Abstract
A new series of 1,4-dihydropyridine derivatives (2a–h, 3a–e, and 4a–e) were systematically designed and synthesized via ultrasound irradiation methods with easy work-up and good yields. Compounds structures were confirmed by IR, 1H NMR, 13C NMR, and mass spectra. The synthesized compounds were screened for both antimicrobial and anticoagulant activities. Compound 2e (MIC: 0.25 μg/mL) was highly active against Escherichia coli and compound 2c (MIC: 0.5 μg/mL) was also highly active against Pseudomonas aeruginosa compared with ciprofloxacin. (MIC: 1 μg/mL) The antifungal activity of 2c (MIC: 0.5 μg/mL) against Candida albicans was high relative to that of clotrimazole (MIC: 1 μg/mL). Anticoagulant activity was determined by activated partial thromboplastin time (APTT) and prothrombin time (PT) coagulation assays. Compound 4-(4-hydroxyphenyl)-2,6-dimethyl-N3,N5-bis(5-phenyl-1,3,4-thiadiazol-2-yl)-1,4-dihydropyridine-3,5-dicarboxamide 3d (>1000 s in APTT assays) was highly active in anticoagulant screening compared with the reference of heparin.
Cytotoxicity was evaluated using HepG2 (liver), HeLa (cervical), and MCF-7 (breast) cancer cell lines, with high toxicities observed for 2c (GI50 = 0.02 μm) against HeLa cell line and 2e (GI50 = 0.03 μm) equipotant against MCF-7 cell line. Therefore, the compounds 2e, 2c and 3d can serve as lead molecules for the development of new classes of antimicrobial and anticoagulant agent.
Keywords: Ultrasound irradiation; 1,4-Dihydropyridine derivatives; Anticoagulant activity; Antimicrobial activity; Structure activity relationship
1. Introduction
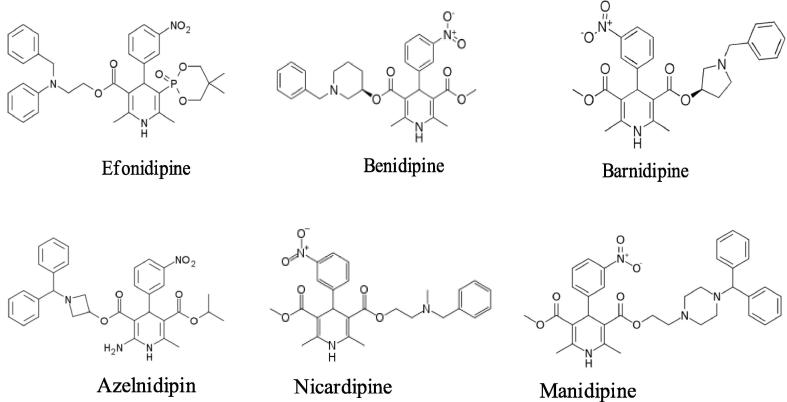
The development of multidrug resistance is a major obstacle in pharmacology, requiring the constant development of new therapeutics. 1,4-Dihydropyridine derivatives display a broad spectrum of pharmacological activities such as antitumor (Boer and Gekeler, 1995), antihypertensive (Wenzel et al., 2000), anticonvulsant (Surendra Kumar et al., 2010), cytotoxic (Miri et al., 2011), and significant of analgesic activities (Agudoawu et al., 1999, Warren and Knaus, 1981, Ulloora et al., 2013). Fig. 1 shows that other important calcium channel blockers of 1,4-dihydropyridine derivatives such as efonidipine, benidipine, barnidipine, azelnidipine, nicardipine, and manidipine. A large number of organic reactions were carried out in higher yield, shorter reaction time or milder conditions under ultrasonic irradiation (Balaji et al., 2014, Palakshi Reddy et al., 2015, He et al., 2015), for example recently reported that 1,4-dihydropyridine for ultrasound reactions and other important ultrasound reaction (Safari et al., 2015).
Fig. 1.
Multidrug calcium channel blockers.
Several methods have been described for the synthesis of 1,4-dihydropyridines (Tsuruo et al., 1983, Wan et al., 2009) and previous report of 1,4-dihydropyridine with derivatives modify at the 3- and 5-positions was exhibited significant anticoagulant and antimicrobial activities (Surendra Kumar et al., 2011a, Surendra Kumar et al., 2011ba).
Thiazole, thiadiazole, and oxadiazole derivatives are also display a wide range of pharmacological activities such as anaesthetic (Geronikaki and Theophilidis, 1992) and anti-inflammatory (Giridhar et al., 2001) properties. Oxadiazole and thiadiazole derivatives have been evaluated and proved for a wide range of pharmacological and clinical uses (Tawfeeq et al., 2012), particularly the activity focused by antibacterial, antifungal (Liu et al., 2008), and analgesic (Almasirad et al., 2014) activities.
Thiazoles are useful structural units in the field of medicinal chemistry and have reported antifungal (Tsuruoka et al., 1998) and analgesic (Argyropoulou et al., 2009) activities. The thiazole nucleus appears frequently in the structure of various natural products and biologically active compounds, like thiamine (vitamin-B), also in some antibiotics drugs like penicillin, micrococcin (Rogers et al., 1966), and many metabolic products of fungi and primitive marine animal etc. Based on above literature collections, the current study describes the ultrasound irradiation synthesis of novel 1,4-dihydropyridine connected with thiazole, thiadiazole, and oxadiazole compounds and their evaluation of anticoagulant, antimicrobial, and cytotoxicity activities.
2. Experimental section
2.1. Chemistry
All the chemicals were synthetic grade and commercially procured from Sigma Aldrich. The melting point was determined in an open capillary tube and it is uncorrected. The IR spectra were recorded in KBr on a shimadzu 8201pc (4000–400 cm−1). The 1H NMR and 13C NMR spectra were recorded on a Bruker. The elemental analysis (C, H and N) was recorded using an elemental analyzer model (Varian EL III). The purity of the compounds was checked by thin layer chromatography (TLC) with silica gel plates.
2.1.1. General method for preparation of 4-(furan-2-yl)-2,6-dimethyl-N3,N5-bis(4-phenyl thiazol-2-yl)-1,4-dihydropyridine-3,5-dicarboxamide (2a)
A mixture of compound, diethyl-4-(furan-2-yl)-2,6-dimethyl-1,4-dihydropyridine-3,5-dicarboxylate 1a (0.01 mol, 3.19 g) and 2-amino-4-phenylthiazole (0.02 mol, 3.52 g) were added in ethanol solvent stirred by 5 min on ultrasound irradiation. After 5 minute the reaction was completed, the product was confirmed by TLC. The product was washed with distilled water and recrystallized by ethyl acetate to give pure product. The above procedure was followed for the synthesis of compounds 2b–h.
