Abstract
Donor-specific antibodies (DSA) to mismatched human leukocyte antigens (HLA) are associated with worse outcomes after lung transplantation. To determine the incidence and characteristics of DSA early after lung transplantation, we conducted a prospective multi-center observational study that used standardized treatment and testing protocols. Among 119 transplant recipients, 43 (36%) developed DSA: 6 (14%) developed DSA only to class I HLA, 23 (53%) developed DSA only to class II HLA, and 14 (33%) developed DSA to both class I and class II HLA. The median DSA mean fluorescence intensity (MFI) was 3197. We identified a significant association between the Lung Allocation Score and the development of DSA (HR = 1.02, 95% CI: 1.001 – 1.03, p = 0.047) and a significant association between DSA with an MFI ≥ 3000 and acute cellular rejection (ACR) grade ≥ A2 (HR = 2.11, 95% CI: 1.04 – 4.27, p = 0.039). However, we did not detect an association between DSA and survival. We conclude that DSA occur frequently early after lung transplantation, and most target class II HLA. DSA with an MFI ≥ 3000 have a significant association with ACR. Extended follow-up is necessary to determine the impact of DSA on other important outcomes.
INTRODUCTION
Lung transplantation may prolong survival and improve quality of life for patients who have advanced lung disease (1–5). However, long-term outcomes after lung transplantation remain disappointing; according to the latest International Society for Heart and Lung Transplantation (ISHLT) Registry report, recipients from the most recent era had a median survival of approximately 6 years (6). The most common causes of death in the first year after transplantation are infection and allograft failure related to primary graft dysfunction (PGD), but allograft failure due to chronic lung allograft dysfunction (CLAD), including bronchiolitis obliterans syndrome (BOS) and restrictive allograft syndrome (RAS), accounts for over 40% of deaths and remains the main obstacle to better long-term outcomes after transplantation (6–10).
Conventional immunosuppression has primarily targeted T-cell proliferation and function for the prevention of allograft rejection in solid-organ transplantation. However, evidence supporting a role for humoral immunity in allograft rejection has grown over time. Hyperacute rejection due to preformed donor-specific antibodies (DSA) to mismatched human leukocyte antigens (HLA) has long been recognized as a cause of early allograft failure, and an association between HLA antibodies and CLAD has been recognized since the late 1990s (11–14). More recently, clinicians and investigators have better recognized antibody-mediated rejection (AMR) after lung transplantation, and an ISHLT working group has developed a consensus definition (15–20).
In previous retrospective single-center studies, the incidence of DSA after lung transplantation has varied between 13% and 61%, and these studies have not used a standardized approach to testing and management (21–28). We sought to examine the incidence of DSA and their characteristics in a prospective multi-center study that used a standardized approach to clinical management, monitoring, and DSA testing.
METHODS
Study Design and Eligibility Criteria
We conducted a prospective observational cohort study of lung transplant recipients at 6 centers in the United States to determine the cumulative incidence of post-transplant DSA, describe their characteristics, and assess their impact on clinical outcomes. The National Institutes of Health funded the study as a 2-year R34 grant (HL 10542). Each site aimed to prescreen/screen all lung transplant candidates placed on the United Network for Organ Sharing (UNOS) waiting list at the institution. Study personnel screened patients for eligibility (Table E1 in the online data supplement) at the time of listing for transplantation or within the first 10 days after transplantation. Enrollment was initiated on December 1, 2011 and completed on June 30, 2012; follow-up was completed on October 28, 2012. Local Institutional Review Boards at each center approved the study protocol, and all participants provided written informed consent.
Endpoints and Outcomes
Primary and secondary endpoints were defined during protocol development. The study used HALT-defined DSA (Table 1) as the primary endpoint, and the cumulative incidence of first DSA during the first 4 months after transplantation as the primary outcome. Protocol-defined secondary endpoints included antibody mediated rejection (AMR), acute cellular rejection (ACR), lymphocytic bronchiolitis (LB), and death. ACR and LB were defined according to the standard ISHLT criteria (29). However, for the diagnosis of AMR, we used a central blinded adjudication committee because the ISHLT definition was proposed after the study was completed (20). As part of the secondary endpoints, we also examined the association between DSA and the development of ACR and LB.
Table 1.
HALT Study definition of post-transplant donor-specific HLA antibody (DSA) positivity.
| De novo post-transplant DSA |
Compared to pre-transplant bead reactivity*:
|
| Pre-transplant DSA with MFI: 1000 – 5000 and negative flow cytometry crossmatch |
Compared to pre-transplant bead reactivity:
|
DSA: donor-specific HLA antibody
MFI: mean fluorescence intensity
LABScreen® Single Antigen assay (One Lambda, Canoga Park, CA)
HLA Testing
Before transplantation, candidates underwent low resolution HLA-A, B, C, DRB1, DRB3, DRB4, DRB5, and DQB typing by the transplant center’s histocompatibility laboratory using DNA-based methods. Donors underwent similar typing at the transplant center. In cases where testing detected anti-DQA, DP or allele-specific antibodies, the lab conducted additional HLA-DQA, DP or allele level typing of donors and recipients. Before transplantation, candidates were tested for HLA antibodies using the LABScreen® Single Antigen assay (One Lambda, Canoga Park, CA), and donor organs were accepted for an allosensitized patient if the patient did not have HLA antibodies against the donor type (i.e., negative virtual crossmatch). However, the mean fluorescence intensity (MFI) cut-off to define pre-transplant HLA antibody positivity varied among sites. Candidates with pre-transplant DSA were eligible for enrollment if the DSA MFI < 5000 and the direct donor-recipient flow cytometry crossmatch was negative at the time of transplantation. The histocompatibility laboratory at the sites used a direct final prospective or a retrospective flow cytometry T- and B-cell crossmatch to determine donor-recipient HLA compatibility. The laboratories tested pre-transplant serum, and sera were tested in duplicate. Each laboratory used their protocols to perform sample preparation and standardization of instrument settings; instruments used by different laboratories were not cross-calibrated using reference materials. Flow crossmatch results were reported using median channel shift (MCS). The interpretation of the T- and B-cell crossmatch compatibility was based on cut off values established by each laboratory as defined in the HALT manual of operation. Prior to reporting, the crossmatch results were reviewed for consistency with past and current HLA antibody specificities, presence of autoantibody reactivity, or other potentially interfering substances. All laboratories considered patients with “positive” flow crossmatches according to the HALT eligibility criteria to participate in the study. In some centers, weak DSA (MFI value in the range of 1000–3000) may be undetected by the flow cytometry crossmatch if the sensitivity of the crossmatch is low due sample preparation or instrument setting. The HALT HLA Adjudication Committee adjudicated all flow crossmatch results as positive or negative.
