Abstract
In the United States, the Centers for Medicare and Medicaid Services (CMS) use Systems Improvement Agreements (SIAs) to require transplant programs repeatedly flagged for poor-performance to improve performance or lose CMS funding for transplants. We identified 14 kidney transplant (KT) programs with SIAs and 28 KT programs without SIAs matched on waitlist volume and characterized kidney acceptance using SRTR data from 12/2006–3/2015. We used difference-in-differences linear regression models to identify changes in acceptance associated with an SIA independent of program variation and trends prior to the SIA. SIA programs accepted 26.9% and 22.1% of offers pre- and post-SIA, while non-SIA programs accepted 33.9% and 44.4% of offers in matched time periods. After adjustment for donor characteristics, time-varying waitlist volume, and secular trends, SIAs were associated with a 5.9 percentage-point (22%) decrease in kidney acceptance (95% CI: −10.9 to −0.8, p=0.03). The decrease in acceptance post-SIA was more pronounced for KDPI 0–40 kidneys (12.3 percentage-point decrease, p=0.007); reductions in acceptance of higher KDPI kidneys occurred pre-SIA. Programs undergoing SIAs substantially reduced acceptance of kidney offers for waitlisted candidates. Attempts to improve post-transplant outcomes might have the unintended consequence of reducing access to transplantation as programs adopt more restrictive organ selection practices.
INTRODUCTION
Since 2007, the Centers for Medicare and Medicaid Services (CMS) have conducted transplant program quality regulation under the Conditions for Participation (COPs), using OPTN data to flag programs with worse than expected post-transplant patient or graft survival (1). Programs that receive two consecutive flags within a one-year period and fail to successfully argue that this is due to unmeasured patient or practice characteristics (mitigating factors) undergo the most extreme form of quality regulation used by the CMS; a Systems Improvement Agreement (SIA). SIAs require transplant programs to demonstrate improved measured performance within a specified time or lose CMS as a payer.
While studies have examined the response to a poor performance flag (2–4), transplant program responses to SIAs, which are a much more aggressive regulatory action, have not been studied. Transplant programs might respond to SIA requirements to improve post-transplant outcomes by becoming more conservative in their transplant practices (5–7). One way to change behavior is to change patterns of acceptance of organs offered for transplantation. In particular, transplant programs might avoid organs that they perceive will be associated with worse than expected post-transplant outcomes. Since CMS participation depends only on post-transplant outcomes, programs may let transplant rates and waitlist mortality rates worsen while taking steps to ensure high rates of patient and graft survival post-transplant.
Because SIAs represent the final opportunity for programs to demonstrate improved performance before losing their largest payer (and potentially other payers as a result), we tested whether these strong incentives to improve post-transplant outcomes influence kidney acceptance patterns (6, 8). Specifically, we used a difference-in-differences approach, a robust econometric method previously used to identify changes associated with regulatory and policy changes (9–11), to isolate the net effect of undergoing an SIA on program-level practices. Using this method, we characterized kidney offer acceptance before and after the start of the SIA among 14 programs undergoing SIAs and compared changes to patterns observed irrespective of the SIA among 28 non-SIA programs matched on waitlist volume between 2009 and 2013.
METHODS
Data source
This study used data from the Scientific Registry of Transplant Recipients (SRTR) external release made available in October 2017. The SRTR data system includes data on all donors, waitlisted candidates, and transplant recipients in the United States, submitted by members of the OPTN, and has been described elsewhere (12). The HRSA, US Department of Health and Human Services, provides oversight to the activities of the OPTN and SRTR contractors. We obtained details on KT program SIAs from 12/2009–3/2013 through a Freedom of Information Act request to the Centers for Medicare and Medicaid Services.
