRT affords high rates of tumor control while avoiding severe toxic effects in the majority of patients with early-stage lung cancer unable to tolerate surgical resection (ie, medically inoperable). While surgery has long been the standard treatment in patients with operable disease, the convenience, noninvasiveness, and favorable outcomes experienced by patients with medically inoperable disease have logically led to interest in using SBRT for all patients with stage I lung cancer.
To our knowledge, the NRG Oncology RTOG 0618 trial was the first North American multicenter, cooperative group study to test SBRT in treating patients with operable early-stage lung cancer. In this report, we describe the 4-year results from RTOG 0618.
Methods
Patient Eligibility
Prior to enrollment, the trial was approved by each enrolling site’s local institutional review board and written informed consent was obtained for each patient. Cytologic or histologic proof of non–small cell cancer was required for entry. Eligible patients could have American Joint Committee on Cancer stages T1, T2, or T3 (primary tumor ≤5 cm, peripheral tumors only), N0, M0 cancer based on both mandatory computed tomography and positron emission tomography (PET) screening. Only patients with operable disease suitable at least for a sublobar resection as determined by a thoracic surgeon were eligible according to all of the following baseline characteristics: forced expiratory volume in 1 second and diffusing capacity of the lung for carbon monoxide greater than 35% predicted, arterial oxygen tension greater than 60 mm Hg, arterial carbon dioxide tension less than 50 mm Hg, and absent severe underlying medical problems.
Radiotherapy Specifications
Radiotherapy planning and delivery were identical to the requirements of the RTOG 0236 study described previously.6 Patients effectively received 54 Gy in 3 fractions prescribed to the edge of the tumor target.7
Follow-up and End Points
Patients were observed at least every 6 months for 4 years after treatment with computed tomography imaging. Tumor measurements at each follow-up were carried out using the Response Evaluation Criteria in Solid Tumors.8
The primary end point of the study was 2-year actuarial primary tumor control (avoiding recurrence). Primary tumors were said to have recurred if they showed local enlargement (≥20% increase in tumor diameter) and evidence of tumor viability. Marginal failure within 2 cm of the treated tumor was included in determining primary tumor control. The protocol urged centers to confirm tumor viability and distinguish from local masslike treatment effects by repeated biopsy when possible; however, it also could be affirmed by PET.
Secondary end points included assessments of treatment-related toxic effects, disease-free survival (DFS), and overall survival (OS). Disease-free survival included separate assessments of local-regional failure and disseminated recurrence. The protocol design included instructions urging speedy referral to a thoracic surgeon in the event of an isolated local or regional recurrence so that surgical salvage might be considered.
The National Cancer Institute’s Common Toxicity Criteria, version 3.0, was used for grading of adverse events (AEs).9 All AEs reported by participating centers were collected and assessed. In addition, information regarding morbidity following surgical salvage was collected centrally as a secondary protocol end point.
Statistical Design
An acceptable tumor control rate at 2 years was considered to be 90% (monthly hazard of 0.00439), and an unacceptable rate was 70% (monthly hazard of 0.01486). With a 1-sided type I error of .05 and statistical power of 90%, a sample of 27 patients was required. A 1-sided z test was used to determine whether the difference between the logarithm of the observed hazard rate and the logarithm of the hypothesized hazard rate of 0.01486 was statistically significant. All outcomes were measured from the start of SBRT until the date of first failure or, if no failure, the date of death or last known alive. Both OS and DFS were estimated using the Kaplan-Meier method10; all other end points were estimated using the cumulative incidence method,11 in which death without an event was considered as a competing risk. The 95% confidence intervals were calculated using the method described by Gaynor et al.12 All analyses were performed using SAS, version 9.2.
Results
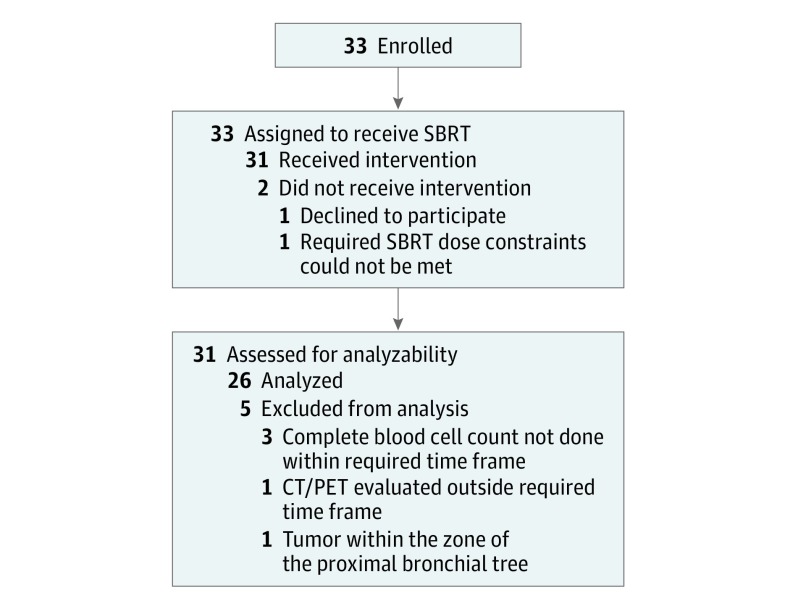
Between December 2007 and May 2010, 33 patients were enrolled to the study. The CONSORT diagram is shown in Figure 1. Pretreatment characteristics are presented in the Table. Median follow-up for evaluable patients (n = 26) was 48.1 months (range, 15.4-73.7 months).
