Abstract
Rice is the staple food for more than half of the world's population, but selenium (Se) is low in many rice growing countries. Water management model affects rice soil pH and Eh, and then affects the bioavailability of Se in soil. A pot experiment was conducted to investigate the effects of water management on soil Se species, dynamics and selenium uptake by rice plants. Sodium selenite was added to the soil so that the soil selenium content reached 0.5 mg kg−1 to study the effects of 3 different water management modes on soil selenium uptake by rice plants. These three modes are flood irrigation (F), aerobic irrigation (A) and alternate flood and aerobic irrigation (AFA). The results showed that flooded irrigation treatment increased the soil soluble selenium concentration, and the selenium in soil solution mainly existed in the form of selenite and selenomethionine selenium oxide. The content of selenium in grain was 2.44 and 1.84 times that of flooded irrigation treatment under A and AFA respectively. The content of selenium in straw was 1.32 and 1.58 times that of flooded treatment under A and AFA respectively. After rice grain enzyme hydrolysis, HPLC-ICP-MS analysis showed that Selenomethionine was the main selenium speciation in rice grains. This study showed that aerobic flooded treatment is one of the most effective ways to increase selenium content in rice field.
Keywords: Water management, Se, Rice
1. Introduction
Selenium (Se) is an essential micronutrient in human body, and a component of glutathione peroxidase (GSH-Px) system, whose antioxidant capacity is more than 200 times that of vitamin E, which can reduce excessive oxidative damage and immune damage to the human body by free radicals (Taneja, 2016, Halim et al., 2017). Selenium plays a very important role in maintaining the immune system of human health and reducing the risk of cancer (Wallace et al., 2009).
Soil selenium content varies greatly in China. The content of selenium in soil of Enshi in Hubei is the highest, which can reach 45.5 mg kg−1. According to the Soil Selenium Environmental Quality Standard of China, more than 72% of the areas in China are low selenium areas, but the content of available selenium in soil is very low, accounting for about 1.7%–5.5% of the total selenium content. The plant availability of selenium in soil is very low (Li et al., 2016). It is the most ideal strategy to increase selenium in human beings and the fundamental way to control selenium nutrition from the source if it is possible to control the bioavailability of selenium in soil and increase the content of selenium in rice plants by reasonable agronomic measures (Navarro-Alarcon and Cabrera-Vique, 2008).
Irrigation is one of the main measures for rice production. With the shortage of available water resources, the water management in rice cultivation has become the focus of agricultural research. In recent years, to reduce the rice irrigation water, various water management modes (such as alternate wetting drying irrigation and soil wetting irrigation) have been developed and promoted, which can not only reduce irrigation water consumption, but also become an important measure for high yield and high efficiency agriculture of rice (Mostafazadeh-Fard et al., 2011). Based on increasing rice yield and rice quality, these water management modes reduce irrigation water use and reduce fertilizer use, and have good environmental, ecological and economic benefits (Hamad et al., 2017).
Water management has great influence on the redox potential (Eh) and pH of rice fields, which may eventually control the selenium species and bioavailability in rice fields. The chemical forms of soil selenium are mainly selenate (+6 valence), selenite (+4 valence), elemental selenium (0 valence), selenide (−2 valence) and organic selenium. The Eh and pH play a major role in the chemical transformation of soil selenium forms (Masscheleyn et al., 1990). Therefore, scientists are trying to find an economical and effective way to increase selenium content in rice grains. Some available data show that in low selenium soil, compared with aerobic dry farming, the whole growth period flooding cultivation can significantly increase the Se content in rice stem and grain (Li et al., 2010), which may be because the iron oxide/hydroxide reduction dissolution in the flooded soil can release the adsorbed metal/nonmetal ions into the soil liquid phase (Grybos malgorzata et al., 2007). So, how water management affects soil selenium bioavailability, and then affects selenium content in rice grain, and the specific mechanism is still unknown. Therefore, 3 kinds of water management mode are set in this study: flood irrigation (Control), aerobic irrigation and alternate flooding and aerobic irrigation to analyze the change of soil redox potential and the variety and concentration of selenium in soil solution under different water management modes. How does the water management mode affect the allocation and accumulation of selenium in different organs of rice plants in low selenium soil? It is expected to select effective water management measures to promote the transfer of Se in soil to rice (Noor and Ashraf. 2017).
