Abstract
Craniomaxillofacial congenital anomalies comprise approximately one third of all congenital birth defects and include deformities such as alveolar clefts, craniosynostosis, and microtia. Current surgical treatments commonly require the use of autogenous graft material which are difficult to shape, limited in supply, associated with donor site morbidity and cannot grow with a maturing skeleton. Our group has demonstrated that 3D-printed bio-ceramic scaffolds can generate vascularized bone within large, critical-sized defects (defects too large to heal spontaneously) of the craniomaxillofacial skeleton. Furthermore, these scaffolds are also able to function as a delivery vehicle for a new osteogenic agent with a well-established safety profile. The same 3D printers and imaging software platforms have been leveraged by our team to create sterilizable patient-specific intraoperative models for craniofacial reconstruction. For microtia repair, the current standard of care surgical guide is a two-dimensional drawing taken from the contralateral ear. Our laboratory has used 3D-printers and open source software platforms to design personalized microtia surgical models. In this review, we report on the advancements in tissue engineering principles, digital imaging software platforms and 3D printing that have culminated in the application of this technology to repair large bone defects in skeletally immature transitional models and provide in-house manufactured, sterilizable patient-specific models for craniofacial reconstruction.
Background
Approximately one third of all birth defects affect the craniofacial region (Gorlin RJ, 1990). Many of these anomalies result in cranial deformities, bony defects of the midface, and auricular deformity. An alveolar cleft is a bony defect of the maxilla and is frequently seen in patients suffering from cleft lip and palate. Craniosynostosis is a condition of the skull defined by premature fusion of one or more of the cranial sutures. Microtia is a range of congenital abnormalities of the auricle or ear that can range from minute structural abnormalities to a total absence of the external ear structure. Without advanced reconstructive techniques to restore form and function, patients with these congenital abnormalities could suffer from increased intracranial pressure, malocclusion, dysphagia, speech abnormalities and significant social stigma (Teven, Fisher, Ameer, He, & Reid, 2015). Defects of the pediatric craniofacial skeleton are commonly reconstructed with autogenous bone grafts which possess many ideal characteristics for bone healing such as: osteogenicity, osseoinductivity, and osseoconductivity (Myeroff & Archdeacon, 2011). However, autogenous bone grafts are limited by donor site morbidity, limited bone stock, difficultly in shaping, and absorption over time (Ahlmann, Patzakis, Roidis, Shepherd, & Holtom, 2002; Younger EM, 1989). As autografts used in these procedures typically lack vascular nourishment, resorption and the need for revision surgery are common occurrences. These limitations have provided the impetus for tissue engineering-based approaches to pediatric craniomaxillofacial treatments. Recently, safe and personalized approaches to craniomaxillofacial reconstruction using 3D-printed bio-ceramic scaffolds and osteogenic agents have shown significant advancements.
3D printing allows clinicians and scientists to construct individualized, patient-specific bone substitutes (Bauermeister, Zuriarrain, & Newman, 2016). In order to regenerate bone, these constructs must be osseoconductive (guide directional bone formation/ingrowth on a surface), osteogenic, mechanically stable, and facilitate vascular in-growth and stem cell recruitment/differentiation (Kim et al., 2017). Ideally, they are progressively resorbed as the construct is replaced by native tissue (Hutmacher, Schantz, Lam, Tan, & Lim, 2007).
This review will describe: 1) a 3D-printed bio-ceramic scaffold with favorable material properties, which can be precisely designed from radiographic images to fit and fill any defect; 2) A novel osteogenic agent which enhances bone generation of the described bio-ceramic scaffold while demonstrating a favorable safety profile for pediatric bone tissue engineering; 3) the utility of 3D-printing in creating superior surgical models for microtia repair.
Advances in Material Science
The introduction of pediatric alloplastics or biological materials for implantation purposes requires that the material properties and safety profile are thoroughly investigated. Biomaterials made from calcium phosphate are clinically favorable as their safety properties and biocompatibility have been well-documented for decades (Inzana et al., 2014; Simon et al., 2007). In fact, calcium-phosphate bio-ceramics are used instead of autogenous bone grafts in more than fifty percent all bone grafting procedures annually (Roberts & Rosenbaum, 2012). However, mixed outcomes associated with calcium-phosphate ceramics have provided the impetus for improving construct designs. In order to maximize bone healing, communication at the interface of bone defect margins and synthetic material borders must be given thorough consideration to optimize osseoconduction, or directionally guided bone growth on a surface (Albrektsson & Johansson, 2001), vascular in-growth, absorption and macro-geometry of the defect itself. A customized approach cannot be achieved when using the block-shaped or granular materials currently used for patient care. While these biomaterials are produced in mass quantity to accommodate a multitude of standardized defects, this comes at the expense of an inability for constructs to be personalized for patient-specific defects. Defect fit and fill precision and scaffold bulk spatial organization are needed to maximize bone formation.
3D Printing & Tissue Engineering: Essential Geometric Considerations
Deliberate geometric design affects biomaterial osseoconductivity. Studies assessing bone healing around endosteal metallic implants have demonstrated that osseoconduction depends on various physical properties that can be altered. This includes macro-, meso-, micro-, and nanometer level changes in construct design (Coelho & Jimbo, 2014; Steigenga, Al-Shammari, Nociti, Misch, & Wang, 2003; Teixeira, Tovar, Vandeweghe, & … dentistry, 2013; Wilson, de Bruijn, van Blitterswijk, Verbout, & Dhert, 2004). By manipulating these geometric levels, stages of both early and late bone healing can be altered to maximize new bone formation. These principles have recently demonstrated robust healing capacity at large bony defects when applied to calcium-phosphate based bio-ceramics (Bekisz et al., 2017; Ishack, Mediero, Wilder, Ricci, & Cronstein, 2015; Lopez et al., 2018b; Simon et al., 2007).
The success of this translation can be attributed to advances in additive manufacturing (3D printing). Calcium phosphate bio-ceramic materials usually exist in powder form and manufacturing personalized skeletal fit and fill designs is a significant challenge. However, biomedical research has leveraged 3D-printing and created protocols capable of printing calcium-phosphates as viscous inks, which can then be sintered at high temperatures to solidify constructs that can structurally withstand fitting and filling bony defects to facilitate bone regeneration. Consequently, 3D-printing is being investigated as a powerful tool in tissue-engineering, regenerative medicine, and surgery.
3D printing allows for unique scaffold designs based on imaging modalities such as CT, thereby offering a personalized approach to patient-specific skeletal defects. Computer aided design and manufacturing (CAD/CAM) permits scaffold designs of any shape, size, and porous design, down to micrometer level resolution (Figure 1). Even though 3D printing has been used in biomedical research for over a decade, robust bone regenerative outcomes have not been achieved until recently (Brunello et al., 2016; Cai, Xi, & Chua, 2012; Castilho et al., 2013; Cooke, Fisher, Dean, Rimnac, & Mikos, 2003; Cox, Thornby, Gibbons, Williams, & Mallick, 2015; Farzadi, Solati-Hashjin, Asadi-Eydivand, & Osman, 2014; Gonçalves et al., 2015; Hollister et al., 2015; Luo et al., 2015; Provaggi, Leong, & Kalaskar, 2016; Seitz, Rieder, Irsen, Leukers, & Tille, 2005; Wang, Schröder, & Müller, 2014; Xinning et al., 2016; Yao et al., 2015; Zhou, Buchanan, Mitchell, & Dunne, 2014). The paucity of promising translational outcomes is attributed to 3D-printing approaches that do not leverage the principles of bone healing gleaned from endosteal implant bone healing. It is well established that metallic biomaterials heal differently in response to geometric considerations ranging from macrogeometric to nanogeometric levels. The 3D printing investigations utilizing these principles remain limited (Simon et al., 2007; Wilson et al., 2004).