Figure 1. CONSORT Diagram.
CT/PET indicates computed tomography/positron emission tomography; SBRT, stereotactic body radiation therapy.
Table. Pretreatment Characteristics of 26 Patients in NRG Oncology RTOG 0618.
| Characteristic | Value |
|---|---|
| Age, median (range), y | 72.5 (54-88) |
| FEV1 percent predicted, median (range) | 72.5 (38-136) |
| DLCO percent predicted, median (range) | 68 (22-96) |
| Racial category, No. (%) | |
| Asian | 1 (4) |
| Black or African American | 4 (15) |
| White | 20 (77) |
| Unknown | 1 (4) |
| Sex, No. (%) | |
| Male | 15 (58) |
| Female | 11 (42) |
| Zubrod performance status, No. (%) | |
| 0 | 19 (73) |
| 1 | 7 (27) |
| Stage, No. (%) | |
| IA | 23 (88) |
| IB | 3 (12) |
| Histologic type, No. (%) | |
| Squamous cell carcinoma | 5 (19) |
| Adenocarcinoma | 12 (46) |
| Non–small cell carcinoma not otherwise specified | 9 (35) |
Abbreviations: DLCO, diffusing capacity of the lung for carbon monoxide; FEV1, forced expiratory volume in 1 second.
Local Control
One patient experienced a documented recurrence/progression at the primary site, while none had marginal or involved lobe recurrences. The 2-year rate of both primary tumor and local control was 96% (95% CI, 83%-100%), with a monthly hazard of 0.002. The corresponding P value from the comparison to the hypothesized monthly hazard rate of 0.015 is .02. Control of all components of local control–defining sites (primary, marginal, and involved lobe) was durable to this value out to 4 years of median follow-up.
Regional Failure, Disseminated Recurrence, and Survival
Regional failures were reported in 3 patients, occurring at 11, 14, and 29 months following the start of protocol therapy. Combining local and regional failures, the 4-year local-regional control rate was 88% (95% CI, 73%-97%). Disseminated recurrence was observed in 5 patients, giving a 4-year rate of disseminated failure of 12% (95% CI, 3%-27%).
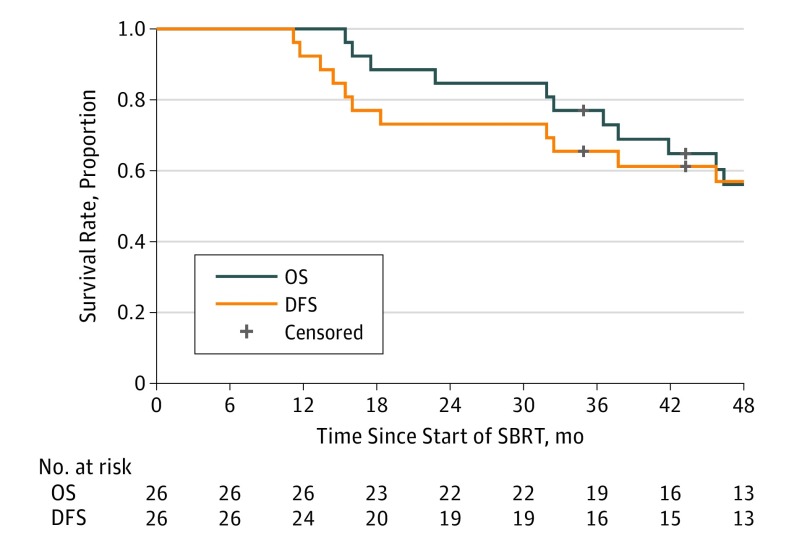
The DFS and OS at 4 years was 57% (95% CI, 36% to 74%) and 56% (95% CI, 35% to 73%), respectively, as shown in Figure 2. Median DFS and OS for all patients was 55.2 months (95% CI, 31.9 months to not reached) and 55.2 months (95% CI, 37.7 months to not reached), respectively.
Figure 2. Overall Survival (OS) and Disease-Free Survival (DFS).
Of 26 patients evaluated for OS and DFS, 12 experienced failure and 14 were censored for each outcome. Median OS was 55.2 months (95% CI, 37.7 months to not reached), and median DFS was 55.2 months (95% CI, 31.9 months to not reached). SBRT indicates stereotactic body radiation therapy.
Adverse Events
No grade 4 to 5 treatment-related AEs were reported. Four patients (15%) were reported to experience grade 3 AEs assigned definitely, probably, or possibly related to protocol therapy. Two of these AEs were related to posttreatment decline in diffusing capacity, 1 to peripheral sensory neuropathy, and 1 to chest wall complications (rib fracture).