2. Materials and methods
2.1. Experimental soil
The neutral purple soil with the largest distribution in Sichuan Pot and the low phosphorus soil of 20–40 cm in the experimental field of Southwest University, Beibei District, Chongqing city was collected. The soil was dried to remove organic matter, debris and debris, and sieved over 2 mm after grinding for use. The tested purple paddy soil, pH 5.12, had organic matter 21.3 g/kg, total nitrogen 0.38 g kg−1, total phosphorus 0.35 g kg−1, alkali hydrolyzable nitrogen 83.65 mg kg−1, available phosphorus 7.5 mg kg−1 and available potassium 97.8 mg kg−1. To facilitate the study, the total soil selenium reached 0.50 mg/kg by adding sodium selenite. The tested rice variety was Yuxiang 203.
2.2. Experiment design
Rice seeds were sterilized with 15% sodium hypochlorite for 15 min, washed with deionized water and sown in selenium free sand cultures, cultured in greenhouse (with 28/14 h light and 20/10 h dark, illumination intensity 260–350 Mu mol m−2 s−1, and relative humidity 60%–70%). Rice seedlings grew in quartz sand for 30 d. Rice seedlings were transplanted when they were about 15 cm. The soil was packed two weeks before transplanting 10 kg per pot. 0.29 g ammonium phosphate, 0.20 g urea, 0.22 g potassium carbonate as base fertilizer were applied per kg. Irrigation was made after base fertilizer and soil were mixed evenly. After transplanting rice plants, different water management measures were carried out: (1) Flooded treatment (F): During the whole growth period of rice plants, the water in the pot remains about 2.0 cm; (2) Aerobic treatment (A): Paddy topsoil remained free from water, and soil moisture content remained at around 35%; (3) Alternate flood and aerobic treatment (A-F-A): Irrigation kept the surface water layer at about 2 cm, and then fell naturally to the surface soil without aquifer. Now, the soil moisture content was about 35%, and irrigation was made again until the surface water layer was about 2 cm, and then cycled until the rice plants were mature. Each water management mode was set up 4 times, a total of 12 pots of rice were planted in a solar greenhouse in randomized blocks. Redox (Eh) and pH values of soil were measured at the 10th, 30th, 60th and 90th days respectively, and the soil solution was extracted at the 10th and 60th days, for determining the contents of selenium, iron, manganese and selenium in soil solution. Rice plants were harvested after ripening. The biomass and Se content of root, stem and leaf, rice husk and brown rice, as well as selenium form of brown rice were determined.
2.3. Analytical method
Soil Eh was measured using a pH meter (pHS-3C). The saturated calomel electrode and a platinum electrode were inserted into the soil surface at 2 cm, read after numerically stable. It was repeated 3 times to get the average.
A porous cup sampler was used to extract 20 ml soil solution, with 1 mL EDTA solution (0.1 mol L−1) added in advance to prevent the transformation of Se with different valence states. After reaching 10 mL, the solution was filtered with a syringe-driven filter with the specification of 0.45 m, and kept at 0–4 °C for determination. The morphology of Se in soil solution was determined by high performance liquid chromatography inductively coupled plasma mass spectrometry (HPLC/ICP/MS) (Agilent 1200, Agilent, Technology, Agilent, 7500, USA) (Li et al., 2008, Tavakoli et al., 2016).
Determination of iron and manganese content in soil solution: 20 ml soil solution was extracted with a porous cup sampler. Each time the soil solution was extracted, it was immediately divided into two parts. 5 ml of soil solution was taken and diluted to 10 ml with 10% nitric acid for the determination of total selenium as well as iron and manganese content in soil solution. The remaining soil solutions were directly determined by atomic fluorescence spectrophotometer for selenium and pH (Li et al., 2016, Samad et al., 2017).
Methods for determination of Se content in various parts of rice followed the methods of Zhang et al., and selenium content in solution to be determined after digestion was determined by AF-610A atomic fluorescence spectrometry. Determination conditions: PMT voltage of 280 V; HCl full cathode current of 80 Ma; carrier gas flow rate of 800 mL min−1; sample volume of 1 mL; atomizer height of 7 mm, room temperature; sampling pump of 100 r min−1; sampling time of 18 s; pump stop time of 5 s. Soil samples (GBW07403) and blank samples were digested simultaneously, and selenium recovery was 90%–105% (Zhang et al., 2006).