Figure 1.
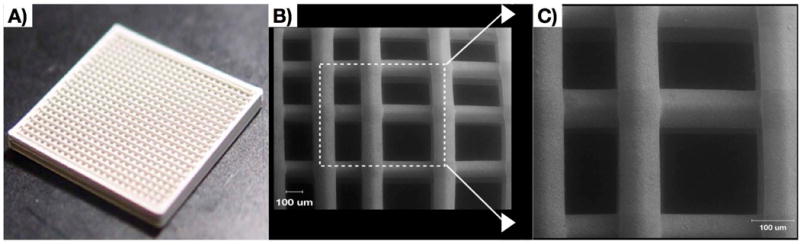
3D-Printed bio-ceramic scaffolds made of 100% beta tricalcium phosphate a) Bulk construct b) Scanning electron microscopy image of scaffold interstices c) Higher magnification image of scaffold strut surface.
Bioactive Ceramics
Bio-ceramics have historically been used for bone regeneration due to their similarities in composition to the inorganic facets of bone. The most investigated material is hydroxyapatite (HA), which is also the predominant inorganic aspect of bone. HA has been proven to be both biocompatible and osseoconductive, making it a rational choice for bone regeneration. However, since an ideal bone tissue engineering scaffold should facilitate regeneration and eventual degradation without the need for surgical retrieval, the unfavorable resorption kinetics of HA, calculated at ~1-2% per year in vivo (Moore, Graves, & Bain, 2001), makes this material not ideal; this all but ensures incomplete bony regeneration due to incomplete material degradation.
β-Tri-Calcium Phosphate (β-TCP) is an alternative bio-ceramic with more favorable degradation kinetics than HA. Like HA, β-TCP is both biocompatible and osseoconductive, but unique to β-TCP is the capacity to regenerate bone while becoming resorbed in a much shorter period of time. By utilizing the viscous ink synthesis properties used in 3D printing, β-TCP can be printed in a lattice structure of deliberate pore size, and degradation rate can be altered by mesogeometric changes in lattice porosity size, strut circumference, or any other change that alters surface area. Nanometer level changes, involving alterations in ink formulation and the sintering process, can alter porosity and affect scaffold degradation rate.
Osteogenic Agents
Adenosine Receptor Activation
Many bioactive agents have been assessed as potential bone regenerative therapeutics. Amongst these molecules, those that activate purinergic receptors have recently been reported to have significant therapeutic potential without undesired side effects that often accompany other notable compounds such as recombinant human bone morphogenetic protein (rhBMP-2) (Bekisz et al., 2017; M. A. Costa et al., 2011; Mediero & Cronstein, 2013; A. Mediero et al., 2012; Mediero, Wilder, Perez-Aso, & Cronstein, 2015; Mediero et al., 2016).
Adenosine, a purinergic receptor agonist, has recently become an osteogenic compound of significant interest (Evans et al., 2006). Historically termed the “retaliatory metabolite,” adenosine has a unique function as it accumulates consequent to ATP dephosphorylation: it reflects metabolic status, and its accumulation attenuates metabolic activity for many cell types, thereby serving as a protective mechanism. Sepsis illustrates this physiology: tissues that are not stressed have extracellular concentrations of adenosine of approximately 1uM/L, but during sepsis, this concentration increases by up to ten times (Martin, Leone, Viviand, & Ayem, 2000). Adenosine can bind to cell surface receptors and modulate exuberant responses from immune cells. For example, adenosine receptor activation on neutrophils results in the attenuation of superoxide anion (Cronstein, Kramer, Weissmann, & Hirschhorn, 1983). This modulatory effect has been observed in many different systems for decades. In 1929, Drury and Szent-Gyorgi discovered adenosine’s vasodilatory and negative inotropic effect on stressed cardiac vessels and tissue, which continues to be utilized today for cardiac stress testing.
Adenosine is soluble and stable at physiologic pH ranges, and in the body it has a half-life of less than ten seconds (Gabrielian, 1977; Kazemzadeh-Narbat et al., 2015). Therefore, it is unsurprising that the factors and processes determining adenosine bioavailability are both highly regulated and interdependent (Haskó & Cronstein, 2004). Hypoxia and ischemia at the cellular level highlight this: in these settings, ATP is rapidly dephosphorylated to adenosine by the enzyme 5′ nucleotidase while suppressing the phosphorylating enzyme adenosine kinase, which is essential for adenosine rephosphorylation (Haskó & Cronstein, 2004). Adenosine amasses immediately outside of the cell by two likely mechanisms. The first begins with precursor nucleotides such as ATP, ADP, and AMP, which upon being released from cells are catabolized by ectonucleosidase enzymes into adenosine. The second is described by adenosine presence intracellularly, and it is transported extracellularly by specialized nucleoside transporters such as equilibrative nucleoside transporter 1 (ENT1) (Haskó & Cronstein, 2004).
Adenosine binds to four receptors: A1, A2A, A2B, and A3 receptors. A1 and A3 receptors are inhibitory (Gi coupled signal transduction), and A2 receptors are stimulatory (G-alpha-s-linked with downstream activation of adenyl cyclase/cAMP) (Haskó, Pacher, Deitch, & Vizi, 2007). Recently, it has been reported that adenosine receptor activation impacts differentiation and function of both osteoblasts and osteoclasts (Evans et al., 2006). The following relationships of adenosine receptor function on bone formation have been reported and are briefly summarized:
A1 Receptor (A1R): Suggested to be involved in osteoclast formation. When investigated both in vitro and in vivo, blocking the A1R or creating A1R knockout models resulted in defects of both colony stimulating factor 1 (CSF-1) and receptor activator of Nuclear Factor κ-B ligand (RANK-L), both of which are essential for osteoclastogenesis (Kara et al., 2010).
A2A Receptor (A2AR): Suggested to be involved in both osteoblast function and osteoclastogenesis. In vitro and in vivo work demonstrates that A2AR activation attenuates osteoclastogenesis, but blocking A2AR upregulates it (Mediero, Kara, Wilder, & Cronstein, 2012). A2ARs also contribute to osteoblast maturation and phenotype expression (Gharibi, Abraham, & Ham, 2011). While A2AR activation does not alter bone formation genetic markers (e.g. RUNX-2 or ALP) at early stages, it does do so at later stages (Gharibi et al., 2011).
A2B Receptor (A2BR): Suggested to be involved in osteoblast differentiation. Osteogenic differentiation of human primary osteoblast-like cells is robust when subjected to A2BR ligation (A. M. Costa et al., 2011), suggesting A2BRs establish stem cell commitment and differentiation towards an osteoblast lineage. A2BRs are predominant relative to other adenosine receptors on the surface of undifferentiated mesenchymal stem cells (MSCs). Their activation on MSCs induces osteoblastogenesis osteoblast function.
A3A Receptor (A3AR): Has not been demonstrated to have any direct effects on osteoclast generation or osteoblast function. However, A3A activation has been reported to attenuate bone resorption and osteoclast number in an arthritis model (Rath-Wolfson et al., 2006).
