Abstract
Mikania cordata is widely used for the treatment of cuts, wounds, and dengue fever in Bangladesh. In the present study, essential oil (12.5, 25 and 50 mg/kg) and two extracts, viz., chloroform and ethyl acetate extracts (200, 400, 800 mg/kg b.w.) were tested for peripheral and central anti-nociceptive activity by acetic acid-induced writhing and hot plate method, respectively. Carrageenan-induced rat paw edema assay and yeast-induced hyperthermia assay were also carried out to evaluate anti-inflammatory and antipyretic properties of oil and extracts, respectively at aforesaid doses. The essential oil (50 mg/kg), chloroform extract (800 mg/kg) and ethyl acetate extract (800 mg/kg) showed potent peripheral anti-nociceptive activity having 47.33%, 29.33% and 16.65% of writhing inhibition, respectively, comparable with standard diclofenac (52.0%). Essential oil (50 mg/kg), chloroform extract (800 mg/kg) and ethyl acetate extract (800 mg/kg) presented promising central anti-nociceptive activity as well having 95.86%, 79.18% and 42.37% elongation of reaction time, respectively, at 90 min after administration of essential oil, ethyl acetate extract and 60 min after administration of chloroform extract. In anti-inflammatory activity screening, the essential oil (50 mg/kg) produced the highest 72.80% edema inhibition at 4 h after administration of carrageenan which was comparable with that of standard phenylbutazoe (87.87%). On the other hand, chloroform extract (800 mg/kg) and ethyl acetate extract (800 mg/kg) showed up to 34.31% and 15.27% of edema inhibition, respectively, at 4 h after administration of carrageenan. In antipyretic assay, the essential oil and chloroform extract displayed a strong antipyretic effect in yeast-induced rats, whereas the ethyl acetate extract had no antipyretic activity. The present study revealed anti-nociceptive, anti-inflammatory and antipyretic potential of M. cordata which could be the therapeutic option against fever, inflammations as well as painful conditions and confirmed the traditional use of M. cordata.
Keywords: Mikania cordata, Essential oil, Chloroform extract, Ethyl acetate extract, Anti-nociceptive, Anti-inflammatory, Antipyretic activity
1. Introduction
Mikania cordata (Burm. f.) Robinson (Asteraceae) is a twining herb, characterized by its cordate leaves, capitulum inflorescence, whitish flowers, narrowly oblong cypsela type of fruits and white pappus. In Bangladesh, M. cordata is locally known as Assamlata or Tarulata, and is widely distributed throughout the country. This plant is used as ethnomedicine in treating cuts and wounds in Bangladesh. A decoction of leaves is administered in the treatment of indigestion, dysentery, and gastrointestinal sore (Ghani, 1998). The Mandi ethnic people in Tangail district of Bangladesh eat young fried leaves of M. cordata in gastric pain (Partha and Hossain, 2007). The Garo tribal people of Netrokona district use this plant in cuts and wounds (Rahmatullah et al., 2009a). The Santals tribal people residing in Thakurgaon district use the leaf of M. cordata for the treatment of cuts and wounds, and as remedy of dengue fever (Rahmatullah et al., 2009b). The plant is also used as leafy vegetable and applied against coughs, eye sores and gastro-intestinal disorders. In Assam (India), the local herbal practitioners (Kabiraj) use the leaf juice of M. cordata in the case of insect and scorpion sting (Sastri, 1962). M. cordata is also indigenously used by the tribe Seminoles to treat itchy skin as well as for circumcision, wounds, and tumors (Sturtevant, 1954). It is reported to have psycho-pharmacological, neuro-pharmacological, antibacterial, antifungal activities and therapeutic properties against pain, inflammation, hyperthermia, ulcer and carcinogenesis (Mosaddik and Alam, 2000). It contains different biologically active compounds e.g. mikanin, friedelin, butyryloxy kaurenic acid, benzoyloxy kaurenic acid, stigmasterol and beta-sitosterin (Ghani, 2003).
The CNS depressant activity with potential antioxidant properties are found in different plant aerial extracts (Hyun et al., 2016) and it is also common in M. cordata (Hasan et al., 2009). The hydroalcoholic leaf extract of this species also found to have neuropharmacological as well as CNS-depressant activity (Dey et al., 2011). Crude ethanolic extract of M. cordata found to have anti-nociceptive, cytotoxicity, and antibacterial activities in animal model (Nayeem et al., 2011). However, no study was carried out on the anti-nociceptive, anti-inflammatory and antipyretic potential of the essential oil as well as chloroform and ethyl acetate extracts of M. cordata leaves so far. Therefore, this study aimed at investigating the anti-nociceptive, anti-inflammatory and antipyretic effects of leaf essential oil and extracts of M. cordata occurring in Bangladesh.
2. Materials and methods
2.1. Plant material
The aerial parts of Mikania cordata were plucked from Muksudpur of Gopalganj district, Bangladesh in the months of June and July 2010. The plant material was collected and identified by Prof. Dr. M. Oliur Rahman of the University of Dhaka, Bangladesh. The voucher specimen has been preserved in Bangladesh National Herbarium (DACB – Voucher No. 37894).
2.2. Extraction of essential oil
After drying of the fresh leaves at room temperature, those were converted into powdered form using pestle and mortar, and the essential oil was extracted by steam distillation for 3 h. The yielding percentage was 0.65 (w/w) and the density at 25 °C was 0.98 g/ml. In this study, one thousand and nine hundred gram (1900 g) of the powder was hydrodistilled and 12.35 g of the essential oil was yielded. The oils was preserved in refrigerator in a lightproof bottle. In order to determine the relative density of the oil 10 ml capacity bottle was employed.