After transplantation, recipients were tested for DSA using the LABScreen® Single Antigen assay at 10 (± 3), 30, 60, 90, and 120 (± 7) days after transplantation, and if they had clinical signs or symptoms of allograft dysfunction. The study classified post-transplant DSA as de novo or pre-formed (Table 1). De novo DSA was defined as a donor-specific antibody with an MFI > 1000, and if the antibody was detectable pre-transplant with an MFI < 1000, the post-transplant MFI had to increase by ≥ 500 and > 30% compared to the pre-transplant MFI (Table 1). Among patients with pre-formed DSA (i.e., DSA MFI between 1000 – 5000 and a negative direct flow cytometry crossmatch), post-transplant DSA was defined as an MFI increase ≥ 500 and > 30% compared to the pre-transplant MFI (Table 1). The HALT HLA Adjudication Committee adjudicated all post-transplant DSA test results as present or absent. Additional details of HLA testing methodology are provided in the online data supplement.
Standardized Clinical Care
Prior to initiation of study enrollment, the sites agreed upon a standardized general clinical approach to induction and maintenance immunosuppression, infection prophylaxis, monitoring with pulmonary function testing (PFT) and bronchoscopy, and ACR treatment.
Statistical Analyses
The study used standard descriptive analyses for reporting. For comparisons of characteristics between subgroups, we used t-tests (or Wilcoxon-Rank sum tests if the data were not normally distributed) and chi-square (or Fisher’s exact) tests. For the primary endpoint of the study (i.e., post-transplant DSA), we used the Kaplan-Meier method to estimate time to DSA development. We used Cox proportional hazards regression to identify risk factors for the development of DSA and to assess for associations between the development of DSA and ACR and LB. We performed these analyses post-hoc, and did not adjust for multiple comparisons. In the analyses of risk factors for the development of DSA, we only included episodes of ACR and LB that occurred before DSA, and in the analyses of the impact of DSA on ACR and LB, we only included episodes of ACR and LB that occurred after DSA. We confirmed model assumptions and performed regression diagnostics, and censored for death, re-transplant, end of study, and loss to follow-up. We used SPSS 22.0 (SAS, Inc., Chicago, IL, USA) for all analyses. The online data supplement provides additional details on data management and power calculations.
RESULTS
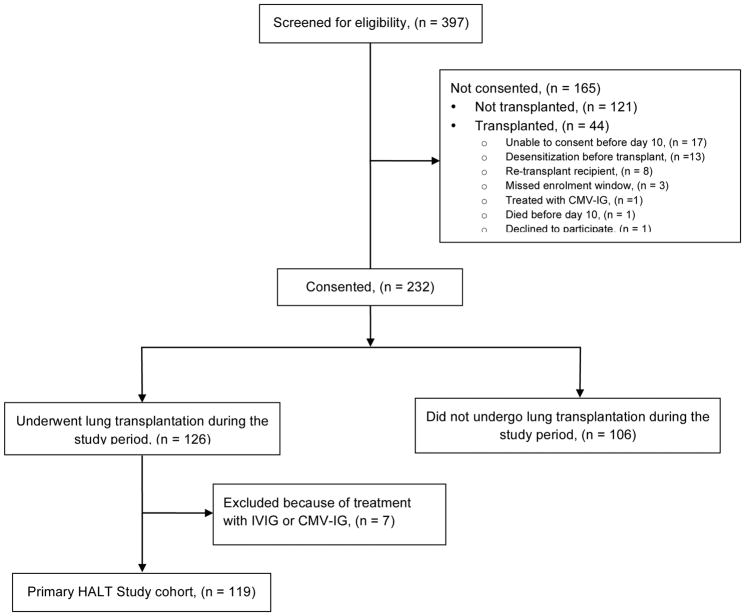
During the enrollment period, study personnel pre-screened 397 transplant candidates and recipients among the centers. Among the 397 potential participants, 227 (57%) were not transplanted during the study period. Of the 232 potentially eligible and consented patients, 126 underwent lung transplantation (Figure 1). Of these, the study excluded 7 patients because of treatment with intravenous immune globulin (IVIG) or cytomegalovirus (CMV) immune globulin for hypogammaglobulinemia or high-risk CMV status. The remaining 119 lung transplant recipients comprised the primary HALT study cohort (Figure 1). Only 51 of 397 (13%) potential participants were transplanted but not included in the study cohort. In general, study participants had similar demographics to contemporary U.S. lung transplant recipients (Table 2) (30). Interstitial lung disease was the most common indication for lung transplantation and accounted for 44% of patients. The mean Lung Allocation Score (LAS) at transplant was 45.59 ± 16.93 (median = 39.99). The median number of donor-recipient HLA mismatches at the A, B, C, DRB1, and DQB loci was 8 (Table 2).
Figure 1.
Flow diagram: study screening and eligibility. IVIG: intravenous immune globulin. CMV-IG: cytomegalovirus immune globulin.
Table 2.
Demographics of HALT study participants.