An important finding is that adenosine receptor-mediated changes to bone healing only occur when adenosine concentrations are greater than those at normal physiologic levels (A. M. Costa et al., 2011). Even when enzymes that break down adenosine (i.e. adenosine deaminase) are blocked, adenosine levels cannot reach levels sufficient to activate its receptors in non-stressed cellular environments. This, in turn, has prompted exploration of alternative approaches to adenosine receptor activation.
Fortunately, pharmacologic manipulation can achieve adenosine concentrations necessary to affect its receptors without inducing a stressful cellular environment. The most notable of these agents is Dipyridamole, an adenosine A2A receptor indirect agonist with a safety profile that has been well established over decades in cardiac clinical care. Dipyridamole is currently indicated for cardiac stress testing and anti-thrombotic therapy, and even has established guidelines in the pediatric population (FitzGerald, 1987; Monagle et al., 2012; Patrono et al., 1998). More recently, Dipyridamole has been shown to attenuate osteoclast formation while upregulating osteoblast function (Ishack et al., 2015; A. Mediero et al., 2012; Aránzazu Mediero et al., 2012), and translational studies have begun to report the application of these principles in highly translational preclinical models (Bekisz et al., 2017).
The Integration of Tissue Engineering Principles
Adenosine’s effects are systemic because its receptors can be found at almost every cellular system, but its limited extracellular presence consequent to tight regulatory pathways ensure a brief half-life and limited extracellular concentrations. Therefore, an ideal approach to adenosine-induced bone regeneration includes a local delivery mechanism that can facilitate extracellular concentrations necessary to activate adenosine receptors and induce bone formation. This is feasible with 3D-printed bio-ceramic scaffold carriers. Scaffold-mediated delivery allows treatments to circumvent the potential side effects that can accompany systemic delivery of any compound. This approach also simplifies issues related to patient compliance: patients, especially pediatric patients, will not need to concern themselves with ingesting medication using this localized approach or be subjected to multiple injections to the bone healing site over time. For Dipyridamole, the dosages needed to induce bone regeneration are much lower when delivered via scaffold compared to intraorally. Furthermore, the dose needed to achieve bone regeneration is already lower than established systemic doses for pediatric patients (Bekisz et al., 2017).
A small but growing body of work has begun to demonstrate the effects of localized adenosine receptor activation via 3D-printed, geometrically-tailored osseoconductive scaffold carriers. Our group reported the first successful deployment of a bio-ceramic scaffold with deliberate geometric design in a translational model (Lopez et al., 2018a): by regeneration of a critical-sized, full-thickness mandibulectomy defect in a skeletally mature rabbit model. We demonstrated that bridging bone formation at the mandibular body could be achieved at eight weeks in vivo, even without osteogenic agents. When these principles were applied to an even more highly translational sheep calvarial defect model, device osseoconduction was both affirmed and augmented when Dipyridamole was delivered locally (Bekisz et al., 2017). These bone regeneration outcomes are promising, but what is notable is the absence of undesired side effects. Neither study reported exuberant bone growth, ectopic bone formation, or osteolysis, all of which have been reported in more commonly used osteogenic agents, such as rhBMP-2 (Carragee, Hurwitz, & Weiner, 2011; Kinsella Jr et al., 2011; Spiro et al., 2010). This exciting result has led to studies assessing the regenerative potential of these principles in skeletally immature translational models, of which preliminary findings are reported here. Given Dipyridamole’s safe and promising bone regenerative potential via scaffold delivery, the influence of these principles on skeletally immature bone formation warrants continued investigation.
Alveolar Clefts
Approximately three out of four cleft lip and palate patients present with an alveolar osseous defect, or an alveolar cleft (Guo et al., 2011). This results in facial asymmetry, insufficient osseous support of dentition, and nasal regurgitation through a nasolabial fistula (Waite & Waite, 1996). Autogenous bone grafting, the choice procedure for reconstruction of the alveolar segment (Boyne & Sands, 1972; Kortebein, Nelson, & Sadove, 1991), is typically performed during the mixed dentition stage of dental development at 6-11 years of age (Kang, 2017). Successful treatment provides structural support for tooth eruption, closure of the nasolabial fistula, enhanced speech ability, and augmentation of the dental arch for improved aesthetic outcome (Kyung & Kang, 2015). However, despite reports of excellent long-term outcomes, the procedure is associated with donor site morbidity and the requirement for hospitalization due to pain in the graft donor site. In addition, graft resorption is well documented, and many studies have reported revision rates from 12-40% depending on cleft type (Goudy, Lott, Burton, Wheeler, & Canady, 2009; Montian Manosudprasit, Chaiyasang, & Chowchuen, 2011; Shirani, Abbasi, & Mohebbi, 2012) . Poor prognosis is more highly associated with patients that have wider or bilateral clefts.
Our group has investigated the skeletally immature New Zealand White Rabbit (NZWR) as a model for alveolar cleft regeneration (Figure 2A). By creating a 3.5 mm × 3.5 mm critical-size bone defect at the alveolus, negative controls were established at t=8 weeks in vivo. 3D-printed bio-ceramic scaffolds designed with the same deliberate geometric considerations as those utilized in previous skeletally mature models were used. These scaffolds were then coated with cross-linked bovine collagen, which functioned as the carrier for localized Dipyridamole delivery.
Figure 2.
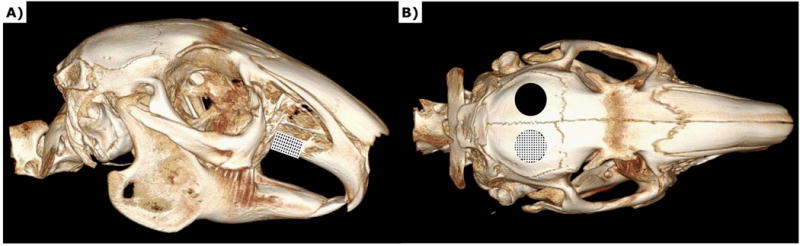
MicroCT image of rabbit skull a) Schematic depicting alveolar cleft model b) Schematic depicting calvarial defect model
Findings in vivo demonstrate bone formation bridging the established critical-sized defect (Figure 3). More importantly, the quality of this new bone demonstrates significant promise. Unlike the random bone formation that occurs when granular grafts are placed, new bone formation is observed both within the scaffold and at the interstices between osteotomy and construct; the newly formed bone is highly cellular and vascularized (Figure 3A). Furthermore, bone formation is robust but not exuberant, suggesting that the scaffold design’s osseoconductive principles are both effective and enhanced by Dipyridamole’s local effects, but not overwhelmed by Dipyridamole’s osteogenic potential (Figure 3). Perhaps most importantly, the suture at the interface between the maxilla and premaxilla remained patent–this is in contrast to compounds such as rhBMP-2, which are currently contraindicated in pediatric patients as per FDA guidelines due to a wide array of adverse events (Administration, 2008).
Figure 3.