Pale yellow solid; Yield 86%; mp 201–205 °C; IR (cm−1): 3174 (NH), 3074 (CHstr), 3034 (Ar-H), 1652 (OCNH), 1488 (C=N), 738 (C-S-C); 1H NMR (300 MHz, DMSO-d6): δ 13.60 (s, 2H, -CONH), 8.84 (s, 1H, NH), 7.89–7.68 (m, 10H, Ar-H), 7.48 (s, 2H, thiazole-2-yl, H-3), 7.29 (d, 1H, J = 6.77 Hz, furan), 6.45 (d, 1H, J = 6.98 Hz, furan), 6.28 (dd, 1H, J = 6.70 Hz, J = 6.92 Hz, furan), 5.85 (s, 1H, 4-CH), 2.50 (s, 6H, 2,6-CH3); 13C NMR (75 MHz, DMSO-d6): 164.2 (2C, thiazol-2-yl, C-1), 163.1 (2C, C=O), 152.5 (1C, furan-C-1), 150.2 (2C, thiazol-2-yl, C-4), 149.8 (2C, 1,4-dihydropyridine, C-2, C-6), 143.2 (1C, furan-C-4), 129.1 (2C, Ar-C′-4), 128.2 (4C, Ar-C′-3, C′-5), 126.9 (4C, Ar-C′-2, C′-6), 126.3 (2C, Ar-C′-1), 113.6 (1C, furan-C-3), 106.4 (1C, furan-C-2), 105.1 (2C, thiazol-2-yl, C-3), 102.8 (2C, 1,4-dihydropyridine, C-3, C-5), 32.4 (1C, 1,4-dihydro pyridine, C-4), 18.0 (2C, 1,4-dihydropyridine, C-2-CH3, C-6-CH3); EI-MS: m/z 579.23 (M+,10%), 503.59, 427.49 (100%); 397.45, 367.40, 343.38, 315.32, 289.32, 261.27, 231.24, 203.19. Elemental analysis: Calcd. for C31H25N5O3S2: C, 64.23%; H, 4.35%; N, 12.08%; S, 11.06%. Found: C, 64.27%; H, 4.40%; N, 12.14%; S, 11.10%.
2.1.2. 2,6-Dimethyl-4-phenyl-N3,N5-bis(4-phenylthiazol-2-yl)-1,4-dihydropyridine-3,5-dicar boxamide (2b)
Yellow solid; Yield 60%; mp:238–241 °C; IR (cm−1): 3162 (NH), 3045 (CHstr), 3034 (Ar-H), 1612 (OCNH), 1485 (C=N), 734 (C-S-C); 1H NMR (300 MHz, DMSO-d6): δ 13.62 (s, 2H, -CONH), 8.80 (s, 1H, NH), 7.88–7.64 (m, 10H, Ar-H), 7.46 (s, 2H, thiazole-2-yl, H-3), 7.30 (d, 2H, J = 7.09 Hz, Ar-C-3, C-5), 7.22 (d, 1H, J = 7.12 Hz, Ar-C-4), 7.21 (d, 2H, J = 7.17 Hz, Ar-H-2, H-6), 5.81 (s, 1H, 4-CH), 2.47 (s, 6H, 2,6-CH3); 13C NMR (75 MHz, DMSO-d6): 164.8 (2C, thiazole-2-yl, C-1), 163.9 (2C, C=O), 150.7 (2C, thiazole-2-yl, C-4), 149.2 (2C, 1,4-dihydro pyridine, C-2, C-6), 144.4 (1C, Ar-C-1), 129.6 (2C, Ar-C′-4), 128.9 (2C, Ar-C-3, C-5), 128.7 (4C, Ar-C′-3, C′-5),127.6 (2C, Ar-C-2, C-6), 126.8 (4C, Ar-C′-2, C′-6), 126.0 (2C, Ar-C′-1), 125.2 (1C, Ar-C-4), 105.4 (2C, thiazole-2-yl, C-3),103.1 (2C, 1,4-dihydropyridine, C-3, C-5), 32.7 (1C, 1,4-dihydro pyridine, C-4), 18.2 (2C, 1,4-dihydropyridine, C-2-CH3, C-6-CH3); EI-MS: m/z 589.72; Elemental analysis: Calcd. For (C33H27N5O2S2): C, 67.21%; H 4.61%; N, 11.88%; S, 10.87%. Found: C, 67.25%; H, 4.67%; N, 11.94%; S, 10.91%.
2.1.3. 4-(4-Chlorophenyl)-2,6-dimethyl-N3,N5-bis(4-phenylthiazol-2-yl)-1,4-dihydro pyridine-3,5-dicarboxamide (2c)
Yellow solid; Yield 63%; mp:113–145 °C; IR (cm−1):3174 (NH), 3074 (CHstr), 3031 (Ar-H), 1642 (OCNH), 1480 (C=N), 827 (C-Cl), 736 (C-S-C); 1H NMR (300 MHz, DMSO-d6): δ 13.58 (s, 2H, -CONH), 8.89 (s, 1H, NH), 7.91–7.66 (m, 10H, Ar-H), 7.48 (s, 2H, thiazole-2-yl, H-3), 7.30 (d, 2H, J = 6.98 Hz, Ar-H-3, H-5), 7.21 (d, 2H, J = 6.87 Hz, Ar-H-2, H-6), 5.89 (s, 1H, 4-CH), 2.44 (s, 6H, 2,6-CH3); 13C NMR (75 MHz, DMSO-d6): 163.8 (2C, C=O), 163.1 (2C, thiazole-2-yl, C-1), 151.1 (2C, thiazole-2-yl, C-4), 148.5 (2C, 1,4-dihydropyridine, C-2, C-6), 142.4 (1C, Ar-C-1), 131.2 (1C, Ar-C-4), 130.6 (2C, Ar-C-2, C-6), 129.3 (2C, Ar-C′-4), 128.3 (4C, Ar-C′-3, C′-5), 126.7 (2C, Ar-C-3, C-5), 126.3 (2C, Ar-C′-1), 126.2 (4C, Ar-C′-2, C′-6), 105.1 (2C, thiazole-2-yl, C-3), 102.2 (2C, 1,4-dihydropyridine, C-3, C-5), 32.4 (1C, 1,4-dihydropyridine, C-4), 18.4 (2C, 1,4-dihydropyridine, C-2-CH3, C-6-CH3); EI-MS: m/z 624.17, Elemental analysis: Calcd. for (C33H26ClN5O2S2): C, 63.50%; H, 4.20%; N, 11.22%; S, 10.27%. Found: C, 63.55%; H, 4.28%; N, 11.27%; S, 10.31%.