| Variable | N = 119 |
|---|---|
| Age, mean ± standard deviation (SD) | 57 ± 13 |
| Female recipient, n (%) | 49 (41%) |
| Diagnosis, n (%) | |
| COPD | 38 (32%) |
| Interstitial lung disease / pulmonary fibrosis | 53 (44%) |
| Cystic fibrosis (CF) or non-CF bronchiectasis | 18 (15%) |
| Pulmonary hypertension | 2 (2%) |
| Other | 8 (7%) |
| Lung allocation score at transplant, mean ± SD | 45.59 ± 16.93 |
| Pre-transplant ECMO support | 2 (2%) |
| Bilateral lung transplant, n (%) | 90 (76%) |
| Single lung transplant, n (%) | 29 (24%) |
| Intraoperative cardiopulmonary bypass, n (%) | 69 (58%) |
| Ischemic time (minutes), mean ± standard deviation | 285 ± 80 |
| Post-transplant ECMO support, n (%) | 1 (1%) |
| Identical donor-recipient ABO status, n (%) | 107 (90%) |
| Compatible donor-recipient ABO status, n (%) | 12 (10%) |
| Recipient CMV seropositive, n (%) | 53 (45%) |
| Recipient CMV seronegative and donor seropositive, n (%) | 35 (29%) |
| Pre-transplant DSA, n (%) | 12 (10%) |
| Number of donor-recipient mismatches at HLA A, B, C, DRB1, and DQB loci, median (IQR) | 8 (3) |
| Number of donor-recipient mismatches at HLA-DRB1 locus, median (IQR) | 2 (1) |
| Number of donor-recipient mismatches at HLA-DQB locus, median (IQR) | 1 (1) |
| Positive Flow Cytometry B-cell crossmatch, n (%) | 17 (15%) |
| PGD grade at time 0*, n (%) | |
| PGD 0 | 69 (60%) |
| PGD 1 | 26 (23%) |
| PGD 2 | 10 (9%) |
| PGD 3 | 10 (9%) |
| PGD grade at 72 hours**, n (%) | |
| PGD 0 | 49 (47%) |
| PGD 1 | 49 (47%) |
| PGD 2 | 3 (3%) |
| PGD 3 | 4 (4%) |
| Blood product transfusion during transplant hospitalization, n (%) | 63 (53%) |
| Induction immunosuppression, n (%) | |
| None | 45 (38%) |
| Basiliximab | 72 (60%) |
| Rabbit anti-thymocyte globulin | 2 (2%) |
COPD: chronic obstructive pulmonary disease, with or without alpha 1-antitrypsin deficiency
ECMO: extracorporeal membrane oxygenation
CMV: cytomegalovirus
PGD: primary graft dysfunction
Immediately after transplantation (time 0), 115 recipients had sufficient data to calculate PGD grades
At 72 hours, 105 recipients had sufficient data to calculate PGD grades
Among the 119 study participants, 12 (10%) had pre-transplant DSA; 7 had DSA only to class I HLA, 3 had DSA only to class II HLA, and 2 had DSA to class I and class II HLA. The median class I DSA MFI was 1496, and the median class II DSA MFI was 1922. In the study cohort, 116 of 119 patients had a direct flow cytometry crossmatch performed. The T-cell crossmatch was negative in 115 (99%) recipients and positive in 1 (1%) with a median channel shift of 38. The B-cell crossmatch was negative in 99 (85%) recipients and positive in 17 (15%) with a mean MCS of 102. None of the recipients who had a positive T-cell or B-cell crossmatch had pre-transplant DSA, and the sites’ clinical teams and the study’s blinded adjudicators deemed all transplants HLA compatible.
Although the sites agreed upon a standardized general clinical approach to immunosuppression, deviations from this approach were allowed for patient-specific indications. Of the 119 recipients, 72 (60%) were treated with basiliximab and 2 (2%) were treated with rabbit anti-thymocyte globulin for induction immunosuppression, while 45 (38%) did not receive induction immunosuppression because they were deemed to be at increased risk of infectious complications (e.g., chronic infection with multi-drug resistant organisms, pre-transplant immunosuppression, or frailty). The maintenance immunosuppressive regimen on day 10 after transplantation consisted of prednisone (119/119; 100%), tacrolimus (116/119; 98%), and mycophenolate mofetil (112/119; 94%) or azathioprine (1/119; 1%).
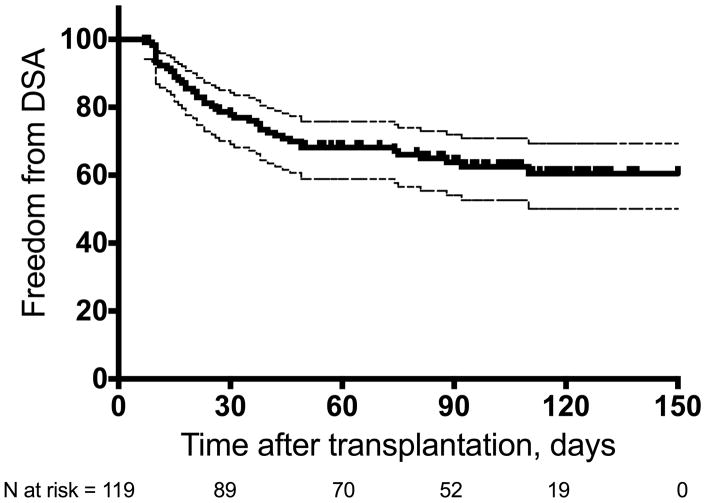
During the 4 months of post-transplant follow-up, the cohort of lung transplant recipients had a first DSA cumulative incidence of 36% (43/119; 95% CI 28% – 45%; Figure 2). There was no statistically significant difference in the incidence of DSA between the 6 sites (p = 0.119, Table E2 in the online data supplement). Of the 43 patients who developed DSA, 26 (60.5%) developed DSA within the first 30 days of transplantation and 17 (39.5%) developed DSA beyond the first 30 days. Among those who developed DSA, 6 (14%) developed DSA only to class I HLA, 23 (53%) developed DSA only to class II HLA, and 14 (33%) developed DSA to class I and class II HLA. Overall, the mean number of DSA specificities among those who developed DSA was 2.0 ± 1.68 (median = 1). Of the 43 transplant recipients who developed DSA, 23 (54%) developed a single DSA, 12 (28%) developed 2 DSA, and 8 (18%) developed 3 or more DSA during follow-up (Figure 3A). DSA specificity for HLA-DQ was most frequent (n = 26, Figure 3B). The median MFI of the immunodominant DSA was 3197 (range 1068 – 18150); the DSA MFI stratified by HLA locus is shown in Figure 3C. Nine of the 43 (21%) recipients who developed DSA had detectable pre-transplant DSA; among these, 5 developed DSA with new specificities (de novo DSA) after transplant and 4 had the same pre-transplant specificities that increased in MFI and met the study definition of post-transplant DSA. We explored the incidence of DSA using different definitions of positivity. Among the 119 study participants, 32 (27%, 95% CI: 19% – 35%) developed DSA with an MFI ≥ 2000, and 24 (20%, 95% CI: 13% – 28%) developed DSA with an MFI ≥ 3000 (Table E3 in the online data supplement). During the study period, 9 patients cleared all DSA on follow-up testing, and all 9 had DSA initially identified in the first 30 days after transplantation.