2.4. Statistical analysis
Excel 2003 and SPSS 18 software were used for statistical analysis and drawing. Duncan statistical method was used for significance analysis.
3. Result analysis
3.1. Dynamic changes of soil Eh and pH under different water management
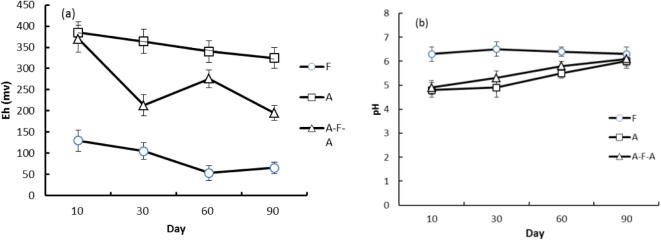
Compared with A, the soil redox potential was significantly reduced by F and AFA, but the soil pH increased (Fig. 1a and b). Soil Eh range was 325–385 mV, F had the lowest Eh, the value range was 53.3–130 mV. The AFA range was 125–370 mV, respectively, and about 300 mV for F and A. During the whole rice growth period, the pH of the soil solution was maintained at about 6.3, the pH values of A and AFA were 0.9–1.5 units lower than that of F. At the end of the test, the pH of the three treatments was basically the same.
Fig. 1.
Dynamic changes of soil Eh (a) and pH (pH) in the culture cycle.
3.2. Dynamic changes of selenium in soil solution under different water management
Different water management significantly affected the total selenium content in soil solution, and the selenium content of F soil solution was 3.0–4.4 and 1.5–4.3 times of A and AFA, respectively. With the pass of sampling time, the content of selenium in F soil solution decreased sharply. Selenium in A soil solution decreased slowly, while selenium in AFA soil solution increased first and then decreased.
Analysis of selenium species in soil solution by HPLC-ICP-MS revealed three kinds of selenium species, including sodium selenite, sodium selenate and SeOMet, and different selenium species in different soil management soil solutions. SeOMet was only found at F10d sampling, and no SeOMet was found at 60d sampling. In the 10d sampling A and AFA soil solution, there was a higher amount of sodium selenate, significantly higher than F; At 60d sampling, selenium contains only sodium selenate in A and AFA soil solutions.
3.3. Dynamic change of iron and manganese in soil solution under different water management
It can be seen from Fig. 4 that the concentration of Fe in soil solution increased first and then decreased under the condition of continuous flooding, and the maximum concentration of Fe was at 30d. The concentration of Fe in soil solution is higher under the condition of flooding, while gradually reducing to stabilize with the passage of time; while Fe concentration remained at a very low level under A and AFA.
Fig. 4.
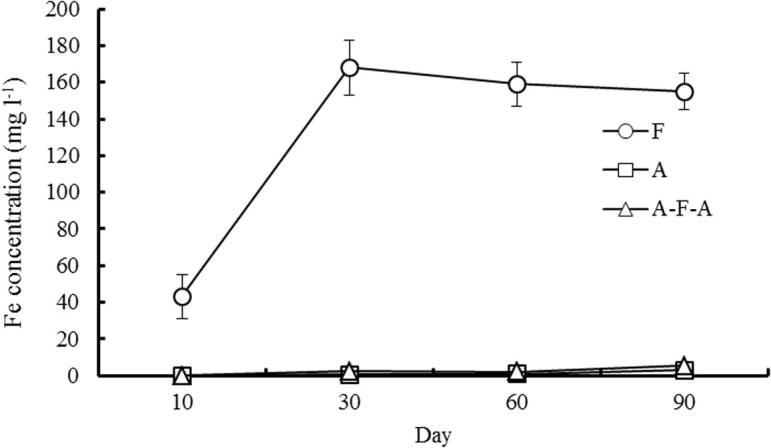
Changes of Fe concentration in soil solution under different water management.
