Abstract
Hibernation is characterized by reduced metabolism and body temperature during torpor bouts. Energy reserves available during winter play an important role for hibernation and some species respond to high energy reserves with reduced torpor expression. Common hamsters are food-storing hibernators and females hibernate for shorter periods than males, probably related to larger food stores. In this study, we provided free-ranging common hamsters with sunflower seeds shortly before winter and recorded body temperature using subcutaneously implanted data loggers. We compared hibernation patterns and body mass changes between individuals with and without food supplements and analysed reproductive onset in females. Supplemented males delayed hibernation onset, hibernated for much shorter periods, and emerged in spring with higher body mass than unsupplemented ones. Additional food did not affect hibernation performance in females, but supplemented females emerged earlier and preceded those without food supplements in reproductive onset. Thus, males and females differently responded to food supplementation: access to energy-rich food stores enabled males to shorten the hibernation period and emerge in better body condition, probably enhancing mating opportunities and reproductive success. Females did not alter hibernation patterns, but started to reproduce earlier than unsupplemented individuals, enabling reproductive benefits by an extended breeding period.
Introduction
Hibernating animals save energy by reducing body temperature and metabolic rate during multiday torpor bouts1–3. During winter, they rely on body fat or food stores as energy reserves, which are accumulated before immergence into the hibernacula4–8. Accordingly, hibernators show a strict timing of the annual cycle as well as sex- and age-specific sequences in vernal emergence and autumnal immergence9–15. Males usually appear above ground in spring before females and shortly thereafter the reproductive period starts with females usually producing one litter per season12,16–18. At the end of the active season, adult males usually terminate above ground activity before adult females and, finally, juveniles enter their hibernacula13. In addition, some hibernating species show sex differences in torpor expression as males usually start to hibernate later, spend less time in torpor, or terminate hibernation earlier than females15,19–29.
In common hamsters (Cricetus cricetus), emergence and immergence sequences resemble those of other hibernators30–32. Likewise, the reproductive period starts shortly after female emergence in April, but in contrast to other hibernators, female hamsters can produce up to three litters per season, resulting in an extended reproductive period31,33–35. Furthermore, common hamsters show exceptional sex differences in hibernation patterns in that adult females delayed hibernation onset for several months and hibernated for shorter periods than males36. This could be related to larger food stores due to much more pronounced food caching activities in females compared to males37.
The availability of energy reserves was found to affect hibernation performance in several hibernating species and might relate to a cost-benefit trade-off of torpor expression6. Despite its apparent advantage of saving energy and reduced predation risk3,38–42, hibernation could also have negative effects such as immune depression43,44, ischemia45, reduced synaptic efficacy46, impaired memory retention47, oxidative stress48, or shortened telomeres49. Correspondingly, hibernating individuals with high internal or external energy reserves were found to spend less time in torpor, reduced the depth of torpor, or showed longer euthermic periods5,27,29,50–52.
In line with these findings, common hamsters were found to adjust torpor expression in relation to the availability and quality of food stores. We previously demonstrated that hamsters were less likely to hibernate when they had access to food stores compared to individuals facing unpredictable food availability53 and, furthermore, hamsters provided with energy-rich food stores almost abandoned deep torpor under laboratory condition54. In the field, however, information on the actual quantity and quality of individual food stores is lacking. In this experiment, we therefore provided free-ranging common hamsters with additional food of high energetic content (sunflower seeds) shortly before autumnal immergence and compared the timing of hibernation, torpor patterns, and body mass changes to that of unsupplemented individuals. Among female hamsters, we additionally analysed the timing of reproductive onset (first conception and parturition) in the subsequent season.
