Abstract
Pterocarpus is often used to make high-grade furniture, with beneficial health functions on the human body. Therefore, this article with large fruit red sandalwood as an example, to explore its extract on the human body beneficial health care ingredients. FT-IR analysis, in the 2855–3421 cm−1 wave segment, ethyl acetate after extraction of large fruit red sandalwood powder infrared transmittance increased the maximum value; GC–MS analysis, large fruit red sandalwood in the human body with a cough and phlegm, detoxification and enhance human immunity and other effects. Among them, Homopterocarpin in the inhibition and killing of cancer cell activity outstanding performance. Cryptomeridiol is a natural product with anti-Alzheimer's disease and antispasmodic properties, with significant medicinal value.
Keywords: Pterocarpus, Pterocarpus Macarocarpus Kurz, FT-IR, GC–MS, Extractives, Health care ingredients
1. Introduction
Pterocarpus Macrocarpus Kurz mainly grows in Myanmar, Laos and Thailand, belonging to the butterfly-shaped flower Pterocarpus, Rosewood mahogany. Pterocarpus Macrocarpus Kurz is a deciduous tree species and it has strong adaptability to growth environment, with the litter of the leaves to deal with drought or low temperature growth conditions (Azratul et al., 2017). When the growth environment is appropriate, deciduous period will become shorter and the grain growth rate will increase, Such as Pterocarpus Macrocarpus Kurz has straight trunk, branches few in hot and humid summer and it is excellent wood tree species (Francis, 2000).
The application value of Pterocarpus Macrocarpus Kurz lies in heartwood. Pterocarpus Macrocarpus Kurz heartwood is reddish-brown, texture clear and diverse, moreover it can give people visual enjoyment and psychological pleasure. It is more important that the Pterocarpus Macrocarpus Kurz heartwood has a high density, large hardnes and a unique woody flavor (Wisittipanich et al., 2012). Therefore, Pterocarpus Macrocarpus Kurz in life commonly used to create high-grade mahogany furniture, sculpture art and musical instruments and so on. Some of the ingredients in the wood aroma can improve the function of the human spleen through the respiratory organs or skin contact, so it play a role in promoting human blood circulation and enhancing the body's immune function (Li et al., 2009, Jalal et al., 2017)). For example, it has an impact on human behavior that essential oil extracted and concentrated in the mahogany wood volatilize gases, stimulating the brain through the olfactory nerve. These effects include regulating human emotions, enhancing physical strength, improving memory and so on. Therefore, the compounds or functional groups before and after extraction of three kinds of extractants were analyzed by FT-IR spectroscopy. The active molecules of the three extracts are analyzed by GC–MS to further explore the health function of the Pterocarpus Macrocarpus Kurz.
2. Materials and method
2.1. Materials
Pterocarpus Macrocarpus Kurz that used Experiments produced and exported in Myanmar. Pterocarpus Macrocarpus Kurz must be crushed, and then the powder is dried. The methanol, ethyl acetate, ethanol/benzene used in the experiment are the chromatographically pure. Quantitative filter paper should be extracted with ethanol/benzene (1:1 by volume) solution for 12 h. The three extractants used in the experiment were methanol, ethyl acetate and 1:1 volume ratio of ethanol/benzene solution.
2.2. Experimental methods
2.2.1. Extraction process
After drying process, the Pterocarpus Macrocarpus Kurz powder weigh 3 parts and the quality of 15 g respectively (accuracy of 1.0 mg). They were placed in three round bottom flasks with solid mass/liquid volume = 1:20. Next 300 ml of methanol, ethyl acetate and ethanol/benzene solution (1:1 by volume) were added and then refluxed at 65 °C to 80 °C for 6 h. The obtained extract was subjected to suction filtration on a circulating water type vacuum pump using a quantitative filter paper subjected to an ethanol/benzene extraction treatment for 12 h. Finally, the extract that obtained by suction filtration was steam-concentrated by a rotary evaporator.
2.2.2. FT-IR analysis
The powder of the Pterocarpus Macrocarpus Kurz and the powder after the reflux of the three kinds of extracts were subjected to FT-IR detection (Thermo Fisher Nicolet, 670FT-IR). Scanning of each powder was collected at a spectral resolution of 4 cm−1 and the spectral range was 400–4000 cm−1.
2.2.3. GC–MS analysis
The extracts obtained from the three extractants were analyzed using a gas chromatography-mass spectrometer (Agilent GC–MS 7890B 5977). Elastic quartz capillary column DB-5MS (30 m × 250 μm × 0.25 μm). The carrier gas used was high purity helium and the constant flow rate of helium was 1 mL/min. Split injection ratio was 20:1. The temperature of the GC started at 50 °C, raised to 170 °C at a rate of 20 °C/min for 3 min and then raised to 200 °C at 5 °C/min for 5 min and then to 280 °C at 10 °C/min for 20 min. MS program scanned quality range of 30–600 amu, ionization voltage of 70 eV, ionization current of 150 μA (EI). The ion source and the quadrupole temperature were set at 230 °C and 150 °C respectively.