The trend of Mn concentration in soil solution is basically the same as that of Fe, and it can be seen from Fig. 5 that the concentration of Mn in soil solution gradually increased after continuous flooding, and the concentration of Mn in soil solution was higher under the condition of flooding, while gradually reducing to stabilize with the passage of time; while Mn concentration remained at a very low level under A and AFA.
Fig. 5.
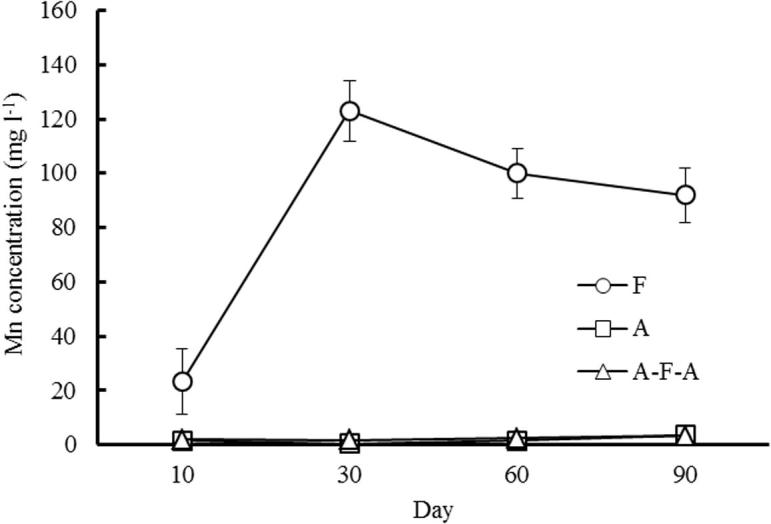
Changes of Mn concentration in soil solution under different water management.
3.4. Effects of different water management on selenium content and selenium forms in rice grains
Rice grew better in the aerobic than in the flooded treatments and A-F-A treatments, Rice grew better in the aerobic than in the F and A-F-A treatment, A rice grain yield and straw weight were significantly higher than the F and A-F-A treatment.
The water management measure significantly affected the grain selenium content (P < 0.01). The selenium content of F and AFA were 2.44 and 1.84 times of that of F, respectively. The selenium contents of F and AFA stalks were 1.32 and 1.58 times of F (Fig. 7), respectively. It can be seen that A is very important for improving the grain selenium content in rice.
Fig. 7.
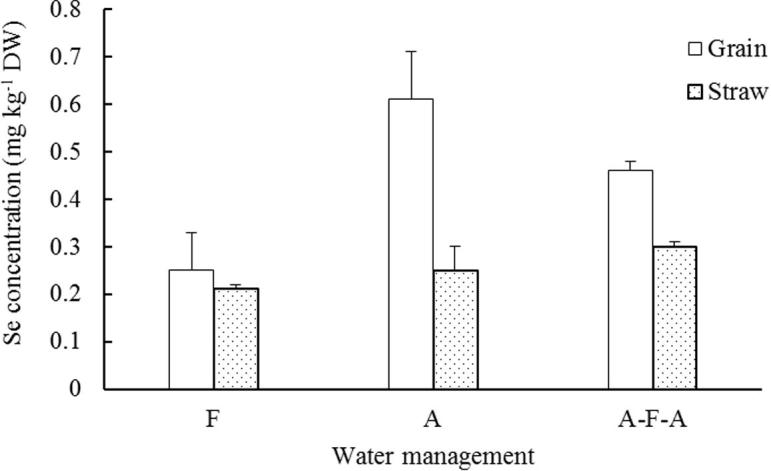
Effects of different water treatments on selenium content in grain and straw of rice.
After the rice seeds were hydrolyzed by enzyme, the contents of ASeMet were higher than those of F and AFA by HPLC-ICP-MS. SeMet is the main selenium type of rice grain, accounting for 63.0% −72.9% of total selenium, in fact it is MeSeCys, MeSeCys accounting for 5.0% −5.3% of the total selenium (Table 1).
Table 1.