Results
Immergence and emergence sequences and hibernation patterns
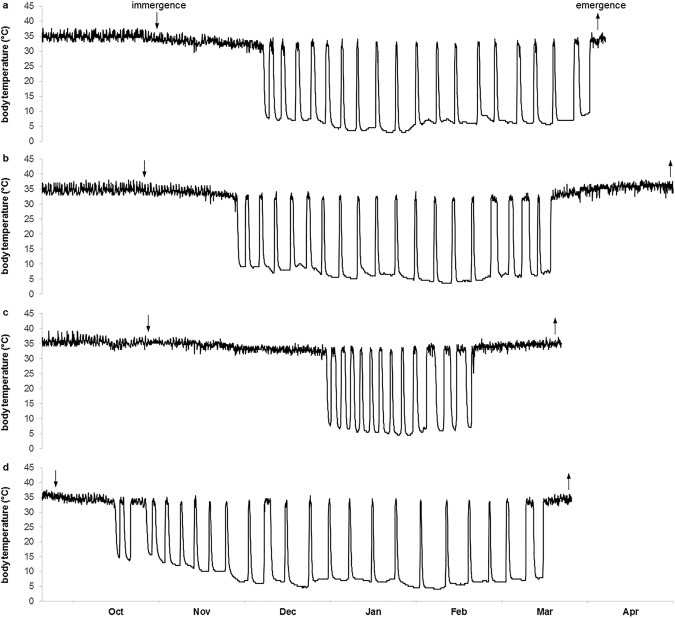
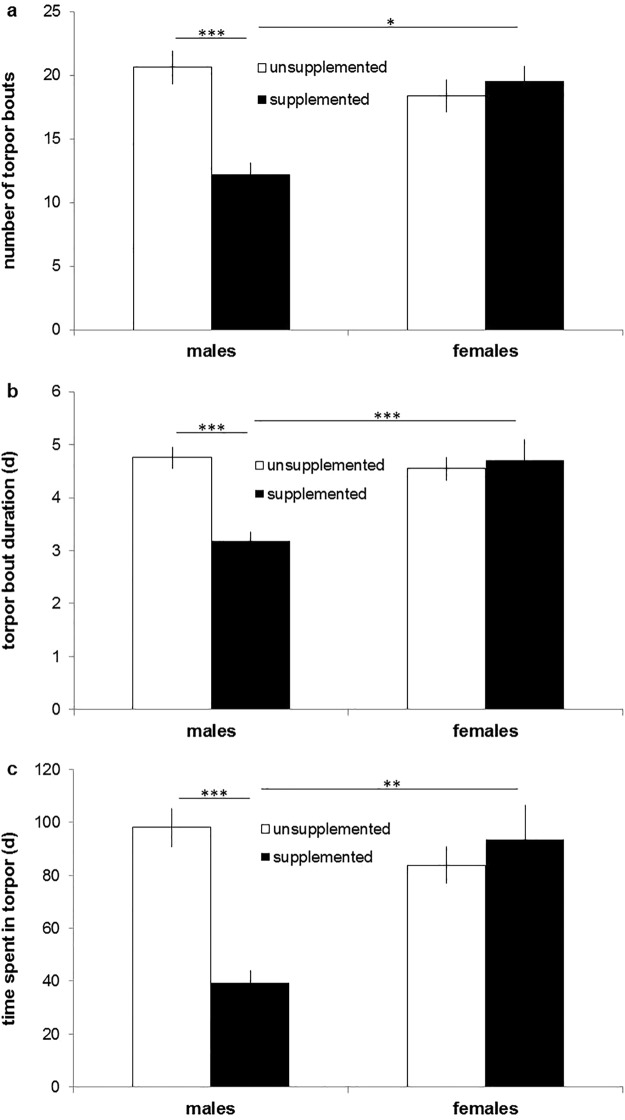
Body temperature patterns varied between the groups (Fig. 1) and sex-dependent effects of food supplementation were found in most of the parameters (Table 1). Among males, supplemented individuals immerged into their burrows later in the season (F1,33 = 9.635, p = 0.008), stayed longer euthermic inside their hibernacula (F1,33 = 10.746, p = 0.005), and, correspondingly, delayed hibernation onset (F1,33 = 21.847, p < 0.001) compared to unsupplemented ones (Fig. 2). Furthermore, supplemented males showed fewer torpor bouts (F1,33 = 16.871, p = 0.001) of shorter durations (χ2 = 21.613, p < 0.001), and, accordingly, spent less time in torpor (F1,33 = 22.633, p < 0.001) than unsupplemented males (Fig. 3). Minimum and mean body temperature during torpor did not differ between the groups (minimum: supplemented males: 7.8 ± 0.7 °C, unsupplemented males: 8.2 ± 0.5 °C, χ2 = 0.300, p > 0.999; mean: supplemented males: 11.1 ± 0.6 °C, unsupplemented males: 10.4 ± 0.4 °C, χ2 = 0.626, p = 0.857). Supplemented males terminated hibernation in early March, similar to unsupplemented ones (F1,33 = 2.636, p = 0.228), but, due to the delayed onset, hibernated for shorter periods (F1,33 = 25.677, p < 0.001; Fig. 2). The duration of post-hibernation euthermy was similar in both groups (F1,29 = 0.710, p = 0.812), but supplemented males tended to emerge earlier in spring (F1,29 = 5.001, p = 0.066) and, finally, spent less time inside the hibernaculum than unsupplemented ones (F1,29 = 14.489, p = 0.001; Fig. 2).
Figure 1.
Body temperature patterns of a representative female and male hamster for each group. (a) Supplemented female, (b) unsupplemented female, (c) supplemented male, (d) unsupplemented male.
Table 1.
ANOVA (Type III) table representing effects of sex and group (supplemented/unsupplemented) on annual timing and hibernation performance in common hamsters. Presented values are corrected for age. Tb…body temperature.
| Response variable | Predictor variable | DF | F value | p value |
|---|---|---|---|---|
| Immergence datea | Sex | 1 | 9.470 | 0.004 |
| Group | 1 | 0.006 | 0.938 | |
| Sex × group | 1 | 5.032 | 0.032 | |
| Prehibernation euthermy (days)a | Sex | 1 | 1.829 | 0.185 |
| Group | 1 | 0.013 | 0.910 | |
| Sex × group | 1 | 5.717 | 0.023 | |
| First torpor bout (date)a | Sex | 1 | 9.536 | 0.004 |
| Group | 1 | 0.021 | 0.886 | |
| Sex × group | 1 | 11.541 | 0.002 | |
| Number of torpor boutsa | Sex | 1 | 2.052 | 0.161 |
| Group | 1 | 0.245 | 0.624 | |
| Sex × group | 1 | 10.534 | 0.003 | |
| Torpor bout duration (hours)a | Sex | 1 | 0.261 | 0.613 |
| Group | 1 | 0.496 | 0.486 | |
| Sex × group | 1 | 14.566 | 0.001 | |
| Time spent in torpor (hours)a | Sex | 1 | 1.842 | 0.184 |
| Group | 1 | 0.672 | 0.418 | |
| Sex × group | 1 | 15.472 | <0.001 | |
| Minimum Tb during torpor (°C)a | Sex | 1 | 2.379 | 0.133 |
| Group | 1 | 1.006 | 0.323 | |
| Sex × group | 1 | 1.189 | 0.284 | |
| Mean Tb during torpor (°C)a | Sex | 1 | 1.455 | 0.236 |
| Group | 1 | 0.365 | 0.550 | |
| Sex × group | 1 | 0.020 | 0.888 | |
| Last torpor bout (date)a | Sex | 1 | 2.903 | 0.098 |
| Group | 1 | 0.651 | 0.426 | |
| Sex × group | 1 | 2.941 | 0.096 | |
| Hibernation duration (days)a | Sex | 1 | 3.049 | 0.090 |
| Group | 1 | 0.354 | 0.556 | |
| Sex × group | 1 | 15.943 | <0.001 | |
| Post-hibernation euthermy (days)b | Sex | 1 | 1.340 | 0.257 |
| Group | 1 | 9.513 | 0.005 | |
| Sex × group | 1 | 7.857 | 0.009 | |
| Emergence dateb | Sex | 1 | 22.887 | <0.001 |
| Group | 1 | 10.744 | 0.003 | |
| Sex × group | 1 | 0.642 | 0.429 | |
| Time inside hibernaculum (days)b | Sex | 1 | 0.003 | 0.960 |
| Group | 1 | 2.501 | 0.125 | |
| Sex × group | 1 | 2.279 | 0.142 |
aSample size supplemented/unsupplemented: males = 6/13, females = 6/13.
bSample size supplemented/unsupplemented: males = 6/12, females = 6/10.
Figure 2.
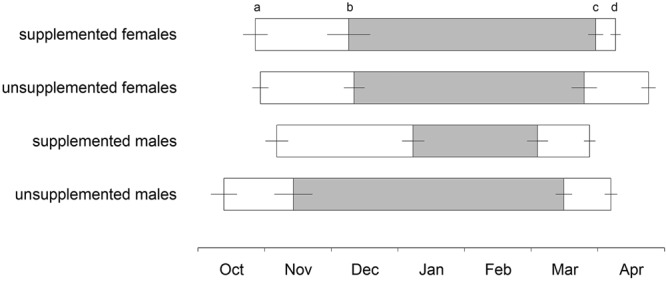
Annual timing and duration of hibernation (grey bars) in unsupplemented and supplemented males and females. (a) Indicates immergence into the hibernaculum (date when an individual terminated above-ground activity), (b) indicates hibernation onset (date of first torpor bout), c indicates hibernation end (date of last torpor bout), and (d) indicates vernal emergence from the hibernaculum (date when an individual resumed above-ground activity). Means ± SE; for statistical results and sample sizes see Table 1 and text.
Figure 3.
