Abstract
Purpose
Patients with cancer frequently experience symptoms such as fatigue and pain that can influence their ability to maintain their usual physical activity (PA). This study aimed to evaluate whether symptoms of fatigue and pain are associated with decreased PA among patients with cancer.
Methods
We recruited patients with a cancer diagnosis from one academic medical center and 11 affiliated community hospitals to participate in a cross-sectional survey. Multivariate logistic regression models were used to examine the association between symptoms, demographics, and clinical characteristics and decreased PA since cancer diagnosis.
Results
Among 629 participants, 499 (79%) reported a decreased level of PA since their cancer diagnosis. In the past seven days from the time of the survey, 78% of participants reported moderate to very severe fatigue, and 68% reported a pain level 4 or greater on a scale of 0 to 10. Adjusted for covariates, patients with fatigue (Adjusted Odds Ratio, AOR 4.01, 95% CI 2.41-6.65) and pain (AOR 1.89, 95% CI 1.14-3.12) had higher odds of reporting decreased PA since diagnosis. Receipt of chemotherapy or currently receiving active cancer treatment was also associated with decreased PA (p<0.05).
Conclusions
Fatigue and pain are associated with decreased PA among patients with cancer, even after adjusting for cancer treatment. Interventions focused on managing these symptoms may help promote maintenance of PA throughout cancer treatment and beyond.
Keywords: physical activity, fatigue, pain, cancer, supportive care
INTRODUCTION
As of January 2016, 15.5 million Americans were living with a cancer diagnosis;[1] many of whom experience bothersome symptoms, such as fatigue and pain, after their cancer diagnosis.[2–4] Previous research has shown that physical activity is important for cancer prevention;[5,6] in addition, emerging data from clinical trials highlight that exercise during and after cancer treatment may improve cancer-related fatigue, quality of life, and other cancer-related outcomes.[7–11] Physical activity can be defined as any movement of the skeletal muscles that results in energy expenditure; whereas, exercise is a type of PA that is planned, structured and repetitive and has a set objective of improving or maintaining physical fitness.[12] Many professional clinical societies and cancer organizations, such as the American Cancer Society (ACS) and the American College of Sports Medicine, have made PA recommendations for cancer patients.[7,13] Among these recommended guidelines, cancer patients are encouraged to engage in 150 minutes of moderate aerobic activity or 75 minutes of vigorous aerobic activity per week, including strength training exercises at least 2 days per week.[7]
Despite the recognized benefits of PA, many cancer patients are not meeting recommended guidelines and are less likely than the general population to engage in PA.[14–16] Previous research in primarily breast and colorectal cancer populations has found that the majority of these patients decrease their PA levels after a cancer diagnosis.[17–20] Cancer and its related treatments are often accompanied by significant symptom burden, including fatigue,[2,21] pain,[21,22] and psychosocial distress,[23,24] which may influence PA levels among cancer patients. While growing research exists examining PA levels following a cancer diagnosis among cancer patients, this research has mainly focused on breast and colorectal cancer patients and is often limited to patients seen in academic centers or in the context of formal exercise intervention studies.
Much less is known about patients with diverse cancer types seen in community cancer care settings or outside the confines of an exercise clinical trial, which by its nature recruits already motivated patients to participate in PA. Using a health services perspective of PA as a desired health behavior, this study examines PA level after cancer among patients diagnosed with different cancer types in both academic and community cancer care settings. Specifically, the objectives of this study were 1) to evaluate the relationship between current symptoms (i.e., fatigue, pain, and emotional distress) and a self-reported decrease in PA level following cancer diagnosis, and 2) to identify other risk factors associated with decreased PA.
MATERIALS AND METHODS
Study Design and Population
We conducted a cross-sectional survey study among cancer patients treated at the Abramson Cancer Center (ACC) at the University of Pennsylvania in Philadelphia and 11 ACC-affiliated community hospitals in Pennsylvania and New Jersey. Eligible patients were 18 years of age or older, had a primary diagnosis of any cancer type, were ambulatory (Karnofsky functional score of ≥ 60), understood written English, and reported experiencing non-zero (>0) pain (on a scale of 0 to 10) in the last seven days. Trained research staff met with patients in clinic waiting rooms, obtained written informed consent, and administered the written paper survey. The institutional review board of the University of Pennsylvania and the Clinical Trials Scientific Review and Monitoring Committee of the ACC approved the study protocol and survey.
Questionnaire Content
Patient demographics, cancer and treatment characteristics, current symptoms, and physical activity questions were included in the study questionnaire completed by the patients. Demographic data included: age at study enrollment, sex, race/ethnicity, relationship status, education, and body mass index (BMI) (calculated from patient-reported height and weight). Participants reported information about cancer type, cancer treatments (e.g., radiation, surgery, chemotherapy), treatment status at time of the questionnaire (i.e., completed cancer treatments, currently receiving cancer treatments, or about to start cancer treatments), and year of cancer diagnosis. Cancer type was verified and cancer stage was determined from chart abstractions.