2.3. Preparation of plant organic extracts
Fifty grams of powdered form of the leaves was used separately for chloroform and ethyl acetate solvent extraction at room temperature for 7 days. After evaporation of solvents using vacuum rotary evaporator at 50 °C, 6.2 g of chloroform extract and 7.4 g ethyl acetate extracts were obtained. The extracts were suspended in normal saline using 5.0% Tween 80 before administration.
2.4. Experimental animal
The animal (Swiss albino mice of 25–30 g and Wister albino rats of 150–200 g) were collected from ICDDR, B (International Centre for Diarrheal Diseases and Research, Bangladesh). Standard polypropylene cages were used to keep the animal at temperature 25 ± 1 °C where humidity remains 60–70% with normal day/night cycle (12 h each). Standard pellets as basal diet (ICDDR, B formulated) and water ad libitum were supplied to the animals for one week. After fasting for overnight, the animal were weighted prior to the experiment. Guidelines of the National Institute of Health for the Care and Use of Laboratory Animals (NIH Publication 80-23, revised in 1996) were strictly followed in carrying out the experiments.
2.5. Drugs and chemicals
Diclofenac sodium manufactured by ACI Pharmaceutical Ltd., Dhaka and morphine manufactured by Gonoshasthaya Pharmaceuticals Ltd, Dhaka, Bangladesh were purchased for the experiments. Acetic acid (Merck, Germany) and carrageenan (BDH, UK.) were used in this study. For other chemicals, only the analytical grade chemicals (Sigma and Merck) were employed in the experiments.
2.6. Anti-nociceptive activity study
2.6.1. Peripheral anti-nociceptive activity assay
Acetic acid writhing inhibition method as developed by Koster et al. (1959) was used to identify peripheral anti-nociceptive activity of the plant extracts and essential oil of M. cordata. Three different groups of mice were tested for each type of the test sample and each group included 5 randomly selected mice. Mice of a group were received 5% Tween 80 (10 ml/kg), while three different groups of mice received essential oil of 12.5, 25.0 and 50.0 mg/kg, other three different groups received chloroform extracts of 200, 400, 800 mg/kg and another three different groups received ethyl acetate extracts of 200, 400, 800 mg/kg. Thirty minutes later, the mice were received 3% acetic acid to induce pain. After 5 min of acetic acid administration, writhing count was started and continued over a period of 20 min. The writhing of the group received tested specimens and diclofenac sodium were compared to the writhing response of the control group and considered as index of analgesia. The percentage of inhibition was calculated as per following equation:
2.6.2. Central anti-nociceptive activity assay
For hot plate experiment, the method of Ojewole (2006) with slight modification was followed. The mice which showed responses to the hot plate (Columbus, USA) thermal stimulation after 4 s were selected for the test. In this case the hot plate temperature was kept at 55 ± 1.0 °C and the time period taken to show the response by hind paw licking or jumping was documented in seconds. The test samples (12.5, 25.0 and 50.0 mg/kg of oil, and 200, 400 and 800 mg/kg of extracts) were given orally to mice. Each of mouse under tests was also considered as the own control. Morphine (10 mg/kg) was employed as positive control, and administrated subcutaneously. The primary response time (control latency) was determined by placing the mice on the hot analgesiometer and the time (in seconds) taken to respond was recorded. The average time of the three consecutive experiment at 1 h interval was recorded as control latency. On the other hand, every group of mice underwent the same testing procedure at 30, 60, and 90 min after receiving the test samples. To avoid tissue impairment a latency period of 30 s was considered as complete analgesia cut off time (Heidari et al., 2009).
.
2.7. Carrageenan-induced paw edema assay
The efficacy of M. cordata in reducing induced inflammation was evaluated by acute inflammation method in rats as designated by Winter et al. (1962). Each type of the test samples was applied on three different groups of rat (n = 5). The basal volume (Co) of the right paw (average volume) of each rat was recorded a plethysmometer (model 7150, UgoBasile, Italy). The control group was administrated orally with 5% Tween 80 (10 mg/kg), while animal from other were given the essential oil and extracts at specified doses. Phenylbutazone (PBZ) (100 mg/kg) was given to each animal of the positive control group. After 30 min, all the animal of each group received 1% carrageenan (0.1 ml) into the sub-planter surface of right hind paw. The volume of the right paw edema was measured at 0 h, 1 h, 2 h, 3 h and 4 h. Inhibitory activity was calculated by the following formula:
where Ct refers to paw size after a specific time interval in hours after carrageenan injection and Co denotes paw size before carrageenan injection.
2.8. Yeast-induced hyperthermia assay
The primary rectal temperature of each of the rats were measured by digital electric thermometer (EXACON, Denmark). After taking this initial temperature, each of the rats was given subcutaneously 20 ml/kg (20%) suspension of brewer’s yeast in distilled water to induce pyrexia (Turner et al., 1965). The animals were chosen for the experiment which displayed an increase of 0.3–0.5 °C after 18 h. Then different groups of rats received the tests samples (oils and extracts) in specified doses as described earlier. The control group was given 0.3 ml normal saline. In this study, we have taken paracetamol (100 mg/kg orally) as standard drug. After treatment with the test samples and standard drug, the rectal temperature of all rats under experiment were measured at 1 h, 2 h, 3 h and 4 h. The average temperature of every group was compared with mean temperature of the group treated with standards drug.