Figure 2.
Kaplan-Meier freedom from post-transplant donor-specific HLA antibodies (DSA) during the study period. Thin dashed lines depict upper and lower bounds of 95% confidence interval.
Figure 3.
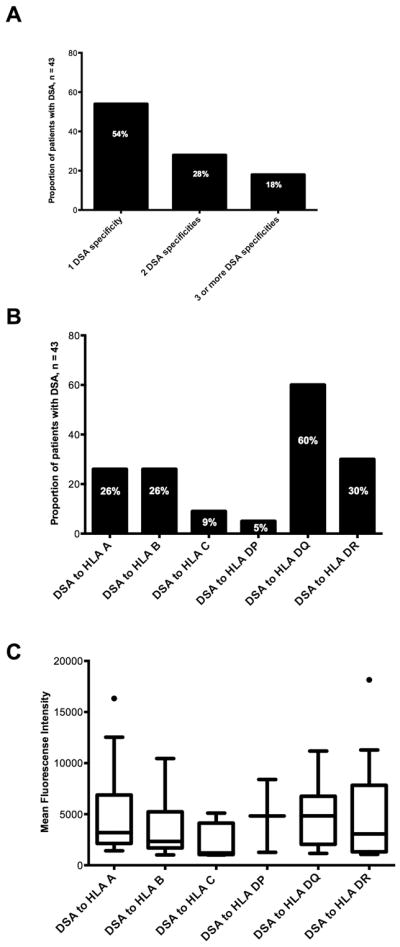
A. The proportion of patients who developed post-transplant donor-specific HLA antibodies (DSA) stratified by the number of DSA specificities.
B. The proportion of patients who developed post-transplant DSA stratified by DSA specificity.
C. Post-transplant DSA mean fluorescence intensity (MFI) stratified by HLA locus. Boxes extend from the 25th to the 75th percentiles, whiskers extend to the largest and smallest values within 1.5 times box lengths, and the line within the boxes represents the median. Solid circles represent outlier cases between 1.5 and 3 box lengths from the upper edge of the boxes.
Statistically significant univariable predictors of post-transplant DSA in Cox proportional hazards regression included pre-transplant DSA and a higher LAS at transplant (Table 3). Although the magnitude of risk associated with LAS is small, this signifies a 2% higher risk of DSA development for every incremental increase in the LAS by 1. To make this relationship more easily interpretable, we analyzed the risk of DSA development with incremental increases of 10 in the LAS: HR = 1.22, 95% CI: 1.02 – 1.38, p = 0.027. We did not evaluate positive T-cell crossmatch as a risk factor for post-transplant DSA because only 1 patient had a positive T-cell crossmatch and developed DSA 16 days after transplantation. In a multivariable Cox proportional hazards regression model, LAS at transplant was a significant predictor of post-transplant DSA but pre-transplant DSA was not (Table 4). In separate analyses, we forced positive B-cell crossmatch, the number of donor-recipient mismatches at the HLA-DR and HLA-DQ loci, LB, ACR, PGD grade, age, and gender into the final model, but none of these covariates improved the model’s −2 log likelihood or showed statistical significance (data not shown). In these models, the proportional hazards assumptions were met and we excluded nonlinearity using partial residual plots.
Table 3.
Univariable Cox proportional hazards models of risk factors for post-transplant donor-specific HLA antibody (DSA) development.
| Variable | HR | 95% CI | p value |
|---|---|---|---|
| Recipient age | 1.01 | 0.99 – 1.04 | 0.297 |
| Recipient female gender | 0.91 | 0.49 – 1.67 | 0.753 |
| Diagnosis (reference = COPD) | 0.381 | ||
| Interstitial lung disease | 1.28 | 0.65 – 2.52 | 0.469 |
| Cystic fibrosis or bronchiectasis | 0.38 | 0.11 – 1.35 | 0.135 |
| Pulmonary hypertension | 1.25 | 0.16 – 9.55 | 0.831 |
| Other | 0.80 | 0.18 – 3.54 | 0.766 |
| Lung allocation score at transplant | 1.02 | 1.002 – 1.03 | 0.027 |
| Pre-transplant ECMO support | 1.19 | 0.16 – 8.64 | 0.865 |
| Pre-transplant DSA | 2.34 | 1.08 – 5.05 | 0.030 |
| Single lung transplant (reference = bilateral) | 0.93 | 0.46 – 1.89 | 0.931 |
| Ischemic time | 1.00 | 0.99 – 1.01 | 0.589 |
| Intraoperative cardiopulmonary bypass | 1.18 | 0.64 – 2.18 | 0.595 |
| Compatible donor-recipient ABO status (reference = identical) | 1.78 | 0.79 – 4.00 | 0.162 |
| Number of donor-recipient HLA mismatches | 0.99 | 0.83 – 1.17 | 0.881 |
| Number of donor-recipient mismatches at HLA-DRB1 locus | 1.34 | 0.78 – 2.29 | 0.289 |
| Number of donor-recipient mismatches at HLA-DQB locus | 1.12 | 0.70 – 1.78 | 0.636 |
| Positive B-cell crossmatch | 1.75 | 0.84 – 3.67 | 0.136 |
| PGD grade at time 0 (reference = PGD 0) | 0.968 | ||
| PGD 1 | 1.27 | 0.62 – 2.59 | 0.514 |
| PGD 2 | 1.07 | 0.32 – 3.57 | 0.907 |
| PGD 3 | 1.16 | 0.40 – 3.33 | 0.790 |
| PGD grade at 72 hours (reference = PGD 0) | 0.999 | ||
| PGD 1 | 1.05 | 0.54 – 2.06 | 0.887 |
| PGD 2 | 0.98 | 0.13 – 7.40 | 0.988 |
| PGD 3 | 1.11 | 0.15 – 8.32 | 0.923 |
| Blood product transfusion | 0.76 | 0.42 – 1.39 | 0.378 |
| Induction agent (reference = none) | 0.177 | ||
| Basiliximab | 0.65 | 0.35 – 1.19 | 0.162 |
| Rabbit anti-thymocyte globulin | 1.94 | 0.45 – 8.32 | 0.371 |
| Acute cellular rejection grade ≥ A1* | 1.43 | 0.54 – 3.82 | 0.470 |
| Acute cellular rejection grade ≥ A2* | 1.88 | 0.65 – 5.46 | 0.244 |
| Lymphocytic bronchiolitis grade ≥ B1R* | 2.43 | 0.80 – 7.39 | 0.117 |
Analyzed as a time-dependent variable.