The PROMIS global health 10-item validated instrument assesses general domains of health and functioning including perceived quality of life, overall physical health, mental health, emotional distress, pain, and fatigue.[25,26] This instrument has been shown to demonstrate adequate reliability with the item-scale correlations for the 10 items ranging from 0.53 to 0.80, and internal consistency reliability equal to 0.92 in community and clinical samples.[25] Specifically, 3items from this instrument were used to assess current symptoms of fatigue, pain and emotional distress in the past 7 days,. For fatigue, participants were asked “How would you rate your fatigue on average?” on a 5-point Likert scale from “none” to “very severe”. To assess pain, participants answered “How would you rate your pain on average?” on a scale of “0 - no pain” to “10 - worst pain imaginable”. For emotional distress, participants were asked to rate on 5-point Likert scale “How often have you been bothered by emotional problems such as feeling anxious, depressed, or irritable?” with response choices ranging from “never” to “always”.
To assess level of PA since cancer diagnosis, participants were asked “Since my cancer diagnosis, I have…” with these three answer options “decreased my level of physical activity”, “maintained my level of physical activity”, or “increased my level of physical activity”. Participants were also asked to report if they had met the ACS physical activity recommendations of 150 minutes of moderate activity or 75 minutes of vigorous activity per week on average in the past month with answer choices of “yes” or “no”.
Statistical Analysis
Descriptive statistics were calculated as frequencies and percentages. The primary outcome was level of physical activity since cancer diagnosis, for which we dichotomized responses as “decreased” or “maintained/increased” level of PA. Pearson chi-square tests were conducted examining demographic, clinical characteristics and current symptoms associated with level of PA since cancer diagnosis. Explanatory variables associated with the primary outcome at p<0.20 in the univariate analyses were included in the multivariate regression models.
Logistic regression models were used to estimate odds ratios (OR) for characteristics associated with decreased level of PA. For demographics, we dichotomized race as “white” and “non-white”, education as “high school or less” and “some college or above”, and relationship status as “partnered” or “not partnered”. For cancer characteristics, cancer type was dichotomized as “breast” versus “other” diagnosis, stage as “metastatic” or “non-metastatic”, and cancer treatment status as “currently receiving treatment” or “completed treatment”. For current symptoms, pain was dichotomized as “level 0 (no pain) to 3” or “level 4 to 10 (worst pain)”, fatigue as “none to mild” or “moderate to very severe”, and emotional distress as “never to rarely” or “sometimes to always”. Statistical significance for the multivariate regression models was set at p<0.05. All statistical analyses were conducted using SPSS (Windows version 24.0, IBM Corporation, Armonk, NY) software.
RESULTS
From September 2014 through September 2015, 706 patients with diverse cancer diagnoses were approached and of these, 668 patients (95%) were consented and enrolled to participate in the survey. Of the 668 patients, 6 individuals withdrew from the study prior to completing the survey for the following reasons: changed mind (n=4), time constraints (n=1) and health issue (n=1). Of the 662 participants who completed the survey, 33 (5%) chose not to report their level of physical activity since cancer diagnosis (primary outcome of interest) leaving 629 (89% of the overall sample) included in the analyses.
Characteristics of Study Participants
The mean age of participants was 60.4±11.6 years (range 23-90), 66% were female, 83% were white, 67% had completed at least some college, and 66% were in a partnered relationship. Over half of the patients (52%) were seen in community hospitals. The most common cancer types reported were breast (32%), thoracic/lung (15%) and hematologic (15%), and 54% of the patients had a non-metastatic cancer diagnosis. Eighty percent of patients were currently receiving some form of active cancer treatment, and the most commonly reported cancer treatments received were chemotherapy (88%), surgery (53%), and radiation (53%). In terms of symptoms in the past 7 days, 78% reported moderate to very severe fatigue, 68% had a pain level 4 or greater on a scale of 0 to 10, and 63% reported experiencing emotional distress sometimes to always (see Table 1).
Table 1.
Participant characteristics by change in physical activity (PA) level since cancer diagnosis
| Characteristic | Overall N=629 |
Decreased PA N=499 |
p value |
|---|---|---|---|
| N (% of total) | N (% within each category) | ||
| Demographics | |||
| Age | 0.66 | ||
| < 45 | 63 (10.0) | 51 (79.7) | |
| 45-54 | 120 (19.1) | 100 (83.3) | |
| 55-64 | 207 (32.9) | 160 (77.7) | |