Study population
We identified 14 kidney transplant programs that underwent an SIA between 12/25/2009 and 3/25/2013. We followed each SIA KT program for the two years prior to and two years following SIA implementation. To account for time-trends occurring independent of quality regulation, we identified KT programs that did not undergo an SIA during the study period and that were performing transplants during the four-year periods identified for each SIA KT program. We calculated the waitlist volume of each SIA program two years prior to the SIA and identified all non-SIA programs with waitlist volumes within ±60 candidates of this value. From this set, we used random sampling without replacement to identify two KT programs (matched on waitlist volume) for each SIA program and followed them over the same four-year period. Changes in waitlist volume over the study period are reported. To characterize kidney acceptance, we identified kidney offers made to active candidates listed at all identified programs in the two years prior to the SIA (pre-SIA) and in the two years following the SIA (post-SIA), or the equivalent time periods at non-SIA programs. For example, if Program A entered an SIA on 1/1/2012, we identified all offers made to Program A and to the two matched non-SIA KT programs between 1/1/2010–12/31/2011 (pre-SIA) and between 1/1/2012–12/31/2014 (post-SIA). The time periods are referred to as pre-SIA and post-SIA for both the SIA and non-SIA programs.
Kidney offer acceptance
We modeled organ acceptance at the program level, defined as acceptance and subsequent transplantation of an offered organ for any candidate on the program’s match run list. In other words, if the program declined the offer for one or more of their candidates but eventually accepted it for a different candidate, we considered this an acceptance and did not include the previous declines in our organ-level model. To avoid analyzing acceptance of offers that could not have been accepted on behalf of a candidate, bypassed offers were excluded from the analysis. Offers made under administrative or special circumstances were also excluded as they represent a subset of kidney offers that were administratively distinct from typical deceased-donor offers. Offers that were accepted but not ultimately transplanted were considered declines. Finally, we aimed to characterize acceptance of offers deemed acceptable by at least one center, and thus, only offers of kidneys that were eventually transplanted were included in the analysis.
Changes in kidney offer acceptance following an SIA
To determine the change in probability of offer acceptance following an SIA, we used difference-in-difference (DID) linear probability regression models. DID models are an econometrics technique increasingly used to study the effects of policy interventions, including efforts to improve surgical quality (9–11, 13–15). In the context of this study, there are persistent differences between KT programs and common time trends affecting all programs that must be accounted for in order to isolate the effect of the SIA. Thus, by including both treated (SIA) and control programs (non-SIA) in a pre-post design with program fixed effects, we can account for underlying differences across SIA vs. non-SIA programs and differences and for common acceptance trends over time to estimate the net effect of an SIA on offer acceptance. The fixed effect for each program controls for persistent but unobserved program characteristics (such as patient socio-economic status and surgeon practice style). The model also accounts for clustering at the program level. We adjusted for donor factors associated with refusal and discard (16): donor age, sex, race, stroke, diabetes, hypertension, increased infectious risk status, donation after cardiac death, creatinine>1.5, hepatitis C-infection, and if the offer was a national or regional share. The model included calendar time in months to account for common time trends affecting all centers and time-varying waitlist volume to account for changes in program waitlists that might directly affect organ offers made to each program. Because our visual inspection suggested that trends in organ acceptance and transplant volume may have been diverging prior to SIA start, we included a separate time trend for SIA programs centered around the SIA start date. Thus, the difference-in-difference estimates can be interpreted as the change in probability of acceptance associated with the SIA, independent of pre-existing trends. To determine whether potential changes in the probability of acceptance following an SIA varied by quality of the deceased-donor kidney (determined using the Kidney Donor Profile Index [KDPI]), we tested the interaction between post-SIA acceptance and KDPI. We visually compared the probability of acceptance across KDPI using Lowess curves and created three KDPI strata based on the distribution of the data (0–40, 41–80, and 81–100). Offer acceptance over time is shown using Lowess curves regressed over days. Because changing acceptance patterns have implications for transplant volume, we also report the average number of DDKTs per month over the study period for SIA and non-SIA programs using Lowess curves regressed over months.
Robustness check
To determine whether our inferences were dependent on 2:1 matching, we performed a sensitivity analysis and identified 10 non-SIA programs with replacement for each SIA program. In this analysis, rather than match on waitlist volume, the programs were matched solely on follow-up time.
