Abstract
Congenital central hypoventilation syndrome (CCHS) is a disorder of ventilatory control and autonomic dysregulation that can be caused by mutations in the paired-like homeobox 2B (PHOX2B) gene. The majority of CCHS cases are caused by polyalanine repeat mutations (PARMs) in PHOX2B; however, in rare cases, non-polyalanine repeat mutations (NPARMs) have been identified. Here, we report two patients with NPARMs in PHOX2B. Patient 1 has a mild CCHS phenotype seen only on polysomnogram, which was performed for desaturations and stridor following a bronchiolitis episode, and characterized by night-time hypoventilation and a history of ganglioneuroblastoma. She carried a novel de novo missense variant, p.R102S (c.304C > A), in exon 2. Patient 2 has an atypical CCHS phenotype including micrognathia, gastroesophageal reflux, stridor, hypopnea, and intermittent desaturations. Sleep study demonstrated that Patient 2 had daytime and night-time hypercarbia with obstructive sleep apnea, requiring tracheostomy. On PHOX2B sequencing, she carried a recently identified nonsense variant, p.Y78* (c.234C > G), in exon 1. In summary, we present two patients with CCHS and identified NPARMs in PHOX2B who have distinct differences in phenotype severity, further elucidating the range of clinical outcomes in CCHS and illustrating the necessity of considering PHOX2B mutations when encountering atypical CCHS presentations.
Keywords: congenital central hypoventilation syndrome, neuroblastoma, neurocristopathy, non-polyalanine repeat mutations, PHOX2B
1 |. INTRODUCTION
Pathogenic mutations in paired-like homeobox 2B (PHOX2B) lead to congenital central hypoventilation syndrome (CCHS), a disorder of ventilatory control and autonomic dysfunction manifested by alveolar hypoventilation. PHOX2B is a gene that encodes the paired mesoderm homeobox 2B protein, a vertebrate homeodomain transcription factor that is essential in the development of central autonomic ganglia and peripheral neural crest derivatives (Weese- Mayer, Pallavi, Carroll, & Rand, 2011). Approximately 90% of CCHS cases are associated with polyalanine repeat mutations (PARMs) that cause expansion of the 20-residue polyalanine tract in exon 3 of the PHOX2B gene, while approximately 10% of cases are associated with missense, nonsense, or frameshift non-polyalanine repeat mutations (NPARMs) (Weese-Mayer et al., 2017). Thus far, 177 cases associated with NPARMs have been reported, with 97 unique variants identified (Weese-Mayer et al., 2017). Both PARMs and NPARMs impair PHOX2B function, leading to a disruption in the development of the neuronal circuits responsible for CO2 sensitivity (Ramanantsoa & Gallego, 2013).
CCHS varies in severity, ranging from mild hypoventilation during sleep to marked hypoxemia and hypercarbia during wakefulness and sleep associated with central apneas due to impaired respiratory center responses (Ramanantsoa & Gallego, 2013). CCHS can occur in isolation or in association with Hirschsprung disease and/or neural crest tumors (Croaker, Shi, Simpson, Cartmill, & Cass, 1998; Ramanantsoa & Gallego, 2013). Compared to patients with PARMs, patients with NPARMs have been shown to have an increased incidence of both Hirschsprung disease (87–100% in patients with NPARMs versus 13–20% in patients with PARMs) (Weese-Mayer et al., 2010) and neuroblastoma (50% versus 1%) (Lombardo, Kramer, Cnota, Sawnani, & Hopkin, 2017).
Currently, there is a well-known genotype-phenotype correlation for PARMs in which patients with a higher number of polyalanine repeats have a more severe CCHS phenotype and clinical course (Weese-Mayer et al., 2010). However, the genotype-phenotype correation for NPARMs is not as well described. While there is evidence that NPARMs are associated with a more severe CCHS phenotype, several studies have identified novel NPARMs in CCHS patients who have a milder clinical presentation and delayed diagnosis, suggesting that NPARM-associated phenotypes may depend on the type and location of the mutation (Cain et al., 2017; Lombardo et al., 2017; Magal- haes et al., 2015; Parodi et al., 2008; Trochet et al., 2009). In this clinical report, we present two patients with NPARMs who have distinct differences in phenotype severity with mild and atypical presentations. “Mild” CCHS is illustrated by Patient 1 who presented during early infancy with mild hypercarbia only during sleep requiring non- invasive positive pressure ventilation (and not tracheostomy), and “atypical” presentation refers to obstructive sleep apnea (OSA)-like symptoms as seen in Patient 2. This further elucidates the range of clinical outcomes in CCHS, and illustrates the need for PHOX2B sequencing and evaluation for NPARMs when there is suspicion for CCHS. Moreover, it is important to note that a clinically milder degree of hypercarbia does not necessarily rule out CCHS and there needs to be a very high index of suspicion to evaluate the patient accordingly.