| ≥ 65 | 239 (38.0) | 188 (78.7) | |
| Sex | 0.48 | ||
| Male | 215 (34.2) | 174 (80.9) | |
| Female | 414 (65.8) | 325 (78.5) | |
| Race | 0.008 | ||
| White | 519 (82.5) | 422 (81.3) | |
| Non-White | 110 (17.5) | 77 (70.0) | |
| Body Mass Index (kg/m2) | 0.68 | ||
| < 25 | 218 (34.8) | 170 (78.0) | |
| 25 – 29.9 | 215 (34.4) | 169 (78.6) | |
| ≥ 30 | 193 (30.8) | 157 (81.3) | |
| Education | 0.42 | ||
| High School or Less | 199 (31.9) | 154 (77.4) | |
| Some College or Above | 424 (68.1) | 340 (80.2) | |
| Relationship Status | 0.27 | ||
| Partnered | 413 (65.7) | 333 (80.6) | |
| Not Partnered | 216 (34.3) | 166 (76.9) | |
| Cancer Characteristics | |||
| Cancer Type | 0.010 | ||
| Breast | 199 (31.6) | 144 (72.4) | |
| Thoracic/Lung | 95 (15.1) | 77 (81.1) | |
| Hematologic | 92 (14.7) | 71 (77.2) | |
| Other* | 243 (38.6) | 207 (85.2) | |
| Cancer Stage | 0.016 | ||
| Non-Metastatic | 310 (53.7) | 235 (75.8) | |
| Metastatic | 267 (46.3) | 224 (83.9) | |
| Cancer Treatment Status | <0.001 | ||
| Currently Receiving Treatment | 480 (80.3) | 404 (84.2) | |
| Completed Treatment | 104 (17.4) | 65 (62.5) | |
| About to Start Treatment | 14 (2.3) | 9 (64.3) | |
| Cancer Treatment Received | |||
| Chemotherapy | 552 (87.8) | 455 (82.4) | <0.001 |
| Radiation | 336 (53.4) | 266 (79.2) | 0.91 |
| Surgery | 334 (53.1) | 270 (80.8) | 0.32 |
| Hospital Treatment Location | 0.21 | ||
| Academic | 302 (48.0) | 246 (81.5) | |
| Community | 327 (52.0) | 253 (77.4) | |
| Symptoms in Past 7 Days | |||
| Fatigue | <0.001 | ||
| None to Mild | 137 (22.2) | 77 (56.2) | |
| Moderate to Very Severe | 481 (77.8) | 416 (86.5) | |
| Pain | 0.001 | ||
| Level of 0 (No Pain) to 3 | 201 (32.5) | 144 (71.6) | |
| Level of 4 to 10 (Worst Pain) | 418 (67.5) | 349 (83.5) | |
| Emotional Distress | 0.007 | ||
| Never to Rarely | 227 (36.6) | 168 (74.0) | |
| Sometimes to Always | 394 (63.4) | 327 (83.0) |
Due to missing data, some variables do not add up to 629.
Other cancer type includes but is not limited to: gastrointestinal, gynecological, genitourinary, head and neck, sarcoma, and skin.
Physical Activity Level Since Cancer Diagnosis
Since their cancer diagnosis, 499 (79%) reported that they had decreased their level of PA, whereas 17% maintained and 4% increased their level of PA. Of those who decreased their level of PA, 82% reported not having met the ACS physical activity recommendations of 150 minutes of moderate activity or 75 minutes of vigorous activity per week on average in the past month. In unadjusted analyses, patients with moderate to very severe fatigue compared to those with none to mild fatigue were more likely to report decreased PA (87% vs. 56%, p<0.001) and not meeting ACS recommendations (77% vs. 66%, p=0.010). Similar findings were found for those experiencing emotional distress sometimes to always (decreased PA: 83% vs. 74%, p=0.007; not meeting ACS recommendations: 78% vs. 70%, p=0.029) compared to those who reported emotional distress never to rarely. Participants with a pain rating of 4 or greater compared to those with a rating of ≤3 were more likely to report decreased PA (84% vs. 72%, p=0.001), yet no significant differences in meeting ACS recommendations were found (75% vs. 73%, p=0.574).
Additionally, race, cancer type, cancer stage, cancer treatment status (currently receiving versus completed treatment), and receipt of chemotherapy were significantly associated with a decreased PA at p<0.05 (Tables 1 and 2). When examining decreased PA level by cancer type, the highest percentages of decreased PA were reported by those with gastrointestinal (89%), gynecological (88%) and head/neck (87%) cancer (Figure 1). Age, sex, body mass index, surgery, radiation treatment, or hospital treatment location were not significantly associated with decreased PA (Table 1).
Table 2.
Participant characteristics associated with decreased physical activity levels since cancer diagnosis (N=629)
| Unadjusted Models | Multivariate Model | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Characteristic | Un-AOR | (95% CI) | p value | AOR | (95% CI) | p value |
| Fatigue (vs. None to Mild) |
4.99 | (3.25 – 7.64) | <0.001 | 4.01 | (2.41 – 6.65) | <0.001 |
| Pain Level 4 to 10 (vs. Level 0 to 3) |
2.00 | (1.34 – 2.99) | 0.001 | 1.89 | (1.14 – 3.12) | 0.013 |
| Emotional Distress (vs. Never to |
1.71 | (1.15 – 2.55) | 0.008 | 1.62 | (0.99 – 2.65) | 0.06 |
| Rarely) Race Non-White (vs. White) |
0.54 | (0.34 – 0.85) | 0.008 | 0.58 | (0.32 – 1.07) | 0.08 |
| Cancer Type Breast (vs. Other) |
0.55 | (0.37 – 0.82) | 0.004 | 0.74 | (0.44 – 1.23) | 0.24 |
| Cancer Stage Metastatic (vs. Non-Metastatic) |
1.66 | (1.10 – 2.52) | 0.017 | 1.17 | (0.70 – 1.95) | 0.55 |
| Cancer Treatment Status Currently Receiving (vs. Completed) |
3.19 | (2.00 – 5.09) | <0.001 | 2.71 | (1.55 – 4.73) | <0.001 |
| Chemotherapy (vs. No Chemotherapy) |
3.52 | (2.13 – 5.81) | <0.001 | 3.54 | (1.77 – 7.08) | <0.001 |
AOR, adjusted odds ratio; CI, confidence interval
The final logistic regression model was adjusted for all variables shown in the table.
