Abstract
This study was conducted with the aim of determining the chemical, biochemical properties, and antimicrobial capabilities of some of the monofloral honeys produced in Turkey. In this study, 23 different monofloral honey samples were obtained from diverse geographical regions of Turkey. Floral origin of the honey samples was determined by melissopalinological analyses. Additionally, antioxidant properties were determined. To determine the antioxidant properties of honey samples, four test methods of total phenolic content, DPPH, iron reduction power and β-carotene linoleic acid emulsion method were used. As a result of the antioxidant activity analysis among the honey samples, rhododendron and parsley honey showed most prominent results in terms of the amount of phenolic compounds and antioxidant activity. On the other hand, acacia and citrus honey samples showed least antioxidant activity. A positive correlation was determined between four methods. Differences between antioxidant activities of honey samples were significantly found (P < 0.01).
Keywords: Monofloral honey, Antioxidant, DPHH, TPC
1. Introduction
Honey is a natural dietary antioxidant whose components are responsible for the redox properties are likely to be flavonoids, phenolic acids, enzymes, vitamins and minerals such as copper and iron (Chua et al., 2013). Honey can originate from single or multiple plant species, and its biochemical composition is affected by the floral source (Elbanna et al., 2014). Due to features such as its geographical position, climatic conditions and three seasons of the year being suited to honey production, Turkey is one of the richest regions of the world in terms of honey production and variety. It is home to a wide variety of nectar and honeydew honey types, both unifloral and multifloral (Can et al., 2015).
The melissoplaynological study is an effective method to determine the pollen inside the honey sample. Taxa of the pollen are usually used to indicate the floral nectar sources utilized by bees to produce honey (Louveaux et al., 1978, Moar, 1985). The relative pollen frequency is usually used to verify a honey sample as to the major and minor nectar sources.
A free radical is an atom, molecule or compound that is highly unstable because of its atomic or molecular structure. Free radicals are very reactive as they attempt to pair up with other molecules, atoms, or even individual electrons to create a stable compound (Wu and Cederbaum, 2003). Therefore reactive oxygen species (ROS) occur and free radicals cause molecular transformations and gene mutations in many types of organisms. This is called oxidative stress and is well known to cause many diseases (Küçük et al., 2007). Honey has been an important food for humans since the beginning. The relation between bees and humans started as early as the stone age. It has been used in alternative medicine since that time, and its role was to treat burns, gastrointestinal disorders, asthma, infections and some chronic wounds. Honey maintains an important place in terms of nutrients as it is known to be rich in antioxidants, including glucose oxidase, catalase, ascorbic acid, phenolic compounds, carotenoids, organic acids, amino acids and proteins. The botanical origin of honey is one of its main quality parameters, and it has been reported that the composition and antioxidant capacity of honey depend on the floral source used to collect nectar, seasonal and environmental factors, as well as processing(Kıvrak and Kıvrak, 2017). These factors may also have an effect on the honey composition and antioxidant activity. Price depends on the quality and is also related to the floral origin. Over the past two decades, intensive studies have been conducted on the effects of free oxygen radicals, known as experimental and clinical cues, reactive oxygen species (ROS) and reactive nitrogen species (RNS) (Halliwell and Gutteridge, 2015). Many studies have shown that oxidative stress causes many diseases such as cancer formation, inflammation, aging (Di Mascio et al., 1991), the pathogenesis and progression of diabetes (Lau et al., 2013), cardiovascular diseases, weakening of the immune system, degenerative diseases of the nervous system (Diplock et al., 1998, Koca and Karadeniz, 2003), heart and lung diseases, cataracts (Aras, 2006).
The adverse effects on human health of synthetic drugs and chemicals in the age of technology have encouraged the use of more traditional and natural methods (Can et al., 2015). Consuming nutrients rich in natural antioxidants, may be effective in the prevention and treatment of chronic diseases that have increased in recent years. These antioxidant compounds can function as an endogenous cellular antioxidant defense against free radicals (Bozdogan Konuskan and Mungan, 2016, Cardozo et al., 2013, Kang et al., 2008, Kelsey et al., 2010, Lau et al., 2013). Honey with rich antioxidant content is one of the characteristics according to the studies (Alves et al., 2013, Da Silva et al., 2013, Isla et al., 2009, Wilczyńska, 2014). Honey presents several biological activities and its use as a medicine has been known since ancient times. Honey has antimicrobial, antiviral, antiparasitic, antiinflammatory, antimutagenic, anticancer, and immuno-suppressive activities (Bogdanov et al., 2008).
Therapeutic techniques utilizing and integrating bee products that protect and strengthen the immune system are known as apitherapy. Honey, a rediscovered natural product, has also begun being used for numerous purposes (Can et al., 2015). Honey is a natural product and consists of a highly concentrated complex solution of sugars. Honey contains other minor constituents such as minerals, proteins, amino acids, enzymes, vitamins, organic acids, phenolic compounds, volatile compounds, and carotenoids and the composition depends mainly on its botanical and geographical origin and, to a lesser extent, on its processing, handling, and storage (Manyi-Loh et al., 2011).
The goal of this study was to determine the antioxidant capacity of some monofloral honey types produced from the diverse regions of Turkey. To determine the origin of honey, melissoplaynological analyses were done before determining the antioxidant capacity of samples.
2. Materials and methods
2.1. Chemicals and instruments
Unless otherwise noted, all reagents and chemicals used were analytical grade from Sigma Chemical Company (St. Louis, MO, USA). A Shimadzu UV-1208 model UV–VIS spectrophotometer (UV-1280 Multipurpose UV-Visible Spectrophotometer, Shimadzu) was used for absorbance measurements.
2.2. Honey samples
Honey samples were directly obtained from the beekeepers during the period of 2015–2016, living across different locations throughout Turkey and registered to the Turkish Beekeeping Associations. A total of 23 monofloral honey samples, not subjected to any heating process, were collected for analyses. Honey samples of different floral sources were obtained from the local beekeepers living in the different regions of Turkey as apparent in Table 1 and Fig. 1. All honey samples were kept at room temperature (24 ± 2) throughout the process of analysis.
Table 1.
Honey samples, location, pollen frequency (%).
| No | Honey types | Location | Pollen frequency (%) |
|---|---|---|---|
| H1 | Anise (Pimpinella sp.) | Antalya | 0.45 |
| H2 | Chestnut (Castanea sp.) | Ordu | 0.75 |
| H3 | Astragalus (Astragalus sp.) | Konya | 0.52 |
| H4 | Sainfoin (Onobrychis sp.) | Van | 0.47 |
| H5 | Wild Mint (Mentha sp.) | Istanbul | 0.51 |
| H6 | Cesme thyme (C. Capitatus) | Izmir | 0.63 |
| H7 | Acacia (R. pseudoacacia) | Ordu | 0.54 |
| H8 | Cedrus (C. libani) | Antalya | 0.58 |
| H9 | Cotton (Gossypium sp.) | Diyarbakır | 0.53 |
| H10 | Thyme (Thymus sp.) | Batman | 0.48 |
| H11 | Euphorbia (Euphorbia sp.) | Mardin | 0.60 |
| H12 | Linden (Tilia sp.) | Ordu | 0.66 |
| H13 | Eucalyptus (Eucalyptus sp.) | Adana | 0.73 |
| H14 | Ferula (Ferula sp.) | Mersin | 0.47 |
| H15 | Yellowstar-thistle (C. solstitialis) | Diyarbakır | 0.46 |
| H16 | Parsley (Petroselinum sp.) | Hatay | 0.79 |
| H17 | Chasteberry (V. agnus-castus) | Izmir | 0.86 |
| H18 | Sunflower (H. Annuus) | Adana | 0.86 |
| H19 | Citrus (Citrus sp.) | Adana | 0.57 |
| H20 | Rhododendron (Rhododendron sp.) | Ordu | 0.56 |
| H21 | Strawberry tree (Arbutus sp.) | Mersin | 0.61 |
| H22 | Carob bean (C. siliqua) | Mersin | 0.46 |
| H23 | Pine honey (Marchalina hellenica) | Muğla | – |
Fig. 1.