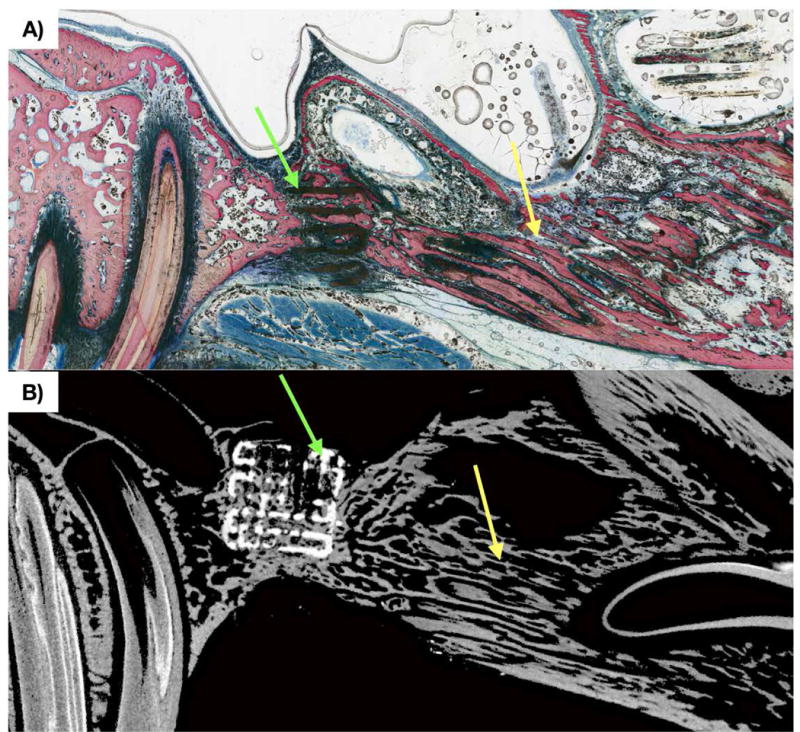
a) Non-decalcified histologic section in sagittal orientation of alveolar cleft defect replaced by scaffold at t = 8 weeks. New bone formation (pink) is observed through scaffold (black, green arrow) porosity. b) MicroCT slice corresponding to histologic slice, with scaffold construct (white) and new bone formation (grey) throughout scaffold (white, green arrow) interstices. On both histology and microCT, the suture at the interface of the premaxilla and maxilla (yellow arrow) is patent.
Craniosynostosis
Craniosynostosis is the premature fusion of the cranial sutures resulting in abnormal shape and development of the skull. The prevalence is approximately 1 in 2,500 births with risk factors consisting of multiple birth, infant’s weight, and maternal age.(Boulet, Rasmussen, & Honein, 2008; Johnson & Wilkie, 2011) Premature suture fusion leads to facial asymmetry, increased intracranial pressure, and restriction of brain expansion (Kabbani & Raghuveer, 2004). Current treatment options include intracranial vault remodeling, distraction osteogenesis (bone lengthening by surgical sectioning of a bone segment and gradually distracting/lengthening the defect so that new bone callus can fill/grow defect space (McCarthy, Stelnicki, Mehrara, & Longaker, 2001)), strip craniectomy followed by use of expanding or molding appliance (Governale, 2015).
Our group again utilized the skeletally immature NZWR as a pediatric defect translational model. Using circular drill attachment termed a trephine burr 10 mm in diameter, full thickness calvarial defects were created in skeletally immature NZWRs with contralateral, internal controls while avoiding injury of the cranial sutures (Figure 2B). Outcomes at 8 weeks demonstrated robust bone regeneration with intramembranous-like healing within the implant (Figure 4). Again, the more prominent finding here was the realization of vascularized bone that grew within scaffold interstices guided by osseoconduction, rather than exuberantly without direction. Furthermore, cranial sutures remained patent, unlike what has been reported in other translational studies using molecules such as rhBMP-2.
Figure 4.
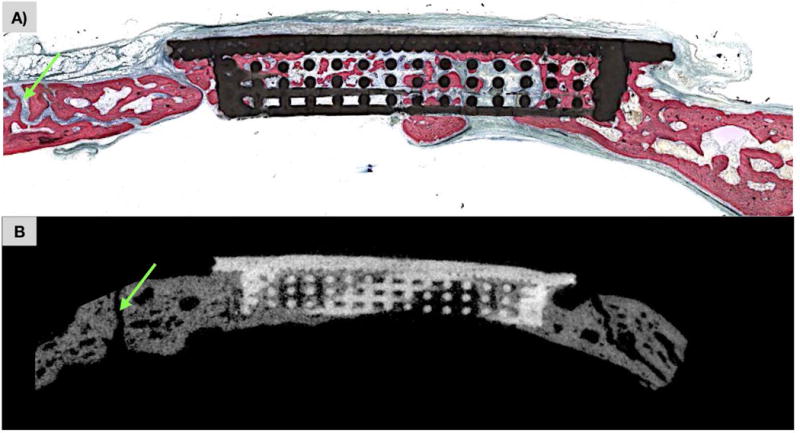
a) Non-decalcified histologic section calvarial defect replaced by scaffold at t=8 weeks. New bone formation (pink) is observed through scaffold (black) porosity with patent cranial suture (green arrow). b) MicroCT slice corresponding to histologic slice, with scaffold construct (white) and new bone formation (grey) throughout scaffold interstices with patent cranial suture (green arrow).
Microtia
A birth defect of the ear resulting in malformation of the auricle is known as microtia. The prevalence of microtia is estimated at about 1.5 in 10,000 births, and although rates vary geographically (Luquetti, Leoncini, & Mastroiacovo, 2011), almost ninety percent of cases are unilateral (Staffenberg, 2003). This congenital anomaly places pediatric patients at increased risk of psychological and social challenges, illustrating an important motivation for effective reconstruction (Du et al., 2007). In fact, reconstructive surgery has proven to increase psychosocial attitude and improve patient self-confidence (Horlock, Vogelin, Bradbury, Grobbelaar, & Gault, 2005; Steffen, Wollenberg, Konig, & Frenzel, 2010). Like other congenital defects, autogenous grafting remains a standard of care. For the ear, autologous costal cartilage graft has been a long-standing technique for decades (Brent, 1992; Tanzer, 1959). Other options include prosthetic reconstruction through osseointegrated implants (Tjellstrom, 1990) and implant reconstruction via porous polyethylene implant and/or temporoparietal fascial flaps (Reinisch & Lewin, 2009). While these tools are powerful, auricular reconstruction presents a unique challenge to reconstructive surgeons: the ear’s unique and complex anatomy. A satisfactory aesthetic outcome is dependent on a surgeon’s ability to accurately rebuild the anatomic subunits of the ear and place them in the correct visuospatial orientation relative to one another (Flores et al., 2017). Despite the plethora of tools available to create reconstructive models for these complex anatomic body parts, the current standard of care involves drawing the intended outcome in two-dimensions, on paper. Although this is useful, drawings are also imprecise (Lee & Boahene, 2013) and make achieving good three-dimensional outcomes tedious and unnecessarily challenging.
3D-printing offers a more realistic model to design/sculpt a new ear that uses patient imaging. By using normal auricular anatomy images captured with 3D digital photographs from a patient’s contralateral or unaffected side, AMIRA (FEI Company, Hillsboro, Oregon, USA) software can read these photographs and convert them to digital models. In order to then refine these models and sculpt essential landmarks, open-access software programs such as Blender™ (version 2.77, Amsterdam, The Netherlands) can digitally modify data. Using an array of selection and brush tools to isolate and refine shape, an ear model can be isolated from the rest of the cranium, “flipped” to guide the replacement of the microtia-affected ear, uploaded to a computer aided design and manufacturing (CAD/CAM) program, and printed in three dimensions to provide 1) identical mirror images of unaffected patient ears and 2) printable subunits for surgeon convenience (Figure 5A-E) (Flores et al., 2017). Indeed our group has already reported promising early outcomes when using these 3D-printed constructs (Figure 6) (Flores et al., 2017; Witek, Khouri, Coelho, & Flores, 2016). By using 3D-printed constructs as guides based on clinical imaging, auricular reconstruction can be done with a reference of an ideal outcome (Figure 5F).