2 |. RESULTS
2.1 |. Patient 1
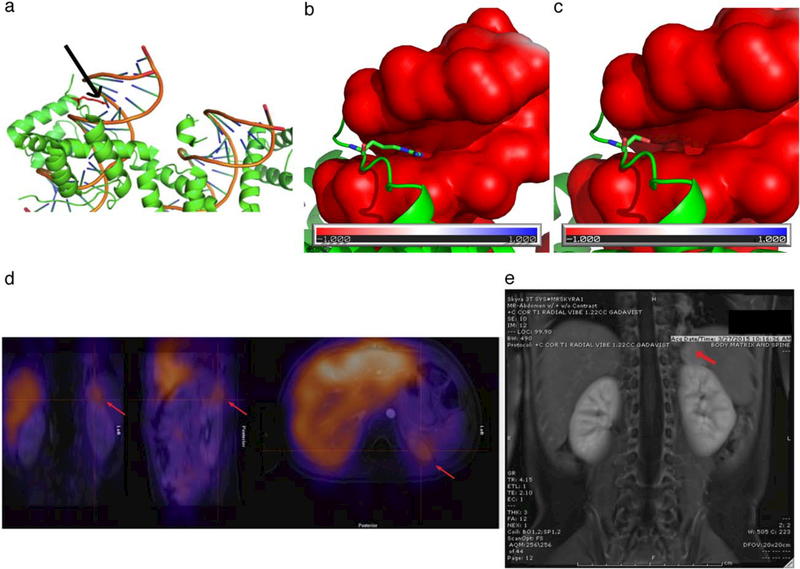
Patient 1 is a 4-year-old female of Vietnamese descent. She was born full term following an uncomplicated pregnancy and discharged home without respiratory issues. She then presented at 5 weeks of age with recurrent desaturations and stridor following bronchiolitis. Venous blood gas during her acute illness demonstrated an elevated CO2 (partial pressure of CO2 [PaCO2] 77 mmHg). Physical examination and chest radiograph were otherwise normal. Bedside flexible rhinoscopy to evaluate her stridor revealed mild laryngomalacia. She was initially managed conservatively with bronchodilators, steroids, and oxygen. Due to persistent hypercarbia and hypoxemia after she recovered from bronchiolitis, an overnight polysomnogram was performed. The first sleep study at 1 month of age revealed persistent sleep hypoventilation (end tidal CO2 [ETCO2] 52–56 torr) associated with moderate to severe brief desaturation (nadir oxygen saturation [SaO2] 81%) but no obstructive or central sleep apnea (Table 1). Neurological evaluation and brain magnetic resonance imaging (MRI) were normal and PHOX2B genetic testing initially revealed a normal number of polyalanine repeats (20/20) in exon 3. A repeat sleep study at 4 months of age revealed excessive periodic breathing (>25% of total sleep time), severe central sleep apnea and sleep hypoventilation (ETCO2 48–65 torr). At this time further genetic testing identified a novel de novo heterozygous missense NPARM, p.R102S (c.304C>A), in exon 2, categorized as a variant of unknown significance (VUS). Molecular modeling suggested that the variant affected PHOX2B function by altering DNA binding (Figure 1a-c). This variant was not present in publicly available databases, including ExAC, gnomAD, dbSNP, NHLBI Exome Sequencing Project, and 1000 Genomes Project, and was predicted to be deleterious by multiple in silico tools, including Polyphen-2, SIFT, and Mutation Taster.
Table 1.