Collected monofloral honey types produced across Turkey.
2.3. Melissopalynological analysis of honey samples
Floral nectar sources of honey samples were determined by melissapalinological analyses according to Louveaux et al., 1978, Sorkun, 2008. Accordingly, 10 g of each honey sample were dissolved in 20 mL of distilled water in a test tube. Each diluted sample was then shaken by a stirrer for 10 min. The solution was then centrifuged at 3500 rpm for 15 min and the supernatant fraction was poured off. The decanted sediment was washed with 10 ml of distilled water and re-centrifuged. Following the second centrifugation, the precipitate remaining at the bottom of the tube was infused with an added quantity of basicfucose glycerin-gelatin taken from the needle tip, and this material was then transferred onto the slide. Then, a lamella was placed on top for examination through a microscope. When one pollen type represented >45% of the total number of pollen grains, the sample was identified as a monofloral honey (Sorkun, 2008). Moar (1985) points out that since 45% of a single pollen type is the “universal minimal limit” needed for a honey to be identified as monofloral (Moar, 1985). Honey samples which had 45% or more dominant pollen, were selected and analyzed for antioxidant capacity.
2.4. Determination of antioxidant activity
2.4.1. Determination of total phenolic content (TPC)
The Folin-Ciocalteu method was used to determine the total phenolic content (TPC) (Singleton and Orthofer, 1999, Yorulmaz and Konuskan, 2017) with some modifications. Briefly, each honey sample (1 g) was dissolved in methanol (5ml) and filtered through Whatman No: 1. This solution was used (40 μl) and mixed with 2.4 ml of distilled water and 200 μl non-diluted Folin–Ciocalteu reagents for 3 min and then 0.6 ml of sodium carbonate was added (20%, Na2CO3). After incubation in the dark at 25 °C for 2 h, the absorbance of the reaction mixture was measured at 760 nm against a methanol blank using a UV-VIS Spectrophotometer (Hitachi U-1900, Japan). All measurements were made in triplicate. Gallic acid (0–1000 mg/L) was used as a standard to derive the calibration curve. The total phenolic content was expressed as mg gallic acid equivalents (GAE) per kg of honey.
2.4.2. DPPH (1,1-diphenyl-2-picrylhydrazyl) assay
The DPPH radical scavenging activity of honey samples was determined as described by Brand-Williams et al. (1995) with some modification. Briefly, eah honey sample (1 g) was dissolved in methanol (5 ml) and filtered through Whatman No: 1. Next, a 0.1 ml aliquot of each honey sample (12.5–200 mg/ml), BHT and BHA in methanol was added to 2.9 ml of 6 × 10−5 M methanolic solution of DPPH. The mixtures were shaken vigorously and left at 25 °C in the dark for 60 min. The absorbance of the solution was measured at 517 nm, using a Spectrophotometer (Hitachi U-1900, Japan) against a methanol blank. All measurements were made in triplicate. The radical scavenging activity was expressed as % DDPH inhibition, calculated by a linear regression analysis.
2.4.3. Ferric reducing/antioxidant power assay (FRAP)
The ferric reduction antioxidant power (FRAP) assay is a spectrophotometric method for the evaluation of the ability of the antioxidant for Fe3+-tripyridyltriazine complex reduction to Fe2+-tripyridyltriazine form (intense blue color) that absorbs at 593 nm. The ferric reducing power of honey samples was determined based on the method described by Oyaizu (1986) (Khiati, 2014, Oyaizu, 1986) with minor modification. The principle of this method is based on the reduction of a ferric 2,4,6-tripyridyl-s-triazine complex to its ferrous, colored form (Fe2+-TPTZ) in the presence of antioxidants (Ahmed et al., 2014). The FRAP reagent was prepared by mixing 2.5 mL of a 10 mM TPTZ (2,4,6-tripyridyl-s-triazine) solution in 40 mM HCl, 2.5 mL of 20 mM FeCl3, and 25 mL of 0.3 M acetate buffer at the pH of 3.6. It was prepared daily and was warmed to 37 °C before use. Honey (1 g) was well dissolved in 10 mL of the n-hexane-acetone mixture (6:4), and the honey solution was filtered through Whatman number 4 filter paper. An aliquot of 200 μL of honey solution was mixed with 1.8 mL of FRAP reagent, and the absorbance of the reagent mixture was measured spectrophotometrically at 593 nm after incubation for 10 min. Trolox was used for the calibration curve, and the results were expressed as milligram of Trolox equivalent (TE) per 100 g of honey.
2.4.4. β-Carotene-linoleic acid emulsion method
The antioxidant activity of methanolic honey solutions was evaluated by the β-carotene linoleate model system (Amarowicz et al., 2004, Silva et al., 2013). A solution of β-carotene (0.2 mg/ml) was prepared in chloroform and two milliliters of this solution was pipetted into a small (100 ml) round-bottom flask. After removing the chloroform under vacuum at 40 °C; 20 mg of linoleic acid, 200 mg of Tween 40 and 50 ml of distilled water were added to the flask with vigorous shaking. Aliquots (4.8 ml) of the prepared emulsion were transferred to a series of tubes containing 0.2 ml of honey samples. After placing the test tubes in a water bath at 50 °C; the absorbance of each tube steadily was measured using a spectrophotometer (Hitachi U-1900, Japan) at 470 nm by starting zero time absorbance (t = 0 min) and at 15-min intervals until the end (t = 120 min), of the experiment. BHA and BHT were used as standards. The β-carotene bleaching was calculated using the following equation:
| (1) |
where “A0” is the initial absorbance of the emulsion at time 0; “At” is the absorbance at 120 min, and “t” is the time in min. The absorbances of all the sample solutions were measured at 470 nm. The antioxidant activity was described as the mean percent inhibition of β-carotene bleaching using the equation:
| (2) |
where Rcontrol and Rsample are the bleaching rates of β-carotene in the emulsion without antioxidant and with honey samples, respectively.
2.5. Statistical analysis
All analyses were run in triplicate and the results were expressed as means ± standard deviation (SD). Statistical analyses were done by using SPSS statistical package (SPSS 22.0 for Windows; SPSS, Chicago, IL, USA). Significant differences were calculated by ANOVA test followed by the least significant difference (DUNCAN) test (p ≤ 0.05) and groups were shown with different letters as depicted in Table 2.
Table 2.
TPC, DPPH, FRAP values and β-Karoten values of analyzed honey samples (Mean ± SE).