(a) Number of torpor bouts, (b) mean torpor bout duration, and (c) time spent in torpor in unsupplemented and supplemented males and females. Means ± SE; sample size unsupplemented/supplemented: males = 13/6, females = 13/6. *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001.
Among females, we found neither differences in timing of immergence nor hibernation performance between supplemented and unsupplemented individuals (p > 0.628 in all cases; Table 1, Figs 2 and 3; minimum body temperature: supplemented females: 8.2 ± 1 °C, unsupplemented females: 7.3 ± 0.4 °C; mean body temperature: supplemented females: 10.4 ± 0.9 °C, unsupplemented females: 9.8 ± 0.5 °C). Supplemented females, however, had a shorter duration of post-hibernation euthermy (F1,29 = 9.513, p = 0.009) and emerged from their hibernacula earlier in spring (F1,29 = 10.744, p = 0.005) than unsupplemented females (Table 1, Fig. 2). The time inside the hibernaculum was similar in females with and without food supplements (F1,29 = 2.501, p = 0.249).
Sex differences among supplemented individuals
Supplemented males and females immerged into their hibernacula and started to hibernate at similar dates (immergence: F1,33 = 0.363, p > 0.99; hibernation onset: F1,33 = 3.881, p = 0.115; Table 1, Fig. 2). Males, however, terminated hibernation earlier (F1,33 = 10.174, p = 0.006) and hibernated for shorter periods compared to females (F1,33 = 12.835, p = 0.002; Fig. 2), showed fewer and shorter torpor bouts (number: F1,33 = 8.429, p = 0.013; duration: χ2 = 17.575, p < 0.001), and, correspondingly, spent less time in torpor than females (F1,33 = 14.239, p = 0.001; Fig. 3). Minimum and mean body temperature did not differ between the sexes (minimum: χ2 = 0.075, p > 0.99; mean: χ2 = 0.933, p = 0.668). Finally, males showed a longer euthermic phase after hibernation (F1,29 = 6.541, p = 0.032) and emerged earlier from their burrows than females (F1,29 = 6.669, p = 0.030; Fig. 2). The time spent inside the hibernaculum, however, was similar in both sexes (F1,29 = 3.531, p = 0.141; Fig. 2).
Body condition
Body mass at immergence was similar in supplemented and unsupplemented individuals of both sexes (supplemented males: 339.3 ± 25.6 g, n = 6, unsupplemented males: 333.9 ± 16.3 g, n = 13, F1,33 = 0.210, p > 0.99; supplemented females: 294.7 ± 16.7 g, n = 6, unsupplemented females: 330.2 ± 23 g, n = 13, F1,33 = 3.847, p = 0.117). In spring, supplemented males emerged with higher body mass than unsupplemented males (supplemented males: 419.7 ± 26.1 g, n = 6, unsupplemented males: 319.9 ± 18.8 g, n = 12, F1,28 = 11.140, p = 0.005), while no differences were found among females (supplemented females: 259.5 ± 21.9 g, n = 6, unsupplemented females: 317.2 ± 23.1 g, n = 9, F1,28 = 4.028, p = 0.109). Body mass change over winter significantly differed between supplemented and unsupplemented males (F1,28 = 8.565, p = 0.014) as supplemented males increased their body mass during winter, while the others lost mass (Fig. 4). In females, however, body mass loss was similar in both groups (F1,28 = 0.615, p = 0.880; Fig. 4).
Figure 4.
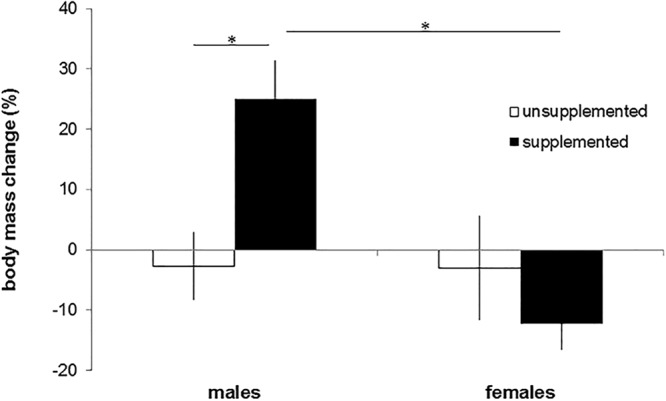
Body mass change (%) in unsupplemented and supplemented males and females. Means ± SE; sample size unsupplemented/supplemented: males = 12/6, females = 9/6. *p ≤ 0.05.
Female subsequent reproductive timing
When comparing females from the same season exclusively, we again found that supplemented females emerged earlier in spring than unsupplemented ones (t = 2.646, p = 0.018; Fig. 5). Females of both groups started to reproduce about one week after vernal emergence (t = −0.177, p = 0.862; Fig. 5). Consequently, females provided with additional food mated earlier in the season (t = 3.457, p = 0.009) and also gave birth to their first litter earlier than those without food supplements (t = 2.623, p = 0.027; Fig. 5).
Figure 5.
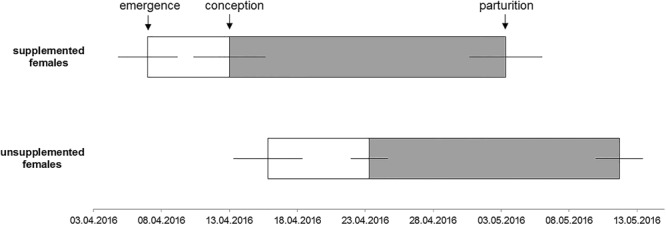
Timing of reproduction after vernal emergence as indicated by first conception and first parturition date in the respective season in unsupplemented and supplemented females. Means ± SE; sample size (p-values) unsupplemented/supplemented: emergence = 13/6 (p = 0.018), conception = 14/6 (p = 0.009), parturition = 13/6 (p = 0.027). For detailed statistical results see text.