Fig. 1.
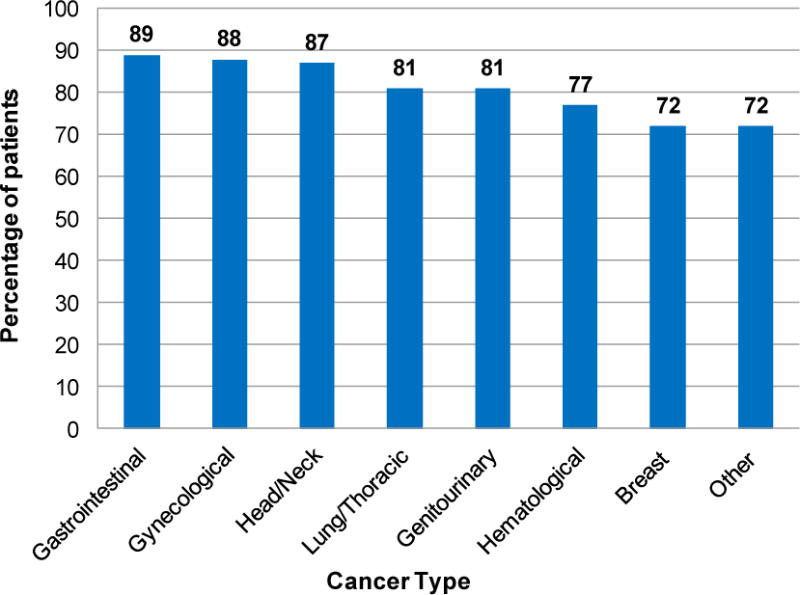
Percentage of patients who reported decreased physical activity since cancer diagnosis by cancer type (N=629)
Estimates from multivariable modeling of decreased level of PA since cancer diagnosis are shown in Table 2. In the fully adjusted model, participants who reported moderate to very severe fatigue had four times higher odds [Adjusted Odds Ratio (AOR) 4.01, 95% Confidence Interval (CI) 2.41, 6.65, p<0.001] of reporting a decreased level of PA than those who did not report fatigue. Participants reporting pain levels ranging from 4 to 10 (worst pain) had a 1.89 times higher odds of decreased PA levels than those with pain levels ranging from 0 to 3 [AOR 1.89, 95% CI 1.14, 3.12, p=0.013]. Participants who were currently receiving cancer treatment had almost three times the odds of decreased PA levels [AOR 2.71, 95% CI 1.55, 4.73, p<0.001] and those who reported having received chemotherapy had 3.5 higher odds [AOR 3.54, 95% CI 1.77, 7.08, p<0.001]. The fully adjusted logistic regression model (including symptoms) in Table 2 explained 26% of the variance in decreased level of PA (Nagelkerk R2 = 0.26); whereas, the model that included only race and clinical characteristics explained 13% of the variance (Nagelkerk R2 = 0.13).
Further, the results of sensitivity analyses (excluding participants who maintained their PA level) conducted among participants who reported a decreased PA level (N=499) compared to those who increased PA level (N=24) confirmed the association between fatigue and pain symptoms and level of PA (data not shown).
DISCUSSION
Engaging in physical activity after a cancer diagnosis has numerous benefits for cancer patients.[7–11] Unfortunately, in this study of over 600 cancer patients seen in academic and community clinical settings, four out of five patients reported a decreased level of PA following their cancer diagnosis. Fatigue and pain were significantly associated with reduced PA since cancer diagnosis. More research and clinical innovations are needed to manage current symptoms and encourage maintenance of PA among cancer patients.
Our study findings provide evidence that patients with fatigue or pain are more likely to report decreased PA since cancer diagnosis, after adjusting for covariates. By contrast, Schmidt and colleagues (2017) found that neither fatigue nor pain were significantly associated with physical inactivity among breast cancer survivors participating in exercise intervention trials.[19] These disparate findings may reflect that patients who enroll in exercise clinical trials are more motivated and have fewer comorbidities and symptoms than patients in the general clinical population, such as those in our study.[27,28] Another possible explanation is that these symptoms may not be associated with absolute levels of physical activity but rather changes in physical activity during cancer treatment and beyond. Because our analyses are cross-sectional, prospective studies are needed to elucidate the temporal relationship between fatigue and pain symptoms and PA.
While the mechanisms of how fatigue and pain influence PA are not fully understood, the observed decreased level of PA may be explained within the “sickness behavior” theoretical framework. This framework posits that the body will exhibit sickness behavior, such as fatigue, as a reaction to the body’s inflammatory response to fighting infection or a disease, such as cancer.[29,30] This heightened inflammatory response can affect a variety of cellular pathways that can potentially influence neurotransmitter systems in the brain that regulate behavior.[29] For example, in animal models, rhesus monkeys with elevated inflammatory biomarkers will crouch into a depressive-like, huddled position and exhibit anhedonic behavior.[31,32] In a recent population-based study in colorectal cancer survivors, Thong et al. report that survivors with fatigue and distress were more likely to experience anhedonia compared to survivors with no fatigue and distress.[33] Since cancer and its related treatments often trigger a heightened inflammatory response in the body,[30] this “sickness behavior” reaction may explain how symptoms can contribute to decreased PA following a cancer diagnosis.