| Honey types | TPC (mg/100 g GAE) | DPPH (mg/ml) | FRAP (mg/100 g honey) | β-Karoten (% OE) |
|---|---|---|---|---|
| H1-Anise | 113.22 ± 0.46k | 18.93 ± 0.33k | 0.0033 ± 3.3 × 10−5m | 71.57 ± 0.52h |
| H2-Chestnut | 327.60 ± 0.88d | 43.77 ± 1.22d | 0.0049 ± 8.3 × 10−5h | 85.51 ± 1.41bcd |
| H3-Astragalus | 73.08 ± 1.13m | 14.45 ± 0.60l | 0.0027 ± 3.2 × 10−5o | 86.22 ± 0.59bcd |
| H4-Sainfoin | 241.99 ± 1.97h | 23.10 ± 0.84ij | 0.0032 ± 2.1 × 10−5n | 67.17 ± 0.88ij |
| H5-Wild Mint | 34.37 ± 0.44p | 23.16 ± 1.00ij | 0.0033 ± 1.2 × 10−5mn | 72.22 ± 0.47h |
| H6-Çesme thyme | 99.76 ± 0.82k | 24.73 ± 1.06hi | 0.0033 ± 2.0 × 10−5mn | 80.15 ± 1.60e |
| H7-Acacia | 51.91 ± 1.32o | 12.72 ± 0.39l | 0.0022 ± 2.3 × 10−5p | 32.09 ± 0.13m |
| H8-Cedrus | 62.67 ± 1.76n | 24.53 ± 1.26hi | 0.0038 ± 2.7 × 10−5l | 94.87 ± 0.51a |
| H9-Cotton | 45.42 ± 1.67o | 21.73 ± 0.76j | 0.0041 ± 3.5 × 10−5jk | 84.06 ± 1.12cd |
| H10-Thyme | 89.23 ± 0.56l | 25.14 ± 0.87ghi | 0.0053 ± 2.6 × 10−5g | 68.41 ± 1.84I |
| H11-Euphorbia | 278.98 ± 4.18e | 26.46 ± 0.44gh | 0.0073 ± 2.5 × 10−5c | 75.64 ± 1.64g |
| H12-Linden | 268.81 ± 1.82f | 27.48 ± 1.05g | 0.0064 ± 2.4 × 10−5d | 83.87 ± 0.87d |
| H13-Eucalyptus | 176.81 ± 1.27j | 24.43 ± 1.54hi | 0.0041 ± 2.2 × 10−5j | 66.73 ± 1.14ij |
| H14-Ferula | 258.43 ± 1.36g | 23.00 ± 1.28ij | 0.0046 ± 2.5 × 10−5I | 50.29 ± 0.72k |
| H15-Yellowstar-thistle | 183.10 ± 2.78j | 35.22 ± 0.44f | 0.0056 ± 2.7 × 10−5e | 65.32 ± 0.99j |
| H16-Parsley | 470.70 ± 7.43a | 39.49 ± 0.52e | 0.0064 ± 3.4 × 10−5d | 86.20 ± 1.17bcd |
| H17-Chasteberry | 73.92 ± 0.76m | 18.83 ± 0.18k | 0.0040 ± 4.8 × 10−5k | 88.33 ± 0.28b |
| H18-Sunflower | 77.64 ± 0.86m | 19.24 ± 0.67k | 0.0047 ± 2.8 × 10−5i | 49.89 ± 0.36k |
| H19-Citrus | 86.00 ± 1.21l | 12.01 ± 0.35l | 0.0028 ± 2.2 × 10−5o | 44.97 ± 1.23l |
| H20-Rhododendron | 408.35 ± 4.71b | 48.95 ± 0.62c | 0.0077 ± 4.6 × 10−5b | 87.13 ± 1.26bc |
| H21-Strawberry tree | 231.52 ± 3.28I | 54.25 ± 0.71b | 0.0054 ± 4.8 × 10−5f | 78.76 ± 1.03ef |
| H22-Carob bean | 336.31 ± 3.91c | 41.53 ± 0.86de | 0.0091 ± 2.4 × 10−5a | 77.10 ± 0.38fg |
| H23-Pine honey | 283.91 ± 3.54e | 65.52 ± 0.88a | 0.0064 ± 3.0 × 10−5d | 75.15 ± 0.47g |
| Mean | 185.81 ± 13.01 | 29.07 ± 1.42 | 0.0048 ± 1.8 × 10−4 | 72.68 ± 1.62 |
3. Results
3.1. Melissopalinological analyses
The pollen content of honey samples is depicted in Table 1 with the scientific names and pollen frequency. The highest dominant pollen was measured in Sunflower and Chasteberry as 0.86, with the lowest dominant pollen found in Anise (0.45), Carob (0.46) and yellow star-thistle (0.46) honey samples. A specialty varietal known as Pine honey is produced by honey bees that collect honeydew from a scale insect species which lives on certain varieties of pine trees called “Marchalina hellenica”. A dominant pollen group was not determined by the melissopalinological examination because of its source.
3.2. Antioxidant activity analyses
The results obtained from honey samples showed that the antioxidant content of honey samples determined by the DPPH, TPC, β-Carotene-Linoleic Acid Emulsion and FRAP varied greatly among the honey types, as is apparent from Table 2. Differences between honey samples were statistically significant as presented from Table 2. A positive correlation between DPPH, TPC, β-Carotene-Linoleic Acid Emulsion and FRAP were obtained and have been depicted in Table 3.
Table 3.
The correlations between the results of TPC, DPPH, FRAP and β-Karoten methods.
| Corelation | TPC | β-Karoten | DPPH | FRAP |
|---|---|---|---|---|
| TPC | 1 | |||
| β-Karoten | 0.285** | 1 | ||
| DPPH | 0.637** | 0.377** | 1 | |
| FRAP | 0.704** | 0.326** | 0.648** | 1 |
Significant at p < 0.01.
The relation between the four methods has been found significant in terms of determining the antioxidant activity by TPC, β-Karoten and DPPH and FRAP methods as depicted in Table 2. The total phenolic content of honey samples was positively correlated with the antioxidant activities such as DPPH free radical scavenging activity (r2 = 0.64), ferric reducing antioxidant power (r2 = 0.70), and β-carotene bleaching inhibition (r2 = 0.29). The highest correlation has been found between FRAP and TPC methods as 0.70, but the lower correlation has been determined between the β-Karoten and TPC as 0.29 as shown in Table 3.
3.2.1. Total phenolic content (TPC)
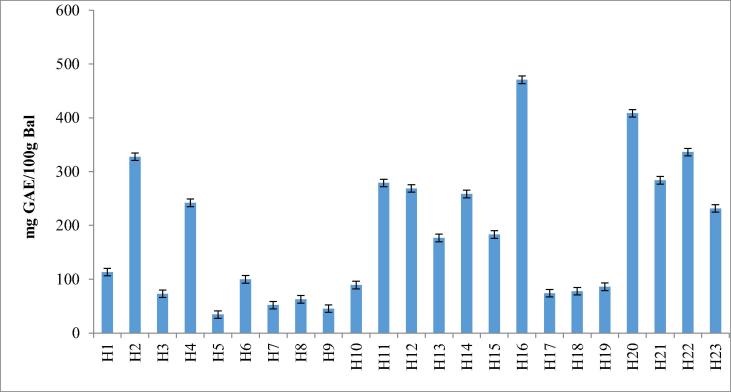
The average total phenolic content (TPC) of honey samples was determined as 185.81 ± 13.01 mg GAE/100 g honey, but varied between 470.70 ± 7.43 and 34.37 ± 0.44. The highest phenolic content of samples was found in parsley, rhododendron, carob, and chestnut honey samples as 470.70 ± 7.43, 408.35 ± 4.71, 336.31 ± 3.91 and 327.60 ± 0.88 GAE/100 g, respectively; and the lowest phenolic content was found in wild mint (34.37 ± 0.44 GAE/100 g) and acacia (51.91 ± 1.32 GAE/100 g) (Table 2, Fig. 2).
Fig. 2.
Total phenolic content of honey samples (mg GAE/100 g honey).
3.2.2. DPPH (1,1-diphenyl-2-picrylhydrazyl) assay
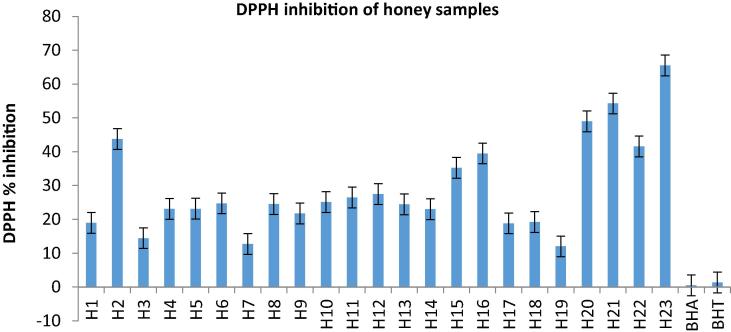
The DPPH radical scavenging effect can provide the overall hydrogen/electron donating activity of honey as well, like other dietary foods. This is based on the measurement of the reducing ability of antioxidants toward DPPH radical. The decreasing of absorbance is also accompanied by a discoloration of DPPH purple color (Alves et al., 2013). The value of the DPPH in honey samples was determined and given in Table 2 and Fig. 3. The mean of the DPPH values were obtained as 29.07 ± 1.42 mg/ml for all honey types within this study. According to the results, citrus (12.01 ± 0.35), acacia (12.72 ± 0.39), and astragalus (14.45 ± 0.60) showed the lowest DPPH value. While pine honey (65.52 ± 0.88), strawberry tree (54.25 ± 0.71), and rhododendron (48.95 ± 0.62) honey samples demonstrated the highest DPPH value(Fig. 3).