Discussion
The provision of energy-rich food shortly before winter produced sex-specific effects on annual timing and hibernation performance in free-ranging common hamsters. Supplemented males immerged into their hibernacula later in the season than unsupplemented ones. In common hamsters, males usually terminate above-ground activity before females, but by extending the active season, supplemented males immerged at a similar time as females. This could have enabled these males to detect and monitor female hibernacula already in autumn, which would be advantageous in the subsequent spring because they could concentrate their activity on areas where oestrous females most likely emerge13. Behavioural observations supported this assumption as the first mating events in spring involved supplemented males. However, due to the large home range sizes of males during the breeding period we were unable to compare mating success of supplemented and unsupplemented individuals. Autumnal weather conditions were relatively mild but similar to previous seasons, indicating that this did not account for the delayed immergence. Furthermore, we found no differences in immergence body mass between supplemented and unsupplemented individuals indicating that the provided food was not consumed prior to immergence. After immergence, supplemented males showed an extended euthermic period inside the hibernaculum, resulting in a delayed hibernation onset and strongly reduced hibernation durations compared to unsupplemented individuals. Accordingly, males provided with additional food showed fewer torpor bouts of shorter duration and spent less time in torpor than unsupplemented ones. Given the remarkably long duration of prehibernation euthermy, males had to consume at least parts of their food stores during this time and expressed torpor only during the coldest winter months (mainly January/February) when this energy-saving state was probably most beneficial. By reducing not only the hibernation period but also the duration of deep torpor bouts, supplemented males probably were able to minimize potential costs of torpor as negative effects might become more intense with increasing torpor bout length. Moreover, supplemented males could benefit from shorter torpor bouts as improved environmental conditions towards the end of the winter period can be detected more quickly. This would be particularly advantageous for the timing of vernal emergence as indicated by an earlier start of the active season in supplemented males compared to those without additional food. We have no information on when the food was consumed in the burrow, but considering the extended prehibernation phase in supplemented males it is likely that they started to hibernate in an improved body condition and with higher body fat proportion compared to autumnal immergence and thus, could afford shorter torpor bout durations during the coldest period in winter.
We previously demonstrated that not only the quantity but particularly the quality of food stores is crucial for hibernation performance. Hamsters with access to large food stores and high sunflower seeds intake virtually abandoned deep torpor under laboratory conditions54. In the presented study, we enlarged and qualitatively improved the natural food stores of both males and females, but only males responded to food supplementation by shortening hibernation. Sunflower seeds resemble food items stored by common hamsters at our study site (e.g., beechnuts, hazelnuts, acorn or black walnuts) in the caloric value and nutrient composition (including fat content)55. The reduced torpor expression in supplemented males, therefore, unlikely resulted from sunflower seeds per se, because in this case one would expect a shortened hibernation period in supplemented females as well. Our results rather indicate that male common hamsters, which usually have limited access to external energy reserves as indicated by less pronounced food caching activities37, minimize hibernation when sufficient, energy-rich food is available.
Furthermore, access to high-quality food stores not only enabled supplemented males to emerge about two weeks earlier in spring than unsupplemented ones but, particularly, to gain body mass over winter, which could be a considerable advantage regarding reproductive success. Common hamsters have a promiscuous mating system and males face a high intrasexual competition, particularly at the onset of the mating period shortly after female emergence56. Furthermore, males usually have scrotal testes at vernal emergence, but testes size continuously increases until shortly after mating onset57. Early emerging males, therefore, might be able to complete testes growth prior to mating onset and, particularly, a better body condition in spring would enhance their competitive potential57,58 and be beneficial for enduring the long and exhausting mating period, lasting until August, which, ultimately, might increase their reproductive success.
Interestingly, food supplementation did not affect the timing of autumnal immergence and patterns of torpor expression in females, indicating that the additional food was not used to shorten the hibernation period. As a result, food supplementation largely reversed the previously documented sex differences in hibernation performance36 as now males hibernated for shorter periods, expressed fewer and shorter torpor bouts, and spent less time in torpor than females. Supplemented females, however, strongly shortened the duration of post-hibernation euthermy and emerged earlier in spring than unsupplemented ones. Moreover, females provided with additional food preceded those without supplements in the date of first conception and parturition. This temporal advantage is of major importance as female common hamsters can produce up to 3 litters per season and it has been previously shown that the earlier a female emerged and started to reproduce in spring, the more litters and more offspring were produced33, which was also found in all seasons investigated at our study site to date (Siutz et al., unpublished data). Thus, females seem to have used the supplemented food for an earlier start of the active season and reproductive onset, most likely resulting in a higher seasonal reproductive output. This is supported by similar emergence body mass and body mass losses over winter in supplemented and unsupplemented females, indicating that supplemented individuals conserved at least parts of the provided food. In contrast to males, which frequently change their burrows during the active season, female hamsters are more philopatric and could, therefore, benefit from remaining food stores as energy source during gestation and lactation, allowing them to decrease above-ground activity and, hence, exposure to predation risk, as well as to have access to high-quality food when seasonally not available.
A limiting factor of this study might be that hibernation data of supplemented and unsupplemented individuals were not collected in the same year. Although annual effects cannot be completely excluded, it is unlikely that our findings resulted from seasonal variations in environmental or climatic conditions. First, we could already show in unsupplemented individuals that timing of hibernation and hibernation performance itself were not affected by different years36. Although immergence and emergence sequences follow sex- and age-specific patterns, annual variations within these groups are usually low, which is not surprising as the seasonal timing of hibernation is mainly triggered by an endogenous circannual clock3. Since in our season of food supplementation, ambient temperatures during autumn, winter, and spring as well as food availability and snow cover were similar to the previous ones, it seems unlikely that environmental or climatic conditions caused the differing hibernation patterns in supplemented hamsters. Second, body temperature during torpor usually drops close to ambient temperature inside the hibernaculum2. We found, however, no differences in minimum and mean body temperature during torpor between supplemented and unsupplemented individuals, indicating that ambient temperatures inside the hibernaculum were similar in all years.
In conclusion, our results highlight sex-specific responses to food supplementation as males and females differently used additional food, which might reflect the sex-specific benefits regarding reproductive success, i.e. body condition in males and vernal timing in females. Moreover, the availability of high-energy food could increase an individual’s subsequent reproductive output, which would be of particular importance in this endangered species. Common hamsters face an ongoing population decline, particularly in Western Europe, and are strictly protected by the Bern Convention (Appendix II) and the Fauna-Flora-Habitat (Appendix IV) directives. Several reasons for the decline have been suggested including high overwinter mortality caused by insufficient food stores and, recently, a considerable decline in reproductive output was documented32,59,60. Our findings, therefore, could provide valuable information for agricultural management and reintroduction projects as enabling access to sufficient food of high quality at critical time periods could have the potential to counteract the population decline in common hamsters.
Methods
Ethical statement
All procedures performed on animals were carried out in accordance with EU guidelines for the protection of animals used for scientific purposes (Directive 2010/63/EU) and were approved by the ethics committee of the Faculty of Life Sciences, University of Vienna (2015-010), the Austrian Federal Ministry of Education, Science and Research (GZ: BMWFW-66.006/0013-WF/V/3b/2015), and the City of Vienna (MA22-2484/10, MA22-310/11).