Selenium speciation in rice grain (Determined by Enzymatic Hydrolysis and HPLC-ICP-MS) as influenced by water management.
| Treatment | SeMet determined by HPLC-ICP-MS (mg of Se kg−1) | MeSeCys determined by HPLC-ICP-MS (mg of Se kg−1) |
|---|---|---|
| F | 0.18 ± 0.02 b | 0.01 ± 0.00 a |
| A | 0.40 ± 0.04 a | 0.03 ± 0.00 a |
| A-F-A | 0.29 ± 0.02 b | 0.02 ± 0.00 a |
4. Discussion
Soil Eh and pH are the key factors affecting the selenium valence and its solubility in paddy soil. It can be seen from Fig. 1, Fig. 2 that the concentration of selenium in soil solution decreased with the decrease of soil Eh, whether it is continuous flooding or intermittent F. Li Huiyong et al. have shown that when the soil Eh is below 147 mV, about 90% of the soil solution Se remained active, with about 10% selenite, and under 450 mV (high Eh value) conditions, selenate valence state was stable, 57.2% −83.5% of external addition of selenate maintained selenate (Li et al., 2001). Xiong Yuanfu and other studies have shown that the majority of paddy soil selenium is less effective, may be relevant to the low Eh, original form of selenium (Xiong et al., 1999). This is consistent with our conclusion, the soil Eh in the A range is 325–385 mV, AFA range is 125–370 mV, respectively, and Eh under F was the lowest, the value of 53.3–130 mV. Eh under A and AFA paddy soil is higher than under F, soil selenate mainly was selenate form, more conducive to the absorption of rice for the use of selenium.
Fig. 2.
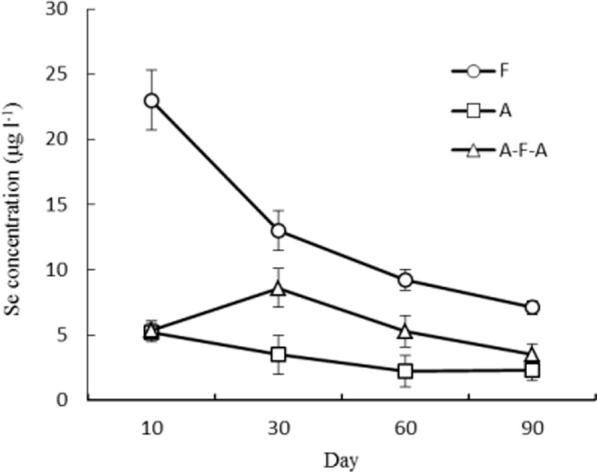
Dynamic changes of selenium content in soil solution during culture time.
Plant root uptake and nutrient mainly comes from soil solution, and the selenium species in soil pore water largely control the bioavailability of plant selenium. The study also carried out the analysis of selenate, selenite, SeMet and Seomet (Fig. 2, Fig. 3). Stroud et al. found that the selenium species quantified using HPLC-ICP-MS was only 17–48% of the dissolved selenium extracted with 0.016 M KH2PO4. This shows that some of the selenium and dissolved organic matter combined to form organic selenium and were not determined, the total amount of various selenium species in the solution is lower than that of the dissolved selenium. Water management greatly affects the selenium species and selenium concentrations in soil solutions (Fig. 2, Fig. 3), the selenium and selenium species and concentrations in the soil solution decreased after 60 days of transplanting (Shahidah et al., 2017). The reason for the decrease was mainly due to plant uptake, microbial assimilation, or soil adsorption. Selenites are easily adsorbed by soil solid phase, such as soil iron hydroxide oxides (Fendorf et al., 2008, Sadeghi et al., 2016), this results in the conversion of a portion of the selenite in the soil solution to other insoluble forms (eg. elemental Se or metal-selenide precipitates). Gammelgaard B and other studies suggest that selenite is the main form of soil inorganic selenium in paddy soil, which can be used for rice, but it is less soluble in paddy soil and accumulate in iron-rich and manganese-rich iron layer or profile (Gammelgaard et al., 2011). From Fig. 2, Fig. 4, Fig. 5, the soil is in a state of reducing water and the soil is in a reduced state, and the concentration of selenium in the soil solution is low and the content of Fe and Mn is higher (See Fig. 6). From the trend of change, the concentration of iron and manganese in soil solution increased steadily after the initial period, and there was a negative correlation between the change of iron and manganese concentration and the change of selenium concentration in soil solution.
Fig. 3.