Statistical analysis
Analyses were performed using Stata 14.2 (College Station, Texas). We used a two-sided α of 0.05 to indicate a statistically significant difference.
RESULTS
Waitlist volume
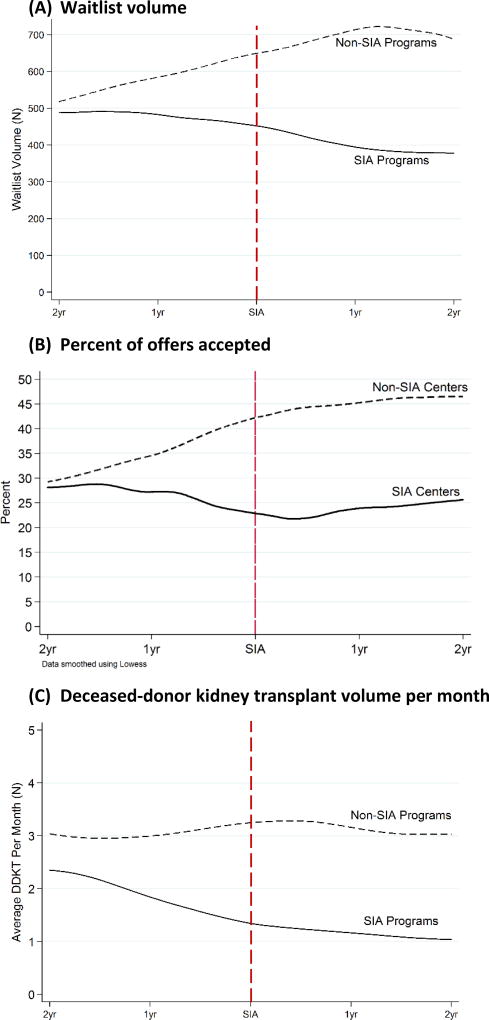
Two years prior to undergoing an SIA, the 14 selected SIA programs had a mean waitlist volume of 531 (252) and the 28 matched non-SIA programs had a mean (SD) waitlist volume of 572 (238). Mean waitlist volume then decreased over time to 348 (159) among SIA programs and increased to 819 (315) among non-SIA programs (Figure 1A).
Figure 1.
(A) Waitlist volume, (B) Probability of offer acceptance, and (C) Monthly deceased-donor kidney transplant volume among SIA and matched non-SIA programs in the two years pre- and post-SIA. Non-SIA and SIA programs matched 2:1 on waitlist volume two-years prior to the SIA.
Kidney offer acceptance
The mean number of offers made to SIA programs decreased from an average of 244 (141) to 188 (136) per program pre and post-SIA. Similarly, the number of offers made to non-SIA programs decreased from an average of 259 (145) to 202 (111) per program in the matched time periods. The number of offers accepted by SIA programs decreased from a mean of 66 (33) to 41 (20) per program pre- and post-SIA, and remained unchanged from 88 (56) to 90 (63) at non-SIA programs (Table 1). SIA programs accepted a total of 920 (26.9%) and 583 offers (22.1%) in the two years pre and two years post-SIA, respectively. Non-SIA programs accepted a total of 2,453 (33.9%) and 2,509 (44.4%) in matched time periods. Two years prior to the SIA, kidney offer acceptance was similar between SIA and non-SIA programs (28.1% and 29.3%, Figure 1B). However, two years after the SIA, SIA programs accepted 25.6% of offers and non-SIA programs accepted 46.4%. After adjustment for persistent program characteristics, donor factors, time-varying waitlist volume, and secular trends, SIA programs reduced acceptance of kidney offers by 5.3 percentage points (95% CI: −8.9 to −1.7), and non-SIA programs increased acceptance of kidney offers by 0.6 percentage points (95% CI: −3.0 to 4.1) (Table 1). Thus, independent of any declines in acceptance pre-SIA, entering an SIA was associated with a net 5.9 percentage point decrease in kidney offer acceptance (95% CI: −10.9 to −0.8, p=0.03). This decrease represents 21.9% of the percent of offers accepted pre-SIA at SIA programs (9.8/26.9). The decline in acceptance was observed in deceased-donor kidney transplant volume at SIA programs (Figure 1C). SIA programs performed a mean of 2.4 DDKT per month two years prior to the SIA and 1.25 per month two years after the SIA, while DDKT volume per month remained unchanged among non-SIA programs (Figure 1C).