2.1.4. 4-(4-Hydroxyphenyl)-2,6-dimethyl-N3,N5-bis(4-phenylthiazol-2-yl)-1,4-dihydro pyridine-3,5-dicarboxamide (2d)
Light yellow solid; Yield 69%; mp:207–210 °C; IR(cm−1): 3164 (NH), 3066 (CHstr), 3028 (Ar-H), 1660 (OCNH), 1472 (C-OH), 1470 (C=N), 737 (C-S-C); 1H NMR (300 MHz, DMSO-d6): δ 13.55 (s, 2H, -CONH), 9.32 (s, 1H, –OH), 8.81 (s, 1H, NH), 7.84–7.61 (m, 10H, Ar-H), 7.45 (s, 2H, thiazole-2-yl, H-3), 7.01 (d, 2H, J = 6.57 Hz, Ar-C-2, C-6), 6.75 (d, 2H, J = 6.88 Hz, Ar-H-3,5 Ar-C-3, C-5), 5.80 (s, 1H, 4-CH), 2.56 (s, 6H, 2,6-CH3); 13C NMR (75 MHz, DMSO-d6): 164.6 (2C, thiazole-2-yl, C-1), 163.2 (2C, C=O), 155.2 (1C, Ar-C-4), 149.2 (2C, thiazole-2-yl, C-4), 149.1 (2C, 1,4-dihydropyridine, C-2, C-6), 137.2 (1C, Ar-C-1), 131.1 (2C, Ar-C-2, C-6), 128.2 (2C, Ar-C′-4), 129.1 (4C, Ar-C′-3, C′-5), 127.6 (4C, Ar-C′-2, C′-6), 126.5 (2C, Ar-C′-1), 116.7 (2C, Ar-C-3, C-5), 105.3 (2C, thiazole-2-yl, C-3), 102.4 (2C, 1,4-dihydropyridine, C-3, C-5), 32.4 (1C, 1,4-dihydropyridine, C-4), 17.6 (2C, 1,4-dihydropyridine, C-2-CH3, C-6-CH3); EI-MS:m/z 605.72 Elemental analysis: Calcd. For (C33H27N5O3S2): C, 65.43%; H, 4.49%; N, 11.56%; S, 10.59%. Found: C, 65.49%; H, 4.52%; N, 11.61%; S, 10.65%.
2.1.5. 4-(4-Nitrophenyl)-2,6-dimethyl-N3,N5-bis(4-phenylthiazol-2-yl)-1,4-dihydropyri dine-3,5-dicarboxamide (2e)
Yellow solid; Yield 71%; mp:193–195 °C; IR (cm−1): 3172 (NH), 3074 (CHstr), 3041 (Ar-H), 1652 (OCNH), 1530 (C-NO2), 1479 (C=N), 738 (C-S-C); 1H NMR (300 MHz, DMSO-d6): δ 13.60 (s, 2H, -CONH), 8.84 (s, 1H, NH), 8.15 (d, 2H, J = 6.78 Hz, Ar-H-3, H-5), 7.91–7.72 (m, 10H, Ar-H), 7.43 (s, 2H, thiazole-2-yl, H-3), 7.41 (d, 2H, J = 6.17 Hz, Ar-C-2, C-6), 5.85 (s, 1H, 4-CH), 2.42 (s, 6H, 2,6-CH3); 13C NMR (75 MHz, DMSO-d6): 163.6 (2C, thiazole-2-yl, C-1), 163.1 (2C, C=O), 149.1 (2C, thiazole-2-yl, C-4), 148.5 (2C, 1,4-dihydropyridine, C-2, C-6), 150.4 (1C, Ar-C-1), 143.2 (1C, Ar-C-4), 135.0 (2C, Ar-C′-1), 130.6 (4C, Ar-C′-2, C′-6), 129.5 (4C, Ar-C′-3, C′-5), 128.8 (2C, Ar-C′-4), 126.1 (2C, Ar-C-2, C-6), 123.7 (2C, Ar-C-3, C-5), 105.1 (2C, thiazole-2-yl, C-3), 103.6 (2C, 1,4-dihydropyridine, C-3, C-5), 32.0 (1C, 1,4-dihydropyridine, C-4), 17.5 (2C, 1,4-dihydropyridine, C-2-CH3, C-6-CH3); EI-MS: m/z 634.72 (M+, 22%), Elemental analysis: Calcd. For (C33H26N6O4S2): C, 62.44%; H, 4.13%; N, 13.24%; S, 10.10%. Found: C, 62.49%; H,4.20%; N, 13.26%; S, 10.15%.
2.1.6. 4-(4-Methoxyphenyl)-2,6-dimethyl-N3,N5-bis(4-phenylthiazol-2-yl)-1,4-dihydro pyrid ine-3,5-dicarboxamide (2f)
White solid; Yield 65%; mp 231 °C; IR (cm−1): 3154 (NH), 3070 (CHstr), 1642 (OCNH), 1482 (C=N), 808 (Ar-H), 728 (C-S-C); 1H NMR (300 MHz, DMSO-d6): δ 13.56 (s, 2H, -CONH), 8.82 (s, 1H, NH), 7.79–7.60 (m, 10H, Ar-H), 7.45 (s, 2H, thiazole-2-yl, H-3), 7.16 (d, 2H, J = 6.70 Hz, Ar-H-2, H-6), 6.81 (d, 2H, J = 6.75 Hz, Ar-H-3, H-5), 5.81 (s, 1H, 4-CH), 3.81 (s, 3H, -OCH3), 2.47 (s, 6H, 2,6-CH3); 13C NMR (75 MHz, DMSO-d6): 164.6 (2C, thiazole-2-yl, C-1), 163.4 (2C, C=O), 150.6 (2C, thiazole-2-yl, C-4), 157.8 (1C, Ar), 148.6 (2C, 1,4-dihydropyridine, C-2, C-6), 136.2 (1C, Ar-C-1), 133.2 (2C, Ar- C′-1), 130.2 (2C, Ar-C-2, C-6), 129.8 (4C, Ar- C′-3, C′-5), 128.4 (2C, Ar- C′-4), 127.2 (4C, Ar- C′-2, C′-6),113 (2C, Ar-C-3, Ar-C-6), 105.1 (2C, thiazole-2-yl, C-3), 103.1 (2C, 1,4-dihydro pyridine, C-3, C-5), 32.4 (1C, 1,4-dihydropyridine, C-4), 17.9 (2C, 1,4-dihydropyridine, C-2-CH3, C-6-CH3); EI-MS: m/z 619.75 (M+, 20%); Elemental analysis: Calcd. For (C34H29N5O3S2) C, 65.89%; H, 4.72%; N, 11.30%; S, 10.35%. Found: C, 65.95%; H, 4.78%; N, 11.37%; S, 10.40%.