3. Results and discussion
3.1. The changes in functional groups after extraction
As shown in Fig. 1, Fig. 2, Fig. 3, there is a comparison of the infrared spectra of Pterocarpus Macrocarpus Kurz powder and Pterocarpus Macrocarpus Kurz powder refluxed with methanol, ethyl acetate and ethanol/benzene solution. The infrared spectra of 3421 cm−1, 3415 cm−1 and 3405 cm−1 are phenolic compounds, acid compounds and alcohol compounds in the —OH stretching vibration (Li et al., 2015, Noor and Ashraf, 2017). The infrared spectrum of 2924 cm−1, 2855 cm−1, 1462 cm−1, 1458 cm−1, 1382 cm−1, 1386 cm−1 are the stretching vibration of methylene and methyl (Lu et al., 2005). The infrared spectra of 1600 cm−1, 1510 cm−1 and 1330 cm−1 are the peak band of co-phenolic compounds (Rezaee et al., 2006, Thaldiri et al., 2017). The absorption peak at 1270 cm−1 is the G-ring telescopic vibration, the carbon-oxygen double bond stretching vibration (Paganelli et al., 1991); The infrared spectrum of 1220 cm−1, 1125 cm−1, 1030 cm−1 and 878 cm−1 is O—C—O—CHCH2— on the carbon-oxygen single bond stretching vibration, C—C single bond stretching vibration, aromatic compounds deformation bending vibration of carbon–hydrogen of aromatic compounds bonds on a certain plane, the β-bond absorption vibration of the cellulose-based compound (Evans et al., 2009, Dekkers et al., 2010).
Fig. 1.
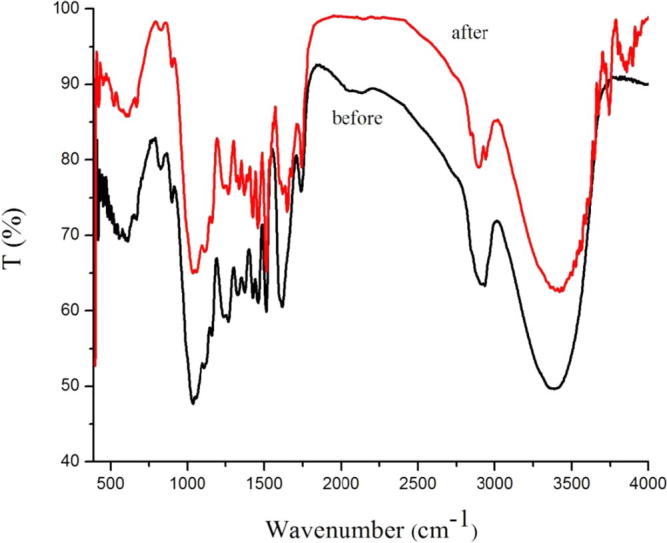
FT-IR spectrums of Pterocarpus macarocarpus Kurz before and after methanol extraction.
Fig. 2.
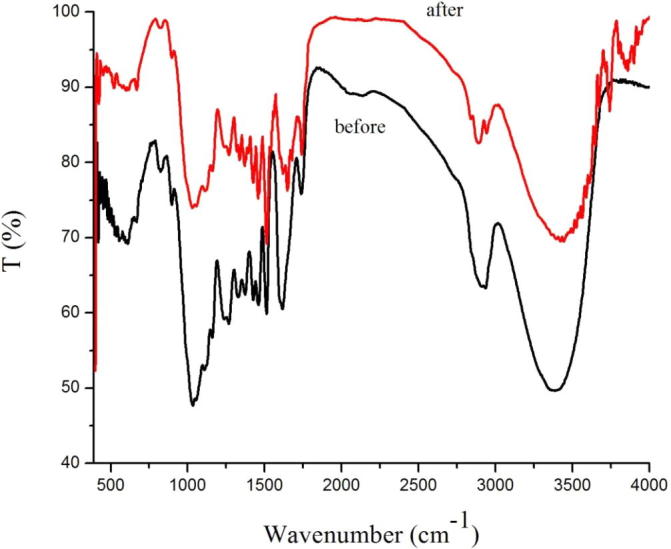
FT-IR spectrums of Pterocarpus macarocarpus Kurz before and after Ethyl acetate extraction.
Fig. 3.