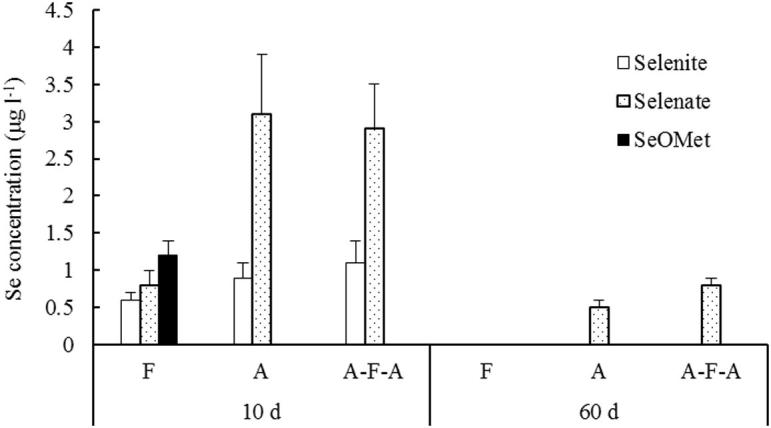
Changes of selenium in soil solution under different water management.
Fig. 6.
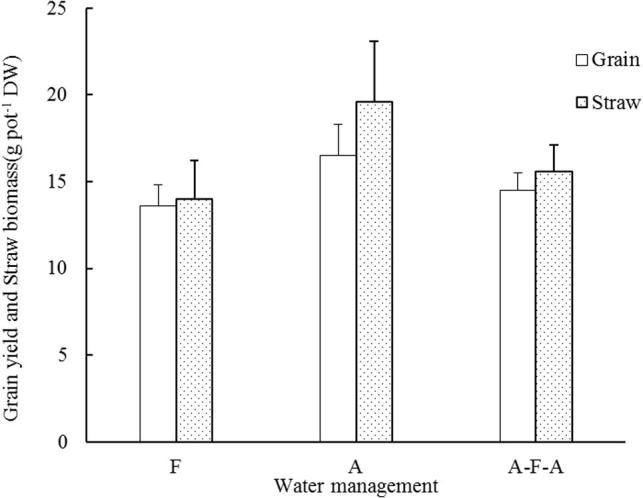
Effect of water management on grain and straw weight of rice.
Compared to A and AFA, F increases the selenium concentration in soil pore water, which has two explanations why flooding increases soil selenium solubility. First, the flooding of the soil results in the dissolution of the ferric hydroxide oxides, thereby reducing the adsorption of the selenite on it, since ferric hydroxide is a very important adsorbent for selenite (Balistrieri and chao, 1990). The iron content in the flooded soil solution is much higher than that in the moist soil, which is about 2 times (Fig. 4). Secondly, more organic matter in the flooded soil is dissolved in the soil pore water than the moist soil, which may bring more organic selenium species into the liquid phase. Partial SeOMet was also found in the flooded soil pore water, but was almost absent in aerobic soil and alternate flood and aerobic soil (Fig. 3). However, the flooding resulted in an increase in selenium content in soil pore water, but did not lead to higher selenium uptake by rice, whereas the content of selenium was lower (Fig. 7). This may be since some selenites are oxidized to selenate under moisturizing and AFA conditions, and selenate has higher bioavailability than selenite because of the low binding of selenate to soil solid phase (Barrow and Whelan, 1989), which is easily transported from the roots of rice to the shoots (Li et al., 2008).
Rice grain selenium is very important for the nutritional value of rice (Rayman et al., 2008, Gao et al., 2017). Our study confirmed that the major selenium species in rice grains is SeMet (Table 1). Li et al. also showed that the major selenium species is SeMet, whether potted and field trials, or rice seeds collected from selenium-enriched region in Enshi, China. Moreover, rice assimilation of inorganic selenium into SeMet was not affected by water management, and potted trials of rice had no more than 5 mg/kg selenium (Li et al., 2010). Fang et al. showed that selenium species mainly include inorganic selenium, SeCys2 and Seomet after enzymatic hydrolysis of rice seeds, but no SeMeSeCys (Fang et al., 2009). Cubadda et al. reported that there was a very small amount of SeMeSeCys in wheat kernels (Cubadda et al., 2010). The content of total selenium in rice was higher, and the content of SeMet in grain was also higher, and the content of A SeMet was higher than that of F and AFA. This also means that A cannot only significantly improve the grain selenium content in rice, but also with high content of SeMet in the grain, which shows that reasonable water management measures of rice fields have great practical significance in strengthening the human selenium nutrition from the source.