As postulated by the “sickness behavior” framework and shown by our study findings, patients experiencing fatigue or pain symptoms are the least likely to participate in PA, yet these patients potentially would benefit the most by engaging in PA. Many professional cancer organizations recommend PA as a non-pharmacological treatment intervention for fatigue and pain in cancer populations.[2–4] Additionally, a recent meta-analysis found that exercise, psychological, and combined exercise/psychological interventions were more effective at reducing cancer-related fatigue than pharmaceutical options.[34] Since many patients suffering from fatigue and pain symptoms are sedentary and not motivated to participate in exercise clinical trials, future research needs to focus on developing strategies to enroll sedentary cancer patients and to motivate them to engage in PA at home or in community treatment settings. Clinical care teams should consider referral of patients to cancer fitness specialists, physical therapists or supervised exercise classes in order to help patients learn how to be more physically active, despite their symptoms or physical limitations.[9]
Despite the ability of PA to improve cancer-related symptoms, many cancer patients rely on their health care providers for symptom management and advice regarding PA. The cancer care team can help patients effectively manage cancer-related symptoms by eliciting information about any symptoms their patients may be experiencing and develop symptom management solutions early in the cancer trajectory to help their patients stay physically active. Research has shown that cancer patients who received a health care provider’s recommendation to engage in PA were more likely to participate in PA than those who did not receive such a recommendation.[35–37] However, provider-patient communication regarding PA does not occur on a regular basis. A recent study found that only 35% of 361 medical encounters between oncology providers and patients resulted in communication involving PA or exercise.[38]
A few limitations to our study should be acknowledged. The primary limitation is the reliance on self-report for the main outcomes of interest, which may be subject to recall bias or social desirability bias and result in an over-report of PA levels. However, patients in our study who responded affirmatively to not meeting ACS guidelines also reported decreased PA levels since cancer diagnosis, which suggests that patients accurately reported their PA. Another limitation is that this study did not assess the patients’ level of PA prior to their cancer diagnosis or use an objective and validated measure of PA. Further, our cross-sectional design versus a longitudinal study design limits our ability to establish causal relationships between decreased PA level and the factors, such as fatigue and pain, identified in this study. Finally, while this study enrolled patients from both academic and community centers, the majority of patients were white and reported experiencing at least some pain; therefore, the results may not be generalizable to racial/ethnic minority patients or the larger population of cancer patients.
CONCLUSIONS
Our study included a large cohort of patients with diverse cancer types from both academic and community settings and provides evidence that fatigue and pain are associated with decreased PA among cancer patients, even after adjusting for cancer treatment. Future studies should be conducted to better understand how clinical care teams and patients can work together to manage fatigue and pain to promote maintenance of PA levels after cancer.
STRUCTURED ABSTRACT.
Our study provides evidence that fatigue and pain are significantly associated with decreased physical activity among cancer patients, even after adjusting for cancer treatment. Our findings have important implications for clinical care teams by highlighting the unmet need of effectively managing fatigue and pain symptoms to help cancer patients maintain recommended levels of physical activity.
Acknowledgments
The authors would like to thank the patients, oncologists, nurses, and clinical staff at all study sites for their contributions to this study. Research related to the development of this paper was supported in part by the National Cancer Institute grants to the University of Pennsylvania Abramson Cancer Center (2P30CA016520-40) and the Memorial Sloan Kettering Cancer Center (3P30CA008748-50; 5T32CA9461-32), the Byrne Fund, and the Translational Research and Integrative Medicine Fund at Memorial Sloan Kettering Cancer Center.
Footnotes
ORCID: 0000-0001-6028-4111
COMPLIANCE WITH ETHICAL STANDARDS
Conflict of Interest: The authors declare no potential conflicts of interest with respect to the research, authorship, and/or publication of this article. Dr. Mao has full control of all primary data and agrees to allow the journal to review the data if requested. Research related to the development of this paper was supported in part by the National Cancer Institute grants to the University of Pennsylvania Abramson Cancer Center (2P30CA016520-40) and the Memorial Sloan Kettering Cancer Center (3P30CA008748-50; 5T32CA9461-32), the Byrne Fund, and the Translational Research and Integrative Medicine Fund at Memorial Sloan Kettering Cancer Center. The content is solely the responsibility of the authors and does not necessarily represent official views of the National Institutes of Health.
The content is solely the responsibility of the authors and does not necessarily represent official views of the National Institutes of Health.
Ethical Approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent: Informed consent was obtained from all individual participants included in the study.
Contributor Information
Sally A. D. Romero, Department of Medicine, Memorial Sloan Kettering Cancer Center, New York, NY.
Lee Jones, Department of Medicine, Memorial Sloan Kettering Cancer Center, New York, NY.
Joshua M. Bauml, Department of Medicine, Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA.
Q. Susan Li, Department of Medicine, Memorial Sloan Kettering Cancer Center, New York, NY.
Roger B. Cohen, Department of Medicine, Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA.
Jun J. Mao, Department of Medicine, Memorial Sloan Kettering Cancer Center, New York, NY.