Table 1.
Percentage point change in the probability of kidney offer acceptance at programs before and after undergoing a Systems Improvement agreement (SIA)
| Percent of offers accepted (Mean (SD) offers accepted per-program) |
Percentage Point Changea (95% CI) | |||
|---|---|---|---|---|
|
|
||||
| Pre-SIA | Post-SIA | Pre − Post SIA | Net change associated with SIA |
|
| SIA KT Programs | 26.9% | 22.1% | ||
| 66 (33) | 41 (20) | −5.3 (−8.9, −1.7), p=0.005 | −5.9 (−10.9, −0.8), p=0.03 | |
| Non-SIA KT Programs+ | 33.9% | 44.4% | ||
| 88 (56) | 90 (63) | 0.6 (−3.0, 4.1), p=0.8 | 0 [Reference] | |
The change in probability of acceptance of kidney offers associated with the SIA is the difference-in-differences estimate comparing SIA to non-SIA programs. The non-SIA programs allow for adjustment of time-dependent trends in kidney acceptance occurring independent of regulatory involvement. Difference-in-differences model adjusted for calendar time, time-varying waitlist volume, time-invariant program factors, deceased-donor characteristics, and a linear time trend interacted with SIA status. 95% confidence intervals based on program level cluster-robust standard errors.
Kidney offer acceptance by KDPI
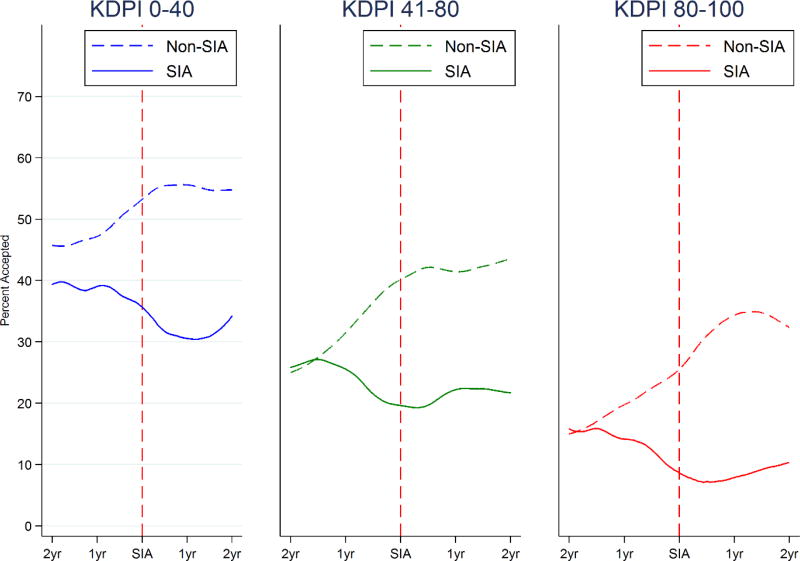
In the pre-SIA period, the 14 SIA programs accepted 38.4%, 25.3%, and 14.3% of kidneys with KDPI 0–40, 41–80, and 81–100, respectively (Table 2). In the post-SIA period, SIA programs accepted 29.4%, 21.9%, and 8.1% of kidneys within each KDPI stratum. Conversely, non-SIA programs accepted 47.0%, 30.4%, and 19.3% of kidneys with KDPI 0–40, 41–80, and 81–100 in matched pre-SIA periods, and 53.4%, 41.2%, and 31.8% in matched post-SIA periods. Two years prior to the SIA, offer acceptance was similar between SIA and non-SIA programs for kidneys with KDPI 41–80 and 81–100 (Figure 2).
Table 2.