5. Conclusion
Soil Eh and pH are important factors to control the selenium uptake by rice. Compared with A, soil redox potential of F and AFA decreased significantly, but soil pH was increased. The total selenium concentration of soil F was significantly higher than that of A and AFA. However, the selenium form of F is mainly in the organic form, while sodium selenate form in A and AFA soil solutions. The iron and manganese contents in the F soil solution were higher, the soil selenite is easy to be fixed, which may have a great relevance with the significant reduce of soil solution selenite content. The contents of selenium and selenium in A and AFA grains were significantly higher than those in F, and Selenomethionine was the main form of selenium in rice. A cannot only improve the grain selenium content in rice, but also with higher content of SeMet in grains, which indicates that water management can be adopted in rice production, which is very important for biologically strengthening human selenium nutrition from the source.
Acknowledgments
This study is financially supported by The National Natural Science Foundation of China (No.31372141; 31672238) and The National Key Research and Development Program of China (The transformation and high efficiency utilization of phosphorus fertilizer in soil-plant system).
Footnotes
Peer review under responsibility of King Saud University.
References
- Balistrieri L.S., Chao T.T. Adsorption of selenium by amorphous iron oxyhydroxide and manganese dioxide. Geochim. Cosmochim. Acta. 1990;54:739–751. [Google Scholar]
- Barrow N.J., Whelan B.R. Testing a mechanistic model. 7. The effects of pH and of electrolyte on the reaction of selenite and selenate with a soil. J. Soil Sci. 1989;40:17–28. [Google Scholar]
- Cubadda F., Aureli F., Ciardullo S., D’Amato M., Raggi A., Acharya R., Reddy R.A.V., Prakash N.T. Changes in selenium speciation associated with increasing tissue concentrations of selenium in wheat grain. J. Agric. Food Chem. 2010;58:2295–2301. doi: 10.1021/jf903004a. [DOI] [PubMed] [Google Scholar]
- Fang Y., Zhang Y.F., Catron B., Chan Q.L., Hu Q.H., Caruso J.A. Identification of selenium compounds using HPLC-ICPMS and nano-ESI-MS in selenium-enriched rice via foliar application. J. Anal. At. Spectrom. 2009;24:1657–1664. [Google Scholar]
- Fendorf S., Herbel M.J., Tufano K.J., Kocar B.D. Biogeochemical processes controlling the cycling of arsenic in soils and sediments. Biophys.-Chem. Process. Heavy Metals Metalloids Soil Environment. 2008:313–338. [Google Scholar]
- Gammelgaard B., Jackson M.I., Gabel-Jensen C. Surveying selenium speciation from soil to cell-forms and transformations. Analyt. Bioanalyt. Chem. 2011;399:1743–1763. doi: 10.1007/s00216-010-4212-8. [DOI] [PubMed] [Google Scholar]
- Gao W. The first multiplication atom-bond connectivity index of molecular structures in drugs. Saudi Pharmaceut. J. 2017;25(4):548–555. doi: 10.1016/j.jsps.2017.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grybos malgorzata S., Davranche M., Gruau G., Petitjean P. Is trace metal release in wetland soils controlled by organic matter mobility or Fe-oxyhydroxides reduction? J. colloid Interface Sci. 2007;314:490–501. doi: 10.1016/j.jcis.2007.04.062. [DOI] [PubMed] [Google Scholar]
- Halim A.A., Sidi S.F.A., Hanafiah M.M. Ammonia removal using organic acid modified activated carbon from landfill leachate. Environment Ecosystem Sci. 2017;1(1):28–30. [Google Scholar]
- Hamad J.R.J., Hanafiah M.M., Abdullah S. Problems and current practices of solid waste management in the city of almarj, Libya. J. CleanWAS. 2017;1(1):01–05. [Google Scholar]
- Li H.F., Lombl E., Stroud J.L., McGrath S.P., Zhao F.J. Selenium speciation in soil and rice:influence of water management and se fertilization. Agricult. Food Chem. 2010;58:11837–11843. doi: 10.1021/jf1026185. [DOI] [PubMed] [Google Scholar]
- Li H.F., McGrath S.P., Zhao F.J. Selenium uptake, translocation and speciation in wheat supplied with selenate or selenite. New Phytol. 2008;178:92–102. doi: 10.1111/j.1469-8137.2007.02343.x. [DOI] [PubMed] [Google Scholar]
- Li, H.Y., Wang, R.W., Yang, Z.H., Liu, J.G., Ge, D.Z., 2001. Valence State Transformation of Added 0215-217 (In Chineses).