COPD: chronic obstructive pulmonary disease, with or without alpha 1-antitrypsin deficiency
ECMO: extracorporeal membrane oxygenation
DSA: donor-specific HLA antibody
HLA: human leukocyte antigen
PGD: primary graft dysfunction
Table 4.
Multivariable Cox proportional hazards model of risk factors for post-transplant donor-specific HLA antibody (DSA) development.
| Variable | HR | 95% CI | p value |
|---|---|---|---|
| Lung allocation score at transplant | 1.02 | 1.001 – 1.03 | 0.047 |
| Pre-transplant DSA | 1.67 | 0.73 – 3.82 | 0.226 |
Model Chi-square = 6.83, −2 log likelihood = 371.02, p = 0.033
During the study period, 112 recipients had transbronchial lung biopsies for evaluation of ACR, while 7 did not because of various clinical contraindications. Among the 112, 72 (64%) had at least one episode of ACR grade ≥ A1, 45 (40%) had at least one episode of ACR grade ≥ A2, and 18 (16%) had at least one episode of LB grade ≥ B1R. We did not detect a significant association between HALT-defined DSA and ACR (Table 5). We examined associations between DSA and ACR using different DSA MFI thresholds, and identified a trend to an increased risk of ACR grade ≥ A1 associated with DSA with an MFI ≥ 2000 and MFI ≥ 3000 and a statistically significant association between DSA with an MFI ≥ 3000 and ACR grade ≥ A2 (Table 5). Furthermore, we detected a significant association between the number of DSA specificities and ACR grade ≥ A2 (Table 5). We examined associations between DSA class and ACR and identified an increased risk of ACR grade ≥ A2 associated with DSA to both class I and class II HLA (Table 5). We did not detect any significant associations between any definition of DSA and LB.
Table 5.
Univariable Cox proportional hazards regression analyses of donor-specific HLA antibody (DSA) as predictors of outcome (acute cellular rejection [ACR] grade ≥ A1, ACR ≥ A2, or lymphocytic bronchiolitis [LB] grade ≥ B1R).
| DSA definition | ACR grade ≥ A1 | ACR grade ≥ A2 | LB grade ≥ B1R | ||||||
|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | p value | HR | 95% CI | p value | HR | 95% CI | p value | |
| HALT-defined DSA* | 1.42 | 0.85 – 2.38 | 0.178 | 1.42 | 0.75 – 2.69 | 0.279 | 1.18 | 0.42 – 3.34 | 0.757 |
| DSA MFI ≥ 2000* | 1.74 | 0.99 – 3.04 | 0.052 | 1.54 | 0.76 – 3.12 | 0.230 | 0.95 | 0.27 – 3.29 | 0.929 |
| DSA MFI ≥ 3000* | 1.80 | 0.98 – 3.28 | 0.057 | 2.11 | 1.04 – 4.27 | 0.039 | 1.19 | 0.34 – 4.12 | 0.788 |
| Number of DSA** specificities | 1.22 | 0.96 – 1.54 | 0.105 | 1.32 | 1.02 – 1.72 | 0.038 | 1.19 | 0.78 – 1.80 | 0.423 |
| DSA Class** | 0.668 | 0.159 | 0.964 | ||||||
| No DSA (reference) | 1.00 | 1.00 | 1.00 | ||||||
| Class I only | 0.84 | 0.33 – 2.11 | 0.705 | 1.01 | 0.31 – 3.31 | 0.993 | 0.82 | 0.11 – 6.33 | 0.851 |
| Class II only | 1.06 | 0.59 – 1.91 | 0.842 | 0.94 | 0.43 – 2.06 | 0.869 | 1.18 | 0.38 – 3.67 | 0.772 |
| Class I and Class II | 1.66 | 0.70 – 3.92 | 0.252 | 2.71 | 1.11 – 6.60 | 0.029 | 0.70 | 0.09 – 5.39 | 0.732 |
ACR: acute cellular rejection
LB: lymphocytic bronchiolitis
MFI: mean fluorescence intensity
Analyzed as a time-dependent variable
DSA defined according to HALT study criteria (i.e., HALT-defined DSA)
During the study period, 4 of the 43 (9%, 95% CI: 1% – 18%) recipients who developed DSA developed AMR (Table 6). All 4 patients had DSA to class I and class II HLA, and the median MFI of the immunodominant DSA was 4939 (range 2167 – 9572). The central blinded adjudication committee evaluated these cases and confirmed the diagnosis of AMR in 2 cases but deemed the other 2 cases as probably not AMR (Table 6). According to the recent ISHLT consensus definition (20), 2 cases were consistent with possible AMR and 2 were consistent with probable AMR. All patients with AMR were treated with various combinations of high-dose corticosteroids, IVIG, and rituximab, and all experienced a clinical improvement. Because study follow-up was limited to 4 months after transplantation, we did not examine the impact of DSA on BOS or CLAD development. We did not detect a significant difference in survival during the study period between recipients who developed DSA and those who did not; 4 of the 76 (5%) recipients who did not develop DSA died, and none of the 43 recipients who developed DSA died during the study period (log rank p = 0.128). However, study follow-up was limited to 4 months after transplantation.
Table 6.
Clinical presentation and diagnostic certainty of cases of suspected antibody-mediated rejection (AMR).