References
- 1.Society AC. Cancer Treatment & Survivorship Facts & Figures 2016-2017 2016 [Google Scholar]
- 2.Bower JE, Bak K, Berger A, Breitbart W, Escalante CP, Ganz PA, Schnipper HH, Lacchetti C, Ligibel JA, Lyman GH, Ogaily MS, Pirl WF, Jacobsen PB, American Society of Clinical Oncology Screening, assessment, and management of fatigue in adult survivors of cancer: an American Society of Clinical Oncology clinical practice guideline adaptation. J Clin Oncol. 2014;32(17):1840–1850. doi: 10.1200/JCO.2013.53.4495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berger AM, Mooney K, Alvarez-Perez A, Breitbart WS, Carpenter KM, Cella D, Cleeland C, Dotan E, Eisenberger MA, Escalante CP, Jacobsen PB, Jankowski C, LeBlanc T, Ligibel JA, Loggers ET, Mandrell B, Murphy BA, Palesh O, Pirl WF, Plaxe SC, Riba MB, Rugo HS, Salvador C, Wagner LI, Wagner-Johnston ND, Zachariah FJ, Bergman MA, Smith C, National Comprehensive Cancer Network Cancer-related fatigue, Version 2.2015. J Natl Compr Cancer Netw. 2015;13(8):1012–1039. doi: 10.6004/jnccn.2015.0122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Swarm RA, Abernethy AP, Anghelescu DL, Benedetti C, Buga S, Cleeland C, Deleon-Casasola OA, Eilers JG, Ferrell B, Green M, Janjan NA, Kamdar MM, Levy MH, Lynch M, McDowell RM, Moryl N, Nesbit SA, Paice JA, Rabow MW, Syrjala KL, Urba SG, Weinstein SM, Dwyer M, Kumar R, National Comprehensive Cancer Network Adult cancer pain. J Natl Compr Cancer Netw. 2013;11(8):992–1022. doi: 10.6004/jnccn.2013.0119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moore SC, Lee IM, Weiderpass E, Campbell PT, Sampson JN, Kitahara CM, Keadle SK, Arem H, Berrington de Gonzalez A, Hartge P, Adami HO, Blair CK, Borch KB, Boyd E, Check DP, Fournier A, Freedman ND, Gunter M, Johannson M, Khaw KT, Linet MS, Orsini N, Park Y, Riboli E, Robien K, Schairer C, Sesso H, Spriggs M, Van Dusen R, Wolk A, Matthews CE, Patel AV. Association of leisure-time physical activity with risk of 26 types of cancer in 1.44 million adults. JAMA Intern Med. 2016;176(6):816–825. doi: 10.1001/jamainternmed.2016.1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kushi LH, Doyle C, McCullough M, Rock CL, Demark-Wahnefried W, Bandera EV, Gapstur S, Patel AV, Andrews K, Gansler T, American Cancer Society Nutrition, Physical Activity Guidelines Advisory Committee American Cancer Society guidelines on nutrition and physical activity for cancer prevention: Reducing the risk of cancer with healthy food choices and physical activity. CA Cancer J Clin. 2012;62(1):30–67. doi: 10.3322/caac.20140. [DOI] [PubMed] [Google Scholar]
- 7.Rock CL, Doyle C, Demark-Wahnefried W, Meyerhardt J, Courneya KS, Schwartz AL, Bandera EV, Hamilton KK, Grant B, McCullough M, Byers T, Gansler T. Nutrition and physical activity guidelines for cancer survivors. CA Cancer J Clin. 2012;62(4):243–274. doi: 10.3322/caac.21142. [DOI] [PubMed] [Google Scholar]
- 8.Jones LW, Alfano CM. Exercise-oncology research: Past, present, and future. Acta Oncol. 2013;52(2):195–215. doi: 10.3109/0284186x.2012.742564. [DOI] [PubMed] [Google Scholar]
- 9.Wolin KY, Schwartz AL, Matthews CE, Courneya KS, Schmitz KH. Implementing the exercise guidelines for cancer survivors. J Support Oncol. 2012;10(5):171–177. doi: 10.1016/j.suponc.2012.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Buffart LM, Kalter J, Sweegers MG, Courneya KS, Newton RU, Aaronson NK, Jacobsen PB, May AM, Galvao DA, Chinapaw MJ, Steindorf K, Irwin ML, Stuiver MM, Hayes S, Griffith KA, Lucia A, Mesters I, van Weert E, Knoop H, Goedendorp MM, Mutrie N, Daley AJ, McConnachie A, Bohus M, Thorsen L, Schulz KH, Short CE, James EL, Plotnikoff RC, Arbane G, Schmidt ME, Potthoff K, van Beurden M, Oldenburg HS, Sonke GS, van Harten WH, Garrod R, Schmitz KH, Winters-Stone KM, Velthuis MJ, Taaffe DR, van Mechelen W, Kersten MJ, Nollet F, Wenzel J, Wiskemann J, Verdonck-de Leeuw IM, Brug J. Effects and moderators of exercise on quality of life and physical function in patients with cancer: An individual patient data meta-analysis of 34 RCTs. Cancer Treat Rev. 2017;52:91–104. doi: 10.1016/j.ctrv.2016.11.010. [DOI] [PubMed] [Google Scholar]
- 11.van Vulpen JK, Peeters PH, Velthuis MJ, van der Wall E, May AM. Effects of physical exercise during adjuvant breast cancer treatment on physical and psychosocial dimensions of cancer-related fatigue: A meta-analysis. Maturitas. 2016;85:104–111. doi: 10.1016/j.maturitas.2015.12.007. [DOI] [PubMed] [Google Scholar]