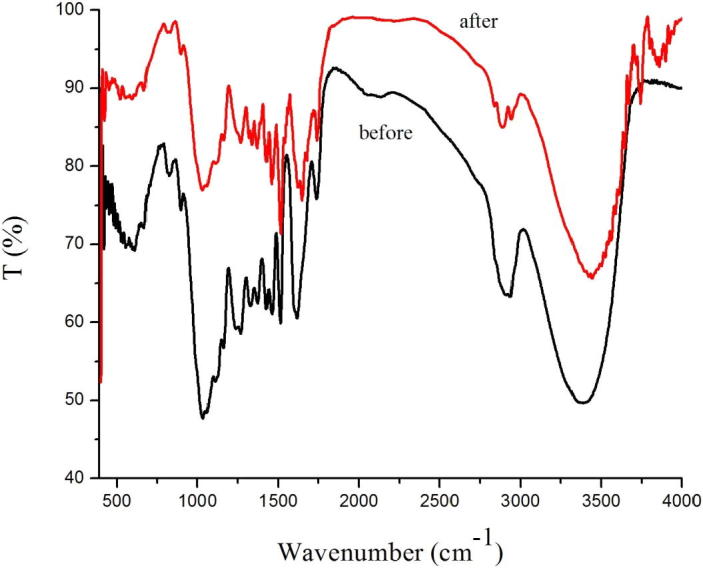
FT-IR spectrums of Pterocarpus macarocarpus Kurz before and after Ethanol/benzene solution extraction.
In Fig. 1, we can see the change of the peak transmittance of the Pterocarpus Macrocarpus Kurzby methanol was as follows: the infrared transmittance increases from 50.3% to 62.6% at 3421 cm−1, 3415 cm−1 and 3405 cm−1. The infrared transmittance increased from 63.6% to 79.4% at 2924 cm−1 and 2855 cm−1. The infrared transmittance increased from 62.6% to 73.2% at 1462 cm−1, 1458 cm−1, 1382 cm−1, 1386 cm−1. The infrared transmittance increased from 80.5% to 86.7%, 60.3% to 65.8%, and 64.2% to 80.1% at 1600 cm−1, 1510 cm−1, 1330 cm−1 respectively. The infrared transmittance increased from 59.4% to 75.7%, 60.3% to 76.5%, 79.6% to 97.7% at 1270 cm−1, 1220 cm−1, 875 cm−1 respectively. The infrared transmittance increased from 47.8% to 65.6% at 1125 cm−1, 1030 cm−1 respectively.
In Fig. 2, we can see the change of the peak transmittance of the Pterocarpus Macrocarpus Kurz by Ethyl acetate was as follows: The infrared transmittance increased from 50.3% to 70.6% at 3421 cm−1, 3415 cm−1 and 3405 cm−1. The infrared transmittance increased from 63.6% to 83.5% at 2924 cm−1 and 2855 cm−1. The infrared transmittance increased from 62.6% to 75.2% at 1462 cm−1, 1458 cm−1, 1382 cm−1, 1386 cm−1. The infrared transmittance increased from 80.5% to 90.2%, 60.3% to 68.4%, and 64.2% to 84.7% at 1600 cm−1, 1510 cm−1, 1330 cm−1 respectively. The infrared transmittance increased from 59.4% to 82.5%, 60.3% to 83.5%, and 79.6% to 98.5% at 1270 cm−1, 1220 cm−1, 875 cm−1 respectively. The infrared transmittance increased from 47.8% to 74.3% at 1125 cm−1, 1030 cm−1.
In Fig. 3, we can see the change of the peak transmittance of the Pterocarpus Macrocarpus Kurz by Ethanol/Benzene was as follows: The infrared transmittance increased from 50.3% to 66.7% at 3421 cm−1, 3415 cm−1 and 3405 cm−1. The infrared transmittance increased from 63.6% to 68.5% at 2924 cm−1 and 2855 cm−1. The infrared transmittance increased from 62.6% to 83.3% at 1462 cm−1, 1458 cm−1, 1382 cm−1, 1386 cm−1. The infrared transmittance increased from 80.5% to 88.6%, 60.3% to 71.4%, and 64.2% to 86.7% at 1600 cm−1, 1510 cm−1, 1330 cm−1 respectively. The infrared transmittance increased from 59.4% to 84.6%, 60.3% to 85.6%, and 79.6% to 96.3% at 1270 cm−1, 1220 cm−1, 875 cm−1 respectively. The infrared transmittance increased from 47.8% to 76.3% at 1125 cm−1, 1030 cm−1.
The results showed that before and after extraction of the Pterocarpus Macrocarpus Kurz powder, the infrared transmittance of each peak is different; After the different extractants are extracted, the infrared transmittance of each peak is also different.