| Case | Time After Transplant | Allograft Dysfunction | DSA Locus & MFI* | Pathology | C4d Deposition | Adjudication Committee Determination | ISHLT Consensus Statement Diagnostic Certainty |
|---|---|---|---|---|---|---|---|
| 1 | 46 days | Dyspnea, decline in spirometry | A (MFI = 1451) DRB1 (MFI = 2167) |
Acute cellular rejection (A2B0), capillaritis | Negative | Definitely AMR | Probable AMR |
| 2 | 22 days | Hypoxemic respiratory failure | B (MFI = 3380) DQB1 (MFI = 3705) DQB1 (MFI = 3320) |
Acute lung injury, capillaritis | Negative | Definitely AMR | Probable AMR |
| 3 | 92 days | Dyspnea, decline in spirometry | A (MFI = 9572) DQB1 (MFI =1461) |
Acute cellular rejection (A2B0) | Negative | Probably not AMR | Possible AMR |
| 4 | 75 days | Hypoxemic respiratory failure | A (MFI = 3008) B (MFI = 2245) DQB1 (MFI = 4312) |
Not done | Not done | Probably not AMR | Possible AMR |
MFI: mean fluorescence intensity
DISCUSSION
In this prospective multicenter study that utilized a standardized approach to patient management and DSA testing, we examined the incidence and characteristics of DSA after lung transplantation. DSA developed frequently and early after transplantation, with an incidence of 36% in the first 4 months. The majority of DSA were directed at class II HLA, and a minority was directed solely at class I HLA. We identified higher LAS at transplant as an independent risk factor for the development of DSA. The early development of DSA, particularly in the first 2 weeks after transplantation, suggests a memory response though most patients did not have identifiable DSA before transplantation. Nonetheless, although pre-transplant DSA was a significant risk factor for developing DSA after transplantation in univariable analysis, it was no longer significant after adjusting for LAS. We detected an association between DSA with an MFI ≥ 3000 and ACR, though we did not detect a significant association between DSA with a lower MFI and ACR, and DSA had no significant impact on allograft survival. However, we note that the short follow-up period in this study limits our ability to fully characterize the impact of DSA on CLAD and allograft survival.
The cumulative incidence of DSA of 36% in this study is similar to previous single-center reports where the incidence ranged between 13% and 61% (21–28). Differences in the incidence of DSA among studies are likely related to different DSA testing protocols, DSA definitions, patient characteristics, and clinical regimens (e.g., immunosuppression, blood product transfusion). We used a low threshold MFI to define DSA positivity in this study in an attempt to detect DSA early. Previous studies have consistently shown an association between DSA and adverse clinical outcomes after lung transplantation, and we reasoned that early detection would identify patients at increased risk for ACR, LB, CLAD, and allograft failure (23–28, 32, 33). Not surprisingly, changing the definition of DSA positivity by increasing the threshold MFI resulted in a lower incidence of DSA. Ultimately, the definition of DSA should be based on the association between DSA development and clinical outcomes, and our results suggest that a threshold MFI ≥ 3000 is associated with a significantly increased risk of ACR. However, longer follow-up may demonstrate that DSA with lower MFI may increase the risk of ACR and LB. In addition, up to 9% of recipients who develop DSA develop possible or probable AMR according to the ISHLT definition. Nonetheless, a longer duration of follow-up is necessary to identify the MFI threshold that may be associated with the development of CLAD. Although our findings identified a significant association between DSA and ACR, this does not indicate that DSA have a causal role in the development of ACR. Rather, this underscores a synergy between humoral and cellular immune responses. Furthermore, the association between the number of DSA specificities and the risk of ACR suggests that the breadth of the humoral immune response correlates with the risk of cellular rejection. We did not identify a significant association between DSA to class II HLA and ACR as described in some previous studies, likely due to the relatively small sample size. In contrast, we did find an association between DSA to both class I and class II HLA and an increased risk of ACR, and we suspect the number of DSA specificities drove this relationship.
The study protocol did not include antibody-directed therapy after DSA identification because this remains controversial (21, 34, 35). Although retrospective cohort studies have suggested that treatment of asymptomatic DSA may improve clinical outcomes, the optimal regimen is unknown and spontaneous DSA clearance may occur (27–28, 34, 35). Thus, we propose that the efficacy and safety of treatment for asymptomatic DSA should be investigated in the context of a randomized controlled trial.
In contrast to previous studies, we did not identify an association between PGD and DSA (36, 37). However, the cohort had a small number of patients who developed PGD grade 2 or grade 3, and the study did not have sufficient power to detect a small but potentially important association. Nevertheless, our findings demonstrate a significant association between the LAS at transplant and DSA development. This is consistent with a recent study that reported a more than 2-fold higher risk of DSA among patients with an LAS > 60 (28). The LAS reflects severity of illness before transplantation, and a higher LAS is associated with increased morbidity that includes PGD, acute kidney injury, infections, respiratory failure, and prolonged critical illness early after transplantation (38–40). These comorbidities may upregulate pro-inflammatory mediators that increase the risk of DSA development (36, 41). Unfortunately, we did not collect data regarding these early post-transplant complications, and we are unable to examine their impact on the risk of DSA development in this cohort.
Despite the standardization of DSA testing, patient assessments, treatment approaches, and data collection among sites, some methodology limitations likely affected the results of the HALT study. The short follow-up duration, limited to 4 months after transplantation, almost certainly underestimates the incidence of DSA after transplantation. Although most patients developed DSA within 3 months of transplantation in some studies (21, 25–26), a significant number of patients developed DSA beyond the first year after transplantation in other studies (23, 24, 27). In addition, the short follow-up did not allow an appropriate assessment of the impact of DSA on CLAD and survival. The study duration also likely underestimated the incidence of AMR. Furthermore, the short follow-up duration limited our ability to assess the frequency of DSA persistence, resolution, recurrence, MFI changes, and profile switching. The study sample size influenced the precision of our estimates of the cumulative incidence of first DSA, though the 95% CI (28% – 45%) suggests that DSA occur commonly. The small sample size likely also impacted our ability to identify risk factors for DSA development. Lastly, we did not validate antibody testing results in a separate or core laboratory.
We conclude that DSA occur commonly early after lung transplantation, and that DSA are more frequently directed at class II HLA. We strongly support the move toward standardized DSA testing and reporting (42). Future studies should further clarify the association between DSA and long-term outcomes after lung transplantation and the potential role of preemptive antibody-directed therapy for transplant recipients who develop DSA.
Supplementary Material
Acknowledgments
This study was funded by the National Institutes of Health, National Heart, Lung, and Blood Institute (Grant Number: HL105412).
ABBREVIATIONS
- ACR
Acute cellular rejection
- AMR
Antibody-mediated rejection
- BOS
Bronchiolitis obliterans syndrome
- CLAD
Chronic lung allograft dysfunction
- CMV
Cytomegalovirus
- DSA
Donor-specific antibodies
- HLA
Human leukocyte antigens
- ISHLT
International Society for Heart and Lung Transplantation
- IVIG
Intravenous immunoglobulin
- MCS
Median channel shift
- MFI
Mean fluorescence intensity
- PFT
Pulmonary function testing
- PGD
Primary graft dysfunction
- RAS
Restrictive allograft syndrome
- UNOS
United Network for Organ Sharing
Footnotes
DISCLOSURE
The authors have no conflicts of interest to disclose as described by the American Journal of Transplantation.