Field techniques
We investigated a free-ranging population of common hamsters inhabiting urban areas in southern Vienna by applying capture-mark-recapture techniques during the active season (March/April–October/November 2015) using Tomahawk live traps baited with peanut butter. Hamsters were examined by using cone-shaped cotton sacks, laterally equipped with Velcro fasteners, allowing animal handling without anaesthesia. We subcutaneously implanted a transponder (Data Mars) and fur-marked each individual in different, distinguishable patterns. Sex and age, classified as adult (hibernated at least once) or juvenile (born in the current season) were determined and body mass (±1 g) was recorded at each capture. Further details of our field methods are described elsewhere33,36,56. We monitored all hamsters until trapping and observation failure in autumn, indicating immergence into the hibernaculum, and defined the date when the individual was trapped or observed for the last time during the pre-hibernation period as immergence date. To confirm that an individual terminated above-ground activity, we plugged the burrows with leaves to detect potential activity and monitored the burrow entrances at daily intervals. Immergence body mass referred to the individual’s mass recorded within 1 week before its immergence into the hibernaculum. During winter, we checked the burrows (open/closed) weekly, but detected no signs of activity outside the burrow until spring. Beginning in early March, the burrow monitoring was done at daily intervals and active individuals were recaptured. The date when an individual was observed above-ground or trapped for the first time was defined as emergence date, which coincided with the day when an individual had removed its burrow plug. Emergence body mass corresponded to an individual’s weight measured within 1 week after emergence from the hibernaculum. The percentage of body mass change over winter was defined as the difference between an individual’s immergence and emergence body mass.
Food supplementation
The hamsters were supplemented in late autumn shortly before immergence into the hibernaculum (September/October) to ensure that the food was used for the winter period. We placed 500 g sunflower seeds (Dehner Natura, Dehner GmbH, Germany) in front of an individual’s hibernaculum and continuously observed the burrow until the hamster had carried all seeds inside. Thus, no other than the focal individual collected the seeds. Sunflower seeds were chosen because of their storability and high energetic content (2.45 MJ/100 g, 51.5 g total fat/100 g)55. The caloric value and nutrient composition of sunflower seeds is similar to the natural winter diet of common hamsters at our study site55, which consists of, e.g., beechnuts, hazelnuts, acorn, and black walnuts61. In addition, hamsters in urban habitats to some extent have access to sunflower seeds (which either are naturally growing or comprised in birdseeds that are occasionally provided by humans) and due to their relatively small size they can be cached more quickly compared to, e.g., hazelnuts, by that facilitating our experimental procedure.
As common hamsters are strictly protected by the Bern Convention (Appendix II) and the Fauna-Flora-Habitat (Appendix IV) directives, we were permitted to implant temperature data loggers (see methods section ‘hibernation patterns’) in 20 individuals (GZ BMWFW-66.006/0013-WF/V/3b/2015). The monitoring of individuals throughout the active season and particularly in autumn allowed us to identify the hibernacula of 18 hamsters. Due to this relatively small sample size as well as considering potential overwinter mortality, all 18 individuals were supplemented. Among these individuals were also young of the year, but all of them were born in spring, had a body mass within the range of adults in autumn, and had completed sexual maturation as indicated by developed, scrotal testes (in case of males) and an opened vagina (in case of females). These individuals were defined as subadult. In the subsequent spring, all individuals sampled in this study were reproductively active. Unsupplemented individuals with available hibernation data from previous years served as control group (7 adult females, 6 subadult females, 5 adult males, 8 subadult males). This data set was already analysed and published36 and showed that hibernation performance and seasonal timing were similar in these years so that annual effects can be excluded. Furthermore, we documented climatic conditions (ambient temperature (Ta) and snow cover) in each year and found no variations between years (Ta autumn: 13.7 ± 0.3 °C, F3,103 = 0.289, p = 0.833; Ta winter: 2.9 ± 0.8 °C, F3,12 = 0.798, p = 0.519; Ta spring: 10.6 ± 0.3 °C, F3,99 = 0.157, p = 0.925; date of first snow cover: 24.12 ± 19 d; date of last snow cover: 25.02. ± 16 d). Moreover, the season of food supplementation did not differ from previous ones (Student’s t tests, Ta autumn: unsupplemented: 13.8 ± 0.4 °C, supplemented: 13.4 ± 0.7 °C, p = 0.589; Ta winter: unsupplemented: 2.4 ± 0.8 °C, supplemented: 4.5 ± 1.7 °C, p = 0.238; Ta spring: unsupplemented: 10.7 ± 0.4 °C, supplemented: 10.4 ± 0.7 °C, p = 0.707).
Hibernation patterns
Body temperature during winter was recorded at 90-min intervals using temperature data loggers (iButtons, DS1922L-F5#, range: −40 °C to + 85 °C, accuracy: ± 0.5 °C, Maxim Integrated Products International, Dublin, Ireland). Immediately after trapping in the morning, the hamsters were transported to a veterinary clinic (~20 min) where the iButtons (coated in Elvax ethylene vinyl acetate resins, DuPont, and paraffin; gas-sterilised; potted mass: ~4.5 g) were implanted subcutaneously in the neck region (dorsal, between the scapulae) under inhalation anaesthesia (isoflurane) and Metacam (0.2 mg/kg body mass) was administered as analgesic before surgery. As soon as the individuals had recovered from anaesthesia (within 5–10 min), we returned them to the field site and released them in front of their burrows (1–2 h after trapping), which were blocked in the meantime to prevent other hamsters from entering the burrows. In spring, iButtons were removed using the same technique, which has proved successful in this species36,53 and was also applied to all individuals used in the analyses as control (unsupplemented) group. We could recover iButtons of 12 supplemented individuals (4 adult females, 2 subadult females, 2 adult males, 4 subadult males).
Torpor bouts were defined as periods of reduced body temperature for longer than 24 h, beginning from the sampling interval when body temperature decreased below 30 °C to at least 15 °C until it had reached 30 °C again. To characterize hibernation patterns, the following parameters were determined: hibernation onset (date of the first torpor bout onset), duration of prehibernation euthermy (days from immergence until hibernation onset), number of torpor bouts, torpor bout duration (calculated in hours, expressed as days), time spent in torpor (total duration of all torpor bouts; calculated in hours, expressed as days), minimum body temperature (lowest value of body temperature during a torpor bout), mean body temperature (beginning from the sampling interval when body temperature decreased below 30 °C until it had reached 30 °C again), hibernation end (date of the last torpor bout termination), hibernation duration (days from the onset of the first to the termination of the last torpor bout), duration of post-hibernation euthermy (days from hibernation end until emergence), and time spent inside the hibernaculum (days from immergence to emergence).