3.2. Ingredients of the Pterocarpus Macrocarpus Kurz extraction
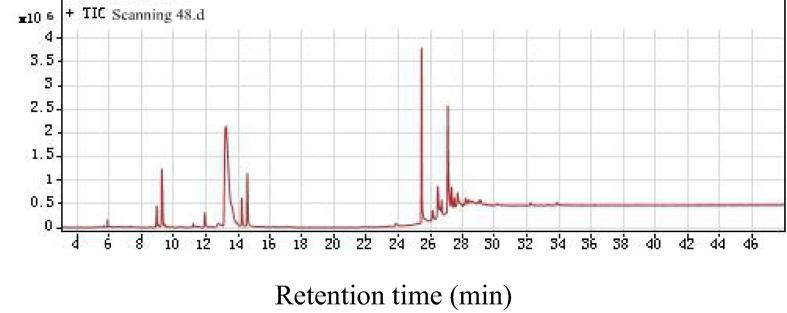
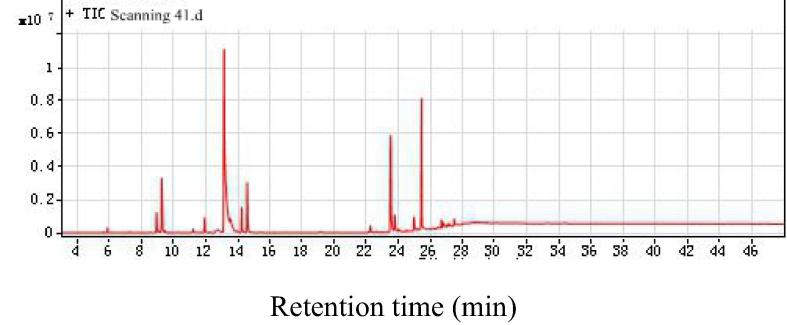
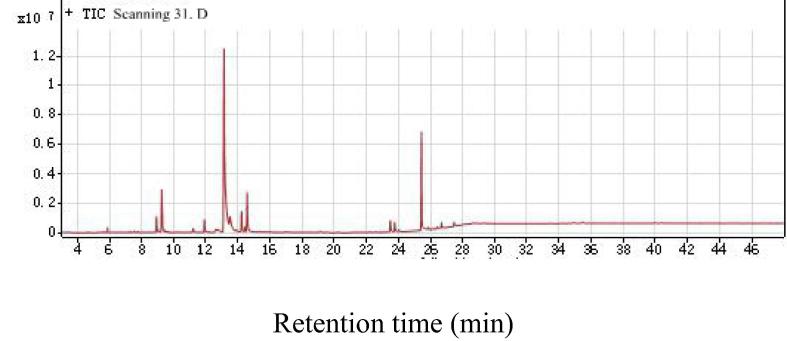
As shown in Fig. 4, Fig. 5, Fig. 6, respectively, total ion chromatograms of methanol, ethyl acetate and ethanol/benzene extractions. The GC–MS qualitative analysis technique can be used to determine the chemical composition of extract of the Pterocarpus Macrocarpus Kurz in methanol, ethyl acetate and ethanol/benzene solution. The chromatographic ion spectrum of the extract obtained by GC–MS analysis can be used to calculate the relative percentage of each component by area normalization method (Peng et al., 2014). The extracts were analyzed by GC–MS, and the NIST standard library and the available data were used to accurately determine the chemical constituents in the extract. Table 1, Table 2, Table 3 are GC–MS analysis results for different extracts.
Fig. 4.
Total ion chromatogram of methanol extract of Pterocarpus macarocarpus Kurz.
Fig. 5.
Total ion chromatogram of Ethyl acetate extract of Pterocarpus macarocarpus Kurz.
Fig. 6.
Total ion chromatogram of Ethanol/benzene solution extract of Pterocarpus macarocarpus Kurz.
Table 1.
Methanol extract of GC–MS analysis results.
| Peak number | Keep time (min) | Area percentage (%) | Chemical formula |
|---|---|---|---|
| 1 | 8.941 | 3.64 | 2-Naphthalenemethanol, 1,2,3,4,4a,5,6,7-octahydro-.alpha.,.alpha.,4a,8-tetramethyl-, (2R-cis)- |
| 2 | 9.265 | 12.17 | 2-Naphthalenemethanol, decahydro-.alpha.,.alpha.,4a-trimethyl-8-methylene-, [2R-(2.alpha.,4a.alpha.,8a.beta.)]- |
| 3 | 9.485 | 0.6 | 5-Azulenemethanol, 1,2,3,3a,4,5,6,7-octahydro-.alpha.,.alpha.,3,8-tetramethyl-, [3S-(3.alpha.,3a.beta.,5.alpha.)]- |
| 4 | 11.238 | 0.72 | Cryptomeridiol |
| 5 | 11.93 | 3.31 | (1R,4aR,7R,8aR)-7-(2-Hydroxypropan-2-yl)-1,4a-dimethyl decahydronaphthalen-1-ol |
| 6 | 12.784 | 1.88 | Alloaromadendrene oxide-(1) |
| 7 | 13.243 | 10 | (-)-Spathulenol |
| 8 | 13.909 | 1.32 | 2,3,3-Trimethyl-2-(3-methylbuta-1,3-dienyl)-6-methylenecyclohexanone |
| 9 | 25.449 | 23.94 | Homopterocarpin |
| 10 | 27.067 | 23.36 | 10,11-Dihydro-10-hydroxy-2,3,6-trimethoxydibenz(b,f)oxepin |
Table 2.