References
- 1.Charman SC, Sharples LD, McNeil KD, Wallwork J. Assessment of survival benefit after lung transplantation by patient diagnosis. J Heart Lung Transplant. 2002;21:226–232. doi: 10.1016/s1053-2498(01)00352-7. [DOI] [PubMed] [Google Scholar]
- 2.De Meester J, Smits JM, Persijn GG, Haverich A. Listing for lung transplantation: Life expectancy and transplant effect, stratified by type of end-stage lung disease, the Eurotransplant experience. J Heart Lung Transplant. 2001;20:518–524. doi: 10.1016/s1053-2498(01)00241-8. [DOI] [PubMed] [Google Scholar]
- 3.Hosenpud JD, Bennett LE, Keck BM, Edwards EB, Novick RJ. Effect of diagnosis on survival benefit of lung transplantation for end-stage lung disease. Lancet. 1998;351:24–27. doi: 10.1016/S0140-6736(97)06405-2. [DOI] [PubMed] [Google Scholar]
- 4.Singer JP, Singer LG. Quality of life in lung transplantation. Semin Respir Crit Care Med. 2013;34:421–430. doi: 10.1055/s-0033-1348470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Singer LG, Chowdhury NA, Faughnan ME, et al. Effects of recipient age and diagnosis on health-related quality of life benefit of lung transplantation. Am J Respir Crit Care Med. 2015;192:965–973. doi: 10.1164/rccm.201501-0126OC. [DOI] [PubMed] [Google Scholar]
- 6.Chambers DC, Yusen RD, Cherikh WS, et al. The Registry of the International Society for Heart and Lung Transplantation: Thirty-fourth adult lung and heart-lung transplantation report – 2017; Focus theme: Allograft ischemic time. J Heart Lung Transplant. 2017;36:1047–1059. doi: 10.1016/j.healun.2017.07.016. [DOI] [PubMed] [Google Scholar]
- 7.Estenne M, Maurer JR, Boehler A, et al. Bronchiolitis obliterans syndrome 2001: An update of the diagnostic criteria. J Heart Lung Transplant. 2001;21:297–310. doi: 10.1016/s1053-2498(02)00398-4. [DOI] [PubMed] [Google Scholar]
- 8.Verleden GM, Raghu G, Meyer KC, Glanville AR, Corris P. A new classification system for chronic lung allograft dysfunction. J Heart Lung Transplant. 2014;33:127–133. doi: 10.1016/j.healun.2013.10.022. [DOI] [PubMed] [Google Scholar]
- 9.Sato M, Hwang DM, Waddell TK, Singer LG, Keshavjee S. Progression pattern of restrictive allograft syndrome after lung transplantation. J Heart Lung Transplant. 2013;32:23–30. doi: 10.1016/j.healun.2012.09.026. [DOI] [PubMed] [Google Scholar]
- 10.Verleden SE, de Jong PA, Ruttens D, et al. Functional and computed tomographic evolution and survival of restrictive allograft syndrome after lung transplantation. J Heart Lung Transplant. 2014;33:270–277. doi: 10.1016/j.healun.2013.12.011. [DOI] [PubMed] [Google Scholar]
- 11.Frost AE, Jammal CT, Cagle PT. Hyperacute rejection following lung transplantation. Chest. 1996;110:559–562. doi: 10.1378/chest.110.2.559. [DOI] [PubMed] [Google Scholar]
- 12.Choi JK, Kearns J, Palevsky HI, et al. Hyperacute rejection of a pulmonary allograft – Immediate clinical and pathological findings. Am J Respir Crit Care Med. 1999;160:1015–1018. doi: 10.1164/ajrccm.160.3.9706115. [DOI] [PubMed] [Google Scholar]
- 13.Jaramillo A, Smith MA, Phelan D, et al. Temporal relationship between the development of anti-HLA antibodies and the development of bronchiolitis obliterans syndrome after lung transplantation. Transplant Proc. 1999;31:185–186. doi: 10.1016/s0041-1345(98)01495-x. [DOI] [PubMed] [Google Scholar]
- 14.Jaramillo A, Smith MA, Phelan D, et al. Development of ELISA-detected anti-HLA antibodies precedes the development of bronchiolitis obliterans syndrome and correlates with progressive decline in pulmonary function after lung transplantation. Transplantation. 1999;67:1155–1161. doi: 10.1097/00007890-199904270-00012. [DOI] [PubMed] [Google Scholar]
- 15.Witt CA, Gaut JP, Yusen RD, et al. Acute antibody-mediated rejection after lung transplantation. J Heart Lung Transplant. 2013;32:1034–1040. doi: 10.1016/j.healun.2013.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Otani S, Davis AK, Cantwell L, et al. Evolving experience of treating antibody-mediated rejection following lung transplantation. Transpl Immunol. 2014;31:75–80. doi: 10.1016/j.trim.2014.06.004. [DOI] [PubMed] [Google Scholar]
- 17.Roux A, Bendib Le Lan I, Holifanjaniaina S, et al. Antibody-mediated rejection in lung transplantation: Clinical outcomes and donor-specific antibody characteristics. Am J Transplant. 2016;16:1216–28. doi: 10.1111/ajt.13589. [DOI] [PubMed] [Google Scholar]
- 18.Ensor CR, Yousem SA, Marrarri M, et al. Proteasome inhibitor carfilzomib-based therapy for antibody-mediated rejection of the pulmonary allograft: Use and short-term findings. Am J Transplant. 2017;17:1380–1388. doi: 10.1111/ajt.14222. [DOI] [PubMed] [Google Scholar]
- 19.Aguilar PR, Carpenter D, Ritter J, et al. The role of C4d deposition in the diagnosis of antibody-mediated rejection after lung transplantation. Am J Transplant. 2017 doi: 10.1111/ajt.14534. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Levine DJ, Glanville AR, Aboyoun C, et al. Antibody-mediated rejection of the lung: A consensus report of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant. 2016;35:397–406. doi: 10.1016/j.healun.2016.01.1223. [DOI] [PubMed] [Google Scholar]