Percentage point change in probability of kidney offer acceptance at programs before and after undergoing a Systems Improvement agreement (SIA) stratified by KDPIa of the kidney offer
| Percent of offers accepted (Mean (SD) offers accepted per- program) |
Adjusted Percentage Point Change (95% CI)b | ||||
|---|---|---|---|---|---|
|
|
|||||
| KDPI | Program | Pre-SIA | Post-SIA | Pre − Post SIA | Net change associated with SIA |
| 0–40 | SIA | 38.4% | 29.4% | ||
| 29 (14) | 20 (11) | −14.1 (−20.9, −7.2), p<0.001 | −12.3 (−21.2, −3.5), p=0.007 | ||
| Non-SIA | 47.0% | 53.4% | |||
| 42 (28) | 42 (26) | −1.7 (7.4, 3.9), p=0.5 | 0 [Reference] | ||
|
| |||||
| 41–80 | SIA | 25.3% | 21.9% | ||
| 28 (16) | 18 (10) | −1.6 (−8.3, 4.0), p=0.6 | −1.1 (−10.1, 7.9), p=0.8 | ||
| Non-SIA | 30.4% | 41.2% | |||
| 35 (23) | 37 (28) | −0.5 (−6.6, 5.6), p=0.9 | 0 [Reference] | ||
|
| |||||
| 81–100 | SIA | 14.3% | 8.1% | ||
| 8 (7) | 2 (3) | −6.7 (−11.5, −1.9), p=0.007 | −5.0 (−13.5, 3.6), p=0.2 | ||
| Non-SIA | 19.3% | 31.8% | |||
| 10 (8) | 10 (12) | −1.7 (−8.7, 5.2), p=0.6 | 0 [Reference] | ||
KDPI; kidney donor profile index
Percentage point change results from difference-in-differences model adjusted for time-varying waitlist volume, calendar time, time-invariant program factors, and a linear time trend interacted with SIA status. 95% confidence intervals based on program level cluster-robust standard errors.
Figure 2.
Kidney offer acceptance among SIA and non-SIA programs in the two years pre- and post-SIA stratified by KDPI of the kidney offer
Non-SIA and SIA programs were matched 2:1 on waitlist volume two-years prior to the SIA.
The probability of kidney offer acceptance following an SIA varied by KDPI of the offered kidney (p-value interaction=0.004). After stratification by KDPI and adjustment for program fixed-effects, time-varying waitlist volume, calendar time, and existing trends pre-SIA, SIAs were associated a net decrease of 12.3 (p=0.007), 1.1 (p=0.8), and 5.0 (p=0.2) percentage points in acceptance of 0–40, 41–80, and 81–100 KDPI kidney offers, respectively (Table 2).
Robustness check
In a DID model of 14 SIA programs and 140 non-SIA programs matched on follow-up time alone, SIA programs were associated with a 6.5 percentage point decrease post-SIA (95% CI −10.9, −2.0, p=0.005), non-SIA programs were associated with a 1.6 percentage point increase (95% CI −0.2, 3.5, p=0.08) over matched time periods. Thus, independent of calendar time, pre-SIA trends, changes in waitlist volume, and donor factors, entering an SIA was associated with an 8.1 percentage point decrease in offer acceptance (95% CI −12.9, −3.3, p=0.001). These findings are consistent with those of the primary analysis.
DISCUSSION
In this national level study of kidney offer acceptance, we identified the 14 KT programs that entered into a Systems Improvement Agreement (SIA) for kidney transplant outcomes between 2009 and 2013 and quantified changes in kidney offer acceptance at these programs following SIA implementation. After adjustment for deceased-donor characteristics, program factors, and secular trends, entering an SIA was associated with a 5.9 percentage-point decrease in kidney acceptance (95% CI: −10.9 to −0.8, p=0.03), which represented a 22% reduction in kidney acceptance. Reduction in acceptance varied by KDPI; SIA KT programs reduced acceptance of kidneys with KDPI 0–40, 41–80, and 81–100 by 12.3 (p=0.007), 1.1 (p=0.8), and 5.0 (p=0.2) percentage points, respectively. Our findings indicate that programs narrowed donor selection for more marginal donors prior to the SIA but that following SIA implementation there was a substantial drop in acceptance of higher quality kidneys beyond any changes made prior to the SIA.