2.9. Statistical analysis
All data were analyzed by means of ANOVA test followed by Student’s t-test. Significant levels were determined at P < 0.05 (95% confidence limit).
3. Results
3.1. Anti-nociceptive activity study
3.1.1. Peripheral anti-nociceptive activity assay
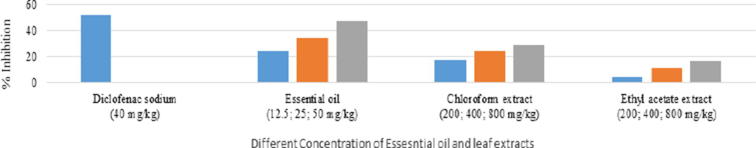
Mikania cordata showed peripheral anti-nociceptive effect by reducing acetic acid induced writhing in mice (Fig. 1). The percentage inhibition of writhing for essential oil at the dose of 12.5, 25.0 and 50.0 mg/kg were 24%, 34.67% and 47.33%, respectively. In contrast, the chloroform extract at 200, 400 and 800 mg/kg inhibited 17.33%, 24.67% and 29.33% and the inhibition percentage for the ethyl acetate extracts at 200, 400 and 800 mg/kg showed 4.66%, 11.3%, 16.65% inhibition, respectively.
Fig. 1.
Effect of the treatment with essential oil (12.5, 25, 50 mg/kg), chloroform leaf extract (200, 400, 800 mg/kg), ethyl acetate extract (200. 400, 800 mg/kg), and diclofenac sodium as control (40 mg/kg) on the intraperitoneal administration of 10 ml/kg of 3% acetic acid. Each group represents the mean of 5 animals. Values are expressed as mean ± SEM, *P < 0.05 and **P < 0.01 compared with control group.
The percentage inhibitions were determined by comparing the animals received test specimen to the animals that received vehicle only. By comparison, 40 mg/kg diclofenac sodium-produced the highest (i.e., 52% effectiveness) anti-nociceptive effect in this nociception model. In this study, the tested samples exhibited dose dependent analgesia and the oil at 50 mg/kg b.w. induced the highest analgesia among the tested doses.
3.1.2. Central anti-nociceptive activity assay
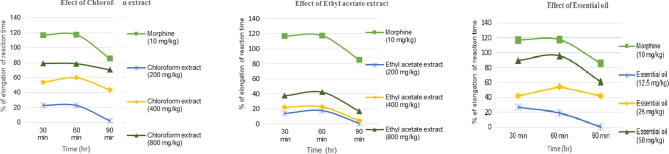
In the hot plate study, the leaf essential oil (12.5, 25.0 and 50.0 mg/kg each), and both of the chloroform and ethyl acetate extracts (200, 400 and 800 mg/kg) of M. cordata elongated the reaction time at different time points. All these elongation in the reaction time was determined by comparing to the corresponding control groups (Table 1; Fig. 2). Morphine significantly prolonged the reaction time of the animals with relatively extended duration of stimulation of 116.99%, 117.52% and 85.55% elongation reaction time at 30 min, 60 min and 90 min, respectively, confirming centrally mediated activity. The essential oil at the dose of 50 mg/kg b.w. induced the highest analgesia among the tested essential oil doses by elongating the reaction time 89.42%, 95.86%, and 60.98% at 30 min, 60 min and 90 min, respectively.
Table 1.
Effects of Mikania cordata on hot plate test in mice.
| Treatment | Dose (mg/kg) | Reaction time (sec) |
||
|---|---|---|---|---|
| 30 min | 60 min | 90 min | ||
| Control (Vehicle) | – | 6.24 ± 0.61 | 6.28 ± 0.28 | 6.92 ± 0.34 |
| Morphine | 10 | 13.54 ± 0.49 | 13.66 ± 0.39** | 12.84 ± 0.56** |
| Essential oil | 12.5 | 7.92 ± 0.52* | 7.48 ± 0.45 | 6.94 ± 0.37* |
| 25.0 | 8.86 ± 0.43 | 9.68 ± 0.19** | 9.84 ± 0.10 | |
| 50.0 | 11.82 ± 0.31** | 12.3 ± 0.31** | 11.14 ± 0.11** | |
| Chloroform extract | 200 | 7.68 ± 0.87 | 7.72 ± 0.55* | 7.08 ± 0.49 |
| 400 | 9.6 ± 0.96 | 10.04 ± 0.57** | 9.94 ± 0.58* | |
| 800 | 11.18 ± 0.48* | 11.22 ± 0.40** | 11.8 ± 0.35** | |
| Ethyl acetate extract | 200 | 7.12 ± 0.26 | 7.4 ± 0.26 | 6.98 ± 0.19* |
| 400 | 7.64 ± 0.79* | 7.72 ± 0.76 | 7.22 ± 0.75 | |
| 800 | 8.58 ± 0.48 | 8.94 ± 0.40** | 8.1 ± 0.35* | |
Each value represents the mean (±S.E.M.) of five observations.
*P < 0.05 and **P < 0.01 compared with control group.
Fig. 2.