- 21.Hachem RR, Yusen RD, Meyers BF, et al. Anti-human leukocyte antigen antibodies and preemptive antibody-directed therapy after lung transplantation. J Heart Lung Transplant. 2010;29:973–980. doi: 10.1016/j.healun.2010.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Snyder LD, Wang Z, Chen DF, et al. Implications for human leukocyte antigen antibodies after lung transplantation. A 10-year experience in 441 patients. Chest. 2013;144:226–233. doi: 10.1378/chest.12-0587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Safavi S, Robinson DR, Soresi S, Carby M, Smith JD. De novo donor HLA-specific antibodies predict development of bronchiolitis obliterans syndrome after lung transplantation. J Heart Lung Transplant. 2014;33:1273–1281. doi: 10.1016/j.healun.2014.07.012. [DOI] [PubMed] [Google Scholar]
- 24.Morrell MR, Pilewski JM, Gries CJ, et al. De novo donor-specific HLA antibodies are associated with early and high-grade bronchiolitis obliterans syndrome and death after lung transplantation. J Heart Lung Transplant. 2014;33:1288–1294. doi: 10.1016/j.healun.2014.07.018. [DOI] [PubMed] [Google Scholar]
- 25.Le Pavec J, Suberbielle C, Lamrani L, et al. De-novo donor-specific anti-HLA antibodies 30 days after lung transplantation are associated with a worse outcome. J Heart Lung Transplant. 2016;35:1067–77. doi: 10.1016/j.healun.2016.05.020. [DOI] [PubMed] [Google Scholar]
- 26.Tikkanen JM, Singer LG, Kim SJ, et al. De novo DQ-donor-specific antibodies are associated with chronic lung allograft dysfunction after lung transplantation. Am J Respir Crit Care Med. 2016;194:596–606. doi: 10.1164/rccm.201509-1857OC. [DOI] [PubMed] [Google Scholar]
- 27.Verleden SE, Vanaudenaerde BM, Emonds MP, et al. Donor-specific and –nonspecific HLA antibodies and outcome post lung transplantation. Eur Respir J. 2017;50:1701248. doi: 10.1183/13993003.01248-2017. [DOI] [PubMed] [Google Scholar]
- 28.Islam AK, Sinha N, DeVos JM, et al. Early clearance vs persistence of de novo donor-specific antibodies following lung transplantation. Clin Transplant. 2017;31:e13028. doi: 10.1111/ctr.13028. [DOI] [PubMed] [Google Scholar]
- 29.Stewart S, Fishbein MC, Snell GI, et al. Revision of the 1996 working formulation for the standardization of nomenclature in the diagnosis of lung rejection. J Heart Lung Transplant. 2007;26:1229–42. doi: 10.1016/j.healun.2007.10.017. [DOI] [PubMed] [Google Scholar]
- 30. [Accessed on 1/13/2016];Scientific Registry of Transplant Recipients. http://srtr.transplant.hrsa.gov/annual_reports/2012/pdf/06_lung_13.pdf.
- 31.Christie JD, Carby M, Bag R, Corris P, Hertz M, Weill D. Report of the ISHLT Working Group on Primary Lung Graft Dysfunction part II: Definition. A consensus statement on the International Society for Heart and Lung Transplantation. J Heart Lung Transplant. 2005;24:1454–9. doi: 10.1016/j.healun.2004.11.049. [DOI] [PubMed] [Google Scholar]
- 32.Girnita AL, McKurry KR, Iacono AT, et al. HLA-specific antibodies are associated with high-grade and persistent-recurrent lung allograft acute rejection. J Heart Lung Transplant. 2004;23:1135–1141. doi: 10.1016/j.healun.2003.08.030. [DOI] [PubMed] [Google Scholar]
- 33.Girnita AL, Duquesnoy R, Yousem SA, et al. HLA-specific antibodies are risk factors for lymphocytic bronchiolitis and chronic lung allograft dysfunction. Am J Transplant. 2005;5:131–138. doi: 10.1111/j.1600-6143.2004.00650.x. [DOI] [PubMed] [Google Scholar]
- 34.Ius F, Sommer W, Tudorache I, et al. Preemptive treatment with therapeutic plasma exchange and rituximab for early donor-specific antibodies after lung transplantation. J Heart Lung Transplant. 2015;34:50–58. doi: 10.1016/j.healun.2014.09.019. [DOI] [PubMed] [Google Scholar]
- 35.Ius F, Sommer W, Kieneke D, et al. IgM-enriched human intravenous immunoglobulin-based treatments of patients with early donor specific anti-HLA antibodies after lung transplantation. Transplantation. 2015 doi: 10.1097/TP.0000000000001027. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bharat A, Kuo E, Steward N, et al. Immunological link between primary graft dysfunction and chronic lung allograft rejection. Ann Thorac Surg. 2008;86:189–197. doi: 10.1016/j.athoracsur.2008.03.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ius F, Sommer W, Tudorache I, et al. Early donor-specific antibodies in lung transplantation: Risk factors and impact on survival. J Heart Lung Transplant. 2014;33:1255–1263. doi: 10.1016/j.healun.2014.06.015. [DOI] [PubMed] [Google Scholar]
- 38.Kozower BD, Meyers BF, Smith MA, et al. The impact of the lung allocation score on short-term transplantation outcomes: A multicenter study. J Thorac Cardiovasc Surg. 2008;135:166–171. doi: 10.1016/j.jtcvs.2007.08.044. [DOI] [PubMed] [Google Scholar]
- 39.Russo MJ, Iribarne A, Hong KN, et al. High lung allocation score is associated with increased morbidity and mortality following transplantation. Chest. 2010;137:651–657. doi: 10.1378/chest.09-0319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Arnaoutakis GJ, Allen JG, Merlo CA, et al. Impact of the lung allocation score on resource utilization after lung transplantation in the United States. J Heart Lung Transplant. 2011;130:14–21. doi: 10.1016/j.healun.2010.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bharat A, Narayanan K, Street T, et al. Early posttransplant inflammation promotes the development of alloimmunity and chronic human lung allograft rejection. Transplantation. 2007;83:150–158. doi: 10.1097/01.tp.0000250579.08042.b6. [DOI] [PubMed] [Google Scholar]
- 42.Reed EF, Rao P, Zhang Z, et al. Comprehensive assessment and standardization of solid phase multiplex-bead arrays for the detection of antibodies to HLA. Am J Transplant. 2013;13:1859–1870. doi: 10.1111/ajt.12287. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.