We report that the probability of kidney offer acceptance was reduced by 5.9 percentage points (22%) above and beyond any changes the programs might have made prior to the SIA. While we did observe new declines in acceptance among higher KDPI kidneys at SIA onset, programs may have initiated such practices in response to CMS flagging well before SIA onset. And, while increased donor selectivity at higher values of KDPI might make sense for centers attempting to improve post-transplant outcomes if programs believed that these organs could not be adequately risk adjusted, we also observed that the probability of accepting KDPI 0–40 kidneys declined by 12.3 percentage points following the SIA. It is important to note that KDPI was not available for each donor until March 2012, and thus, we retrospectively calculated KDPI as a measure of donor quality that these programs were willing to accept and transplant, however programs did have information on donor quality with each offer during the study period.
Our findings are consistent with those of Schold et al. who observed reductions in overall and marginal transplant volume following performance flags (4). The pronounced declines in acceptance that we observed following an SIA were above and beyond any precautionary changes made following receipt of a second poor performance flag. Our findings were also consistent with those of White et al. who observed reductions in extended-criteria, standard criteria, and living donor transplants at programs facing ongoing non-compliance (3). Similarly, we observed declines over the study period in acceptance of poorer quality organs, despite the survival benefit they can provide (17), although we did not observe an additional reduction in acceptance of these organs following the SIA. Similar to White et al’s findings with respect to higher quality organs, we also observed a substantial reduction in acceptance of higher quality kidneys, and this reduction was in excess of any trends occurring prior to the SIA. Beyond changes in transplant volume, however, we were able to use offer data to show that SIA programs were actively turning down organs that were available for their patients and might have provided survival benefit (18).
While lower organ acceptance rates among SIA programs indicates reduced access to transplant among patients waitlisted at SIA KT programs, it is important to note that national transplant volume would not have increased had SIA programs accepted these kidneys, as we studied only kidneys that were eventually accepted and transplanted. Kidney offers that were never transplanted were excluded to avoid punishing programs for turning down organs that no program was willing to use. However, few candidates multilist, even after notification that their program is undergoing an SIA (19), and so it is unlikely that candidates sought improved access elsewhere while their original center accepted fewer kidney offers during the SIA. It is also worth noting that the matched non-SIA KT programs included KT programs with no active flags and programs that might have had at least one poor performance flag but no SIA. These programs were included in the potential set of KT programs as they represent the real world of KT programs with which SIA regulatory involvement should be compared. The non-SIA KT programs might have undergone changes in practice related to flagging or applying for mitigating factors, but changes of this nature would bias our findings towards the null, and yet we still observed a net reduction in acceptance associated with an SIA. Furthermore, our sensitivity analysis indicated that the significant reductions in offer acceptance following an SIA were not dependent on the non-SIA programs included as control programs in the difference-in-differences model.
In addition, the number of offers made to SIA and non-SIA programs decreased over time, which we would expect to drive up the probability of acceptance. Yet the decline in acceptance post-SIA was robust to declining waitlist volume, secular trends, and to any declines occurring at programs prior to the SIA. The methods we used to identify offers (and the definition of acceptance) were similar to those of Wey et al.’s study of organ offers (20); however, we included one decision per donor per program, rather than all declines. This method was designed to isolate the decision to accept a donor at the program-level rather than the candidate level, in which case programs might be penalized for declining the kidney for multiple patients when they ultimately accepted and transplanted the kidney in a candidate lower on their list.