Figure 5.
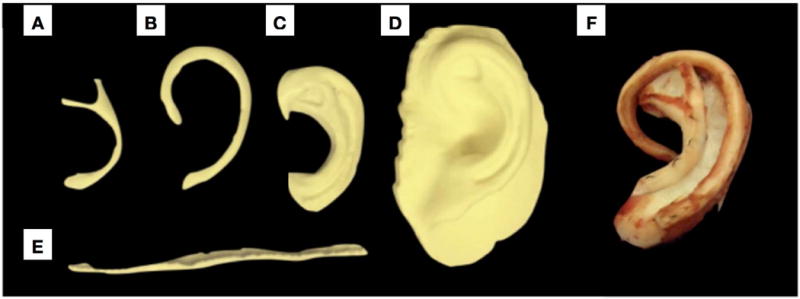
3D printed ear subunits as well as entire ear along from a fused deposition modeling (FDM) printer (Builder Premium 3D Printer, Noordwijkerhout, The Netherlands). Polylactic acid (PLA) filament was used (Batch #: 15099905; Builder Premium 3D Printer, Noordwijkerhout, The Netherlands) over a period of ~4 hours. 3D-printed auricular reconstruction subunits are labeled as follows: a) Anti-helix b) Helix c) Anti tragus to scaphoid fossa d) Pinna e) uncurled helix f) Final post-operative autogenous auricular construct
Figure 6.
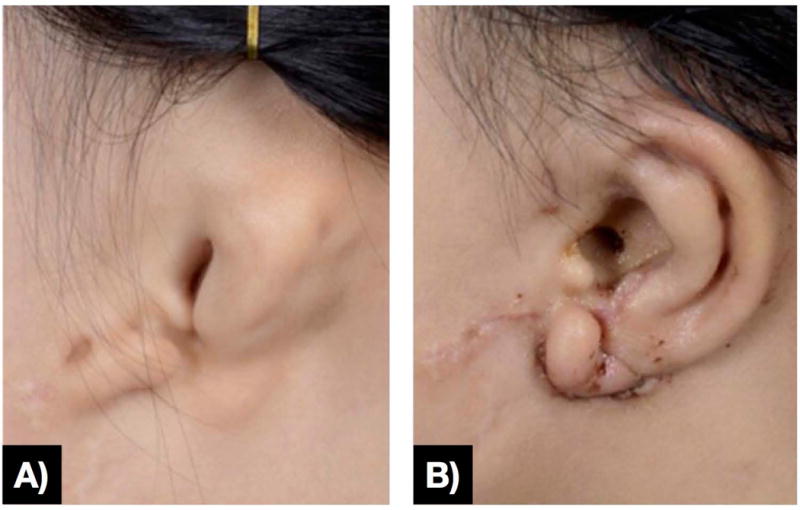
a) Preoperative ear affected by microtia and b) early postoperative result with 3D-printed surgical guide for reference
Conclusions & Future Directions
The promise of rapidly regenerated large bony defects using tissue engineering principles has yet to be realized, but promising outcomes have begun to be reported. The localized delivery of Dipyridamole via 3D-printed bio-ceramic scaffolds made of β-TCP has been established as feasible and successful in both murine and larger, more translational animal models. Building on these findings, preliminary research strongly suggests that these principles can be applied to the growing skeleton.
The immature alveolus and calvaria undergo significant growth before reaching adult size. Therefore, an ideal regenerative structure must be able to replace a defect with new bone that can grow with the native skeleton once a defect is filled. It must also be able to withstand all of the mechanical forces that accompany growth and achieving skeletal maturity (e.g. mastication). Our early findings suggest that cranial and maxillary sutures are not adversely affected by 3D printed β-TCP constructs, and thereby allow for appropriate growth patterns without restrictions that can arise from premature suture fusion. As long-term studies assessing functional outcomes are performed, it will be critical to evaluate the mechanical properties of newly formed bone, as well as whether or not skeletal growth normalcy can be achieved long term.
Acknowledgments
This work was supported by NIH/NIAMS R01AR068593 and NICHD R21HD090664
References
- Administration, U. S. F. D. Important Information on the Infuse/Mastergraft™ Posterolateral Revision Device. 2008 Retrieved from https://www.accessdata.fda.gov/cdrh_docs/pdf4/h040004c.pdf.
- Ahlmann E, Patzakis M, Roidis N, Shepherd L, Holtom P. Comparison of anterior and posterior iliac crest bone grafts in terms of harvest-site morbidity and functional outcomes. J Bone Joint Surg Am. 2002;84-A(5):716–720. doi: 10.2106/00004623-200205000-00003. [DOI] [PubMed] [Google Scholar]
- Albrektsson T, Johansson C. Osteoinduction, osteoconduction and osseointegration. European spine journal. 2001;10(2):S96–S101. doi: 10.1007/s005860100282. %@ 0940-6719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauermeister AJ, Zuriarrain A, Newman MI. Three-Dimensional Printing in Plastic and Reconstructive Surgery: A Systematic Review. Ann Plast Surg. 2016;77(5):569–576. doi: 10.1097/SAP.0000000000000671. [DOI] [PubMed] [Google Scholar]
- Bekisz JM, Flores RL, Witek L, Lopez CD, Runyan CM, Torroni A, Coelho PG. Dipyridamole enhances osteogenesis of three-dimensionally printed bioactive ceramic scaffolds in calvarial defects. Journal of cranio-maxillo-facial surgery : official publication of the European Association for Cranio-Maxillo-Facial Surgery. 2017 doi: 10.1016/j.jcms.2017.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulet SL, Rasmussen SA, Honein MA. A population-based study of craniosynostosis in metropolitan Atlanta, 1989-2003. Am J Med Genet A. 2008;146A(8):984–991. doi: 10.1002/ajmg.a.32208. [DOI] [PubMed] [Google Scholar]
- Boyne PJ, Sands NR. Secondary bone grafting of residual alveolar and palatal clefts. J Oral Surg. 1972;30(2):87–92. [PubMed] [Google Scholar]
- Brent B. Auricular repair with autogenous rib cartilage grafts: two decades of experience with 600 cases. Plast Reconstr Surg. 1992;90(3):355–374. discussion 375-356. [PubMed] [Google Scholar]
- Brunello G, Sivolella S, Meneghello R, Ferroni L, Gardin C, Piattelli A, Bressan E. Powder-based 3D printing for bone tissue engineering. Biotechnology advances. 2016 doi: 10.1016/j.biotechadv.2016.03.009. [DOI] [PubMed] [Google Scholar]
- Cai S, Xi J, Chua CK. A novel bone scaffold design approach based on shape function and all-hexahedral mesh refinement. Computer-Aided Tissue Engineering. 2012:45–55. doi: 10.1007/978-1-61779-764-4_3. [DOI] [PubMed] [Google Scholar]
- Carragee EJ, Hurwitz EL, Weiner BK. A critical review of recombinant human bone morphogenetic protein-2 trials in spinal surgery: emerging safety concerns and lessons learned. The Spine Journal. 2011;11(6):471–491. doi: 10.1016/j.spinee.2011.04.023. [DOI] [PubMed] [Google Scholar]
- Castilho M, Dias M, Gbureck U, Groll J, Fernandes P, Pires I, Vorndran E. Fabrication of computationally designed scaffolds by low temperature 3D printing. Biofabrication. 2013;5(3):035012. doi: 10.1088/1758-5082/5/3/035012. [DOI] [PubMed] [Google Scholar]
- Coelho PG, Jimbo R. Osseointegration of metallic devices: current trends based on implant hardware design. Archives of biochemistry and biophysics. 2014;561:99–108. doi: 10.1016/j.abb.2014.06.033. [DOI] [PubMed] [Google Scholar]
- Cooke MN, Fisher JP, Dean D, Rimnac C, Mikos AG. Use of stereolithography to manufacture critical-sized 3D biodegradable scaffolds for bone ingrowth. Journal of Biomedical Materials Research Part B: Applied Biomaterials. 2003;64(2):65–69. doi: 10.1002/jbm.b.10485. [DOI] [PubMed] [Google Scholar]
- Costa AM, Barbosa A, Neto E, Sousa SA, Freitas R, Neves JM, Sá CP. On the role of subtype selective adenosine receptor agonists during proliferation and osteogenic differentiation of human primary bone marrow stromal cells. Journal of cellular physiology. 2011;226(5):1353–1366. doi: 10.1002/jcp.22458. [DOI] [PubMed] [Google Scholar]