Sleep study findings for Patients 1 and 2
| Patient 1 | Patient 2 | ||
|---|---|---|---|
| Age at sleep study | 1 month | 4 years | 1 month |
| EEG arousal index (per hour), Normal < 14/hour | 13.9 | 22.2 | 21.8 |
| Respiratory arousal index (per hour) | 0.5 | 2.6 | 3.4 |
| Obstructive apnea hypopnea index (per hour), Normal < 1/hour | 0.2 | 3.2 | 23.8 |
| Central apnea hypopnea index (per hour), Normal < 5/hour | 4.4 | 74 | 1.9 |
| Average saturations (SaO2) | 87–93% | 91–95% | 90–93% |
| Nadir saturation (SaO2) | 81% | 65% | 81% |
| % time SaO2 <92% | 7.5% | 61.4% | 13.6% |
| Awake CO2 (mmHg), Normal = 35–45 mmHg | 52–56 | 36–48 | N/A |
| Average ETCO2/TcCO2, Normal = 35–45 mmHg | 52–56 | 52–60 | 60–70 |
| % time CO2 >50 torr during sleep | 80% | 100% | 100% |
| Respiratory rate (per minute) | 30–48 | 8–30 | 24–28 |
| Heart rate (per minute) | 130–158 | 107–136 | 135–155 |
| Electrocardiogram abnormalities | None | None | None |
Note. Electroencephalogram (EEG) arousal index: Number of arousal divided by total sleep duration; Respiratory arousal index (RAI): Total number of arousal due to respiratory events divided by total sleep duration; Apnea hypopnea index (AHI): Total number of apnea and hypopneas divided by total sleep duration. Based on obstructive or central events respective AHI is calculated (Berry et al., 2017)
FIGURE 1.
(a) The side chain of R102 (red, indicated by arrow) points towards the major groove of the DNA helix. (b, c) The surface of the DNA is colored based on its electrostatic potential. A red color indicates a negative charge, while a blue color indicates a positive charge. The protein is shown in green. The residues R102 (b) and S102 (c) are shown in sticks, with the N atoms colored in blue and the O atoms in red. The side chain of R102 points towards the major groove of the DNA helix because of the electrostatic interaction. The mutation R102S disrupts the interaction and affects the binding between PHOX2B and the DNA. (d) Abdominal PET with MIBG avidity and (e) abdominal MRI for Patient 1. Normal uptake noted in the liver, and abnormal uptake noted superior to the left kidney. Arrows indicate abnormal uptake (d) and abnormal mass (e) [Color figure can be viewed at wileyonlinelibrary.com]
Given the confirmed diagnosis of CCHS, tracheostomy and ventilation were recommended. However, her parents refused and opted for noninvasive bi-level ventilation. We agreed with this plan because her CO2 values were relatively normal during daytime. Patient continued on bi-level ventilation during sleep and a follow-up sleep study at 13 months of age revealed progressive normalization in daytime ventilation but persistent mild sleep hypoventilation.
At 21 months of age, an abdominal ultrasound was performed for a history of scant hematochezia. The ultrasound showed a left suprarenal mass, and abdominal positron emission tomography (PET)/MRI demonstrated iodine-131-meta-iodobenzylguanidine (MIBG) avidity consistent with a neuroendocrine tumor (Figure 1d,e). She underwent surgical resection and the surgical pathology revealed a Stage 2B ganglioneuroblastoma.
At 24 months of age, she presented with snoring, choking, and severe OSA secondary to adenotonsillar hypertrophy, and another sleep study revealed severe OSA. She underwent adenotonsillectomy and then developed postoperative complications with post-obstructive pulmonary edema and pneumonitis, requiring a prolonged ICU stay, continuous positive airway pressure (CPAP), diuretics, and oxygen. She was eventually discharged home on bi-level positive airway pressure (BIPAP). Her last sleep study at 4 years of age showed no hypoventilation when awake (ETCO2 36–48 torr) but severe central apnea and persistent sleep hypoventilation (52–60 torr) associated with moderate to severe desaturation (nadir SaO2 65%) during sleep (Table 1). She has no evidence of developmental delays or pulmonary hypertension.