Ethyl acetate extract of GC–MS analysis results.
| Peak number | Keep time (min) | Area percentage (%) | Chemical formula |
|---|---|---|---|
| 1 | 5.888 | 0.55 | 2(3H)-Furanone, 5-butyldihydro-4-methyl- |
| 2 | 8.935 | 5.08 | 2-Naphthalenemethanol, 1,2,3,4,4a,5,6,7-octahydro-.alpha.,.alpha.,4a,8-tetramethyl-, (2R-cis)- |
| 3 | 9.258 | 16.59 | 2-Naphthalenemethanol, decahydro-.alpha.,.alpha.,4a-trimethyl-8-methylene-, [2R-(2.alpha.,4a.alpha.,8a.beta.)]- |
| 4 | 9.478 | 0.71 | 5-Azulenemethanol, 1,2,3,3a,4,5,6,7-octahydro-.alpha.,.alpha.,3,8-tetramethyl-, [3S-(3.alpha.,3a.beta.,5.alpha.)]- |
| 5 | 11.231 | 1.5 | Cryptomeridiol |
| 6 | 11.917 | 4.25 | (1R,4aR,7R,8aR)-7-(2-Hydroxypropan-2-yl)-1,4a-dimethyl decahydronaphthalen-1-ol |
| 7 | 12.7 | 1.33 | 9-Isopropyl-1-methyl-2-methylene-5-oxatricyclo[5.4.0.0(3,8)]undecane |
| 8 | 12.764 | 0.58 | (-)-Spathulenol |
| 9 | 13.146 | 10 | Tricyclo[4.4.0.0(2,7)]dec-8-ene-3-methanol, .alpha.,.alpha.,6,8-tetramethyl-, stereoisomer |
| 10 | 13.515 | 12.82 | (-)-Spathulenol |
| 11 | 13.903 | 0.6 | (4aS,7R)-7-(2-Hydroxypropan-2-yl)-1,4a-dimethyl-4,4a,5,6,7,8-hexahydronaphthalen-2(3H)-one |
| 12 | 25.456 | 27.83 | Homopterocarpin |
Table 3.
Ethanol/Benzene extract of GC–MS analysis results.
| Peak number | Keep time (min) | Area percentage (%) | Chemical formula |
|---|---|---|---|
| 1 | 5.888 | 0.62 | 2(3H)-Furanone, 5-butyldihydro-4-methyl- |
| 2 | 8.928 | 4.66 | 2-Naphthalenemethanol, 1,2,3,4,4a,5,6,7-octahydro-.alpha.,.alpha.,4a,8-tetramethyl-, (2R-cis)- |
| 3 | 9.252 | 15.01 | 2-Naphthalenemethanol, decahydro-.alpha.,.alpha.,4a-trimethyl-8-methylene-, [2R-(2.alpha.,4a.alpha.,8a.beta.)]- |
| 4 | 9.472 | 0.71 | .alpha.-acorenol |
| 5 | 11.225 | 1.37 | Cryptomeridiol |
| 6 | 11.911 | 3.9 | (1R,4aR,7R,8aR)-7-(2-Hydroxypropan-2-yl)-1,4a-dimethyl decahydronaphthalen-1-ol |
| 7 | 12.622 | 1.09 | 2-Propen-1-ol, 3-(2,6,6-trimethyl-1-cyclohexen-1-yl)- |
| 8 | 12.758 | 0.81 | (-)-Spathulenol |
| 9 | 13.133 | 10 | Tricyclo[4.4.0.0(2,7)]dec-8-ene-3-methanol, .alpha.,.alpha.,6,8-tetramethyl-, stereoisomer |
| 10 | 13.502 | 11.42 | (-)-Spathulenol |
| 11 | 14.44 | 1.24 | 1,2-Benzenedicarboxylic acid, butyl 2-methylpropyl ester |
| 12 | 25.443 | 24.42 | Homopterocarpin |
The methanol extract contained 29 peaks in the gas chromatogram of GC–MS and 10 compounds were identified from these peaks. Homopterocarpin (23.94)%, 10, 11-Dihydro-10-hydroxy-2, 3, 6-trimethoxydibenz(b, f) oxepin (23.36%), 2-Naphthalenemethanol, decahydro-.alpha., .alpha., 4a-trimethyl-8-methylene-, [2R-(2.alpha., 4a.alpha., 8a.beta.)]- (12.17%), (-)-Spathulenol (10%), 2-Naphthalenemethanol, 1, 2, 3, 4, 4a, 5, 6, 7-octahydro-.alpha., .alpha., 4a, 8-tetramethyl-, (2R-cis)- (3.64%), (1R, 4aR, 7R, 8aR)-7-(2-Hydroxypropan-2-yl)-1, 4a-dimethyl decahydronaphthalen-1-ol (3.31%), Alloaromadendrene oxide-(1) (1.88%), 2, 3, 3-Trimethyl-2-(3-methylbuta-1, 3-dienyl)-6-methylenecyclohexanone (1.32%), Cryptomeridiol (0.72%), 5-Azulenemethanol, 1, 2, 3, 3a, 4, 5, 6, 7-octahydro-.alpha., .alpha., 3, 8-tetramethyl-, [3S-(3.alpha., 3a.beta., 5.alpha.)]- (0.6%).