Effect of the treatment with chloroform leaf extract (200, 400, 800 mg/kg), ethyl acetate extract (200. 400, 800 mg/kg), essential oil (12.5, 25, 50 mg/kg) and morphine as control (10 mg/kg) on the hot plate anti-nociceptive test. Each mouse is served as its own control. Before treatment, the time for hind paw licking or jumping on the heated plate of analgesiometer was determined thrice at 1 h interval and mean of these were taken as the initial reaction time (control latency). Mice in each group were tested 30, 60 and 90 min after drug treatment. Values are expressed as mean ± SEM, *P < 0.05 and **P < 0.01 compared with control group.
The maximum elongation for the chloroform and ethyl acetate extract was obtained at 800 mg/kg b.w. dose. The percentage of elongation of reaction time for the chloroform at 800 mg/kg were 79.18%, 78.66% and 70.52% at 30 min, 60 min and 90 min, respectively, whereas the percentage of elongation of reaction time for the ethyl acetate extract for the same dose were 37.5%, 42.37% and 17.05% at 30 min, 60 min and 90 min, respectively. All the three specimens, viz. essential oil, chloroform extract and ethyl acetate extract revealed dose dependent central anti-nociceptive activity.
3.2. Carrageenan-induced rat paw edema assay
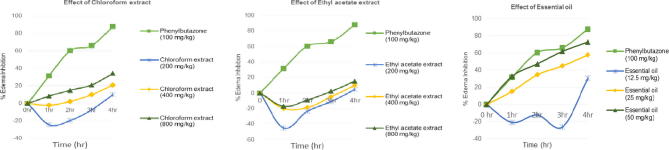
The anti-inflammatory effects of the essential oil and the extracts of M. cordata showed by inhibition of carrageenan-induced paw edema in rat’s are presented in Table 2 and Fig. 3. Ethyl acetate extract showed anti-inflammatory effect in this model only at 4 h and the highest inhibition (15.27%) was found at 800 mg/kg for this extract.
Table 2.
Anti-inflammatory activities of Mikania cordata and phenylbutazone (PBZ) on carrageenan-induced edema in the right hind-limb paw of rats.
| Treatment | Dose (mg/kg) | Time (h) |
Average edema formation | ||||
|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | |||
| EV (ml) | EV (ml) | EV (ml) | EV (ml) | EV (ml) | |||
| Control (saline) edema formation | – | – | 0.35 ± 0.04 | 0.41 ± 0.05 | 0.44 ± 0.050 | 0.48 ± 0.06 | 0.42 |
| Phenylbutazone | 100 | – | 0.24 ± 0.03∗ | 0.16 ± 0.05 | 0.15 ± 0.03∗∗ | 0.06 ± 0.04∗∗ | 0.15 |
| Essential oil | 12.5 | – | 0.46 ± 0.02∗∗ | 0.55 ± 0.02 | 0.33 ± 0.1∗∗ | 0.43 ± 0.02 | 0.44 |
| 25.0 | – | 0.27 ± 0.02∗ | 0.24 ± 0.06∗ | 0.20 ± 0.80∗ | 0.3 ± 0.05∗∗ | 0.25 | |
| 50.0 | – | 0.22 ± 0.06 | 0.17 ± 0.04∗∗ | 0.13 ± 0.04∗∗ | 0.24 ± 0.6∗∗ | 0.19 | |
| Chloroform extracts | 200 | – | 0.44 ± 0.01 | 0.49 ± 0.06∗ | 0.47 ± 0.05 | 0.43 ± 0.09∗ | 0.46 |
| 400 | – | 0.36 ± 0.04 | 0.4 ± 0.44∗ | 0.39 ± 0.22∗ | 0.38 ± 0.12∗∗ | 0.38 | |
| 800 | – | 0.32 ± 0.16∗∗ | 0.35 ± 0.12∗ | 0.34 ± 0.04∗∗ | 0.31 ± 0.22∗∗ | 0.33 | |
| Ethyl acetate extracts | 200 | – | 0.51 ± 0.33 | 0.51 ± 0.16 | 0.49 ± 0.1 | 0.46 ± 0.31∗ | 0.49 |
| 400 | – | 0.43 ± 0.23 | 0.49 ± 0.07∗ | 0.46 ± 0.17∗∗ | 0.43 ± 0.03∗∗ | 0.45 | |
| 800 | – | 0.42 ± 0.31 | 0.45 ± 0.69 | 0.43 ± 0.10∗ | 0.40 ± 0.08 | 0.43 | |
EV: Edema volume.
Values represent the mean ± S.E.M. (n = 5), ∗P < 0.05 and ∗∗P < 0.01 compared with control group.
Fig. 3.
Effect of the treatment with chloroform leaf extract (200, 400, 800 mg/kg), ethyl acetate extract (200, 400, 800 mg/kg), essential oil (12.5, 25, 50 mg/kg) and penylbutazone as control (100 mg/kg) on paw edema induced by intradermal injection of carrageenan (1% 0.1 ml) in rats. Zero (time zero) corresponds to baseline measurement of animals performed 24 h before the test. Each group represents the mean of 5 animals, and the vertical lines indicate the SEM. *P < 0.05 and **P < 0.01 compared with control group.
Chloroform extract (200, 400 and 800 mg/kg) showed suppression of carrageenan-induced paw edema at several time points and at the highest dose of 800 mg/kg, chloroform extract showed noticeable inhibition of 8.47%, 15.12%, 21.10% and 34.31% edema at 1 h, 2 h, 3 h and 4 h, respectively. As shown in Fig. 3, the highest inhibition of paw edema was recorded at 50 mg/kg of essential oil among the tested doses of the plant.