- 12.Caspersen CJ, Powell KE, Christenson GM. Physical activity, exercise, and physical fitness: Definitions and distinctions for health-related research. Public Health Rep. 1985;100(2):126–131. [PMC free article] [PubMed] [Google Scholar]
- 13.Schmitz KH, Courneya KS, Matthews C, Demark-Wahnefried W, Galvao DA, Pinto BM, Irwin ML, Wolin KY, Segal RJ, Lucia A, Schneider CM, von Gruenigen VE, Schwartz AL, American College of Sports Medicine American College of Sports Medicine roundtable on exercise guidelines for cancer survivors. Med Sci Sports Exerc. 2010;42(7):1409–1426. doi: 10.1249/MSS.0b013e3181e0c112. [DOI] [PubMed] [Google Scholar]
- 14.Garcia DO, Thomson CA. Physical activity and cancer survivorship. Nutr Clin Pract. 2014;29(6):768–779. doi: 10.1177/0884533614551969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ottenbacher A, Yu M, Moser RP, Phillips SM, Alfano C, Perna FM. Population estimates of meeting strength training and aerobic guidelines, by gender and cancer survivorship status: Findings from the Health Information National Trends Survey (HINTS) J Phys Act Health. 2015;12(5):675–679. doi: 10.1123/jpah.2014-0003. [DOI] [PubMed] [Google Scholar]
- 16.Mowls DS, Brame LS, Martinez SA, Beebe LA. Lifestyle behaviors among US cancer survivors. J Cancer Surviv. 2016;10(4):692–698. doi: 10.1007/s11764-016-0515-x. [DOI] [PubMed] [Google Scholar]
- 17.Fassier P, Zelek L, Partula V, Srour B, Bachmann P, Touillaud M, Druesne-Pecollo N, Galan P, Cohen P, Hoarau H, Latino-Martel P, Menai M, Oppert JM, Hercberg S, Deschasaux M, Touvier M. Variations of physical activity and sedentary behavior between before and after cancer diagnosis: Results from the prospective population-based NutriNet-Sante cohort. Medicine. 2016;95(40):e4629. doi: 10.1097/md.0000000000004629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Littman AJ, Tang MT, Rossing MA. Longitudinal study of recreational physical activity in breast cancer survivors. J Cancer Surviv. 2010;4(2):119–127. doi: 10.1007/s11764-009-0113-2. [DOI] [PubMed] [Google Scholar]
- 19.Schmidt ME, Wiskemann J, Ulrich CM, Schneeweiss A, Steindorf K. Self-reported physical activity behavior of breast cancer survivors during and after adjuvant therapy: 12 months follow-up of two randomized exercise intervention trials. Acta Oncol. 2017;56(4):618–627. doi: 10.1080/0284186x.2016.1275776. [DOI] [PubMed] [Google Scholar]
- 20.Chung JY, Lee DH, Park JH, Lee MK, Kang DW, Min J, Kim DI, Jeong DH, Kim NK, Meyerhardt JA, Jones LW, Jeon JY. Patterns of physical activity participation across the cancer trajectory in colorectal cancer survivors. Support Care Cancer. 2013;21(6):1605–1612. doi: 10.1007/s00520-012-1703-5. [DOI] [PubMed] [Google Scholar]
- 21.Henry DH, Viswanathan HN, Elkin EP, Traina S, Wade S, Cella D. Symptoms and treatment burden associated with cancer treatment: Results from a cross-sectional national survey in the U.S. Support Care Cancer. 2008;16(7):791–801. doi: 10.1007/s00520-007-0380-2. [DOI] [PubMed] [Google Scholar]
- 22.van den Beuken-van Everdingen MH, Hochstenbach LM, Joosten EA, Tjan-Heijnen VC, Janssen DJ. Update on prevalence of pain in patients with cancer: Systematic review and meta-analysis. J Pain Symptom Manage. 2016;51(6):1070–1090 e1079. doi: 10.1016/j.jpainsymman.2015.12.340. [DOI] [PubMed] [Google Scholar]
- 23.Holland JC, Andersen B, Breitbart WS, Buchmann LO, Compas B, Deshields TL, Dudley MM, Fleishman S, Fulcher CD, Greenberg DB, Greiner CB, Handzo GF, Hoofring L, Hoover C, Jacobsen PB, Kvale E, Levy MH, Loscalzo MJ, McAllister-Black R, Mechanic KY, Palesh O, Pazar JP, Riba MB, Roper K, Valentine AD, Wagner LI, Zevon MA, McMillian NR, Freedman-Cass DA. Distress management. J Natl Compre Cancer Netw. 2013;11(2):190–209. doi: 10.6004/jnccn.2013.0027. [DOI] [PubMed] [Google Scholar]
- 24.Mitchell AJ, Chan M, Bhatti H, Halton M, Grassi L, Johansen C, Meader N. Prevalence of depression, anxiety, and adjustment disorder in oncological, haematological, and palliative-care settings: A meta-analysis of 94 interview-based studies. Lancet Oncol. 2011;12(2):160–174. doi: 10.1016/s1470-2045(11)70002-x. [DOI] [PubMed] [Google Scholar]