- Costa MA, Barbosa A, Neto E, Sa-e-Sousa A, Freitas R, Neves JM, Correia-de-Sa P. On the role of subtype selective adenosine receptor agonists during proliferation and osteogenic differentiation of human primary bone marrow stromal cells. Journal of cellular physiology. 2011;226(5):1353–1366. doi: 10.1002/jcp.22458. [DOI] [PubMed] [Google Scholar]
- Cox SC, Thornby JA, Gibbons GJ, Williams MA, Mallick KK. 3D printing of porous hydroxyapatite scaffolds intended for use in bone tissue engineering applications. Materials Science and Engineering: C. 2015;47:237–247. doi: 10.1016/j.msec.2014.11.024. [DOI] [PubMed] [Google Scholar]
- Cronstein BN, Kramer SB, Weissmann G, Hirschhorn R. A new physiological function for adenosine: regulation of superoxide anion production. Transactions of the Association of American Physicians. 1983;96:384–391. [PubMed] [Google Scholar]
- Du JM, Zhuang HX, Chai JK, Liu GF, Wang Y, Guo WH. Psychological status of congenital microtia patients and relative influential factors: analysis of 410 cases. Zhonghua Yi Xue Za Zhi. 2007;87(6):383–387. [PubMed] [Google Scholar]
- Evans BAJ, Elford C, Pexa A, Francis K, Hughes AC, Deussen A, Ham J. Human Osteoblast Precursors Produce Extracellular Adenosine, Which Modulates Their Secretion of IL-6 and Osteoprotegerin. Journal of Bone and Mineral Research. 2006;21(2):228–236. doi: 10.1359/JBMR.051021. [DOI] [PubMed] [Google Scholar]
- Farzadi A, Solati-Hashjin M, Asadi-Eydivand M, Osman NAA. Effect of layer thickness and printing orientation on mechanical properties and dimensional accuracy of 3D printed porous samples for bone tissue engineering. PloS one. 2014;9(9):e108252. doi: 10.1371/journal.pone.0108252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FitzGerald GA. Dipyridamole. The New England journal of medicine. 1987;316(20):1247–1257. doi: 10.1056/NEJM198705143162005. [DOI] [PubMed] [Google Scholar]
- Flores RL, Liss H, Raffaelli S, Humayun A, Khouri KS, Coelho PG, Witek L. The technique for 3D printing patient-specific models for auricular reconstruction. Journal of Cranio-Maxillo-Facial Surgery. 2017;45(6):937–943. doi: 10.1016/j.jcms.2017.03.022. %@ 1010-5182. [DOI] [PubMed] [Google Scholar]
- Gabrielian AG. Solubility of adenosine in concentrated salt solutions. Biofizika. 1977;22(5):789–793. %@ 0006-3029. [PubMed] [Google Scholar]
- Gharibi B, Abraham AA, Ham J. Adenosine receptor subtype expression and activation influence the differentiation of mesenchymal stem cells to osteoblasts and adipocytes. Adenosine receptor subtype expression and activation influence the differentiation of mesenchymal stem cells to osteoblasts and adipocytes. 2011 doi: 10.1002/jbmr.424. [DOI] [PubMed] [Google Scholar]
- Gonçalves EM, Oliveira FJ, Silva RF, Neto MA, Fernandes MH, Amaral M, Vila M. Three-dimensional printed PCL-hydroxyapatite scaffolds filled with CNTs for bone cell growth stimulation. Journal of Biomedical Materials Research Part B: Applied Biomaterials. 2015 doi: 10.1002/jbm.b.33432. [DOI] [PubMed] [Google Scholar]
- Gorlin RJ, C M, Levin LS. Syndromes of the Head and Neck. Oxford University Press; 1990. [Google Scholar]
- Goudy S, Lott D, Burton R, Wheeler J, Canady J. Secondary alveolar bone grafting: outcomes, revisions, and new applications. The Cleft Palate-Craniofacial Journal. 2009;46(6):610–612. doi: 10.1597/08-126.1. %@ 1055-6656. [DOI] [PubMed] [Google Scholar]
- Governale LS. Craniosynostosis. Pediatr Neurol. 2015;53(5):394–401. doi: 10.1016/j.pediatrneurol.2015.07.006. [DOI] [PubMed] [Google Scholar]
- Guo J, Li C, Zhang Q, Wu G, Deacon SA, Chen J, Ye Q. Secondary bone grafting for alveolar cleft in children with cleft lip or cleft lip and palate. Cochrane Database Syst Rev. 2011;(6):CD008050. doi: 10.1002/14651858.CD008050.pub2. [DOI] [PubMed] [Google Scholar]
- Haskó G, Cronstein BN. Adenosine: an endogenous regulator of innate immunity. Trends in Immunology. 2004;25(1):33–39. doi: 10.1016/j.it.2003.11.003. [DOI] [PubMed] [Google Scholar]
- Haskó G, Pacher P, Deitch EA, Vizi SE. Shaping of monocyte and macrophage function by adenosine receptors. Pharmacology & Therapeutics. 2007;113(2):264–275. doi: 10.1016/j.pharmthera.2006.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollister SJ, Flanagan CL, Zopf DA, Morrison RJ, Nasser H, Patel JJ, Green GE. Design control for clinical translation of 3D printed modular scaffolds. Annals of Biomedical Engineering. 2015;43(3):774–786. doi: 10.1007/s10439-015-1270-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horlock N, Vogelin E, Bradbury ET, Grobbelaar AO, Gault DT. Psychosocial outcome of patients after ear reconstruction: a retrospective study of 62 patients. Ann Plast Surg. 2005;54(5):517–524. doi: 10.1097/01.sap.0000155284.96308.32. [DOI] [PubMed] [Google Scholar]
- Hutmacher DW, Schantz JT, Lam CXF, Tan KC, Lim TC. State of the art and future directions of scaffold-based bone engineering from a biomaterials perspective. Journal of Tissue Engineering and Regenerative Medicine. 2007;1(4):245–260. doi: 10.1002/term.24. %@ 1932-7005. [DOI] [PubMed] [Google Scholar]
- Inzana JA, Olvera D, Fuller SM, Kelly JP, Graeve OA, Schwarz EM, Awad HA. 3D printing of composite calcium phosphate and collagen scaffolds for bone regeneration. Biomaterials. 2014;35(13):4026–4034. doi: 10.1016/j.biomaterials.2014.01.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishack S, Mediero A, Wilder T, Ricci JL, Cronstein BN. Bone regeneration in critical bone defects using three-dimensionally printed β-tricalcium phosphate/hydroxyapatite scaffolds is enhanced by coating scaffolds with either dipyridamole or BMP-2. Journal of Biomedical Materials Research Part B: Applied Biomaterials. 2015 doi: 10.1002/jbm.b.33561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson D, Wilkie AO. Craniosynostosis. Eur J Hum Genet. 2011;19(4):369–376. doi: 10.1038/ejhg.2010.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabbani H, Raghuveer TS. Craniosynostosis. Am Fam Physician. 2004;69(12):2863–2870. [PubMed] [Google Scholar]