The limitations of the study merit consideration. We were unable to determine who made the decision to accept or decline each organ offer; however, the main goal of the analysis was to capture center acceptance of offers, and not to discern particular patient characteristics with each offer decision. It is possible that our findings are not driven by the SIA itself, but an unobserved third factor. However, we controlled for persistent characteristics of SIA KT programs, time-varying waitlist volume, time trends common to all programs and differential rates of acceptance among SIA programs that persist across the pre and post period. It is unlikely that there is another unrelated factor that consistently occurs at the same time as an SIA that could account for these results. We do not have kidney biopsy information and other details made available to the transplant surgeon at the time of the offer through OPTN data; however, the kidneys studied were eventually used for transplant, and thus, these unmeasured clinical factors might explain some but not all of why these kidneys were declined by some centers and not by others. Additionally, programs might have restricted acceptance of donors based on factors not included in risk adjustment in order to improve adjusted outcomes. Thus, there might be additional changes in acceptance by donor quality we were unable to isolate.
In this national study of KT program offer acceptance, we found that KT programs undergoing the most severe form of regulatory involvement, an SIA, significantly reduced their probability of accepting kidney organ offers for candidates on their waitlists. SIAs were associated with a significant reduction in acceptance of kidneys with KDPI 0–40, despite the survival benefit these kidneys can provide. KT programs attempting to improve post-transplant outcomes might be inadvertently reducing access to transplant for candidates on their waitlists.
Acknowledgments
The data reported here have been supplied by the Minneapolis Medical Research Foundation (MMRF) as the contractor for the Scientific Registry of Transplant Recipients (SRTR). The interpretation and reporting of these data are the responsibility of the authors and in no way should be seen as official policy of or interpretation by the SRTR, United Network for Organ Sharing (UNOS/OPTN), or the U.S. Government. This work was supported in part by the John and Laura Arnold Foundation. D.L. Segev and A.B. Massie are supported by grant numbers K24DK101828 and K01DK101677 from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), respectively. L.H. Nicholas is supported by grant number K01AG041763 from the National Institute on Aging.
ABBREVIATIONS
- CI
confidence interval
- CMS
Centers for Medicare and Medicaid Services
- DDKT
deceased-donor kidney transplant
- KDPI
kidney donor profile index
- KT
kidney transplant
- OPTN
Organ Procurement and Transplantation Network
- SIA
Systems Improvement Agreement
- SRTR
Scientific Registry of Transplant Recipients
Footnotes
DISCLOSURE
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
References
- 1.Centers for M, Medicaid Services HHS. Medicare program; hospital conditions of participation: requirements for approval and re-approval of transplant centers to perform organ transplants. Final rule. Fed Regist. 2007 Mar 30;72(61):15197–280. [PubMed] [Google Scholar]
- 2.Schold JD, Buccini LD, Poggio ED, Flechner SM, Goldfarb DA. Association of Candidate Removals From the Kidney Transplant Waiting List and Center Performance Oversight. Am J Transplant. 2016 Apr;16(4):1276–84. doi: 10.1111/ajt.13594. [DOI] [PubMed] [Google Scholar]
- 3.White SL, Zinsser DM, Paul M, Levine GN, Shearon T, Ashby VB, et al. Patient selection and volume in the era surrounding implementation of Medicare conditions of participation for transplant programs. Health Serv Res. 2015 Apr;50(2):330–50. doi: 10.1111/1475-6773.12188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schold JD, Buccini LD, Srinivas TR, Srinivas RT, Poggio ED, Flechner SM, et al. The association of center performance evaluations and kidney transplant volume in the United States. Am J Transplant. 2013 Jan;13(1):67–75. doi: 10.1111/j.1600-6143.2012.04345.x. [DOI] [PubMed] [Google Scholar]