The Ethyl acetate extract contained 29 peaks in the gas chromatogram of GC–MS and 11 compounds were identified from these peaks. Homopterocarpin (27.83%), 2-Naphthalenemethanol, decahydro-.alpha., .alpha., 4a-trimethyl-8-methylene-, [2R-(2.alpha., 4a.alpha., 8a.beta.)]- (16.59%), (-)-Spathulenol (13.40%), Tricyclo[4.4.0.0(2, 7)]dec-8-ene-3-methanol, .alpha., .alpha., 6, 8-tetramethyl-, stereoisomer (10%), 2-Naphthalenemethanol, 1, 2, 3, 4, 4a, 5, 6, 7-octahydro-.alpha., .alpha., 4a, 8-tetramethyl-, (2R-cis)- (5.08%), (1R, 4aR, 7R, 8aR)-7-(2-Hydroxypropan-2-yl)-1, 4a-dimethyl decahydronaphthalen-1-ol(4.25%), Cryptomeridiol (1.5%), 9-Isopropyl-1-methyl-2-methylene-5-oxatricyclo[5.4.0.0(3, 8)]undecane (1.33%), 5-Azulenemethanol, 1, 2, 3, 3a, 4, 5, 6, 7-octahydro-.alpha., .alpha.,3, 8-tetramethyl-, [3S-(3.alpha., 3a.beta., 5.alpha.)] (0.71%), (4aS, 7R)-7-(2-Hydroxypropan-2-yl)-1, 4a-dimethyl-4, 4a, 5, 6, 7, 8-hexahydronaphthalen-2(3H)-one(0.6%), 2(3H)-Furanone, 5-butyldihydro-4-methyl- (0.55%).
The Ethanol/Benzene extract contained 24 peaks in the gas chromatogram of GC–MS and 11 compounds were identified from these peaks.Homopterocarpin(24.42%), 2-Naphthalenemethanol, decahydro-.alpha., .alpha., 4a-trimethyl-8-methylene-, [2R-(2.alpha., 4a.alpha., 8a.beta.)]-(15.01%), (-)-Spathulenol(12.23%), Tricyclo[4.4.0.0(2, 7)]dec-8-ene-3-methanol, .alpha., .alpha., 6, 8-tetramethyl-, stereoismer (10%), 2-Naphthalenemethanol, 1, 2, 3, 4, 4a, 5,6, 7-octahydro-.alpha., .alpha., 4a, 8-tetramethyl-, (2R-cis)- (4.66%), (1R, 4aR, 7R, 8aR)-7-(2-Hydroxypropan-2-yl)-1, 4a-dimethyl decahydronaphthalen-1-ol (3.9%), Cryptomeridiol(1.37%), 1, 2-Benzenedicarboxylic acid, butyl 2-methylpropyl ester(1.24%), 2-Propen-1-ol, 3-(2, 6, 6-trimethyl-1-cyclohexen-1-yl)- (1.09%), .alpha.-acorenol (0.71%), 2(3H)-Furanone, 5-butyldihydro-4-methyl- (0.62%).