Fig. 3.
DPPH inhibition of honey samples (%).
3.2.3. Ferric reducing/antioxidant power assay (FRAP)
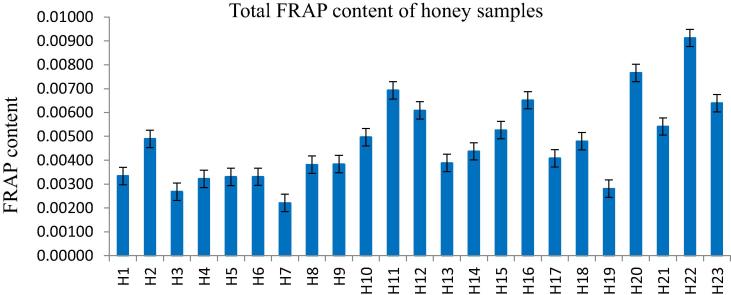
The results showed that the antioxidant content determined by the FRAP assay varied among the honey types between 0.0022 and 0.0091, and the mean of all samples were obtained 0.0048 ± 1.8 × 10−4, as given in Table 2. The FRAP assay showed a difference in antioxidant reduction profiles of honey samples, suggesting that honey samples with higher TP content are the monofloral samples of carob (0.0091 ± 2.4 × 10−5), rhododendron (0.0077 ± 4.6 × 10−5), euphorbia (0.0073 ± 2.5 × 10−5), linden (0.0064 ± 2.4 × 10−5), parsley (0.0064 ± 3.4 × 10−5 ppm), and chestnut (0.0049 ± 8.3 × 10−5). Otherwise, acacia (0.0022 ± 2.3 × 10−5), astragalus (0.0027 ± 3.2 × 10−5), and citrus (0.0028 ± 2.2 × 10−5) demonstrated lower FRAP values (Fig. 4).
Fig. 4.
The FRAP value of honey samples.
3.2.4. β-Carotene-linoleic acid emulsion method
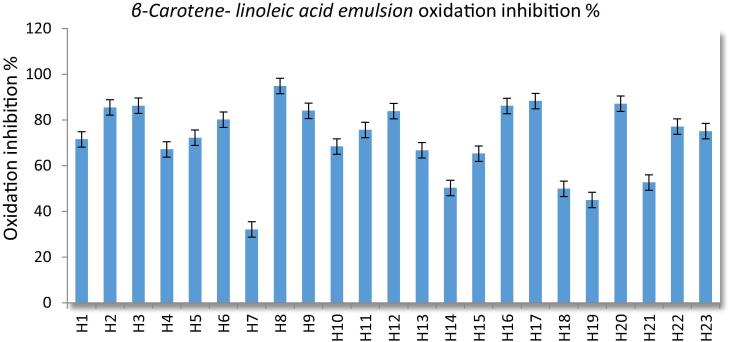
The total content of carotenoids (β-Carotene-Linoleic Acid) ranged between 32.09 and 94.87 and mean of all samples were obtained as 72.68 ± 1.62. The highest β-Carotene-Linoleic Acid content of honey samples obtanined from cedrus, chasteberry and rhododendron honey samples as 94.87 ± 0.51, 88.33 ± 0.28 and 87.13 ± 1.26, otherwise, lowest values obtained from acacia, citrus and sunflower as 32.09 ± 0.13, 44.97 ± 1.23 and 49.89 ± 0.36, respectively (Table 2, Fig. 5).
Fig. 5.
Honey samples and standard inhibition % values.
4. Discussion
4.1. Total phenolic content (TPC)
Total Phenol Content (TPC) is considered a fast and simple method to determine the total phenol content in honey. It was reported that TPC was sensitive enough for total phenol estimation in honey samples (Al et al., 2009). There is a positive correlation coefficient between total phenolic, DPPH, FRAP and β-Carotene methods indicating that these compounds are mainly responsible for the antioxidant capacity of honey.
Chua et al. (2013) has found a positive correlation between the antioxidant capacity of honey samples and their biochemical contents; such as total phenol, total flavonoid content and total water soluble vitamins (B1 vitamin, B2, B3, B9, B12 ve C vitamin) in honey samples produced in Malaysia. Many components (such as catalase, phenolic acids, flavonoids, carotenoids, proteins, organic acids, ascorbic acid, amino acids, B1, B2, B3, B9, B12, C vitamins, and maillard reaction products) of honey samples that are effective in exhibiting antioxidant activity are enclosed in the scientific literatures(Al-Mamary et al., 2002, Frankel et al., 1998, Gheldof et al., 2002, Nasuti et al., 2006, Schramm et al., 2003, Vela et al., 2007, Wang et al., 2011).
Positive relationships were significantly found between TPC and antioxidant activity in honey samples (TPC/DPPH r = 0.637, p < 0.01), (TPC/FRAP r = 0.704, TPC/β-Caroten r = 0.285 p < 0.01) (Table 3). Similar to this study, positive results of the TPC and antioxidant activity relationship have also been declared by various other studies (Alvarez-Suarez et al., 2010, Alves et al., 2013, Beretta et al., 2005, Bertoncelj et al., 2007, Blasa et al., 2006, Chua et al., 2013, Da Silva et al., 2013, Silici et al., 2010). In addition, we found that the total phenolic substances and antioxidant activities were higher in dark colored honey; such as chestnut, euphorbia, and parsley honey samples when compared to the light colored honey samples such as acacia, citrus and cotton honey samples (Table 2). Honey samples from different geographical origins, such as dark honeys (heather, eucalyptus, arbutus and locust), usually demonstrate higher total phenol content than light honeys (i.e. rosemary and orange) which was directly correlated with their higher reduction power (Alves et al., 2013, Ferreira et al., 2009). Similar results were obtained by Küçük et al. (2007), in honey samples as the following the order: chestnut > heterofloral honey > rhododendron honey with respect to TP contents. Attanzio et al. (2016) obtained a similar result with the current study. They found the lowest TP content in acacia honey samples as 21.1 mg AAE/100 g., but obtained high value in dark honey samples like ferula (93.7 ve 110.2) and astragallus (69.4).
The results show that parsley and rhonodendron honey were among the honey samples with the highest TPC among other honey samples and these samples showed significant effects in all antioxidant activity tests. The reason for this high results might be because of both honey samples have been used different antioxidant activity pathway in the experiment (Dorman et al., 2003). Therefore, these honey samples are thought to contain high amounts of various phenolic compounds that operate different antioxidant activity mechanism. However, this was literally different for the astragallus, chasteberry and cedrus honey samples. Although astragalus had lowest TPC, DPPH ve FRAP activity, it was between the honey samples which have high β-caroten linoleic acid emulsion activity. Unlike cedrus honey (whose samples were among the honey samples which had highest DPPH and FRAP activity) which showed the lowest β-Caroten linoleic acid emulsion activity. While chasteberry honey samples had low TPC acticity, it demonstrated high β-caroten linoleic acid emulsion. The TPC in rhododendron honey samples (Table 3) has been found higher when compared to the results determined by Küçük et al. (2007) (132 mg catechin/100 g honey) and Silici et al. (2010) (0.24–141.83 mgGAE/100 g). Similarly, the TPC in parsley honey samples was found higher than results which announced by Bucak (2011) as 12.163 mg GAE/100 g. Although the highest TPC content has been found in parsley honey in the present study, there is limited literature about the antioxidant activity of this honey. The antioxidant activity (51.91 mg GAE/100 g) of acacia honey sample determined in the present study has been found higher than the results determined by Bertoncelj et al., 2007, Wilczyńska, 2014, Kowalski, 2013, Can et al., 2015, Attanzio et al., 2016, as 4.48, 32.54–40.55, 38.29, 16.02, 18.2, respectively. However; the total phenolic content of acacia has been found lower than the results obtained by Al-Mamary et al., 2002, Chua et al., 2013 as 100.12–246.21 mg GAE/100 g and 196.5 mg GAE/100 g from acacia honey samples produced from Yemeni and Malaysia countries, respectively.