3.3. Yeast-induced hyperthermia assay
The antipyretic potential of the essential oil and two extracts of M. cordata leaf were presented in Table 3. Administration of brewer’s yeast to rats produced a noticeable increase in rectal temperature 18 h after yeast injection (Table 3). In this study, although the chloroform extract reduced the rectal temperature right from 1 h onward, however, the ethyl acetate extract showed no antipyretic activity (data not shown). The essential oil showed strong antipyretic effect and pyrexia was reduced after the oil administration. Paracetamol (100 mg/kg orally) significantly reduced the temperature. The reduction in temperature by the essential oil and the extract varied and of different potencies (Table 3).
Table 3.
Antipyretic activities of Mikania cordata and paracetamol on brewer’s yeast–induced pyrexia in rats.
| Treatment | Dose (mg/kg) | Rectal Temperature |
|||||
|---|---|---|---|---|---|---|---|
| Before drug |
After drug |
||||||
| −18 h | 0 h | 1 h | 2 h | 3 h | 4 h | ||
| Control (Saline) | 0.3 ml | 37.22 ± 0.20 | 38.106 ± 0.30 | 38.018 ± 0.28 | 38.00 ± 0.19 | 38.05 ± 0.19 | 38.05 ± 0.20 |
| Essential oil | 12.5 | 36.92 ± 0.17 | 37.98 ± 0.27∗ | 37.91 ± 0.25∗ | 37.79 ± 0.19∗ | 37.70 ± 0.12∗ | 37.3 ± 0.12∗∗ |
| 25.0 | 36.86 ± 0.22 | 38.05 ± 0.21 | 37.91 ± 0.22 | 37.70 ± 0.19∗∗ | 37.2 ± 0.28 | 37.11 ± 0.13 | |
| 50.0 | 36.96 ± 0.15 | 38.19 ± 0.25∗ | 38.04 ± 0.25∗ | 37.61 ± 0.13∗∗ | 37.11 ± 0.33∗∗ | 36.87 ± 0.05∗∗ | |
| Chloroform extract | 200 | 37.13 ± 0.31 | 37.63 ± 0.33 | 37.21 ± 0.12∗ | 37.35 ± 0.15 | 37.31 ± 0.13∗∗ | 37.17 ± 0.17∗∗ |
| 400 | 36.47 ± 0.36 | 37.26 ± 0.27∗ | 37.08 ± 0.4∗∗ | 37.0 ± 0.3 | 37.13 ± 0.15∗∗ | 36.79 ± 0.15∗ | |
| 800 | 37.32 ± 0.27 | 38.49 ± 0.41∗∗ | 38.10 ± 0.22 | 37.53 ± 0.12∗∗ | 37.18 ± 0.12 | 37.0 ± 0.15∗∗ | |
| Paracetamol | 100 | 36.97 ± 0.28 | 38.19 ± 0.32∗ | 37.76 ± 0.12∗ | 37.13 ± 0.19 | 36.98 ± 0.05∗∗ | 36.95 ± 0.09∗ |
Values are mean ± S.E.M. rectal temperature (n = 5), ∗P < 0.05 and ∗∗P < 0.01 compared with control group.
4. Discussion
The long history of traditional uses of M. cordata in treating pain, inflammations, cuts, wounds and dengue fever have been reported in different studies (Ghani, 1998, Rahmatullah et al., 2009a, Rahmatullah et al., 2009b). The objective of our study was to examine the effect of M. cordata on pain, inflammations and pyrexia to ascertain scientific rationales behind using M. cordata as ethnomedicinal plant in treating cuts, wounds as well as dengue fever and its symptoms. Our study demonstrated the efficacy of essential oil and organic extracts of M. cordata against induced pain, inflammation and pyrexia.
The anti-nociceptive activity (both peripheral and central) of the test specimens were investigated by applying two different methods. The most suitable and widely used acetic acid induced writhing method was applied to identify the peripheral effects whereas hot plate method was employed to detect central anti-nociceptive activity of the test samples. Our results clearly indicated that essential oil and the extracts of M. cordata possessed both peripheral (writhe reduction) and central (thermal reaction time prolongation) anti-nociceptive effects. From these observation it could be concluded that the M. cordata could be considered as an alternative source of commercially available analgesic medications.