- Li J., Peng Q., Liang D.L. Effects of aging on the fraction distribution and bioavailability of selenium in three different soil. Chemosphere. 2016;144:2351–2359. doi: 10.1016/j.chemosphere.2015.11.011. [DOI] [PubMed] [Google Scholar]
- Masscheleyn P.H., Delaune R.D., Patrick W.H. Transformations of selenium as affected by sediment oxidation reduction potentail and pH. Environ. Sci. Technol. 1990;24:91–96. [Google Scholar]
- Mostafazadeh-Fard B., Jafari F., Mousavi S.F. Effects of irrigation water management on yeild and water use efficiency of rice in cracked paddy soils. Australian J. Crop Sci. 2011;4(3):136–141. [Google Scholar]
- Navarro-Alarcon M., Cabrera-Vique C. Selenium in food and the human body: a review. Sci. Total Environ. 2008;400:115–141. doi: 10.1016/j.scitotenv.2008.06.024. [DOI] [PubMed] [Google Scholar]
- Noor M.J., Ashraf M.A. Accumulation and tolerance of radio cesium in plants and its impact on the environment. Environment Ecosys. Sci. 2017;1(1):13–16. [Google Scholar]
- Rayman M.P., Infante H.G., Sargent M. Food-chain selenium and human health: spotlight on speciation. Br. J. Nutr. 2008;100:238–253. doi: 10.1017/S0007114508922522. [DOI] [PubMed] [Google Scholar]
- Sadeghi E., Barkhordar S. Determination of zearalenone levels in consumed rice samples in iran by high performance liquid chromatography (2015) Acta Medica Mediterranea. 2016;32(5):1945–1949. [Google Scholar]
- Samad M.N.S.A., Hanafiah M.M., Hasan M.J.A., Ghazali N.F., Harun S.N. Ratio of water withdrawal to availability in Kelantan watersheds Malaysia. J. CleanWAS. 2017;1(1):40–44. [Google Scholar]
- Shahidah N., Hasnah S., Shuhaili S., Syamzany A., Mohd Shukri M.A. Indoor airborne bacteria and fungi atdifferent background area in nurseries and day care centres environments. J. CleanWAS. 2017;1(1):36–39. [Google Scholar]
- Taneja S.S. Difference in association of obesity with prostate cancer risk between US African American and Non-hispanic white men in the selenium and Vitamin E Cancer Prevention Trial (SELECT) J. Urology. 2016;195(3):627–628. doi: 10.1016/j.juro.2015.12.017. [DOI] [PubMed] [Google Scholar]
- Tavakoli H.R., Farsani M.K. A comparative evaluation on the varieties of imported and cultivated high consumption rice in iran in terms of contamination by heavy metals (lead, cadmium and arsenic) and its associated health risk. Acta Medica Mediterranea. 2016;32(4):1543–1547. [Google Scholar]
- Wallace K., Kelsey K.T., Schned A., Morris J.S., Andrew A.S., Karagas M.R. Selenium and risk of bladder cancer: a population-based case-control study. Cancer Prev. Res. 2009;2:70–73. doi: 10.1158/1940-6207.CAPR-08-0046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong Y.F., Li H.Y., Liu J.G., Liu C.M. Study on valence state transformation and solubility of selenium in paddy soils. Environmant. Chem. 1999;18(4):338–342. [Google Scholar]
- Zhang L.H., Shi W.M., Wang X.C. Difference in selenite absorption between high- and low- selenium rice cultivars and its mechanism. Plant Soil. 2006;282(1):183–193. [Google Scholar]