Similar antioxidant activity of TPC content was also apparant in eucalyptus honey samples as well. TPC value of the eucalyptus honey sample measured 176.81 mg GAE/100 g. This value has been found higher when compared to results determined by Attanzio et al. (2016) (36.5–110.3) and Bueno-Costa et al. (2016) (87.83). Eucalyptus honey samples produced in Sicily (Italy) and Brazil, respectively, have been found to be similar with Ouchemoukh et al. (2007) (147–185 mg GAE/100 g) produced in Algeria.
The results revealed by Alves et al. (2013) showed that orange honey samples demonstrated lower TP contents, but thyme honey demonstrated similar TP values with this study. Otherwise noted, dark honey samples like arbutus, locust podshrub, and heather honey samples demonstrated higher TP content. Similar results obtained in the present study where orange honey samples showed lower TP content and dark honey samples, such as chestnut, parsley, strawberry tree, honeydew, and carob demonstrated higher TP value. This indicate that the antioxidant activity of honey was higher for monofloral honey from floral species such as castanea, eucalyptus, parsley, rhododendron, carob, and honeydew (Marchalina hellenica) from different geographical locations in present study.
Kaygusuz et al. (2016) also analyzed several different Turkish monofloral honeys and their antioxidant activities. They determined TPC in the following honeys: chestnut (524–1050 mg GAE/kg), pine (586–746 mg GAE/kg), astragalus (420–751 mg GAE/kg), and acacia (98–122 mg GAE/kg). The results obtained in this review were higher than the results obtained from similar samples demonstrated in this present study. The antioxidant activity of chestnut, astragallus, rhododendron, pine honey, acacia, linden and chasteberry honey samples in a study conducted by Can et al. (2015) across Turkey were found lower than the results demonstrated in the similar samples of this study: H2, H3, H20, H23, H7, H12 and H17 respectively.
4.2. DPPH (1,1-diphenyl-2-picrylhydrazyl) assay
The activity of the DPPH varied significantly among the honey samples (Table 2) (Fig. 3). The highest antioxidant activity was observed in the pine honey sample (H23) as 65.52 mg/ml, whereas the lowest activity was observed in the citrus honey sample (H19) as 12.01 mg/ml. Noor et al. (2014) found similar results (2.85–39.86 mgQEA·100 g−1) in a variety of honey samples across Pakistan. The antioxidant capacity of honey and of its components pose as useful parameters to correlate phytochemical determinations (Alvarez-Suarez et al., 2010). The results obtained by Meda et al. (2005) regarding antioxidant activity of honey originating in Burkina Faso, a country in Africa, was 12.94 mgQEA·100 g−1, and demonstrated sincere similarities to acacia honey samples in this present study. However, the results obtained by Bueno-Costa et al. (2016) in eucalyptos honey samples (9.98 mgQEA/100 g−1) were higher than the results of the eucalyptus honey sample in this study. The DPPH content determined by Attanzio et al. (2016) in astragallus and acacia honey samples as 17.5, and 24.1 μmolTE/100 g were similar with the results obtained in this study; but the DPPH content of the following samples: honeydew (235.3–226.2 μmolTE/100 g), ferula 9150.4–114.2 μmolTE/100 g), citrus (196.4 μmolTE/100 g), and eucalyptus (180.3–194.3 μmolTE/100 g) measured higher than the results obtained in this study.
Estevinho et al. (2008) demonstrated that dark honeys had DPPH inhibition values above 70% and light honeys demonstrated inhibition values below than 40%. Similar to their study, Silici et al. (2008) studied DPPH inhibition for 50 rhododendron honey samples in which about half of those samples revealed DPPH inhibition values higher than 50%. In following studies, Silici et al. (2010) indicated that radical scavenging activity (inhibition %) of the rhododendron honey measured between 2.30% and 90.73%. Kowalski (2013), also found the DPPH (inhibition %) value of the honeydew, linden, and acacia honey samples as 86.91, 62.37 and 23.96, respectively. All results founded by Kowalski (2013) were similar when compared to the results of this study.
Alves et al. (2013) expressed that DPPH inhibitions have been above 50% for analyzed honey samples. However, several samples of rosemary (4.5–59.3%), orange (8.8–23.2%), thyme (35.8–47.3%), and eucalyptus (27.7%) demonstrated DPPH inhibition below 50%. They found the DPPH value in citrus honey to be between 8.8 and 23.2%; thyme honey sample measured between 35.8 and 47.3%; eucalyptus honey sample 27.7%; strawberry tree honey sample 64.2%; and carob honey sample measured 61.6%.
Özcan and Ölmez (2014) determined the DPPH values at 1 and 2 years in honeydew honey samples as 0.38 ve 0.44, cotton honey samples as 0.75 ve 1.29, cedrus honey samples as 0.43 ve 0.34, and yellow-star thistle honey samples as 0.27–0.56 mg/ml. All these honey samples demonstrated higher DPPH content than similar honey samples analyzed for DPPH in this study.
Wilczyńska (2014) analyzed 10 different monofloral and multifloral honey samples and found the DPPH content of honey samples to be higher in respect to antioxidant activity in the following order: acacia < goldenrods < rape < lime < nectar-honeydew < multifloral < buckwheat < honeydew < phacelia < heather.
The antioxidant activity of honey samples of Polish origin were found that the specific content for honeydew honey samples was measured at 58–72 mg GAE/100 g, 72–83% DPPH, 9–12% ABTS+; linden honey samples 45–47 mg GAE/100 g, 63–65% DPPH, 17–27% ABTS+, acacia honey samples measured 32–40 mg GAE/100 g, 25–36% DPPH, 2–13% ABTS+, and for buckwheat honey samples measured 87–180, 56–100% DPPH, 19–30% ABTS+, respectively (Wilczyńska, 2010). In this present study, the total polyphenols content of honeydew was similar. However, total polyphenols content of acacia honey samples and linden honey samples was lower.
4.3. Ferric reducing/antioxidant power assay (FRAP)
The FRAP assay provides a direct estimation of reductants in a sample such as honey; and is based on the ability of the analyte to reduce the Fe3+/Fe2+ couple. The darker honey samples, like carob, showed the highest ferric ion reduction capacity; whereas acacia honey measured as the lowest one, according to the results of this present study. Can et al. (2015) has found the FRAP value in honey samples of chestnut as 4.30 lmol FeSO4_7H2O/g, astragalus 0.66 lmol FeSO4_7H2O/g, rhodendron 0.67 lmol FeSO4_7H2O/g, pine honey 1.48 lmol FeSO4_7H2O/g, acacia 0.64 lmol FeSO4_7H2O/g, linden 0.86 lmol FeSO4_7H2O/g, and chastaberry 0.67 lmol FeSO4_7H2O/g. Their results demonstrate similarities to the FRAP values when compared to similar samples in this present study. It is thought that light color honey contains lower value phenolic contents, while dark honey contains high value phenolic content as mostly seen in honey samples in this study.