Female subsequent reproductive timing
After vernal emergence, we were able to monitor all six supplemented females until the end of their first lactation period (late June). In addition, 14 unsupplemented females (without hibernation data) could be monitored during the same period. Some of these individuals were already trapped in the previous autumn, but immerged into their hibernacula before we could implant temperature data loggers. We, therefore, were able to compare reproductive timing via the dates of conception and parturition in supplemented and unsupplemented females of the same season. All individuals were individually fur-marked for distant recognition and observed at daily intervals. In addition, we captured females at weekly intervals and recorded body mass and teat size based on a 3-point-scale from small (1) to swollen with milk remains33,56. The onset of reproduction (conception date) was defined as the date of observed copulations if followed by cumulative body mass gain indicating gestation. Parturition was defined as distinct body mass loss followed by strongly increasing teat size, partly with milk rests, indicating lactation. By assuming 17–20 d of gestation62, we intensified trapping around the expected day of parturition, which allowed us to determine the date of parturition with an accuracy of ±2 d. As the exact date of litter emergence could only be detected in two supplemented females, we were not able to compare this parameter between supplemented and unsupplemented females.
Statistics
Statistical analyses were performed in R63 by using the packages ‘car’64 for linear models, ‘nlme’65 for linear mixed models (LMEs), and ‘phia’66 for post-hoc analyses of significant interaction effects. We applied LMEs for the parameters torpor bout duration, minimum body temperature, and mean body temperature and included the parameters sex, group (supplemented/unsupplemented), and their interaction as fixed effects and individual identity as a random effect to correct for repeated measurements. All other parameters (Table 1) were analysed using linear models, including the parameters sex, group (supplemented/unsupplemented), and their interaction as predictor variables. In addition, we corrected for differences in age by including the parameter age (adult/subadult) as predictor variable in each model, but omitted its interactions with the parameters sex and group due to small sample sizes among supplemented individuals. To simplify the presentation of these results, we omitted the parameter age in Table 1, but all values presented are corrected for age. We further only stated sex differences among supplemented individuals in the results section since sex differences among unsupplemented individuals were already published36. Model residuals were tested for normality using Shapiro–Wilk tests and for homoscedasticity by Levene-tests. Not normally distributed parameters (immergence body mass and duration of post-hibernation euthermy) were transformed by applying the natural logarithm. Statistics were obtained from ANOVA (Type III) tables; all post-hoc analyses were Bonferroni-corrected. For comparisons of subsequent reproductive timing (dates of emergence, conception, and parturition) between supplemented and unsupplemented females we applied Student’s t tests. Significance level was set at p ≤ 0.05. Results are presented as means ± s.e.m.
Acknowledgements
We would like to thank C. Machowetz, J. Kolar, and M. Rotter for their help in field work. This study was funded by the Austrian Science Fund (FWF, Project: 24280-B20).
Author Contributions
C.S. participated in the design of the study, collected field data, carried out the statistical analyses, and wrote the manuscript; M.V., V.A. and A.N. collected field data and reviewed drafts of the manuscript; E.M. conceived and designed the study, acquired funding of the study, and reviewed drafts of the manuscript. All authors read and approved the final manuscript.
Data Availability
The datasets generated and/or analysed during the current study are available from the corresponding author on reasonable request.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Geiser F. Metabolic rate and body temperature reduction during hibernation and daily torpor. Annu. Rev. Physiol. 2004;66:239–274. doi: 10.1146/annurev.physiol.66.032102.115105. [DOI] [PubMed] [Google Scholar]
- 2.Heldmaier G, Ortmann S, Elvert R. Natural hypometabolism during hibernation and daily torpor in mammals. Respir. Physiol. Neurobiol. 2004;141:317–329. doi: 10.1016/j.resp.2004.03.014. [DOI] [PubMed] [Google Scholar]
- 3.Ruf T, Geiser F. Daily torpor and hibernation in birds and mammals. Biol. Rev. 2015;90:891–926. doi: 10.1111/brv.12137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Florant GL, Healy JE. The regulation of food intake in mammalian hibernators: a review. J. Comp. Physiol. B. 2012;182:451–467. doi: 10.1007/s00360-011-0630-y. [DOI] [PubMed] [Google Scholar]
- 5.French AR. Interdependency of stored food and changes in body temperature during hibernation of the eastern chipmunk. Tamias striatus. J. Mammal. 2000;81:979–985. doi: 10.1644/1545-1542(2000)081<0979:IOSFAC>2.0.CO;2. [DOI] [Google Scholar]
- 6.Humphries MM, Thomas DW, Kramer DL. The role of energy availability in mammalian hibernation: A cost-benefit approach. Physiol. Biochem. Zool. 2003;76:165–179. doi: 10.1086/367950. [DOI] [PubMed] [Google Scholar]
- 7.Munro D, Thomas DW, Humphries MM. Extreme suppression of aboveground activity by a food-storing hibernator, the eastern chipmunk (Tamias striatus) Can. J. Zool. 2008;86:364–370. doi: 10.1139/Z08-008. [DOI] [Google Scholar]
- 8.Sheriff MJ, Fridinger RW, Tøien Ø, Barnes BM, Buck CL. Metabolic rate and prehibernation fattening in free-living arctic ground squirrels. Physiol. Biochem. Zool. 2013;86:515–527. doi: 10.1086/673092. [DOI] [PubMed] [Google Scholar]
- 9.Barnes, B. M. Relationships between hibernation and reproduction in male ground squirrels. In Adaptations to the cold: 10thInternational Hibernation Symposium (eds Geiser, F., Hulbert, A. J., & Nicol, S. C.) 71–80 (University of New England Press, Armidale, 1996).
- 10.Blumstein DT, Im S, Nicodemus A, Zugmeyer C. Yellow-bellied marmots (Marmota flaviventris) hibernate socially. J. Mammal. 2004;85:25–29. doi: 10.1644/1545-1542(2004)085<0025:YMMFHS>2.0.CO;2. [DOI] [Google Scholar]
- 11.Buck CL, Barnes BM. Annual cycle of body composition and hibernation in free-living arctic ground squirrels. J. Mammal. 1999;80:430–442. doi: 10.2307/1383291. [DOI] [Google Scholar]
- 12.Gür H, Kart Gür M. Annual cycle of activity, reproduction, and body mass of Anatolian ground squirrels (Spermophilus xanthoprymnus) in Turkey. J. Mammal. 2005;86:7–14. doi: 10.1644/1545-1542(2005)086<0007:ACOARA>2.0.CO;2. [DOI] [Google Scholar]
- 13.Michener, G. R. Age, sex, and species differences in the annual cycles of ground-dwelling sciurids: implications for sociality. In The Biology of Ground-dwelling Squirrels (eds Murie, J. O. & Michener, G. R.) 81–107 (University of Nebraska Press, Lincoln, USA, 1984).