2.2 |. Patient 2
Patient 2 is a 2-month-old female born at 39 1/7 weeks by repeat cesarean section to a 31-year-old mother with severe obesity and type II diabetes mellitus, for which she has been taking glyburide. At birth, she presented with hypoglycemia, hypercarbia, stridor, and gastroesophageal reflux disease, and a swallow study demonstrated aspiration of thin liquids. Despite caffeine, lasix, and oxygen Total number of apnea and hypopneas divided by total sleep duration. Based on obstructive or central events respective AHI is calculated (Berry et al., 2017). administration, she continued to demonstrate episodic desaturations with stridor and congestion. Due to her oxygen requirement and ongoing desaturations, a sleep study was performed demonstrating severe obstructive apnea and frequent moderate desaturations (nadir SaO2 81%) (Table 1). Although breathing was quite stable in quiet (non-rapid eye movement) sleep, there was persistent hypercar- bia (transcutaneous CO2 [TcCO2] 60–70 torr), suggestive of significant hypoventilation. She was managed with nasogastric tube feeding and CPAP for OSA. In addition, she underwent sleep endoscopy, which revealed moderate laryngomalacia with severe pharyngeal wall collapse and normal vocal cord movements.
Given ongoing concern for hypercarbia and noted shallow breathing with persistently low respiratory rate (<20 beats per minute), a PHOX2B genetic test was sent. The test revealed a normal number of polyalanine repeats (20/20) in exon 3 and identified a nonsense heterozygous NPARM, p.Y78* (c.234C> G) in exon 1. This variant has been recently reported in another family (Lombardo et al., 2017) and is expected to result in loss of PHOX2B function via premature protein truncation or nonsense-mediated mRNA decay.
Due to severe daytime and nighttime hypercarbia and shallow respiratory efforts, she underwent tracheostomy and placement of a gastrostomy tube. She was initially on 24-hr ventilator support, with which her CO2 levels were less than 40 torr, and is currently on heat and moisture exchanger 2 hr during the day with spot check ETCO2 and remains fully vented at night. She has no cardiac rhythm disturbances. Of note, a liver mass was incidentally found on abdominal ultrasound screening for adrenal masses and followup contrast-enhanced ultrasound showed that the mass is likely a hemangioma.
3 |. DISCUSSION
Unlike the mutations associated with the majority of CCHS cases, the mutations identified in Patients 1 and 2 did not arise from abnormal polyalanine repeat numbers in PHOX2B. Patient 1 has a novel de novo mis- sense NPARM and Patient 2 has a nonsense NPARM only recently identified in one other family. Although there is a well understood genotype-phenotype correlation for PARMs, in which patients with a higher number of polyalanine repeats have a more severe clinical course, it is currently difficult to assess severity and prognosis based on specific NPARMs as more data are needed. Patient 1 has a relatively mild CCHS phenotype. She was asymptomatic at birth and discharged home routinely, and then presented with worsening hypercarbia following an acute respiratory infection in the first weeks of life. Her persistent hypercarbia during sleep, even after resolution of bronchiolitis symptoms, raised suspicion for CCHS. Although initial genetic testing revealed a normal number of polyalanine repeats (20/20) in PHOX2B, further sequencing identified a novel de novo NPARM in the homeodomain, initially classified as a VUS but in silico analysis, molecular modeling and the presence of ganglioneuroblastoma was suggestive of its pathogenicity.
Patient 1 developed ganglioneuroblastoma at 21 months of age, consistent with the increased incidence of ganglioneuroblastoma in patients with NPARMs compared to patients with PARMs. Thus, while some CCHS patients with NPARMs may present with a relatively mild respiratory course, they are still at high risk for neuroendocrine tumors, necessitating close follow up and monitoring. Although there have also been reports of an increased incidence of Hirschsprung disease in CCHS patients with NPARMs compared to patients with PARMs, neither of our patients have been diagnosed with this disease to date. Of note, the nearby p.R100L mutation has been identified in familial neuroblastoma as well as a patient with CCHS, sporadic neuroblastoma, and Hirschprung disease, and the p.R141Q mutation has been identified in a patient with CCHS, Hirschprung disease, and neuroblastoma (Raabe et al., 2008; Tro- chet et al., 2004; Trochet, O'Brien, et al., 2005b); thus, missense mutations in this region may lead to non-isolated CCHS.