3.3. The health care ingredients of the Pterocarpus Macrocarpus Kurz
Pterocarpus is not only a good material for making high-grade furniture, but also considered a treasure of human health care. For example, Pterocarpus has effect on soothe the nerves, speed up the body's metabolism and lower blood pressure, blood lipids. Through the analysis of the Pterocarpus Macrocarpus Kurz extract, we we also got some proven medicinal ingredients. 2-Naphthalenemethanol, 1, 2, 3, 4, 4a, 5, 6, 7-octahydro-. alpha.,. alpha., 4a, 8-tetramethyl-, (2R-cis)- not only can inhibit the growth of Staphylococcus aureus, but also can inhibit the growth of colibacillus. It can also be used as cosmetics and food additives in industrial production (Wang et al., 2013). 2-Naphthalenemethanol, decahydro-. alpha.,. alpha., 4a-trimethyl-8-methylene-, [2R-(2. alpha., 4a. alpha., 8a. beta.)]- has a high value in the application of medicine, what’s more it has cough and phlegm, detoxification and diuretic and other effects (Tu et al., 2009, Gao et al., 2017). Cryptomeridiol is a natural product with anti-Alzheimer's disease and antispasmodic properties, and its medicinal value is significant (Mohamed et al., 2011). 2, 3, 3-Trimethyl-2-(3-methylbuta-1, 3-dienyl)-6-methylenecyclohexanone has the property of inhibiting alpha-amylase activity and it can be used to control alpha-amylase hydrolysis of starch to produce glucose, syrup, beer and monosodium glutamate (Ramsha and Dildar, 2015). Homopterocarpin inhibits and kills cancer cell activity. It can inhibit at low concentrations, kill at high concentrations human laryngeal cancer cells and human hepatocarcinoma cells (Kan et al., 1994, Li et al., 2001). 1, 2-Benzenedicarboxylic acid, butyl 2-methylpropyl ester has the function of heat cough and enhance the body's immune function (Han et al., 2004, Ataa et al., 2017).
4. Conclusion
The infrared transmittance of the extracted Pterocarpus Macrocarpus Kurz powder at each peak position became larger in the FT-IR analysis. However, after the extraction of different extracts, the increase in the infrared transmittance of each peak is different. The increase of the infrared transmittance of ethyl acetate is the highest in the wavenumber range of 2855–3421 cm−1. The increase of the infrared transmittance of ethanol/benzene in 1030–1510 cm−1 wavenumber range is the highest.
By GC–MS analysis, 17 kinds of chemical components are identified in the Pterocarpus Macrocarpus Kurz extract. Some of them have been proven to have ingredients that are beneficial to human health. These beneficial ingredients have cough and phlegm, detoxification and enhance the function of human immunity. The Homopterocarpin is excellent in inhibiting and killing cancer cell activity. Cryptomeridiol is a natural product with anti-Alzheimer's disease and antispasmodic properties and its medicinal value is significant.
Acknowledgments
This research was supported by the Planned Science and Technology Project of Hunan Province, China (No. 2016SK2089; No. 2016RS2011; No. 2016SK2086), Major scientific and technological achievements transformation projects of strategic emerging industries in Hunan Province (2016GK4045).
Footnotes
Peer review under responsibility of King Saud University.
References
- Ataa S., Hazmi I.R., Samsudin S.F. Insect’s visitation on melastomamalabathricum in UKM Bangi forest reserve. Environ. Ecosyst. Sci. 2017;1(1):20–22. [Google Scholar]
- Azratul A.N.M.D., Akbar John B., Kamaruzzaman B.Y., Sheikh Hassan, Jalal K.C.A., Noor Faizul H.N. Biomonitoring selected heavy metal concentration in Nerita Sp. collected from tanjunglumpur mangrove forest. Environ. Ecosyst. Sci. 2017;1(1):04–07. [Google Scholar]
- Dekkers H.F., Srinivasan N.B., Pourtois G. Influence of density on N–H bond stretch vibration in plasma enhanced chemical vapor deposited SiNx:H. Appl. Phys. Lett. 2010;96(1) 011902-011902-3. [Google Scholar]
- Dongli Li, Peng Wanxi, Ge Shengbo, Li Shasha, Mo Bo. Groups characteristics of bioactivator extractives in three poplar woods. Wood Res. 2015:755–762. [Google Scholar]
- Evans M.E., Burke C.L., Yaibuathes S., Clot E., Eisenstein O., Jones W.D. Energetics of C−H bond activation of fluorinated aromatic hydrocarbons using a [Tp′ Rh (CNneopentyl)] complex. J. Am. Chem. Soc. 2009;131(37):13464–13473. doi: 10.1021/ja905057w. [DOI] [PubMed] [Google Scholar]
- Francis, J.K., 2000. Pterocarpus macrocarpus Kurz. Seed Leaflet - Danida Forest Seed Centre.