2.1.7. 4-(4-(Dimethylamino)phenyl)-2,6-dimethyl-N3,N5-bis(4-phenylthiazol-2-yl)-1,4-dihydro pyridine-3,5-dicarboxamide (2g)
Pale yellow solid; Yield 60%; mp:175–178 °C; IR (cm−1): 3170 (NH), 3069 (CHstr), 1652 (OCNH), 1481 (C=N), 810 (Ar-H), 738 (C-S-C); 1H NMR (300 MHz, DMSO-d6): δ 13.08 (s, 2H, -CONH), 8.90 (s, 1H, NH), 7.87–7.65 (m, 10H, Ph), 7.55 (s, 2H, thiazole-2-yl, H-3), 7.14 (d, 2H, J = 7.54 Hz, Ar-H), 7.12 (d, 2H, J = 7.45 Hz, Ar-H), 5.10 (s, 1H, 4-CH), 3.01 (s, 6H, -N(CH3)2), 2.25 (s, 6H, 2,6-C-CH3); 13C NMR (75 MHz, DMSO-d6): 165.7 (2C, thiazol-2-yl, C-1), 164.5 (2C, C=O), 150.4 (2C, thiazol-2-yl, C-4), 144.5 (1C, Ar-C-1), 135.2 (1C, Ar-C-4), 134.3 (2C, Ar- C′-1), 129.1 (4C, Ar- C′-3, C′-5), 128.9 (2C, Ar-C-2,6), 128.7 (2C, Ar-C-3,5), 128.0 (2C, Ar- C′-4), 127.0 (4C, Ar- C′-2, C′-6), 105.6 (2C, thiazol-2-yl, C-3), 103.4 (2C, 3,5-C in 1,4-dihydropyridine), 102.9 (2C, 2,6-C in 1,4-dihydropyridine), 21.2 (2C, Ph-N(CH3)2, 43.0 (1C, 1,4-dihydropyridine in C-4), 17.9 (2C, 2,6-CH3); EI-MS: m/z 632.15 (M+, 34%), Elemental analysis: Calcd. For (C35H32N6O2S2) C, 66.43%; H, 5.10%; N, 13.28%; S, 10.13%. Found: C, 66.40%; H, 5. 13%; N, 13.22%; S, 10.15%.
2.1.8. 2,6-Dimethyl-N3,N5-bis(4-phenylthiazol-2-yl)-4-p-tolyl-1,4-dihydropyridine-3,5-dicarbo xamide (2h)
White Powder; Yield 62%; mp:184–185 °C; IR (cm−1): 3180 (NH), 3072 (CHstr), 1662 (OCNH), 1445 (C=N), 816 (Ar-H), 742 (C-S-C); 1H NMR (300 MHz, DMSO-d6): δ 13.03 (s, 2H, -CONH), 8.90 (s, 1H, NH), 7.87–7.63 (m, 10H, Ph), 7.46 (s, 2H, thiazole-2-yl, H-3), 7.14 (d, 2H, J = 7.54 Hz, Ar-H), 7.12 (d, 2H, J = 7.45 Hz, Ar-H), 5.12 (s, 1H, 4-CH), 2.34 (s, 3H, Ar-CH3), 2.22 (s, 6H, 2,6-C-CH3); 13C NMR (75 MHz, DMSO-d6): 165.1 (2C, thiazol-2-yl, C-1), 164.1 (2C, C=O), 150.3 (2C, thiazol-2-yl, C-4), 144.5 (1C, Ar-C-1), 128.9 (2C, Ar-C-2,6), 128.7 (2C, Ar-C-3,5), 135.2 (1C, Ar-C-4), 134.3 (2C, Ar-C′-1), 129.1 (4C, Ar-C′-3, C′-5), 128.0 (2C, Ar-C′-4), 127.0 (4C, Ar-C′-2, C′-6), 105.0 (2C, thiazol-2-yl, C-3), 104.2 (2C, 1,4-dihydropyridine, C-3, C-5), 102.3 (2C,1,4-dihydropyridine, C-2, C-6), 43.0 (1C, 1,4-dihydropyridine-C-4), 21.1 (1C, Ph-CH3), 18.4 (2C, 2,6-CH3); EI-MS:m/z 603.76 (M+, 29%), Elemental analysis: Calcd. For (C34H29N5O2S2) C, 67.64%; H, 4.84%; N, 11.60%; S, 10.62%. Found: C, 67.38%; H,4.49%; N,11.95%; S, 10.94%.
2.1.9. General method for preparation of 4-(furan-2-yl)-2,6-dimethyl-N3,N5-bis(5-phenyl-1,3,4-thiadiazol-2-yl)-1,4-dihydropyridine-3,5-dicarboxamide (3a)
A mixture of compound, (diethyl-4-(furan-2-yl)-2,6-dimethyl-1,4-dihydropyridine-3,5-dicarboxylate) 1a (0.01 mol, 3.19 g), and 5-phenyl-1,3,4-thiadiazol-2-amine (0.02 mol, 3.54 g) were added in ethanol solvent stirred by 5 min on ultrasound irradiation. After 8 minute the reaction was completed, the product was confirmed by TLC. The product was washed with water and recrystallized by ethyl acetate to give pure product. The above procedure was followed for the synthesis of compounds 3b–e.
Yellow powder; Yield 80%; mp:192–195 °C; IR (cm−1): 3171 (NH), 3065 (Ar-CHstr), 1641 (OCNH), 1471 (C=N), 828 (Ar-H), 730 (C-S-C); 1H NMR (300 MHz, DMSO-d6): δ 13.21 (s, 2H, -CONH), 8.73 (s, 1H, NH), 8.26–7.43 (m, 10H, Ar-H), 7.27 (d, 1H, J = 6.70 Hz, furan), 6.41 (d, 1H, J = 6.93 Hz, furan), 6.24 (dd, 1H, J = 6.72 Hz, J = 6.95 Hz, furan), 5.24 (s, 1H, 4-CH), 2.42 (s, 6H, 2,6-CH3); 13C NMR (75 MHz, DMSO-d6): 174.2 (2C, thiadiazol-2-yl, C-3), 162.2 (2C, C=O), 152.5 (1C, furan, C-1), 152.4 (2C, thiadiazol-2-yl, C-1), 149.1 (2C, 1,4-dihydropyridie,C-2, C-6), 143.2 (1C, furan, C-4), 106.4 (1C furan, C-2), 129.1 (2C, Ar-C′-4), 128.2 (4C, Ar-C′-3, C′-5), 126.5 (4C, Ar-C′-2, C′-6), 126.3 (2C, Ar-C′-1), 113.3 (2C, furan, C-3), 103.6 (2C, 1,4-dihydropyridie, C-3, C-5), 32.4 (1C, 1,4-dihydropyridie, C-4), 18.4 (2C, 2,6-CH3); EI-MS: m/z 581.36 (M+, 24%); Elemental analysis: Calcd. for C29H23N7O3S2: C, 59.88%; H, 3.99%; N, 16.86%; S, 11.03%. Found: C, 59.82%; H, 3.97%; N, 16.82%; S, 11.05%.