Patient 1 also developed severe OSA due to adenotonsillar hypertrophy, and the severity of OSA was likely worsened due to her underlying CCHS, which may have also contributed to her post-operative complications after adenotonsillectomy. She never had a tracheostomy or gastrostomy tube and has been managed with nocturnal BIPAP. To date she is doing well developmentally, and she will continue to require close follow up in case respiratory support is needed during future respiratory illnesses. Evidence from Patient 1 and others suggest that CCHS is not always associated with a severe respiratory phenotype, and in fact can be later diagnosed in patients who are asymptomatic at birth (Cain et al., 2017; Lombardo et al., 2017; Magalhaes et al., 2015; Parodi et al., 2008; Trochet et al., 2009; Weese-Mayer et al., 2010). For example, the majority of NPARMs reported to date are frameshift variants. Frame- shifts in exon 3, which can affect the polyalanine repeat region, are associated with a severe phenotype, but frameshift variants in the end of exon 2 or the beginning of exon 3 are associated with a milder phenotype (Cain et al., 2017; Low et al., 2014; Trochet, Hong, et al., 2005a).
In contrast to Patient 1, Patient 2 presented at birth with a more severe clinical course characterized by persistent severe desaturations, severe hypercarbia while awake and asleep, OSA, and oropharyngeal airway collapse requiring tracheostomy and placement of a gastrostomy tube. PHOX2B genetic testing revealed a normal number of polyalanine repeats and identified a pathogenic nonsense NPARM recently reported in a different family (Lombardo et al., 2017). In that family, the mutation was maternally inherited in two siblings and was associated with a variable phenotype. In terms of respiratory issues, one child was noted to have desaturation episodes, one child was diagnosed with central apnea, and the mother reported possible apneic episodes during sleep. In addition, both children were diagnosed with Hirschsprung's disease, and both children and the mother have mildly dysmorphic facial features and aniso- coria. Interestingly, there is also a history of congenital heart disease, with one child having aberrant origin of the left coronary artery and the mother having a complete vascular ring and right-sided aortic arch. To date, both children have screened negative for neural crest tumors (Lombardo et al., 2017). In comparison, our Patient 2 has a severe phenotype with significant respiratory compromise and an incidental liver hemangioma, but no evidence of Hirschprung's or congenital heart disease. For the nonsense NPARMs reported to date, the location of the mutation appears to be associated with the phenotypic severity. Although a severe clinical presentation was reported in a patient with p.K155*, of p. M18/M21, likely due to translational reinitiation at p.M18 or p.M21 (Cain et al., 2017; Lombardo et al., 2017; Magalhaes et al., 2015; Parodi et al., 2008; Trochet et al., 2009). In the future, additional research is needed on patients with NPARMs to further understand and characterize the variety of associated clinical presentations and disease progression.
In summary, the key learning points from this report are that (a) genetic testing of PHOX2B needs to be done to investigate for NPARMs in addition to PARMs if there is suspicion for CCHS and (b) CCHS patients with NPARMs can present with a diverse range of clinical phenotypes. Thus, it is possible that additional factors besides PHOX2B mutations, such as epigenetic alterations, may contribute to the clinical presentation of these patients.
ACKNOWLEDGMENTS
We acknowledge the patients and their families for their contribution to this research. PBA was supported by NIH/NIAMS (1R01AR068429–01) and NICHD/NHGRI/NIH (U19HD077671). AMD was supported by NIGMS (T32GM007753).
Footnotes
CONFLICT OF INTEREST
None.