Alves et al. (2013) determined the highest FRAP value for the dark honey samples such as carob (1326.7 mM Fe (II)), strawberry tree (1312.8 mM Fe (II)), eucalyptus (953.1 mM Fe (II)), and lowest FRAP value in light honey samples such as citrus (316.8–636.3 mM Fe (II)) and thyme (530.7–785.6 mM Fe (II)) similarly with the present study. Authors indicate that dark honeys such as, heather, arbutus and locust podshrub, showed higher TP content than light honeys (rosemary and orange) which was directly correlated with their higher reduction power. These results are concordant with the TPC and FRAP value results in present study. Similarly, Kus et al. (2014) obtained the 0.6 mmol Fe (II)/kg FRAP value for the acacia honey, and 1.4 mmol Fe (II)/kg FRAP value for the linden honey, and Lachman et al. (2010) determined high FRAP value for linden and honeydew honey samples as 415.59 and 776.05 mg AAE/kg, respectively. These results are similar with previous results of TPC, showing the phenolics that reacts in Folin–Ciocalteu reaction has a similar.
4.4. β-Carotene-linoleic acid emulsion method
The free linoleic acid radical which forms on the abstraction of a hydrogen atom from one of its methylene groups attacked the β-carotene molecules (which then loses the double bonds; and therefore, its characteristic orange color). In term of inhibition of β-carotene bleaching activity, almost all of the honey samples expressed high content of reductant against oxidative damage, except acacia and sunflower samples as seen in Table 2 in this present study. The lowest β-carotene bleaching activity values determined in acacia honey samples were found to be lower than the results obtained by Chua et al. (2013) as 74.66%. Acacia and sunflower honey samples showed not only lower antioxidant activity in the β-carotene-linoleic acid emulsion; but these samples also showed low antioxidant activity in scavenging and ferric-reducing mechanism, total phenolic content, and DPPH values.
Chua et al. (2013) determined the β-Carotene-linoleic acid by the CIB method in tualang, gelam ve acacia honey samples as 35.81%, 67.41% ve 74.66%, respectively. Also, they obtained a positive correlation between the antioxidan activity and the total phenolic content similar with this study.
Boussaid et al. (2013) analyzed total carotene content in 6 monofloral varietals of honey of Tunisian origin: mint, horehound, eucalyptus, thyme, rosemary, and orange- and reported higher contents in orange honey (4.72 mgb-carotene·Kg−1). All monofloral samples in this study provided not only carotenoid contents within the limit of the detection method, but also demonstrated high content in this present study. Carotenoids are a group of pigments that are responsible for the rich colors found in many fruits and vegetables. It may also be related to honey coloring as well. Honeys which normally have extra light colors (between 9 and 17 mm Pfund), have low carotenoid rates (Alvarez-Suarez et al., 2010, Ferreira et al., 2009). This relationship was observed in this current study as honey samples which were slightly colored, such as acacia (32.09), orange (44.97), and sunflower (49.89), demonstrated low TCC. However, the honey samples with strongly colored hues, such as cedrus (94.87), chasteberry (88.33), rhododendron (87.13), astragalus (86.22), parsley (86.20), and chestnut (85.51), demonstrated high TCC values. Antioxidant activities of pine and cedar honeys, respectively, were higher from the darker colored honeys(Özcan and Ölmez, 2014). Pita-Calvo et al. (2017) indicate that the darker the color, the higher the antioxidant properties of honeydews are than those of most blossom honeys. The results of β-Carotene-linoleic acid determined by this study show that total content of carotenoids is not depended on the color of honey samples.
5. Conclusion
It is thought that these differences are due to the utilization of different radical scavenging pathways of the phenolic content and chemical structures of honey samples. As a matter of fact, scientists have announced that the differences in chemical structure of phenolic substances (the number of the OH and CH3O groups added to the aromatic compound, position, and the structure of the side chains) has a key role in the antioxidant activity of phenolic acids (Antolovich et al., 2004, Natella et al., 1999, Pekkarinen et al., 1999). These results indicate that the total phenolic content is not enough by itself to determine antioxidant activity.
According to several scientists, phenolic contents of honey are responsible for antioxidant activity (Beretta et al., 2005, Da Silva et al., 2013, Wang et al., 2011); but others are of the opinion that TPC of honey cannot be related as positive to the its antioxidant activity at all times(Al-Mamary et al., 2002, Küçük et al., 2007). Because the antioxidan activity of honey samples is depend on the floral origine, geogropichal origine, humidity, temperature, climate and environment conditions as cited by scientist. Similar results were found in the rhododendron honey sample in this study, as well. Although the total phenolic content of the rhododendron honey sample was high, β-carotene content was the only highest antioxidant activity test in comparison to the others. This could have originated due to different chemical bonds and structures apart from the different antioxidant content measuring mechanism. Similarly, Küçük et al. (2007) is of the opinion that the incompatibility between the amount of phenolic substance and the antioxidant activity values are explained by the different radical scavenging activities of different types of polyphenols.
At the end of the study, 5 honey samples showing the highest antioxidant activity, and 5 samples showing the lowest antioxidant activity were expressed according to the individual methods and emphasize the antioxidant activities of the diverse honey samples. Ultimately, the most prominent honey samples are listed in the following order: parsley > rhododendron > carob > chestnut > pine honey in terms of the total phenolic contents. The lowest phenolic content was obtained from the honey samples in the following order: wild mint < cotton < acacia < astragallus < chasteberry.
Similarly, the optimum value of DPPH in honey samples was obtained in the following order: pine honey > strawberry > rhododendron > chestnut > parsley. And the lowest DPPH values were obtained in honey samples in the following order: citrus > acacia > astragallus > chasteberry > anise. Similar results were also seen in the FRAP method and β-Carotene, as well. In the FRAP method, honey samples carob > rhododendron > euphorbia > linden > parsley showed the highest FRAP values, respectively; whereas acacia < astragallus < citrus < sainfoin < wild mint honey samples demonstrated the lowest FRAP values, respectively.
The following honey samples demonstrated the highest β-Carotene values: cedar > chasteberry > rhododendron > astragallus > parsley; while acacia < citrus < sunflower < ferula < cedar measured as the lowest β-Carotene values. When the results were evaluated, dark colored honey samples such as parsley, chestnut, strawberry, pine honey, euphorbia, linden, cedar, and astragalus generally showed high activity; whereas light colored honey samples, such as acacia, citrus, sunflower, cotton, and chasteberry demonstrated lower antioxidant activity. Interestingly, although the rhododendron honey sample was not dark, it generally measured high antioxidant activity.
Acknowledgement
This study a part of the PhD thesis and was supported by the MKU BAP “Mustafa Kemal University Scientific Research Project (Project No: 409).
Footnotes
Peer review under responsibility of King Saud University.
References
- Ahmed, M., Sahile, S., Subramanian, C., 2014. Evaluation of Antibacterial Potential of Honey Against Some Common Human Pathogens in North Gondar Zone of Ethiopia 2, 286–295.
- Al-Mamary M., Al-Meeri A., Al-Habori M. Antioxidant activities and total phenolics of different types of honey. Nutr. Res. 2002;22:1041–1047. [Google Scholar]
- Al M.L., Daniel D., Moise A., Bobis O., Laslo L., Bogdanov S. Physico-chemical and bioactive properties of different floral origin honeys from Romania. Food Chem. 2009;112:863–867. [Google Scholar]
- Alvarez-Suarez J.M., Tulipani S., Díaz D., Estevez Y., Romandini S., Giampieri F., Damiani E., Astolfi P., Bompadre S., Battino M. Antioxidant and antimicrobial capacity of several monofloral Cuban honeys and their correlation with color, polyphenol content and other chemical compounds. Food Chem. Toxicol. 2010;48:2490–2499. doi: 10.1016/j.fct.2010.06.021. [DOI] [PubMed] [Google Scholar]
- Alves A., Ramos A., Gonçalves M.M., Bernardo M., Mendes B. Antioxidant activity, quality parameters and mineral content of Portuguese monofloral honeys. J. Food Compos. Anal. 2013;30:130–138. [Google Scholar]
- Amarowicz R., Pegg R.B., Rahimi-Moghaddam P., Barl B., Weil J.A. Free-radical scavenging capacity and antioxidant activity of selected plant species from the Canadian prairies. Food Chem. 2004;84:551–562. [Google Scholar]
- Antolovich M., Bedgood D.R., Bishop A.G., Jardine D., Prenzler P.D., Robards K. LC-MS investigation of oxidation products of phenolic antioxidants. J. Agric. Food Chem. 2004;52:962–971. doi: 10.1021/jf0349883. [DOI] [PubMed] [Google Scholar]
- Aras, Ö., 2006. Üzüm Ve Üzüm Ürünlerinin Toplam Karbonhidrat, Protein, Mineral Madde ve Fenolik Bilesik İçeriklerinin Belirlenmesi.