2.1.10. 2,6-Dimethyl-4-phenyl-N3,N5-bis(5-phenyl-1,3,4-thiadiazol-2-yl)-1,4-dihydro pyridine-3,5-dicarboxamide (3b)
White powder; Yield 69%; mp: 192–195 °C; IR (cm−1): 3174 (NH), 3065 (CHstr), 1644 (OCNH), 1474 (C=N), 832 (Ar-H), 728 (C-S-C); 1H NMR (300 MHz, DMSO-d6): δ 13.24 (s, 2H, -CONH), 8.70 (s, 1H, NH), 8.21–7.41 (m, 10H, Ar-H), 7.30 (d, 2H, J = 7.09 Hz, Ar-C-3, C-5), 7.22 (t, 1H, J = 7.12 Hz, Ar-C-4),7.21 (d, 2H, J = 7.17 Hz, Ar-C-2, C-6), 5.22 (s, 1H, 4-CH), 2.41 (s, 6H, 2,6-CH3); 13C NMR (75 MHz, DMSO-d6): 174.0 (2C, thiadiazol-2-yl, C-3), 162.1 (2C, C=O), 151.8 (2C, thiadiazol-2-yl, C-1), 149.1 (2C, 1,4-dihydropyridie-C-2, C-6), 144.4 (1C, Ar-C-1), 129.6 (2C, Ar-C′-4), 128.9 (2C, Ar-C-3, C-5), 128.7 (4C, Ar-C′-3, C′-5), 127.6 (2C, Ar-C-2, C-6), 126.8 (4C, Ar-C′-2, C′-6), 126.0 (2C, Ar-C′-1), 125.2 (1C, Ar-C-4), 103.5 (2C, 1,4-dihydropyridie, C-3, C-5), 32.1 (1C, 1,4-dihydropyridie, C-4), 18.8 (2C, 2,6-CH3); EI-MS: m/z 591.36 (M+, 43%); Elemental analysis: Calcd. for C31H25N7O2S2: C, 62.93%; H, 4.26%; N, 16.57%; S, 10.84%. Found: C, 64.27%; H, 4.40%; N, 16.14%; S, 11.10%.
2.1.11. 4-(4-Chlorophenyl)-2,6-dimethyl-N3,N5-bis(5-phenyl-1,3,4-thiadiazol-2-yl)-1,4-dihydropyridine-3,5-dicarboxamide (3c)
Yellow solid; Yield 58%; mp:201–205 °C; IR (cm−1): 3175 (NH), 3068 (Ar-CHstr), 1648 (OCNH), 1480 (C=N), 827 (C-Cl), 812 (Ar-H), 736 (C-S-C); 1H NMR (300 MHz, DMSO-d6): δ 13.20 (s, 2H, -CONH), 8.72 (s, 1H, NH), 7.56–7.43 (m, 10H, Ar-H), 7.26 (d, 1H, J = 6.78 Hz, Ar-H), 6.41 (d, 1H, J = 6.91 Hz, Ar-H), 6.29 (t, 2H 1H, J = 6.89 Hz, J = 6.97 Hz, Ar-H-3,5), 5.20 (s, 1H, 4-CH), 2.40 (s, 6H, 2,6-CH3); 13C NMR (75 MHz, DMSO-d6): 174.1 (2C, thiadiazol-2-yl, C-3), 162.5 (2C, C=O), 152.3 (2C, thiadiazol-2-yl, C-1), 148.1 (2C, 1,4-dihydropyridie-C-2, C-6), 142.4 (1C, Ar-C-1), 131.2 (1C, Ar-C-4), 130.6 (2C, Ar-C-2, C-6), 128.3 (4C, Ar-C′-3, C′-5), 129.3 (2C, Ar-C′-4), 126.7 (2C, Ar-C-3, C-5), 126.5 (2C, Ar-C′-1), 126.2 (4C, Ar-C′-2, C′-6), 103.2 (2C, 1,4-dihydropyridie-C-3,5), 32.8 (1C, 1,4-dihydropyridie-C-4), 18.2 (2C, 2,6-CH3); EI-MS: m/z 626.98 (M+, 30%); Elemental analysis: Calcd. for C31H24ClN7O2S2: C, 64.27%; H, 4.40%; N, 12.14%; S, 11.10%. Found: C, 64.35%; H, 4.51%; N, 12.20%; S, 11.15%.
2.1.12. 4-(4-Hydroxyphenyl)-2,6-dimethyl-N3,N5-bis(5-phenyl-1,3,4-thiadiazol-2-yl)-1,4-dihydpyridine-3,5-dicarboxamide (3d)
Pale yellow solid; Yield 63%; mp:165–168 °C; IR (cm−1): 3178 (NH), 3070 (CHstr), 1645 (OCNH), 1477 (C-OH), 1475 (C=N), 815 (Ar-H), 733 (C-S-C); 1H NMR (300 MHz, DMSO-d6): δ 13.21 (s, 2H, -CONH), 9.32 (s, 1H, –OH), 8.72 (s, 1H, NH), 7.60–7.43 (m, 10H, Ar-H), 7.01 (d, 2H, J = 6.57 Hz, Ar-C-2, C-6), 6.75 (d, 2H, J = 6.88 Hz, Ar-C-3, C-5), 5.28 (s, 1H, 4-CH), 2.41 (s, 6H, 2,6-CH3); 13C NMR (75 MHz, DMSO-d6): 174.6 (2C, thiadiazol-2-yl, C-3), 162.8 (2C, C=O), 155.2 (1C, Ar-C-4), 152.1 (2C, thiadiazol-2-yl, C-1), 148.6 (2C, 1,4-dihydropyridie-C-2, C-6), 137.2 (1C, Ar-C-1), 131.1 (2C, Ar-C-2, C-6), 129.1 (4C, Ar-C′-3, C′-5), 128.2 (2C, Ar-C′-4), 127.6 (4C, Ar-C′-2, C′-6), 126.5 (2C, Ar-C′-1), 116.7 (2C, Ar-C-3, C-5), 102.9 (2C, 1,4-dihydropyridie-C-3,5), 32.0 (1C, 1,4-dihydropyridie-C-4), 18.1 (2C, 2,6-CH3); EI-MS: m/z 607.12 (M+, 13%); Elemental analysis: Calcd. for C31H25N7O3S2: C, 64.27%; H, 4.40%; N, 12.14%; S, 11.10%. Found: C, 64.35%; H, 4.50%; N, 12.19%; S, 11.19%.