REFERENCES
- Berry RB, Brooks R, Gamaldo CE, Harding SM, Lloyd RM, Quan SF, ... Vaughn BV for the American Academy of Sleep Medicine. (2017). The AASM manual for the scoring of sleep and associated events: Rules, terminology and technical specifications (Version 2.4) Darien, IL: American Academy of Sleep Medicine. [Google Scholar]
- Cain JT, Kim DI, Quast M, Shivega WG, Patrick RJ, Moser C, ... Landsverk M. (2017). Nonsense pathogenic variants in exon 1 of PHOX2B lead to translational reinitiation in congenital central hypoventilation syndrome. American Journal of Medical Genetics: Part A, 173(5), 1200–1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croaker GD, Shi E, Simpson E, Cartmill T, & Cass DT (1998). Congenital central hypoventilation syndrome and Hirschsprung’s disease. Archives of Disease in Childhood, 78(4), 316–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombardo RC, Kramer E, Cnota JF, Sawnani H, & Hopkin RJ (2017). Variable phenotype in a novel mutation in PHOX2B. American Journal of Medical Genetics: Part A, 173(6), 1705–1709. [DOI] [PubMed] [Google Scholar]
- Low KJ, Turnbull AR, Smith KR, Hilliard TN, Hole LJ, Meecham Jones DJ, ... Donaldson A (2014). A case of congenital central hypoventilation syndrome in a three-generation family with nonpolyalanine repeat PHOX2B mutation. Pediatric Pulmonology, 49(10), E140–E143. [DOI] [PubMed] [Google Scholar]
- Magalhaes J, Madureira N, Medeiros R, Fernandes PC, Oufadem M, Amiel J, ... Reis MG (2015). Late-onset congenital central hypoventilation syndrome and a rare PHOX2B gene mutation. Sleep and Breathing, 19(1), 55–60. [DOI] [PubMed] [Google Scholar]
- Parodi S, Bachetti T, Lantieri F, Di Duca M, Santamaria G, Ottonello G, ... Ceccherini I (2008). Parental origin and somatic mosaicism of PHOX2B mutations in congenital central hypoventilation syndrome. Human Mutation, 29(1), 206. [DOI] [PubMed] [Google Scholar]
- Raabe EH, Laudenslager M, Winter C, Wasserman N, Cole K, LaQuaglia M, ... Maris JM (2008). Prevalence and functional consequence of PHOX2B mutations in neuroblastoma. Oncogene, 27(4), 469–476. [DOI] [PubMed] [Google Scholar]
- Ramanantsoa N, & Gallego J (2013). Congenital central hypoventilation syndrome. Respiratory Physiology and Neurobiology, 189(2), 272–279. [DOI] [PubMed] [Google Scholar]
- Trochet D, Bourdeaut F, Janoueix-Lerosey I, Deville A, de Pontual L, Schleiermacher G, ... Amiel J (2004). Germline mutations of the paired-like homeobox 2B (PHOX2B) gene in neuroblastoma. American Journal of Human Genetics, 74(4), 761–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trochet D, Hong SJ, Lim JK, Brunet JF, Munnich A, Kim KS, ... Amiel J (2005a). Molecular consequences of PHOX2B missense, frameshift and alanine expansion mutations leading to autonomic dysfunction. Human Molecular Genetics, 14(23), 3697–3708. [DOI] [PubMed] [Google Scholar]
- Trochet D, Mathieu Y, Pontual L, Savarirayan R, Munnich A, Brunet JF, ... Amiel J (2009). In vitro studies of non poly alanine PHOX2B mutations argue against a loss-of-function mechanism for congenital central hypoventilation. Human Mutation, 30(2), E421–E431. [DOI] [PubMed] [Google Scholar]
- Trochet D, O’Brien LM, Gozal D, Trang H, Nordenskjold A, Laudier B, ... Amiel J (2005b). PHOX2B genotype allows for prediction of tumor risk in congenital central hypoventilation syndrome. American Journal of Human Genetics, 76(3), 421–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weese-Mayer DE, Berry-Kravis EM, Ceccherini I, Keens TG, Loghmanee DA, Trang H, & ATS Congenital Central Hypoventilation Syndrome Subcommittee. (2010). An official ATS clinical policy statement: Congenital central hypoventilation syndrome: Genetic basis, diagnosis, and management. American Journal of Respiratory and Critical Care Medicine, 181(6), 626–644. [DOI] [PubMed] [Google Scholar]
- Weese-Mayer DE, Pallavi PP, Carroll MS, & Rand CM (2011). Congenital central hypoventilation syndrome In Kothare SV & Kotagal S (Eds.), Sleep in childhood neurological disorders (pp. 295–312). New York, NY: Demos Medical Publishing. [Google Scholar]
- Weese-Mayer DR, Zhou A, Reineke P, Speare V, Dunne E, Niewijk G, ... Jennings LJ (2017). PHOX2B non-polyalanine repeat expansion mutations in congenital central hypoventilation syndrome (CCHS): Advancing understanding of phenotype by mutation type through industry-academic medicine collaboration. American Journal of Respiratory and Critical Care Medicine, 195(IC), A2571. [Google Scholar]