- Gao W., Wang Y., Basavanagoud B., Jamil M.K. Characteristics studies of molecular structures in drugs. Saudi Pharm. J. 2017;25(4):580–586. doi: 10.1016/j.jsps.2017.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jalal K.C.A., Akbar John B., Hassan I., Sheikh Shahbudin S., Nor Hafiza Y.A.A. Study on physicochemical parameters and distribution of phytoplankton in Kuantan estuary, Pahang. Environ. Ecosyst. Sci. 2017;1(1):8–12. [Google Scholar]
- Kai-Fu Li, Peng Wen-Li, Hou Xiao-Jia. Chemical composition and healthy care properties of Cinnamomum camphora by GC/MS. Int. Conf. Bioinformatics Biomed. Eng. 2009;87(5):1–3. [Google Scholar]
- Lu R., Gan W., Wu B.H., Zhang Z., Guo Y., Wang H.F. C-H stretching vibrations of methyl, methylene and methine groups at the vapor/alcohol (N = 1–8) interfaces. J. Phys. Chem. B. 2005;109(29):14118. doi: 10.1021/jp051565q. [DOI] [PubMed] [Google Scholar]
- Noor M.J., Ashraf M.A. Accumulation and tolerance of radiocesium in plants and its impact on the environment. Environ. Ecosyst. Sci. 2017;1(1):13–16. [Google Scholar]
- Paganelli S., Matteoli U., Scrivanti A., Botteghi C. ChemInform abstract: Pt(0) complexes as catalyst precursors for homogeneous carbon-carbon and carbon-oxygen double bond hydrogenation. J. Organometall. Chemistr. 1991;22(6):375–381. [Google Scholar]
- Rezaee M., Assadi Y., Milani Hosseini M.R., Aghaee E., Ahmadi F., Berijani S. Determination of organic compounds in water using dispersive liquid–liquid microextraction. J. Chromatogr. A. 2006;1116(1–2):1. doi: 10.1016/j.chroma.2006.03.007. [DOI] [PubMed] [Google Scholar]
- Saeed Ramsha, Ahmed Dildar. Bioactive compounds from Carissa opaca roots and xanthine oxidase and alpha-amylase inhibitory activities of their methanolic extract and its fractions in different solvents. Pharmacogn. Res. 2015;7(4):295. doi: 10.4103/0974-8490.158440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu Han., Yang P.A.N., Guang-ming Y.A.N.G., Bao-chang C.A.I. Research on components of Cornus officinalis extracted by supercritical carbon dioxide. China J. Chinese Mater. Med. 2004;28(12) 1148-50, 1183. [PubMed] [Google Scholar]
- Tebbaa Mohamed, El Hakmaoui Ahmed, Benharref Ahmed, Akssira Mohamed. Short and efficient hemisynthesis of α-eudesmol and cryptomeridiol. Tetrahedron Lett. 2011;52(29):3769–3771. [Google Scholar]
- Thaldiri N.H., Hanafiah M.M., Halim A.A. Effect of modified micro-sand, poly-aluminium chloride and cationic polymer on coagulation-flocculation process of landfill leachate. Environ. Ecosyst. Sci. 2017;1(1):17–19. [Google Scholar]
- Chunrong Wang, Chengji F.A.N.G., Qingqing Y.U., Peng J.I.A.N.G., Wei T.I.A.N. Gas chromatography and mass spectrometry(GC-MS) analysis of supercritical CO2 extract of flower bud of Chrysanthemum indicum and its antibacterial activity. J. Zhejiang Univ. 2013;39(2):167–172. [Google Scholar]
- Wanxi Peng, Lin Zhi, Chang Junbo, Fangliang Gu, Zhu Xiangwei. Biomedical molecular characteristics of YBSJ extractives from fruit. Biotechnol. Biotechnol. Equip. 2014;27(6):4311–4316. [Google Scholar]
- Weidong Li, KanYuming Hong Min, Quan Zh.u. The inhibitory effects of homopterocarpin and medicarpin on humans liver cancer cells in vitro. J. Shenyang Pharm. Univ. 2001;18(3):211–212. [Google Scholar]
- Wisittipanich, S., Khadee, C., Jintana, P., 2012. Shoot production of plus tree branch log and it's cutting test of Tectona grandis Linn and Pterocarpus macrocarpus Kurz. Kasetsart University Conference, 2580.
- Yong-qi Tu, Rong-ping Yang, Hua-li Zhu, Bin-hao Wang, Teng Peng. GC-MS analysis of the chemical components of essential oils in ligularia. Melanocephala J. Southwest Univ. 2009 [Google Scholar]
- Yu-ming Kan., Quan Z.H.U., Long C.H.E.N., Rong-di W.A.N.G., Xiang L.I., Mei-fang H.O.N.G. Effect of homopterocarpin separated from Glycyrrhiza pallicdiflora onhuman's throat cancer cell(HE_p-2) Chinese Pharm. J. 1994 [Google Scholar]