- Kang NH. Current Methods for the Treatment of Alveolar Cleft. Arch Plast Surg. 2017;44(3):188–193. doi: 10.5999/aps.2017.44.3.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kara FM, Doty SB, Boskey A, Goldring S, Zaidi M, Fredholm BB, Cronstein BN. Adenosine A1 receptors regulate bone resorption in mice: Adenosine A1 receptor blockade or deletion increases bone density and prevents ovariectomy-induced bone loss in adenosine A1 receptor–knockout mice. Arthritis & Rheumatism. 2010;62(2):534–541. doi: 10.1002/art.27219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazemzadeh-Narbat M, Annabi N, Tamayol A, Oklu R, Ghanem A, Khademhosseini A. Adenosine-associated delivery systems. Journal of drug targeting. 2015;23(7–8):580–596. doi: 10.3109/1061186X.2015.1058803. %@ 1061-1186X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HD, Amirthalingam S, Kim SL, Lee SS, Rangasamy J, Hwang NS. Biomimetic Materials and Fabrication Approaches for Bone Tissue Engineering. Adv Healthc Mater. 2017;6(23) doi: 10.1002/adhm.201700612. [DOI] [PubMed] [Google Scholar]
- Kinsella CR, Jr, Cray JJ, Durham EL, Burrows AM, Vecchione L, Smith DM, Losee JE. Recombinant Human Bone Morphogenetic Protein-2–Induced Craniosynostosis and Growth Restriction in the Immature Skeleton. Plastic and reconstructive surgery. 2011;127(3):1173–1181. doi: 10.1097/PRS.0b013e318205f2b4. [DOI] [PubMed] [Google Scholar]
- Kortebein MJ, Nelson CL, Sadove AM. Retrospective analysis of 135 secondary alveolar cleft grafts using iliac or calvarial bone. J Oral Maxillofac Surg. 1991;49(5):493–498. doi: 10.1016/0278-2391(91)90172-i. [DOI] [PubMed] [Google Scholar]
- Kyung H, Kang N. Management of Alveolar Cleft. Arch Craniofac Surg. 2015;16(2):49–52. doi: 10.7181/acfs.2015.16.2.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee LN, Boahene KD. A novel technique for sculpting costal cartilage in microtia repair and rhinoplasty. JAMA facial plastic surgery. 2013;15(5):349–351. doi: 10.1001/jamafacial.2013.1307. %@ 2168-6076. [DOI] [PubMed] [Google Scholar]
- Lopez CD, Diaz-Siso JR, Witek L, Bekisz JM, Cronstein BN, Torroni A, Coelho PG. Three dimensionally printed bioactive ceramic scaffold osseoconduction across critical-sized mandibular defects. journal of surgical research. 2018a;223:115–122. doi: 10.1016/j.jss.2017.10.027. %@ 0022-4804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez CD, Diaz-Siso JR, Witek L, Bekisz JM, Cronstein BN, Torroni A, Coelho PG. Three dimensionally printed bioactive ceramic scaffold osseoconduction across critical-sized mandibular defects. journal of surgical research. 2018b;223:115–122. doi: 10.1016/j.jss.2017.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Y, Zhai D, Huan Z, Zhu H, Xia L, Chang J, Wu C. Three-dimensional printing of hollow-struts-packed bioceramic scaffolds for bone regeneration. ACS applied materials & interfaces. 2015;7(43):24377–24383. doi: 10.1021/acsami.5b08911. [DOI] [PubMed] [Google Scholar]
- Luquetti DV, Leoncini E, Mastroiacovo P. Microtia-anotia: a global review of prevalence rates. Birth Defects Res A Clin Mol Teratol. 2011;91(9):813–822. doi: 10.1002/bdra.20836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin C, Leone M, Viviand X, Ayem ML. High adenosine plasma concentration as a prognostic index for outcome in patients with septic shock. Critical care …. 2000 doi: 10.1097/00003246-200009000-00014. [DOI] [PubMed] [Google Scholar]
- McCarthy JG, Stelnicki EJ, Mehrara BJ, Longaker MT. Distraction osteogenesis of the craniofacial skeleton. Plastic and reconstructive surgery. 2001;107(7):1812–1827. doi: 10.1097/00006534-200106000-00029. %@ 0032-1052. [DOI] [PubMed] [Google Scholar]
- Mediero A, Cronstein BN. Adenosine and bone metabolism. Trends in Endocrinology & Metabolism. 2013;24(6):290–300. doi: 10.1016/j.tem.2013.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mediero A, Frenkel SR, Wilder T, He W, Mazumder A, Cronstein BN. Adenosine A2A receptor activation prevents wear particle-induced osteolysis. Sci Transl Med. 2012;4(135):135ra165. doi: 10.1126/scitranslmed.3003393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mediero A, Kara FM, Wilder T, Cronstein BN. Adenosine A(2A) receptor ligation inhibits osteoclast formation. The American journal of pathology. 2012;180(2):775–786. doi: 10.1016/j.ajpath.2011.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mediero A, Wilder T, Perez-Aso M, Cronstein BN. Direct or indirect stimulation of adenosine A2A receptors enhances bone regeneration as well as bone morphogenetic protein-2. The FASEB Journal. 2015;29(4):1577–1590. doi: 10.1096/fj.14-265066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mediero A, Wilder T, Reddy VS, Cheng Q, Tovar N, Coelho PG, Cronstein BN. Ticagrelor regulates osteoblast and osteoclast function and promotes bone formation in vivo via an adenosine-dependent mechanism. The FASEB Journal. 2016;30(11):3887–3900. doi: 10.1096/fj.201600616R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monagle P, Chan AKCKC, Goldenberg NA, Ichord RN, Journeycake JM, Nowak-Göttl U, Vesely SK. Antithrombotic therapy in neonates and children: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 Suppl) doi: 10.1378/chest.11-2308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montian Manosudprasit DDS, Chaiyasang S, Chowchuen B. Revision rates of alveolar bone grafting in unilateral cleft lip and palate patients with and without orthodontic preparation. J Med Assoc Thai. 2011;94(6):S62–S69. [PubMed] [Google Scholar]
- Moore WR, Graves SE, Bain GI. Synthetic bone graft substitutes. ANZ Journal of Surgery. 2001;71(6):354–361. [PubMed] [Google Scholar]
- Myeroff C, Archdeacon M. Autogenous bone graft: donor sites and techniques. JBJS. 2011;93(23):2227–2236. doi: 10.2106/JBJS.J.01513. %@ 0021-9355. [DOI] [PubMed] [Google Scholar]
- Patrono C, Coller B, Dalen JE, Fuster V, Gent M, Harker LA, Roth G. Platelet-active drugs: the relationships among dose, effectiveness, and side effects. Chest. 1998;114(5 Suppl) doi: 10.1378/chest.114.5_supplement.470s. [DOI] [PubMed] [Google Scholar]