In the peripheral nociception model, the pain is developed by inducing local inflammatory sensation. Following inflammation, there is biogenesis of prostaglandins from cyclooxygenase pathway and leukotrienes from lipoxygenase pathway (Das et al., 2012). The released prostaglandins, mainly prostacyclin (PGI2), and to a lesser extent, prostaglandin E, are supposed to induce pain and abnormal contraction of mice body which is termed as ‘writhing’. The biogenesis process of lipooxygenase and/or cyclooxygenase might be prevented by phyto-constituents of extracts and oil of M. cordata and this might affect the formation of PGE2. Thus the transformation mechanism in the nociceptor is suppressed which results in reduced or no pain perception. Previous studies showed that acetic acid induced writhing model might give rise to false positive results (Le Bars et al., 2001). Therefore, to evaluate the actual anti-nociceptive activity, another test model e.g. hot plate thermal stimulation in combination with the acetic acid induced writhing model is generally suggested. The essential oil and both of the extracts of M. cordata were found to prolong reaction time in hot plate thermal stimulation test. The effect of oil and extracts is comparable with that obtained by morphine. Central nervous system depression properties of morphine resulted in reduced behavioral activities. It has already been established that the exciting states of monoamines neurotransmitters are the initiator of spontaneous motor activity and exploratory behaviors (Rang et al., 1998). Thus, oil and extracts may have properties as like as morphine, which may have some suppressing effect on the secretion of these neurotransmitters. Previous studies revealed that the aerial parts of M. cordata exerted central anti-nociceptive, locomotors depressant, muscle relaxant and sedative potential effect which, in turn, leads to the conclusion that the aerial parts of this species has some central nervous system depression properties (Dey et al., 2011). Moreover, Siddiqui et al. (2017) stated that monoterpene and sesquiterpene compounds are predominant in essential oil composition, while De Sousa (2011) showed that the monoterpenes and sesquiterpenes played very important role as analgesic compound in essential oils. Furthermore, the analgesic role of flavonoids (Rao et al., 1998), tannin and alkaloids (Goyal et al., 2013) in different plant species has been well established, and this particular species M. cordata has been claimed to possess flavonoid, steroid, tannin, and saponin as predominant compounds (Hasan et al., 2009, Nayeem et al., 2011). Thus the anti-nociceptive properties of oil and leaf extracts can be attributed to those compounds available in the M. cordata. The results of our present study thus support the neuro-pharmacological properties of M. cordata as reported by Dey et al. (2011). Previous studies based on hydromethanolic extract (Hasan et al., 2009) and crude extract (Ahmed et al., 2001) also suggested that M. cordata had strong analgesic effect and those findings were further supported by our results.
The inflammation sensation may be induced due to different diseases and establishment of a active therapy for treating inflammation is problematic. Consequently, it is necessary for finding out alternative plant derived compounds to treat inflammations effectively. Several compounds have already been isolated from different plant species which indicated positive therapeutic action on inflammatory conditions. In our study, tested specimens of M. cordata exhibited positive effects in anti-inflammatory and antipyretic tests models. In the carrageenan induced rat paw edema model, essential oil presented the most prominent activities, whereas, chloroform extract displayed moderate effect in controlling induced inflammation. Ethyl acetate extract, however, showed somewhat lower activity. Our results have been found congruent with previous studies in some other genera (Silva et al., 2003, Goyal et al., 2013), which demonstrated strong anti-nociceptive and anti-inflammatory properties. Suyenaga et al. (2002) studied some other species of Mikana and showed that those species have anti-inflammatory and anti-nociceptive properties which support our findings. The result of our study is in good agreement with previous report on anti-inflammatory activity of M. cordata (Chandra et al., 2012).
The mechanism of the anti-inflammatory effects of the oil and extracts found in this study is not so clear as like as anti-nociceptive activities. However, our previous study revealed that essential oil of M. cordata is a rich source of various mono- and sesquiterpenes, e.g., β-caryophyllene, δ-cadinene, β-himachalene, T-cadinol, β-farnesene as well as other monoterpenes and sesquiterpenes (Siddiqui et al., 2017). It has already been shown that the β-caryophyllene and δ-Cadinene can inhibit the biogenesis of leukotriene B2, prostaglandin E2, and other arachidonic acid metabolites in inflammation pathway (Kamatou et al., 2006). However, De Sousa (2011) states that mediators, cytokines responsible for inflammatory sensation is usually inhibited by monoterpene components of the oil. Moreover, monoterpenes compounds have significant suppressing effect on the secretion of several inflammatory mediators (Juergens et al., 1998). Based on these studies we can conclude that monoterpenes compound of the oil sample of M. cordata played an active role to show potent effect against induced inflammation as evidenced in our study. The anti-inflammatory effect of the extracts could be attributed to flavonoid, steroid, tannin, and saponin content. Moreover, M. cordata is a good source of dilactones type mikanolide and miscandenin, and kaurenoic acid, which were found to active against induced pain and inflammation (Ahmed et al., 2001). All these compounds may impart a significant effect in the anti-inflammatory property of the extracts. To our continuous search for natural components with properties of reducing pain and inflammation sensation, our works are in progress to explore active compound(s) from M. cordata responsible for these effects.
The drug or medicinal compound bearing the suppressing effect on biogenesis of prostaglandin generally have antipyretic properties (Vane, 1987). In our study, essential oil and the chloroform extract were found to inhibit pyrexia considerably developed by yeast administration. Essential oil have impacts on central nervous system and the major components of oil (i.e. β-caryophyllene) are likely to inhibit the synthesis of prostaglandin E2 and thus lessen hyperthermia. In this case the compounds like β-caryophyllene exert some inhibitory effect by its action on COX-3 which decrease the amount prostaglandin E2 in the hypothallamus region of brain (Botting and Ayoub, 2005). There is another possibility in which the compounds may have some positive influence to increase the secretion of vasopressin and arginine like antipyretic agents within the body (Chandrasekharan, 2002). In contrast, the phyto-constituents present in the chloroform extract may play a vital role to reduce pyrexia in rats. However, some of our observations regarding incongruent or multiphasic activities of test samples could be correlated with the fact that oils and extracts are complex mixture of different chemical compounds (Jager et al., 1996). These components can vary in kind and concentration which may lead to variation in individual internal activities of the components. The internal interaction of the active components may results in some synergistic or antagonistic effects which may impose interference on pharmacokinetic and pharmacodynamic activities of active ingredients (Juergens et al., 1998).