- 25.Hays RD, Bjorner JB, Revicki DA, Spritzer KL, Cella D. Development of physical and mental health summary scores from the patient-reported outcomes measurement information system (PROMIS) global items. Qual Life Res. 2009;18(7):873–880. doi: 10.1007/s11136-009-9496-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cella D, Riley W, Stone A, Rothrock N, Reeve B, Yount S, Amtmann D, Bode R, Buysse D, Choi S, Cook K, Devellis R, DeWalt D, Fries JF, Gershon R, Hahn EA, Lai JS, Pilkonis P, Revicki D, Rose M, Weinfurt K, Hays R. The Patient-Reported Outcomes Measurement Information System (PROMIS) developed and tested its first wave of adult self-reported health outcome item banks: 2005-2008. J Clin Epidemiol. 2010;63(11):1179–1194. doi: 10.1016/j.jclinepi.2010.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van Waart H, van Harten WH, Buffart LM, Sonke GS, Stuiver MM, Aaronson NK. Why do patients choose (not) to participate in an exercise trial during adjuvant chemotherapy for breast cancer? Psychooncology. 2016;25(8):964–970. doi: 10.1002/pon.3936. [DOI] [PubMed] [Google Scholar]
- 28.Gollhofer SM, Wiskemann J, Schmidt ME, Klassen O, Ulrich CM, Oelmann J, Hof H, Potthoff K, Steindorf K. Factors influencing participation in a randomized controlled resistance exercise intervention study in breast cancer patients during radiotherapy. BMC Cancer. 2015;15:186. doi: 10.1186/s12885-015-1213-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miller AH, Raison CL. The role of inflammation in depression: From evolutionary imperative to modern treatment target. Nature Rev Immunol. 2016;16(1):22–34. doi: 10.1038/nri.2015.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Diakos CI, Charles KA, McMillan DC, Clarke SJ. Cancer-related inflammation and treatment effectiveness. Lancet Oncol. 2014;15(11):e493–503. doi: 10.1016/s1470-2045(14)70263-3. [DOI] [PubMed] [Google Scholar]
- 31.Felger JC, Alagbe O, Hu F, Mook D, Freeman AA, Sanchez MM, Kalin NH, Ratti E, Nemeroff CB, Miller AH. Effects of interferon-alpha on rhesus monkeys: A nonhuman primate model of cytokine-induced depression. Biol Psychiatry. 2007;62(11):1324–1333. doi: 10.1016/j.biopsych.2007.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Felger JC, Mun J, Kimmel HL, Nye JA, Drake DF, Hernandez CR, Freeman AA, Rye DB, Goodman MM, Howell LL, Miller AH. Chronic interferon-alpha decreases dopamine 2 receptor binding and striatal dopamine release in association with anhedonia-like behavior in nonhuman primates. Neuropsychopharmacology. 2013;38(11):2179–2187. doi: 10.1038/npp.2013.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thong MSY, Mols F, van de Poll-Franse LV, Sprangers MAG, van der Rijt CCD, Barsevick AM, Knoop H, Husson O. Identifying the subtypes of cancer-related fatigue: Results from the population-based PROFILES registry. J Cancer Surviv. 2018;12(1):38–46. doi: 10.1007/s11764-017-0641-0. [DOI] [PubMed] [Google Scholar]
- 34.Mustian KM, Alfano CM, Heckler C, Kleckner AS, Kleckner IR, Leach CR, Mohr D, Palesh OG, Peppone LJ, Piper BF, Scarpato J, Smith T, Sprod LK, Miller SM. Comparison of pharmaceutical, psychological, and exercise treatments for cancer-related fatigue: A meta-analysis. JAMA Oncol. 2017;3(7):961–968. doi: 10.1001/jamaoncol.2016.6914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tarasenko YN, Miller EA, Chen C, Schoenberg NE. Physical activity levels and counseling by health care providers in cancer survivors. Prev Med. 2017;99:211–217. doi: 10.1016/j.ypmed.2017.01.010. [DOI] [PubMed] [Google Scholar]
- 36.Jones LW, Courneya KS, Fairey AS, Mackey JR. Effects of an oncologist’s recommendation to exercise on self-reported exercise behavior in newly diagnosed breast cancer survivors: A single-blind, randomized controlled trial. Ann Behav Med. 2004;28(2):105–113. doi: 10.1207/s15324796abm2802_5. [DOI] [PubMed] [Google Scholar]
- 37.Winters-Stone KM, Moe EL, Perry CK, Medysky M, Pommier R, Vetto J, Naik A. Enhancing an oncologist’s recommendation to exercise to manage fatigue levels in breast cancer patients: A randomized controlled trial. Support Care Cancer. 2018;26(3):905–912. doi: 10.1007/s00520-017-3909-z. [DOI] [PubMed] [Google Scholar]
- 38.Nyrop KA, Deal AM, Williams GR, Guerard EJ, Pergolotti M, Muss HB. Physical activity communication between oncology providers and patients with early-stage breast, colon, or prostate cancer. Cancer. 2016;122(3):470–476. doi: 10.1002/cncr.29786. [DOI] [PMC free article] [PubMed] [Google Scholar]