- Attanzio A., Tesoriere L., Allegra M., Livrea M.A. Monofloral honeys by Sicilian black honeybee (Apis mellifera ssp. sicula) have high reducing power and antioxidant capacity. Heliyon 2. 2016 doi: 10.1016/j.heliyon.2016.e00193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beretta G., Granata P., Ferrero M., Orioli M., Maffei Facino R. Standardization of antioxidant properties of honey by a combination of spectrophotometric/fluorimetric assays and chemometrics. Anal. Chim. Acta. 2005;533:185–191. [Google Scholar]
- Bertoncelj J., Doberšek U., Jamnik M., Golob T. Evaluation of the phenolic content, antioxidant activity and colour of Slovenian honey. Food Chem. 2007;105:822–828. [Google Scholar]
- Blasa M., Candiracci M., Accorsi A., Piacentini M.P., Albertini M.C., Piatti E. Raw Millefiori honey is packed full of antioxidants. Food Chem. 2006;97:217–222. [Google Scholar]
- Bogdanov S., Jurendic T., Sieber R., Gallmann P. Honey for nutrition and health: a review. J. Am. Coll. Nutr. 2008;27:677–689. doi: 10.1080/07315724.2008.10719745. [DOI] [PubMed] [Google Scholar]
- Boussaid A., Chouaibi M., Rezig L., Hellal R., Donsì F., Ferrari G., Hamdi S. Physicochemical and bioactive properties of six honey samples from various floral origins from Tunisia. Arab. J. Chem. 2013 [Google Scholar]
- Bozdogan Konuskan D., Mungan B. Effects of variety, maturation and growing region on chemical properties, fatty acid and sterol compositions of virgin olive oils. J. Am. Oil Chem. Soc. 2016;93:1499–1508. [Google Scholar]
- Brand-Williams W., Cuvelier M.E., Berset C. Use of a free radical method to evaluate antioxidant activity. LWT – Food Sci. Technol. 1995;28:25–30. [Google Scholar]
- Bucak S. Mustafa Kemal Üniversitesi/Fen Bilimleri Enstitüsü; Kimya Anabilim Dalı: 2011. Hatay ilinde üretilen salgı, okaliptüs, çiçek ve maydanoz ballarının antioksidan, antimikrobiyal, yağ asidi ve kalıntı analizleri. [Google Scholar]
- Bueno-Costa F.M., Zambiazi R.C., Bohmer B.W., Chaves F.C., da Silva W.P., Zanusso J.T., Dutra I. Antibacterial and antioxidant activity of honeys from the state of Rio Grande do Sul, Brazil. LWT – Food Sci. Technol. 2016;65:333–340. [Google Scholar]
- Can Z., Yildiz O., Sahin H., Akyuz Turumtay E., Silici S., Kolayli S. An investigation of Turkish honeys: their physico-chemical properties, antioxidant capacities and phenolic profiles. Food Chem. 2015;180:133–141. doi: 10.1016/j.foodchem.2015.02.024. [DOI] [PubMed] [Google Scholar]
- Cardozo, L.F.M.F., Pedruzzi, L.M., Stenvinkel, P., Stockler-Pinto, M.B., Daleprane, J.B., M, L.J., Mafra, D., 2013. Nutritional strategies to modulate inflammation and oxidative stress pathways via activation of the master antioxidant switch Nrf2. Biochimie 1525–1533. [DOI] [PubMed]
- Chua L.S., Rahaman N.L.A., Adnan N.A., Eddie Tan T.T. Antioxidant activity of three honey samples in relation with their biochemical components. J. Anal. Methods Chem. 2013 doi: 10.1155/2013/313798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Da Silva I.A.A., Da Silva T.M.S., Camara C.A., Queiroz N., Magnani M., de Novais J.S., Soledade L.E.B., de Lima E. de O., de Souza A.L., de Souza A.G. Phenolic profile, antioxidant activity and palynological analysis of stingless bee honey from Amazonas, Northern Brazil. Food Chem. 2013;141:3552–3558. doi: 10.1016/j.foodchem.2013.06.072. [DOI] [PubMed] [Google Scholar]
- Di Mascio P., Murphy M.E., Sies H. Antioxidant defense systems: the role of carotenoids, tocopherols, and thiols. Am. J. Clin. Nutr. 1991;53:194S–200S. [PubMed] [Google Scholar]
- Diplock A.T., Charuleux J.-L., Crozier-Willi G., Kok F.J., Rice-Evans C., Roberfroid M., Stahl W., Viña-Ribes J. Functional food science and defence against reactive oxidative species. Br. J. Nutr. 1998;80:S77–S112. doi: 10.1079/bjn19980106. [DOI] [PubMed] [Google Scholar]
- Dorman H.J.D., Peltoketo A., Hiltunen R., Tikkanen M.J. Characterisation of the antioxidant properties of de-odourised aqueous extracts from selected Lamiaceae herbs. Food Chem. 2003;83:255–262. [Google Scholar]
- Elbanna K., Attalla K., Elbadry M., Abdeltawab A., Gamal-Eldin H., Fawzy Ramadan M. Impact of floral sources and processing on the antimicrobial activities of different unifloral honeys. Asian Pacific J. Trop. Dis. 2014;4:194–200. [Google Scholar]
- Estevinho L., Pereira A.P., Moreira L., Dias L.G., Pereira E. Antioxidant and antimicrobial effects of phenolic compounds extracts of Northeast Portugal honey. Food Chem. Toxicol. 2008;46:3774–3779. doi: 10.1016/j.fct.2008.09.062. [DOI] [PubMed] [Google Scholar]
- Ferreira I.C.F.R., Aires E., Barreira J.C.M., Estevinho L.M. Antioxidant activity of Portuguese honey samples: different contributions of the entire honey and phenolic extract. Food Chem. 2009;114:1438–1443. [Google Scholar]
- Frankel S., Robinson G.E., Berenbaum M.R. Antioxidant capacity and correlated characteristics of 14 unifloral honeys. J. Apic. Res. 1998;37:27–31. [Google Scholar]
- Gheldof N., Wang X.-H., Engeseth N.J. Identification and quantification of antioxidant components of honeys from various floral sources. J. Agric. Food Chem. 2002;50:5870–5877. doi: 10.1021/jf0256135. [DOI] [PubMed] [Google Scholar]
- Halliwell B., Gutteridge J.M.C. Free radicals in biology and medicine. Free Radical Biol. Med. 2015 doi: 10.1016/0891-5849(92)90062-l. [DOI] [PubMed] [Google Scholar]
- Isla M.I., Zampini I.C., Ordonez R.M., Cuello S., Juarez B.C., Sayago J.E., Moreno M.I.N., Alberto M.R., Vera N.R., Bedascarrasbure E., Alvarez A., Cioccini F., Maldonado L.M. Effect of seasonal variations and collection form on antioxidant activity of propolis from San Juan, Argentina. J. Med. Food. 2009;12:1334–1342. doi: 10.1089/jmf.2008.0286. [DOI] [PubMed] [Google Scholar]
- Kang, C., Hong, C., Youm, H., Choi, S., Heo, T., 2008. Growth inhibitory activities of Siegesbeckiae glabrescens against foodborne pathogens. J. Biotechnol. 733.