- Provaggi E, Leong JJ, Kalaskar DM. Applications of 3D printing in the management of severe spinal conditions. Proceedings of the Institution of Mechanical Engineers, Part H: Journal of Engineering in Medicine. 2016 doi: 10.1177/0954411916667761. 0954411916667761. [DOI] [PubMed] [Google Scholar]
- Rath-Wolfson L, Bar-Yehuda S, Madi L, Ochaion A, Cohen S, Zabutti A, Fishman P. IB-MECA, an A3 adenosine receptor agonist prevents bone resorption in rats with adjuvant induced arthritis. Clinical and Experimental Rheumatology. 2006 [PubMed] [Google Scholar]
- Reinisch JF, Lewin S. Ear reconstruction using a porous polyethylene framework and temporoparietal fascia flap. Facial Plast Surg. 2009;25(3):181–189. doi: 10.1055/s-0029-1239448. [DOI] [PubMed] [Google Scholar]
- Roberts TT, Rosenbaum AJ. Bone grafts, bone substitutes and orthobiologics: the bridge between basic science and clinical advancements in fracture healing. Organogenesis. 2012;8(4):114–124. doi: 10.4161/org.23306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seitz H, Rieder W, Irsen S, Leukers B, Tille C. Three-dimensional printing of porous ceramic scaffolds for bone tissue engineering. Journal of Biomedical Materials Research Part B: Applied Biomaterials. 2005;74(2):782–788. doi: 10.1002/jbm.b.30291. [DOI] [PubMed] [Google Scholar]
- Shirani G, Abbasi AJ, Mohebbi SZ. Need for revision surgery after alveolar cleft repair. Journal of Craniofacial Surgery. 2012;23(2):378–381. doi: 10.1097/SCS.0b013e318240fe7f. %@ 1049-2275. [DOI] [PubMed] [Google Scholar]
- Simon JL, Michna S, Lewis JA, Rekow DE, Thompson VP, Smay JE, Ricci JL. In vivo bone response to 3D periodic hydroxyapatite scaffolds assembled by direct ink writing. Journal of Biomedical Materials Research Part A. 2007;83A(3):747–758. doi: 10.1002/jbm.a.31329. [DOI] [PubMed] [Google Scholar]
- Spiro AS, Timo Beil F, Baranowsky A, Barvencik F, Schilling AF, Nguyen K, Schinke T. BMP-7–induced ectopic bone formation and fracture healing is impaired by systemic NSAID application in C57BL/6-mice. Journal of Orthopaedic Research. 2010;28(6):785–791. doi: 10.1002/jor.21044. [DOI] [PubMed] [Google Scholar]
- Staffenberg DA. Microtia repair. Journal of Craniofacial Surgery. 2003;14(4):481–486. doi: 10.1097/00001665-200307000-00016. %@ 1049-2275. [DOI] [PubMed] [Google Scholar]
- Steffen A, Wollenberg B, Konig IR, Frenzel H. A prospective evaluation of psychosocial outcomes following ear reconstruction with rib cartilage in microtia. J Plast Reconstr Aesthet Surg. 2010;63(9):1466–1473. doi: 10.1016/j.bjps.2009.09.005. [DOI] [PubMed] [Google Scholar]
- Steigenga JT, Al-Shammari KF, Nociti FH, Misch CE, Wang HL. Dental implant design and its relationship to long-term implant success. Implant dentistry. 2003;12(4):306–317. doi: 10.1097/01.id.0000091140.76130.a1. %@ 1056-6163. [DOI] [PubMed] [Google Scholar]
- Tanzer RC. Total reconstruction of the external ear. Plast Reconstr Surg Transplant Bull. 1959;23(1):1–15. doi: 10.1097/00006534-195901000-00001. [DOI] [PubMed] [Google Scholar]
- Teixeira HS, Tovar N, Vandeweghe S, dentistry J-MN. Histomorphometry and bone mechanical property evolution around different implant systems at early healing stages: an experimental study in dogs. … dentistry. 2013 doi: 10.1097/ID.0b013e31829f1f4b. [DOI] [PubMed] [Google Scholar]
- Teven CM, Fisher S, Ameer GA, He TC, Reid RR. Biomimetic approaches to complex craniofacial defects. Ann Maxillofac Surg. 2015;5(1):4–13. doi: 10.4103/2231-0746.161044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tjellstrom A. Osseointegrated implants for replacement of absent or defective ears. Clin Plast Surg. 1990;17(2):355–366. [PubMed] [Google Scholar]
- Waite PD, Waite DE. Bone grafting for the alveolar cleft defect. Semin Orthod. 1996;2(3):192–196. doi: 10.1016/s1073-8746(96)80014-4. [DOI] [PubMed] [Google Scholar]
- Wang X, Schröder HC, Müller WE. Enzymatically synthesized inorganic polymers as morphogenetically active bone scaffolds: application in regenerative medicine. Int Rev Cell Mol Biol. 2014;313:27–77. doi: 10.1016/B978-0-12-800177-6.00002-5. [DOI] [PubMed] [Google Scholar]
- Wilson CE, de Bruijn JD, van Blitterswijk CA, Verbout AJ, Dhert WJA. Design and fabrication of standardized hydroxyapatite scaffolds with a defined macro-architecture by rapid prototyping for bone-tissue-engineering research. Journal of Biomedical Materials Research Part A. 2004;68A(1):123–132. doi: 10.1002/jbm.a.20015. [DOI] [PubMed] [Google Scholar]
- Witek L, Khouri KS, Coelho PG, Flores RL. Patient-specific 3D models for autogenous ear reconstruction. Plastic and Reconstructive Surgery–Global Open. 2016;4(10):e1093. doi: 10.1097/GOX.0000000000001093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xinning Y, Jinghua F, Jianyang L, Xianyan Y, Dongshuang H, Zhongru G, Xuesong D. Fabrication of bioactive tissue engineering scaffold for reconstructing calcified cartilage layer based on three-dimension printing technique. Zhejiang da xue xue bao. Yi xue ban= Journal of Zhejiang University. Medical sciences. 2016;45(2):126–131. doi: 10.3785/j.issn.1008-9292.2016.03.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao Q, Wei B, Guo Y, Jin C, Du X, Yan C, Zhou Z. Design, construction and mechanical testing of digital 3D anatomical data-based PCL–HA bone tissue engineering scaffold. Journal of Materials Science: Materials in Medicine. 2015;26(1):1–9. doi: 10.1007/s10856-014-5360-8. [DOI] [PubMed] [Google Scholar]
- Younger EM, C M. Morbidity at Bone Graft Donor Sites. Journal of Orthopaedic Trauma. 1989;(3):192–195. doi: 10.1097/00005131-198909000-00002. [DOI] [PubMed] [Google Scholar]
- Zhou Z, Buchanan F, Mitchell C, Dunne N. Printability of calcium phosphate: calcium sulfate powders for the application of tissue engineered bone scaffolds using the 3D printing technique. Materials Science and Engineering: C. 2014;38:1–10. doi: 10.1016/j.msec.2014.01.027. [DOI] [PubMed] [Google Scholar]