2.1.13. 2,6-Dimethyl-4-(4-nitrophenyl)–N3,N5-bis(5-phenyl-1,3,4-thiadiazol-2-yl)-1,4-dihydro pyridine-3,5-dicarboxamide (3e)
White powder; Yield 69%; mp:150–152 °C; IR (cm−1): 3180 (NH), 3070 (Ar-CHstr), 1641 (OCNH), 1530 (C-NO2), 1478 (C=N), 820 (Ar-H), 736 (C-S-C); 1H NMR (300 MHz, DMSO-d6): δ 13.21 (s, 2H, -CONH), 8.72 (s, 1H, NH), 8.15 (d, 2H, J = 6.78 Hz, Ar-C-3, C-5), 7.58–7.40 (m, 10H, Ar-H), 7.41 (d, 2H, J = 6.17 Hz, Ar-C-2, C-6), 5.25 (s, 1H, 4-CH), 2.46 (s, 6H, 2,6-CH3); 13C NMR (75 MHz, DMSO-d6): 174.9 (2C, thiadiazol-2-yl, C-3), 162.0 (2C, C=O), 154.3 (2C, thiadiazol-2-yl, C-1), 150.4 (1C, Ar-C-1), 148.4 (2C, 1,4-dihydropyridie-C-2, C-6), 143.2 (1C, Ar-C-4), 135.0 (2C, Ar-C′-1), 130.6 (4C, Ar-C′-2, C′-6), 129.5 (4C, Ar-C′-3, C′-5), 128.8 (2C, Ar-C′-4), 126.1 (2C, Ar-C-2, C-6), 123.7 (2C, Ar-C-3, C-5), 103.61 (2C, 1,4-dihydropyridie-C-3,5), 32.1 (1C, 1,4-dihydropyridie-C-4), 18.5 (2C, 2,6-CH3); EI-MS: m/z 636.36 (M+, 27%); Elemental analysis: Calcd. for C31H24N8O4S2: C, 64.27%; H, 4.40%; N, 12.14%; S, 11.10%. Found: C, 64.31%; H, 4.45%; N, 12.20%; S, 11.13%.
2.1.14. General method for preparation of 4-(furan-2-yl)-2,6-dimethyl-N3,N5-bis(5-phenyl-1,3,4-oxadiazol-2-yl)-1,4-dihydropyridine-3,5-dicarboxamide (4a)
A mixture of compound, (diethyl-4-(furan-2-yl)-2,6-dimethyl-1,4-dihydropyridine-3,5-dicarboxylate) 1a (0.01 mol, 3.19 g), and 5-phenyl-1,3,4-oxadiazol-2-amine (0.02 mol, 3.22 g) were added in ethanol solvent stirred by 5 min on ultrasound irradiation. After 9 minute the reaction was completed, the product was confirmed by TLC. The product was washed with water and recrystallized by ethyl acetate to give pure product. The above procedure was followed by synthesis of remaining compounds 4b–e.
Pale yellow solid; Yield 60%; mp:195–198 °C; IR (cm−1): 3170 (NH), 3054 (CHstr), 1642 (OCNH), 1470 (C=N), 812 (Ar-H), 712 (C-O-C); 1H NMR (300 MHz, DMSO-d6): δ 13.21 (s, 2H, -CONH), 8.75 (s, 1H, NH), 7.78–7.42 (m, 10H, Ar-H), 7.22 (d, 1H, J = 6.65 Hz, furan), 6.45 (d, 1H, J = 6.97 Hz, furan), 6.27 (dd, 1H, J = 6.75 Hz, J = 6.91 Hz, furan), 5.25 (s, 1H, 4-CH), 2.43 (s, 6H, 2,6-CH3); 13C NMR (75 MHz, DMSO-d6): 164.8 (2C, oxadiazol-2-yl, C-3), 164.3 (2C, oxadiazol-2-yl, C-1), 162.2 (2C, C=O), 152.5 (1C, furan-C-1), 148.3 (2C, 1,4-dihydropyridie-C-2, C-6), 143.2 (1C, furan-C-4), 129.1 (2C, Ar-C′-4), 128.2 (4C, Ar-C′-3, C′-5), 126.5 (2C, Ar-C′-2, C′-6), 126.3 (2C, Ar-C′-1), 113.0 (2C, furan-C-3), 106.4 (2C, furan-C-2), 103.3 (2C, 1,4-dihydropyridie-C-3,5), 32.4 (1C, 1,4-dihydropyridie-C-4), 18.9 (2C, 2,6-CH3); EI-MS: m/z 549.36 (M+, 56%); Elemental analysis: Calcd. for C29H23N7O5: C, 63.38%; H, 4.22%; N, 17.84%; Found: C, 63.30%; H, 4.21%; N, 17.85%.
2.1.15. 2,6-Dimethyl-4-phenyl-N3,N5-bis(5-phenyl-1,3,4-oxadiazol-2-yl)-1,4-dihydropy ridine-3,5-dicarboxamide (4b)
Pale yellow solid; Yield 60%; mp:207–210 °C; IR (cm−1): 3175 (NH), 3066 (CHstr), 1644 (OCNH), 1475 (C=N), 821 (Ar-H), 708 (C-O-C); 1H NMR (300 MHz, DMSO-d6): δ 13.22 (s, 2H, -CONH), 8.72 (s, 1H, NH), 7.69–7.41 (m, 10H, Ar-H), 7.30 (d 2H, J = 7.09 Hz, Ar-C-3, C-5), 7.22 (d, 1H, J = 7.12 Hz, Ar-C-4), 7.20 (d, 2H, J = 7.17 Hz, Ar-C-2, C-6), 5.25 (s, 1H, 4-CH), 2.42 (s, 6H, 2,6-CH3); 13C NMR (75 MHz, DMSO-d6): 163.6 (2C, oxadiazol-2-yl, C-3), 165.8 (2C, oxadiazol-2-yl, C-2), 162.2 (2C, C=O), 148.4 (2C, 1,4-dihydropyridie, C-2, C-6), 144.4 (1C, Ar-C-1), 129.6 (2C, Ar-C′-4), 128.7 (2C, Ar-C-3, C-5), 128.5 (4C, Ar-C′-3, C′-5), 127.6 (2C, Ar-C-2, C-6), 126.8 (4C, Ar-C′-2, C′-6), 126.0 (2C, Ar-C′-1), 125.2 (1C, Ar-C-4), 102.5 (2C, 1,4-dihydropyridie, C-3, C-5), 32.2 (1C, 1,4-dihydropyridie, C-4), 17.1 (2C, 2,6-CH3); EI-MS: m/z 559.36 (M+, 46%); Elemental analysis: Calcd. for C31H25N7O4: C, 66.54%; H, 4.50%; N, 17.52%; Found: C, 64.27%; H, 4.40%; N, 17.14%.