5. Conclusion
The present study confirmed the traditional uses of M. cordata, claiming that the essential oil and the leaf extracts of the species have potent anti-nociceptive, anti-inflammatory and antipyretic properties. The usefulness of this species for treatment of pain, inflammations, pyrexia over and above dengue fever could be supported and enhanced if combination of anti-nociceptive, anti-inflammatory and antipyretic properties is taken into consideration. A number of mechanisms including corticosteroid-like effects, discharge of endogenous glucocorticoids, interaction with prostaglandin biosynthesis or some other inflammatory mediators are associated with anti-inflammatory action of medicines (Barnes et al., 1990) and they should be examined for these fractions henceforth. Our phytochemical and biological research on Mikania cordata is ongoing to isolate and characterize the active chemical constituent(s) responsible for these activities.
Acknowledgements
This work was supported by the Centre for Biomedical Research, University of Dhaka and Department of Applied Chemistry & Chemical Technology, Islamic University, Kushtia, Bangladesh. The authors would like to extend their sincere appreciation to the Deanship of Scientific Research at King Saud University for its funding of the research through the research group project number RGP-195.
Footnotes
Peer review under responsibility of King Saud University.
References
- Ahmed M., Rahman M.T., Alimuzzaman M., Shilpi J.A. Analgesic sesquiterpene dilactone from Mikania cordata. Fitoterapia. 2001;72:919–921. doi: 10.1016/s0367-326x(01)00318-5. [DOI] [PubMed] [Google Scholar]
- Barnes P.J., Belivisi M.G., Rogers D.F. Modulation of neurogenic inflammation: novel approaches to inflammatory disease. Trends Pharmacol. Sci. 1990;11:185–189. doi: 10.1016/0165-6147(90)90112-l. [DOI] [PubMed] [Google Scholar]
- Botting R., Ayoub S.S. COX-3 and the mechanism of action of paracetamol/acetaminophen. PLEFA. 2005;72:85–87. doi: 10.1016/j.plefa.2004.10.005. [DOI] [PubMed] [Google Scholar]
- Chandra S., Dey P., Bhattacharya S. Preliminary in vitro assessment of anti-inflammatory property of Mikania scandens flower extract. J. Adv. Pharm. Educ. Res. 2012;2(1):25–31. [Google Scholar]
- Chandrasekharan N.V. COX-3, a cyclooxygenase-1 variant inhibited by acetaminophen and other analgesic/antipyretic drugs: cloning, structure and expression. Proc. Nat. Acad. Sci. USA. 2002;99:13926–13931. doi: 10.1073/pnas.162468699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das S.C., Bhadra S., Roy S., Saha S.K., Islam M.S., Bachar S.C. Analgesic and anti-inflammatory activities of ethanol root extract of Swertia chirata (Gentianaceae) Jordan J. Biol. Sci. 2012;5:31–36. [Google Scholar]
- De Sousa D.P. Analgesic-like activity of essential oils constituents. Molecules. 2011;16:2233–2252. doi: 10.3390/molecules16032233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dey P., Chandra S., Chatterjee P., Bhattacharya S. Neuropharmacological properties of M. scandens (L.) Willd. (Asteraceae) J. Adv. Pharm. Tech. Res. 2011;2:255–259. doi: 10.4103/2231-4040.90883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghani A. first ed. The Asiatic Society of Bangladesh; Dhaka: 1998. Medicinal Plants of Bangladesh; p. 224. [Google Scholar]
- Ghani A. second. ed. The Asiatic Society of Bangladesh; Dhaka: 2003. Medicinal Plants of Bangladesh; p. 603. [Google Scholar]
- Goyal M., Ghosh M., Nagori B.P., Sasmal D. Analgesic and anti-inflammatory studies of cyclopeptide alkaloid fraction of leaves of Ziziyphus nummularia. Saudi J. Biol. Sci. 2013;20:365–371. doi: 10.1016/j.sjbs.2013.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasan S.M.R., Jamila M., Majumder M.M., Akter R., Hossain M.M., Mazumder M.E.H., Alam M.A., Jahangir R., Arif M., Rahman S. Analgesic and antioxidant activity of the hydromethanolic extract of Mikania scandens (L.) Willd. leaves. Am. J. Pharmacol. Toxicol. 2009;41:1–7. [Google Scholar]
- Heidari M.R., Foroumadi A., Noroozi H., Samzadeh-Kermani A., Azimzadeh B.S. Study of the anti-inflammatory and analgesic effects of novel rigid benzofuran-3,4-dihydroxy chalcone by formalin, hot-plate and carrageenin tests in mice. Pak. J. Pharm. Sci. 2009;22(4):395–401. [PubMed] [Google Scholar]
- Hyun T.K., Kim H.-C., Ko Y.-J., Kim J.-S. Antioxidant, α-glucosidase inhibitory and anti-inflammatory effects of aerial parts extract from Korean crowberry (Empetrum nigrum var. japonicum) Saudi J. Biol. Sci. 2016;23:181–188. doi: 10.1016/j.sjbs.2015.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jager W., Nasel B., Nasel C., Binder R., Stimpfl T., Vycudilik W., Buchbauer G. Pharmacokinetic studies of the fragrance compound 1,8-cineol in humans during inhalation. Chem. Senses. 1996;21:477–480. doi: 10.1093/chemse/21.4.477. [DOI] [PubMed] [Google Scholar]