- Kaygusuz H., Tezcan F., Erim F.B., Yildiz O., Sahin H., Can Z., Kolayli S. Characterization of Anatolian honeys based on minerals, bioactive components and principal component analysis. LWT – Food Sci. Technol. 2016 [Google Scholar]
- Kelsey N.A., Wilkins H.M., Linseman D.A. Nutraceutical antioxidants as novel neuroprotective agents. Molecules. 2010 doi: 10.3390/molecules15117792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khiati B. Evaluation of Physicochemical and Antioxidant Properties of Raw Honey from Algeria. J. Microb. Biochem. Technol. s1. 2014 [Google Scholar]
- Kıvrak Ş., Kıvrak İ. Assessment of phenolic profile of Turkish honeys. Int. J. Food Prop. 2017;20:864–876. [Google Scholar]
- Koca N., Karadeniz F. Serbest radikal oluşum mekanizmaları ve antioksidan savunma sistemleri. Gıda Mühendisliği Derg. 2003:32–37. [Google Scholar]
- Kowalski S. Changes of antioxidant activity and formation of 5-hydroxymethylfurfural in honey during thermal and microwave processing. Food Chem. 2013;141:1378–1382. doi: 10.1016/j.foodchem.2013.04.025. [DOI] [PubMed] [Google Scholar]
- Kus P.M., Congiu F., Teper D., Sroka Z., Jerkovic I., Tuberoso C.I.G. Antioxidant activity, color characteristics, total phenol content and general HPLC fingerprints of six Polish unifloral honey types. LWT – Food Sci. Technol. 2014;55:124–130. [Google Scholar]
- Küçük M., Kolayli S., Karaoǧlu Ş., Ulusoy E., Baltaci C., Candan F. Biological activities and chemical composition of three honeys of different types from Anatolia. Food Chem. 2007;100:526–534. [Google Scholar]
- Lachman J., Hejtmánková A., Sýkora J., Karban J., Orsák M., Rygerová B. Contents of major phenolic and flavonoid antioxidants in selected Czech honey. Czech J. Food Sci. 2010;28:412–426. [Google Scholar]
- Lau Y.S., Tian X.Y., Huang Y., Murugan D., Achike F.I., Mustafa M.R. Boldine protects endothelial function in hyperglycemia-induced oxidative stress through an antioxidant mechanism. Biochem. Pharmacol. 2013;85:367–375. doi: 10.1016/j.bcp.2012.11.010. [DOI] [PubMed] [Google Scholar]
- Louveaux J., Maurizio A., Vorwohl G. Methods of melissopalynology. Bee World. 1978;59:139–157. [Google Scholar]
- Manyi-Loh C.E., Ndip R.N., Clarke A.M. Volatile compounds in honey: a review on their involvement in aroma, botanical origin determination and potential biomedical activities. Int. J. Mol. Sci. 2011;12:9514–9532. doi: 10.3390/ijms12129514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meda A., Lamien C.E., Romito M., Millogo J., Nacoulma O.G. Determination of the total phenolic, flavonoid and proline contents in Burkina Fasan honey, as well as their radical scavenging activity. Food Chem. 2005;91:571–577. [Google Scholar]
- Moar N.T. Pollen analysis of New Zealand honey. New Zeal. J. Agric. Res. 1985;28:39–70. [Google Scholar]
- Nasuti C., Gabbianelli R., Falcioni G., Cantalamessa F. Antioxidative and gastroprotective activities of anti-inflammatory formulations derived from chestnut honey in rats. Nutr. Res. 2006;26:130–137. [Google Scholar]
- Natella F., Nardini M., Di Felice M., Scaccini C. Benzoic and cinnamic acid derivatives as antioxidants: structure-activity relation. J. Agric. Food Scem. 1999:1453–1459. doi: 10.1021/jf980737w. [DOI] [PubMed] [Google Scholar]
- Noor N., Sarfraz R.A., Ali S., Shahid M. Antitumour and antioxidant potential of some selected Pakistani honeys. Food Chem. 2014;143:362–366. doi: 10.1016/j.foodchem.2013.07.084. [DOI] [PubMed] [Google Scholar]
- Ouchemoukh S., Louaileche H., Schweitzer P. Physicochemical characteristics and pollen spectrum of some algerian honeys. Food Control. 2007;18:52–58. [Google Scholar]
- Oyaizu, M., 1986. Studies on products of browning reaction – antioxidative activities of products of browning reaction prepared from glucosamine. Eiyogaku zasshi = Jpn. J. Nutr.
- Özcan M.M., Ölmez Ç. Some qualitative properties of different monofloral honeys. Food Chem. 2014;163:212–218. doi: 10.1016/j.foodchem.2014.04.072. [DOI] [PubMed] [Google Scholar]
- Pekkarinen S.S., Stöckmann H., Schwarz K., Heinonen I.M., Hopia A.I. Antioxidant activity and partitioning of phenolic acids in bulk and emulsified methyl linoleate. J. Agric. Food Chem. 1999;47:3036–3043. doi: 10.1021/jf9813236. [DOI] [PubMed] [Google Scholar]
- Pita-Calvo C., Guerra-Rodriguez M.E., Vazquez M. Analytical methods used in the quality control of honey. J. Agric. Food Chem. 2017;65:690–703. doi: 10.1021/acs.jafc.6b04776. [DOI] [PubMed] [Google Scholar]
- Schramm D.D., Karim M., Schrader H.R., Holt R.R., Cardetti M., Keen C.L. Honey with high levels of antioxidants can provide protection to healthy human subjects. J. Agric. Food Chem. 2003;51:1732–1735. doi: 10.1021/jf025928k. [DOI] [PubMed] [Google Scholar]
- Silici S., Sagdic O., Ekici L. Total phenolic content, antiradical, antioxidant and antimicrobial activities of Rhododendron honeys. Food Chem. 2010;121:238–243. [Google Scholar]
- Silici S., Uluozlu O.D., Tuzen M., Soylak M. Assessment of trace element levels in Rhododendron honeys of Black Sea Region, Turkey. J. Hazard. Mater. 2008;156:612–618. doi: 10.1016/j.jhazmat.2007.12.065. [DOI] [PubMed] [Google Scholar]
- Silva T.M.S., dos Santos F.P., Evangelista-Rodrigues A., da Silva E.M.S., da Silva G.S., de Novais J.S., dos Santos F. de A.R., Camara C.A. Phenolic compounds, melissopalynological, physicochemical analysis and antioxidant activity of jandaíra (Melipona subnitida) honey. J. Food Compos. Anal. 2013;29:10–18. [Google Scholar]
- Singleton V.L., Orthofer R., Lamuela-Raventós R.M.B.T.-M. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods Enzymol. 1999;299:152–178. [Google Scholar]
- Sorkun K. Polenleri ve Balları; Palm Yayıncılık: 2008. Türkiye’nin Nektarlı Bitkileri. [Google Scholar]
- Vela L., de Lorenzo C., Pérez R.A. Antioxidant capacity of Spanish honeys and its correlation with polyphenol content and other physicochemical properties. J. Sci. Food Agric. 2007;87:1069–1075. [Google Scholar]
- Wang N.-F., Yan Z., Li C.-Y., Jiang N., Liu H.-J. Antioxidant activity of peanut flour fermented with lactic acid bacteria. J. Food Biochem. 2011;35:1514–1521. [Google Scholar]
- Wilczyńska A. Effect of filtration on colour, antioxidant activity and total phenolics of honey. LWT – Food Sci. Technol. 2014;57:767–774. [Google Scholar]
- Wilczyńska A. Phenolic content and antioxidant activity of different types of polish honey – a short report. Polish J. Food Nutr. Sci. 2010;60:309–313. [Google Scholar]
- Wu D., Cederbaum A. Alcohol, oxidative stress, and free radical damage. Alcohol Res. Heal. 2003;27:277–284. [PMC free article] [PubMed] [Google Scholar]
- Yorulmaz H.O., Konuskan D.B. Antioxidant activity, sterol and fatty acid compositions of Turkish olive oils as an indicator of variety and ripening degree. J. Food Sci. Technol. 2017;54:4067–4077. doi: 10.1007/s13197-017-2879-y. [DOI] [PMC free article] [PubMed] [Google Scholar]