2.1.16. 4-(4-Chlorophenyl)-2,6-dimethyl-N3,N5-bis(5-phenyl-1,3,4-oxadiazol-2-yl)-1,4-dihydr opyridine-3,5-dicarboxamide (4c)
Yellow solid; Yield 64%; mp: 241–244 °C; IR (cm−1): 3171 (NH), 3065 (CHstr), 1641 (OCNH), 1471 (C=N), 827 (C-Cl), 823 (Ar-H), 730 (C-O-C); 1H NMR (300 MHz, DMSO-d6): δ 13.21 (s, 2H, -CONH), 8.71 (s, 1H, NH), 7.74–7.51 (m, 10H, Ar-H), 7.30 (d, 2H, J = 6.98 Hz, Ar-H-3, H-5), 7.21 (d, 2H, J = 6.87 Hz, Ar-H-2, H-6), 5.20 (s, 1H, 4-CH), 2.40 (s, 6H, 2,6-CH3); 13C NMR (75 MHz, DMSO-d6): 165.2 (2C, oxadiazol-2-yl, C-3), 163.6 (2C, oxadiazol-2-yl, C-1), 163.2 (2C, C=O), 148.2 (2C, 1,4-dihydropyridie-C-2, C-6), 142.4 (1C, Ar-C-1), 131.2 (1C, Ar-C-4), 130.6 (2C, Ar-C-2, C-6), 129.3 (2C, Ar-C′-4), 128.3 (4C, Ar-C′-3, C′-5), 126.7 (2C, Ar-C-3, C-5), 126.3 (2C, Ar-C′-1), 126.2 (4C, Ar-C′-2, C′-6), 102.3 (2C, 1,4-dihydropyridie-C-3,5), 33.2 (1C, 1,4-dihydropyridie-C-4), 17.5 (2C, 2,6-CH3); EI-MS: m/z 594.25 (M+, 19%); Elemental analysis: Calcd. for C31H24ClN7O4: C, 62.68%; H, 4.07%; N, 16.51%; Found: C, 62.27%; H, 4.15%; N, 16.14%.
2.1.17. 4-(4-Hydroxyphenyl)-2,6-dimethyl-N3,N5-bis(5-phenyl-1,3,4-oxadiazol-2-yl)-1,4-dihy dropyridine-3,5-dicarboxamide (4d)
White powder; Yield 86%; mp 195–198 °C; IR (cm−1): 3174 (NH), 3061 (CHstr), 1643 (OCNH), 1475 (C=N), 1472 (C-OH), 820 (Ar-H), 732 (C-O-C); 1H NMR (300 MHz, DMSO-d6): δ 13.23 (s, 2H, -CONH), 9.41 (s, 1H, –OH), 8.70 (s, 1H, NH), 7.74–7.42 (m, 10H, Ar-H), 7.01 (d, 2H, J = 6.57 Hz, Ar-H-2, H-6), 6.75 (d, 2H, J = 6.88 Hz, Ar-C-3, C-5), 5.24 (s, 1H, 4-CH), 2.44 (s, 6H, 2,6-CH3); 13C NMR (75 MHz, DMSO-d6): 164.8 (2C, oxadiazol-2-yl, C-2), 164.4 (2C, oxadiazol-2-yl, C-4), 162.1 (2C, C=O), 155.2 (1C, Ar-C-4), 149.6 (2C, 1,4-dihydropyridie, C-2, C-6), 137.2 (1C, Ar-C-1), 131.1 (2C, Ar-C-2, C-6), 129.1 (4C, Ar-C′-3, C′-5), 128.2 (2C, Ar-C′-4), 127.6 (4C, Ar-C′-2, C′-6),126.5 (2C, Ar-C′-1), 116.7 (2C, Ar-C-3, C-5), 103.4 (2C, 1,4-dihydropyridie, C-3, C-5), 32.4 (1C, 1,4-dihydropyridie, C-4), 18.1 (2C, 2,6-CH3); EI-MS: m/z 575.10 (M+, 33%); Elemental analysis: Calcd. for C31H25N7O5: C, 64.27%; H, 4.40%; N, 12.14%; S, 11.06%. Found: C, 64.18%; H, 4.45%; N, 12.18%; S, 11.15%.
2.1.18. 2,6-Dimethyl-4-(4-nitrophenyl)–N3,N5-bis(5-phenyl-1,3,4-oxadiazol-2-yl)-1,4-dihydro pyridine-3,5-dicarboxamide (4e)
White solid; Yield 79%; mp:210–212 °C; IR (cm−1): 3173 (NH), 3065 (CHstr), 1530 (C-NO2), 1641 (OCNH), 1471 (C=N), 818 (Ar-H), 730 (C-O-C); 1H NMR (300 MHz, DMSO-d6): δ 13.20 (s, 2H, -CONH), 8.72 (s, 1H, NH), 7.68–7.42 (m, 10H, Ar-H), 8.15 (d, 2H, J = 6.78 Hz, Ar-H-3, H-5), 7.41 (d, 2H, J = 6.17 Hz, Ar-H-2, H-6), 5.23 (s, 1H, 4-CH), 2.46 (s, 6H, 2,6-CH3); 13C NMR (75 MHz, DMSO-d6): 164.8 (2C, oxadiazol-2-yl, C-3), 165.2 (2C, oxadiazol-2-yl, C-1), 162.0 (2C, C=O), 150.4 (1C, Ar-C-1), 149.3 (2C, 1,4-dihydro pyridie, C-2, C-6), 143.2 (1C, Ar-C-4), 135.0 (2C, Ar-C′-1), 130.6 (4C, Ar-C′-2, C′-6), 129.5 (4C, Ar-C′-3, C′-5), 128.8 (2C, Ar-C′-4),126.1 (2C, Ar-C-2, C-6), 123.7 (2C, Ar-C-3, C-5), 102.6 (2C, 1,4-dihydropyridie, C-3, C-5), 32.1 (1C, 1,4-dihydropyridie-C-4), 17.8 (2C, 2,6-CH3); EI-MS: m/z 604.76 (M+, 17%); Elemental analysis: Calcd. for C31H24N8O6: C, 64.27%; H, 4.00%; N, 18.53%; Found: C, 64.30%; H, 4.48%; N, 12.14%.
2.2. Biological evaluation
2.2.1. Anticoagulant activity
The anticoagulant study was carried out according to the method described in previous literature (De-Zoysa et al., 2008).