- Juergens U.R., Stober M., Schmidt-Schilling L., Kleuver T., Vetter H. Anti-inflammatory effects of eucalyptol (1,8-cineole) in bronchial asthma: inhibition of arachidonic acid metabolism in human blood monocytes ex vivo. Eur. J. Med. Res. 1998;3:407–412. [PubMed] [Google Scholar]
- Kamatou G.P.P., van Zyl R.L., van Vuuren S.F., Viljoen A.M., Figueiredo A.C., Barroso J.G., Pedro L.G., Tilney P.M. Chemical composition, leaf trichome types and biological activities of the essential oils of four related Salvia species indigenous to Southern Africa. J. Essen. Oil Res. 2006;18:72–79. [Google Scholar]
- Koster R., Anderson M., Beer E.J. Acetic acid for analgesic screening. Fed. Proc. 1959;18:412–416. [Google Scholar]
- Le Bars D., Gozariu M., Cadden S.W. Animal models of nociception. Pharmacol. Rev. 2001;53:597–652. [PubMed] [Google Scholar]
- Mosaddik M.A., Alam K.M. The antiulcerogenic effect of an alkaloidal fraction from Mikania cordata on diclofenac sodium-induced gastrointestinal lesions in rats. J. Pharm. Pharmacol. 2000;52:1157–1162. doi: 10.1211/0022357001774930. [DOI] [PubMed] [Google Scholar]
- Nayeem A.A., Khatun A., Rahman M.S., Rahman M. Evaluation of phytochemical and pharmacological properties of Mikania cordata (Asteraceae) leaves. J. Pharmacogn. Phytother. 2011;3(8):118–123. [Google Scholar]
- Ojewole J.A.O. Analgesic, anti-inflammatory and hypoglycaemic effects of ethanol extract of Zingiber officinale (Roscoe) Rhizomes (Zingiberaceae) in mice and rats. Phytother. Res. 2006;20:764–772. doi: 10.1002/ptr.1952. [DOI] [PubMed] [Google Scholar]
- Partha P., Hossain A.B.M.E. Ethnobotanical investigation into the Mandi ethnic community in Bangladesh. Bangladesh J. Plant Taxon. 2007;14(2):129–145. [Google Scholar]
- Rahmatullah M., Mukti I.J., Fahmidul Haque A.K.M., Mollik M.A.H., Parvin K., Jahan R., Chowdhury M.H., Rahman T. An Ethnobotanical Survey and pharmacological evaluation of medicinal plants used by the Garo tribal community living in Netrakona district, Bangladesh. Adv. Nat. Appl. Sci. 2009;3(3):402–418. [Google Scholar]
- Rahmatullah M., Mollik M.A.H., Azam A.T.M.A., Islam M.R., Chowdhury M.A.M., Jahan R., Chowdhury M.H., Rahman T. Ethnobotanical survey of the Santal tribe residing in Thakurgaon district, Bangladesh. Amer.-Euras. J. Sustain. Agric. 2009;3(4):889–898. [Google Scholar]
- Rang H.P., Dale M.M., Ritter J.M. third ed. Churchill Livingstone; London: 1998. Pharmacology; pp. 494–498. [Google Scholar]
- Rao M.R., Rao Y.M., Rao A.V., Prabhkar M.C., Rao C.S., Muralidhar N. Anti-nociceptive and anti-inflammatory activity of a flavonoid isolated from Caralluma attenuata. J. Ethnopharmacol. 1998;62:63–66. doi: 10.1016/s0378-8741(98)00048-8. [DOI] [PubMed] [Google Scholar]
- Sastri, B.N., 1962. The Wealth of India. A Dictionary of Indian Raw Materials and Industrial Products. Raw Materials. vol. 6, Council of Scientific and Industrial Research, New Delhi, India, pp. 483.
- Siddiqui S.A., Islam R., Jamal A.H.M., Parvin T., Rahman A. Chemical composition and antifungal properties of the essential oil and various extracts of Mikania scandens (L.) Willd. Arabian J. Chem. 2017;10(2):2170–2174. [Google Scholar]
- Silva J., Abebe W., Sonsa S.M., Duarte V.G., Machado M.I.L., Matos F.J.A. Analgesic and anti-inflammatory effects of essential oil of Eucalyptus. J. Ethnopharmacol. 2003;89:277–283. doi: 10.1016/j.jep.2003.09.007. [DOI] [PubMed] [Google Scholar]
- Sturtevant, W., 1954. The Mikasuki Seminole: Medical Beliefs and Practices. A Ph.D. Thesis submitted to Yale University, 166 pp.
- Suyenaga E.S., Reche E., Farias F.M., Schapoval E.E.S., Chaves C.G.M., Henriques A.T. Anti-inflammatory investigation of some species of Mikania. Phytother. Res. 2002;16:519–523. doi: 10.1002/ptr.908. [DOI] [PubMed] [Google Scholar]
- Turner R.A. Academic Press; New York & London: 1965. Screening Method in Pharmacology; p. 268. [Google Scholar]
- Vane J. The evolution of non-steroidal anti-inflammatory drugs and their mechanisms of action. Drugs. 1987;33(suppl. 1):18–27. doi: 10.2165/00003495-198700331-00005. [DOI] [PubMed] [Google Scholar]
- Winter C.A., Risley E.A., Nuss G.W. Carrageenin-induced edema in hind paw of the rat as an assay for anti-inflammatory drugs. Proc. Soc. Exp. Bio. Med. 1962;111:544–547. doi: 10.3181/00379727-111-27849. [DOI] [PubMed] [Google Scholar]