- 14.Millesi E, Strijkstra AM, Hoffmann IE, Dittami JP, Daan S. Sex and age differences in mass, morphology, and annual cycle in european ground squirrels. Spermophilus citellus. J. Mammal. 1999;80:218–231. doi: 10.2307/1383222. [DOI] [Google Scholar]
- 15.Sheriff MJ, et al. Phenological variation in annual timing of hibernation and breeding in nearby populations of arctic ground squirrels. Proc. R. Soc. B. 2011;278:2369–2375. doi: 10.1098/rspb.2010.2482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kenagy G, Barnes B. Seasonal reproductive patterns in four coexisting rodent species from the Cascade Mountains, Washington. J. Mammal. 1988;69:274–292. doi: 10.2307/1381378. [DOI] [Google Scholar]
- 17.Michener GR. Sexual differences in reproductive effort of Richardson’s ground squirrels. J. Mammal. 1998;79:1–19. doi: 10.2307/1382838. [DOI] [Google Scholar]
- 18.Millesi E, Huber S, Everts LG, Dittami JP. Reproductive decisions in female European ground squirrels: Factors affecting reproductive output and maternal investment. Ethology. 1999;105:163–175. doi: 10.1046/j.1439-0310.1999.00379.x. [DOI] [Google Scholar]
- 19.Batavia M, Nguyen G, Harman K, Zucker I. Hibernation patterns of Turkish hamsters: influence of sex and ambient temperature. J. Comp. Physiol. B. 2013;183:269–277. doi: 10.1007/s00360-012-0706-3. [DOI] [PubMed] [Google Scholar]
- 20.Buck, C. L., Breton, A., Kohl, F., Toien, O. & Barnes, B. M. Overwinter body temperature patterns in free-living arctic squirrels (Spermophilus parryii). In Hypometabolism in animals: hibernation, torpor and cryobiology (eds Lovegrove, B. G. & McKechnie, A. E.) 317–326 (University of KwaZulu-Natal, 2008).
- 21.Geiser F. Daily torpor and thermoregulation in Antechinus (Marsupialia): influence of body mass, season, development, reproduction, and sex. Oecologia. 1988;77:395–399. doi: 10.1007/BF00378050. [DOI] [PubMed] [Google Scholar]
- 22.Healy JE, Burdett KA, Buck CL, Florant GL. Sex differences in torpor patterns during natural hibernation in golden-mantled ground squirrels (Callospermophilus lateralis) J. Mammal. 2012;93:751–758. doi: 10.1644/11-MAMM-A-120.1. [DOI] [Google Scholar]
- 23.Kart Gür M, Gür H. Age and sex differences in hibernation patterns in free-living Anatolian ground squirrels. Mamm. Biol. 2015;80:265–272. doi: 10.1016/j.mambio.2015.02.006. [DOI] [Google Scholar]
- 24.Körtner G, Rojas AD, Geiser F. Thermal biology, torpor use and activity patterns of a small diurnal marsupial from a tropical desert: sexual differences. J. Comp. Physiol. B. 2010;180:869–876. doi: 10.1007/s00360-010-0459-9. [DOI] [PubMed] [Google Scholar]
- 25.Lee TN, Kohl F, Buck CL, Barnes BM. Hibernation strategies and patterns in sympatric arctic species, the Alaska marmot and the arctic ground squirrel. J. Mammal. 2016;97:135–144. doi: 10.1093/jmammal/gyv163. [DOI] [Google Scholar]
- 26.Michener GR. Sexual differences in over-winter torpor patterns of Richardson’s ground squirrels in natural hibernacula. Oecologia. 1992;89:397–406. doi: 10.1007/BF00317418. [DOI] [PubMed] [Google Scholar]
- 27.Munro D, Thomas DW, Humphries MM. Torpor patterns of hibernating eastern chipmunks Tamias striatus vary in response to the size and fatty acid composition of food hoards. J. Anim. Ecol. 2005;74:692–700. doi: 10.1111/j.1365-2656.2005.00968.x. [DOI] [Google Scholar]
- 28.Young P. Hibernating patterns of free-ranging Columbian ground squirrels. Oecologia. 1990;83:504–511. doi: 10.1007/BF00317201. [DOI] [PubMed] [Google Scholar]
- 29.Zervanos SM, Maher CR, Florant GL. Effect of body mass on hibernation strategies of woodchucks (Marmota monax) Integr. Comp. Biol. 2013;54:443–451. doi: 10.1093/icb/ict100. [DOI] [PubMed] [Google Scholar]
- 30.Franceschini, C. & Millesi, E. Reproductive timing and success in Common hamsters. In The Common hamster (Cricetus cricetus, L. 1758): hamster biology and ecology, policy and management of hamsters and their biotop (ed Losinger, I.) 63–66 (Office National de la Chasse et de la Faune Sauvage, 2005).
- 31.Millesi, E., Hoffmann, I. E., Aschauer, A. & Franceschini, C. Reproduction and hibernation in females: a comparison of two sympatric ground-dwelling rodents. In Life in the cold: Evolution, Mechanisms, Adaptation, and Application. 12thInternational Hibernation Symposium (eds Barnes, B. M. & Carey, H. V.) 127–135 (Biological Papers of the University of Alaska, number 27, 2004).
- 32.Weinhold, U. & Kayser, A. Der Feldhamster. (Westarp Wisenschaften, 2006).
- 33.Franceschini-Zink C, Millesi E. Reproductive performance in female common hamsters. Zoology. 2008;111:76–83. doi: 10.1016/j.zool.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 34.Hufnagl S, Franceschini-Zink C, Millesi E. Seasonal constraints and reproductive performance in female Common hamsters (Cricetus cricetus) Mamm. Biol. 2011;76:124–128. doi: 10.1016/j.mambio.2010.07.004. [DOI] [Google Scholar]
- 35.Niethammer, J. Cricetus cricetus (Linnaeus, 1758) - Hamster (Feldhamster). In Handbuch der Säugetiere Europas, Bd. 2/I. (eds Niethammer, J. & Krapp, F.) 7–28 (Akademische Verlagsgesellschaft Wiesbaden, Germany, 1982).
- 36.Siutz C, Franceschini C, Millesi E. Sex and age differences in hibernation patterns of common hamsters: adult females hibernate for shorter periods than males. J. Comp. Physiol. B. 2016;186:801–811. doi: 10.1007/s00360-016-0995-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Siutz, C., Pluch, M., Ruf, T. & Millesi, E. Sex differences in foraging behaviour, body fat, and hibernation patterns of free-ranging Common hamsters. In Living in a seasonal world: thermoregulatory and metabolic adaptations (eds Ruf, T., Bieber, C., Arnold, W. & Millesi, E.) 155–165 (Springer, 2012).
- 38.Bieber C, Juškaitis R, Turbill C, Ruf T. High survival during hibernation affects onset and timing of reproduction. Oecologia. 2012;169:155–166. doi: 10.1007/s00442-011-2194-7. [DOI] [PubMed] [Google Scholar]
- 39.Geiser, F. & Brigham, R. M. The other functions of torpor. In Living in a seasonal world: thermoregulatory and metabolic adaptations (eds Ruf, T., Bieber, C., Arnold, W., & Millesi, E.) 109–121 (Springer, 2012).