Discussion
As with RTOG 0236, RTOG 0618 also showed a high rate of primary tumor control (96% at 4 years). Unlike SBRT, lobectomy with regional lymph node dissection provides primary tumor, involved lobe, and regional clearance, although survival is profoundly worse if there is extension beyond the primary tumor.13 Even without a regional therapy, SBRT as delivered showed a reasonably low rate of involved lobe or regional failure, possibly indicating that patients were well staged with PET. Indeed, for patients whose disease was clinically stage I based on computed tomography/PET, the negative predictive value is as high as 95%.14
In RTOG 0236, 26% of patients had grade 3 to 4 toxic effects, while only 14% of patients in the present trial had grade 3 events (no grade 4). Because the delivered therapies were identical, one can assume that the selection of healthier patients more capable of withstanding the potent therapy accounts for the difference. Similarly, disseminated recurrence was lower for the report on patients with operable disease. With these and other factors, survival is better in the operable population, with fewer competing causes of death.
Limitations
This was a small pilot study only enrolling 33 patients. With no control or randomized arm, comparisons with other experience should be interpreted with caution.
Conclusions
RTOG 0618 demonstrated that high rates of intrathoracic control can be achieved with a noninvasive, outpatient alternative to surgery in a healthier group of patients with early-stage lung cancer. This tumor control was achieved with a relatively low rate of toxic effects. Collectively, SBRT for this operable population may be a viable alternative to surgical resection, which would ideally be compared in a phase 3, randomized clinical trial. Further attempts to perform such a trial are ongoing (eg, VALOR, Stablemates, and SAbRtooth).
References
- 1.Timmerman RD, Kavanagh BD, Cho LC, Papiez L, Xing L. Stereotactic body radiation therapy in multiple organ sites. J Clin Oncol. 2007;25(8):947-952. [DOI] [PubMed] [Google Scholar]
- 2.Potters L, Steinberg M, Rose C, et al. ; American Society for Therapeutic Radiology and Oncology; American College of Radiology . American Society for Therapeutic Radiology and Oncology and American College of Radiology practice guideline for the performance of stereotactic body radiation therapy. Int J Radiat Oncol Biol Phys. 2004;60(4):1026-1032. [DOI] [PubMed] [Google Scholar]
- 3.Huang L, Park K, Boike T, et al. . A study on the dosimetric accuracy of treatment planning for stereotactic body radiation therapy of lung cancer using average and maximum intensity projection images. Radiother Oncol. 2010;96(1):48-54. [DOI] [PubMed] [Google Scholar]
- 4.Baumann P, Nyman J, Hoyer M, et al. . Outcome in a prospective phase II trial of medically inoperable stage I non-small-cell lung cancer patients treated with stereotactic body radiotherapy. J Clin Oncol. 2009;27(20):3290-3296. [DOI] [PubMed] [Google Scholar]
- 5.Nagata Y, Hiraoka M, Shibata T, et al. . Stereotactic body radiation therapy for T1N0M0 non-small cell lung cancer: first report for inoperable population of a phase II trial by Japan Clinical Oncology Group (JCOG 0403). Int J Radiat Oncol Biol Phys. 2012;84(3 suppl):S46. [Google Scholar]
- 6.Timmerman R, Paulus R, Galvin J, et al. . Stereotactic body radiation therapy for inoperable early stage lung cancer. JAMA. 2010;303(11):1070-1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xiao Y, Papiez L, Paulus R, et al. . Dosimetric evaluation of heterogeneity corrections for RTOG 0236: stereotactic body radiotherapy of inoperable stage I-II non-small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2009;73(4):1235-1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang W, Jones R, Lu W, et al. . Feasibility of non-coplanar tomotherapy for lung cancer stereotactic body radiation therapy. Technol Cancer Res Treat. 2011;10(4):307-315. [DOI] [PubMed] [Google Scholar]
- 9.Trotti A, Colevas AD, Setser A, et al. . CTCAE v3.0: development of a comprehensive grading system for the adverse effects of cancer treatment. Semin Radiat Oncol. 2003;13(3):176-181. [DOI] [PubMed] [Google Scholar]
- 10.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53(282):457-481. [Google Scholar]
- 11.Gray R. A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat. 1988;16(3):1141-1154. [Google Scholar]
- 12.Gaynor JJ, Feuer EJ, Tan CC, et al. . On the use of cause-specific failure and conditional failure probabilities: examples from clinical oncology data. J Am Stat Assoc. 1993;88(422):400-409. [Google Scholar]
- 13.Rusch VW, Hawes D, Decker PA, et al. . Occult metastases in lymph nodes predict survival in resectable non-small-cell lung cancer: report of the ACOSOG Z0040 trial. J Clin Oncol. 2011;29(32):4313-4319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Defranchi SA, Cassivi SD, Nichols FC, et al. . N2 disease in T1 non-small cell lung cancer. Ann Thorac Surg. 2009;88(3):924-928. [DOI] [PMC free article] [PubMed] [Google Scholar]