- 5.Heilman RL, Green EP, Reddy KS, Moss A, Kaplan B. Potential Impact of Risk and Loss Aversion on the Process of Accepting Kidneys for Transplantation. Transplantation. 2017 Jul;101(7):1514–7. doi: 10.1097/TP.0000000000001715. [DOI] [PubMed] [Google Scholar]
- 6.Howard RJ, Cornell DL, Schold JD. CMS oversight, OPOs and transplant centers and the law of unintended consequences. Clin Transplant. 2009 Nov-Dec;23(6):778–83. doi: 10.1111/j.1399-0012.2009.01157.x. [DOI] [PubMed] [Google Scholar]
- 7.Kasiske BL, McBride MA, Cornell DL, Gaston RS, Henry ML, Irwin FD, et al. Report of a consensus conference on transplant program quality and surveillance. Am J Transplant. 2012 Aug;12(8):1988–96. doi: 10.1111/j.1600-6143.2012.04130.x. [DOI] [PubMed] [Google Scholar]
- 8.Schold JD, Segev DL. Increasing the pool of deceased donor organs for kidney transplantation. Nat Rev Nephrol. 2012 Mar 27;8(6):325–31. doi: 10.1038/nrneph.2012.60. [DOI] [PubMed] [Google Scholar]
- 9.Dimick JB, Nicholas LH, Ryan AM, Thumma JR, Birkmeyer JD. Bariatric surgery complications before vs after implementation of a national policy restricting coverage to centers of excellence. JAMA. 2013 Feb 27;309(8):792–9. doi: 10.1001/jama.2013.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nicholas LH, Dimick JB. Bariatric surgery in minority patients before and after implementation of a centers of excellence program. JAMA. 2013;310(13):1399–400. doi: 10.1001/jama.2013.277915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nicholas LH, Dimick JB. Evaluating Changes in Health Care Policy: Methods Matter. JAMA Surg. 2015 Jul;150(7):649. doi: 10.1001/jamasurg.2015.120. [DOI] [PubMed] [Google Scholar]
- 12.Massie AB, Kucirka LM, Segev DL. Big data in organ transplantation: registries and administrative claims. Am J Transplant. 2014 Aug;14(8):1723–30. doi: 10.1111/ajt.12777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Osborne NH, Nicholas LH, Ryan AM, Thumma JR, Dimick JB. Association of hospital participation in a quality reporting program with surgical outcomes and expenditures for Medicare beneficiaries. JAMA. 2015 Feb 3;313(5):496–504. doi: 10.1001/jama.2015.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dimick JB, Ryan AM. Methods for evaluating changes in health care policy: The difference-in-differences approach. JAMA. 2014;312(22):2401–2. doi: 10.1001/jama.2014.16153. [DOI] [PubMed] [Google Scholar]
- 15.Sommers BD, Baicker K, Epstein AM. Mortality and access to care among adults after state Medicaid expansions. N Engl J Med. 2012 Sep 13;367(11):1025–34. doi: 10.1056/NEJMsa1202099. [DOI] [PubMed] [Google Scholar]
- 16.Massie AB, Desai NM, Montgomery RA, Singer AL, Segev DL. Improving distribution efficiency of hard-to-place deceased donor kidneys: Predicting probability of discard or delay. Am J Transplant. 2010 Jul;10(7):1613–20. doi: 10.1111/j.1600-6143.2010.03163.x. [DOI] [PubMed] [Google Scholar]
- 17.Massie AB, Luo X, Chow EK, Alejo JL, Desai NM, Segev DL. Survival benefit of primary deceased donor transplantation with high-KDPI kidneys. Am J Transplant. 2014 Oct;14(10):2310–6. doi: 10.1111/ajt.12830. [DOI] [PubMed] [Google Scholar]
- 18.Schold JD, Buccini LD, Goldfarb DA, Flechner SM, Poggio ED, Sehgal AR. Association between kidney transplant center performance and the survival benefit of transplantation versus dialysis. Clin J Am Soc Nephrol. 2014 Oct 07;9(10):1773–80. doi: 10.2215/CJN.02380314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nicholas LH, Segev DL. Effects of Systems Improvement Agreements on Waitlist Patient Outcomes. Am J Transplant. 2017;17:19. [Google Scholar]
- 20.Wey A, Salkowski N, Kasiske BL, Israni AK, Snyder JJ. Influence of kidney offer acceptance behavior on metrics of allocation efficiency. Clin Transplant. 2017 Sep;31(9) doi: 10.1111/ctr.13057. [DOI] [PMC free article] [PubMed] [Google Scholar]