- 40.Heldmaier G, Ruf T. Body temperature and metabolic rate during natural hypothermia in endotherms. J. Comp. Physiol. B. 1992;162:696–706. doi: 10.1007/BF00301619. [DOI] [PubMed] [Google Scholar]
- 41.Lebl K, et al. Survival rates in a small hibernator, the edible dormouse: a comparison across Europe. Ecography. 2011;34:683–692. doi: 10.1111/j.1600-0587.2010.06691.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Turbill C, Bieber C, Ruf T. Hibernation is associated with increased survival and the evolution of slow life histories among mammals. Proc. R. Soc. B. 2011;278:3355–3363. doi: 10.1098/rspb.2011.0190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Franco M, Contreras C, Nespolo RF. Profound changes in blood parameters during torpor in a South American marsupial. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2013;166:338–342. doi: 10.1016/j.cbpa.2013.07.010. [DOI] [PubMed] [Google Scholar]
- 44.Prendergast BJ, Freeman DA, Zucker I, Nelson RJ. Periodic arousal from hibernation is necessary for initiation of immune responses in ground squirrels. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2002;282:R1054–R1062. doi: 10.1152/ajpregu.00562.2001. [DOI] [PubMed] [Google Scholar]
- 45.Carey HV, Andrews MT, Martin SL. Mammalian hibernation: cellular and molecular responses to depressed metabolism and low temperature. Physiol. Rev. 2003;83:1153–1181. doi: 10.1152/physrev.00008.2003. [DOI] [PubMed] [Google Scholar]
- 46.Strijkstra AM, Hut RA, de Wilde MC, Stieler J, Van der Zee EA. Hippocampal synaptophysin immunoreactivity is reduced during natural hypothermia in ground squirrels. Neurosci. Lett. 2003;344:29–32. doi: 10.1016/S0304-3940(03)00399-9. [DOI] [PubMed] [Google Scholar]
- 47.Millesi E, Prossinger H, Dittami JP, Fieder M. Hibernation effects on memory in European ground squirrels (Spermophilus citellus) J. Biol. Rhythms. 2001;16:264–271. doi: 10.1177/074873001129001971. [DOI] [PubMed] [Google Scholar]
- 48.Carey HV, Frank CL, Seifert JP. Hibernation induces oxidative stress and activation of NF-κB in ground squirrel intestine. J. Comp. Physiol. B. 2000;170:551–559. doi: 10.1007/s003600000135. [DOI] [PubMed] [Google Scholar]
- 49.Hoelzl F, Cornils JS, Smith S, Moodley Y, Ruf T. Telomere dynamics in free-living edible dormice (Glis glis): the impact of hibernation and food supply. J. Exp. Biol. 2016;219:2469–2474. doi: 10.1242/jeb.140871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bieber C, Lebl K, Stalder G, Geiser F, Ruf T. Body mass dependent use of hibernation: why not prolong the active season, if they can? Funct. Ecol. 2014;28:167–177. doi: 10.1111/1365-2435.12173. [DOI] [Google Scholar]
- 51.Humphries MM, Kramer DL, Thomas DW. The role of energy availability in mammalian hibernation: An experimental test in free-ranging eastern chipmunks. Physiol. Biochem. Zool. 2003;76:180–186. doi: 10.1086/367949. [DOI] [PubMed] [Google Scholar]
- 52.Landry-Cuerrier M, Munro D, Thomas DW, Humphries MM. Climate and resource determinants of fundamental and realized metabolic niches of hibernating chipmunks. Ecology. 2008;89:3306–3316. doi: 10.1890/08-0121.1. [DOI] [PubMed] [Google Scholar]
- 53.Siutz C, Millesi E. Torpor patterns in common hamsters with and without access to food stores. J. Comp. Physiol. B. 2017;187:881–888. doi: 10.1007/s00360-017-1093-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Siutz C, et al. Effects of food store quality on hibernation performance in common hamsters. PLoS ONE. 2017;12:e0185913. doi: 10.1371/journal.pone.0185913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.USDA. US Department of Agriculture, Agricultural Research Service, Nutrient Data Laboratory. USDA National Nutrient Database for Standard Reference, Release 28. Version Current: September 2015, slightly revised May 2016 (2016).
- 56.Franceschini C, Siutz C, Palme R, Millesi E. Seasonal changes in cortisol and progesterone secretion in Common hamsters. Gen. Comp. Endocrinol. 2007;152:14–21. doi: 10.1016/j.ygcen.2007.02.008. [DOI] [PubMed] [Google Scholar]
- 57.Lebl, K. & Millesi, E. Yearling male Common hamsters and the trade-off between growth and reproduction. In The Common hamster (Cricetus cricetus): Perspectives on an endangered species. Biosystematics and Ecology Series (eds Millesi, E., Winkler, H., & Hengsberger, R.) 115–126 (Austrian Academy of Sciences Press, 2008).
- 58.Grulich I. The reproduction of Cricetus cricetus (Rodentia) in Czechoslovakia. Acta Sc. Nat. Brno. 1986;20:1–56. [Google Scholar]
- 59.Kayser A, Weinhold U, Stubbe M. Mortality factors of the common hamster Cricetus cricetus at two sites in Germany. Acta Theriol. 2003;48:47–57. doi: 10.1007/BF03194265. [DOI] [Google Scholar]
- 60.Surov A, Banaszek A, Bogomolov P, Feoktistova N, Monecke S. Dramatic global decrease in the range and reproduction rate of the European hamster Cricetus cricetus. Endanger. Spec. Res. 2016;31:119–145. doi: 10.3354/esr00749. [DOI] [Google Scholar]
- 61.Roswag A, et al. Stable isotope analysis as a minimal-invasive method for dietary studies on the highly endangered Common hamster (Cricetus cricetus) Mammalia. 2018 [Google Scholar]
- 62.Vohralik V. Biology of the reproduction of the common hamster, Cricetus cricetus. Vestn. Cesk. Spol. Zool. 1974;38:288–240. [Google Scholar]
- 63.R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL http://www.R-project.org (2015).
- 64.Fox, J. & Weisberg, S. An R Companion to Applied Regression. Second Edition. Thousand Oaks, CA, Sage Publications. http://socserv.socsci.mcmaster.ca/jfox/Books/Companion (2011).
- 65.Pinheiro, J., Bates, D., DebRoy, S., Sarkar, D. & Team, R. C. nlme: Linear and Nonlinear Mixed Effects Models. R package version 3.1–122. https://CRAN.R-project.org/package=nlme (2015).
- 66.De Rosario-Martinez, H. phia: Post-Hoc Interaction Analysis. R package version 0.2-1, https://CRAN.R-project.org/package=phia (2015).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and/or analysed during the current study are available from the corresponding author on reasonable request.