2.2.2. Antibacterial activity
Compounds 2a–h, 3a–e and 4a–e were evaluated for their in vitro antibacterial activity against Escherichia coli (ATCC-25922), Pseudomonas aeruginosa (ATCC-27853), Staphylococcus aureus (ATCC-25923), Staphylococcus epidermidis, and Klebsiella pneumoniae (recultured) by disc diffusion method (Wayne, 2003) with performed using Mueller-Hinton agar (Hi-media) each compounds. Each compound was tested at a concentration of 100 μg/mL in DMSO. Ciprofloxacin was used as the standard. The zone of inhibition was measured after 24 h incubation at 37 °C. The minimum inhibitory concentration (MIC) was considered to be the lowest concentration that completely inhibited the growth on agar plates.
2.2.3. Antifungal screening
The compounds were evaluated for their in-vitro antifungal activity against Aspergillus niger, Candida albicans, Cryptococcus neoformans, and Microsporum audouinii using an agar dilution method (Gillespie, 1994) with sabouraud’s dextrose agar (Hi-Media). Each compound was tested at a concentration of 100 μg/mL in DMSO and samples were carefully placed on the agar culture plates that had been previously inoculated separately with the microorganisms. Clotrimazole was used as the standard. The plates were then incubated for 24 h at 37 °C, and the diameter of the growth inhibition zones was measured and recorded.
2.2.4. Determination of the minimum inhibitory concentration (MIC)
Selected synthesized compounds 2c, 2e, 3b and 4b where determined by the minimum inhibitory concentration (MIC) at a concentration of 64 μg/mL. The two fold dilutions of the solution were prepared (64, 32…, 0.5 μg/mL). The microorganism suspensions at 106 (CFU/mL, colony forming unit/mL) concentrations were inoculated to the corresponding wells. The plates were incubated at 36 °C at 24 h. The (MIC) values were determined as the lowest concentration that completely inhibited visible growth of the microorganisms.
2.2.5. Cytotoxic activity
The newly synthesized compounds (2a–h), (3a–e), and (4a–e) were screened for their anticancer activity according to a previously published procedure (Surendra Kumar et al., 2017). Compounds (100 μM) were incubated in a microtiter plate with three different cell lines for 72 h, and cell viability was assessed by MTT (3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) assay. The three cell lines were HepG2 (liver), MCF-7 (breast), and HeLa (cervical). The percentage of growth of the treated cells compared to that of the untreated control cells was calculated. Compounds that reduced the growth of a cell line by 32% or more were considered to have antitumor activity.
The measured 0.1 mL of the cell suspension (containing 5 × 106 cells/100 μL) and 0.1 mL of the test solution (6.25–100 μg 1% DMSO such that the final concentration of DMSO in media was less than 1%) were added to the 27 well plates and kept in a 5% CO2 incubator at 37 °C for 72 h. The blank contained only cell suspension and control wells contained 1% DMSO and cell suspension. After 72 h, 20 μL of MTT was added and kept in the CO2 incubator for 2 h followed by addition of 100 μL propanol. The plate was covered with aluminum foil to protect from light. Then the 27 well plates were kept in a rotary shaker for 10–20 min. After 10–20 min, the 27 well plates were processed on an ELISA reader for absorption at 562 nm.
3. Results and discussion
3.1. Chemistry
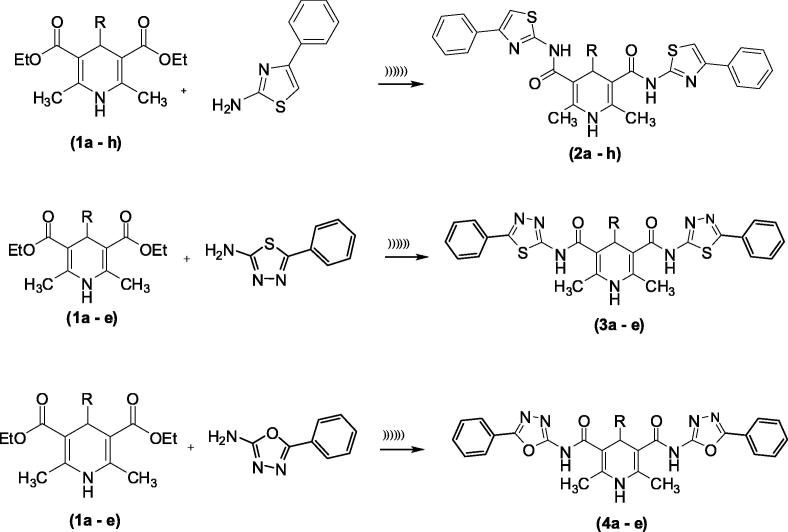
We reported that 1,4-dihydropyridine derivatives 1a–h using the Hantzsch method (Hadizadeh et al., 2002). Compounds 2a–h, 3a–e, and 4a–e were prepared via amination method (Scheme 1).
Scheme 1.
Synthetic route of compounds (2a–h, 3a–e and 4a–e). R = 2a, 3a, 4a: -Furyl; 2b, 3b, 4b: -Ph; 2c, 3c, 4c: 4-Cl-Ph; 2d, 3d, 4d: 4-OH-Ph; 2e, 3e, 4e: 4-NO2–Ph; 2f: 4-CH3O-Ph; 2g: 4-(CH3)2N-Ph; 2h: 4-CH3-Ph.
To recognize the optimization of the reaction conditions, the reaction was studied by employing ethanol medium in ultrasound irradiation the hope to maximize the product good yield in short reaction times than other solvents and conventional method.
The IR spectrum of 2a–h displayed absorption bands range at 3154–3180, 1612–1662, 728–742, 1445–1488, and 3045–3074 cm−1, corresponding to the -NH, -HNCO, -C-S-C, -C=N, and aromatic CH groups, respectively. The 1H NMR spectrum of 2a–h shows that signals range at δ 13.03–13.62, 8.81–8.90, and 5.10–5.89 ppm, corresponding to the CONH, NH, and 4CH protons, respectively. The 13C NMR spectrum of 2a–h shows peaks at δ163.1–164.5, 105.0–105.6, and 32.0–43.4 ppm, corresponding to the C=O, CH-thiazole ring, and 4-C carbon atoms present in 1,4-dihydropyridne, respectively. The EI mass spectrum of 2a showed a molecular ion peak at m/z 579.23 (M+, 10%), confirming the molecular weight of compound 2a.
IR spectrum of compounds 3a–e shows that important characteristics absorption band range at 3171–3180, 1641–1648, 728–736, and 1471–1480 cm−1, corresponding to the -NH, -HNCO, -C-S-C, and C=N group present in 1,3,4-thiadiazol-2-yl, respectively. The 1H NMR spectrum of compounds 3a–e shows that signals range at δ 13.20–13.24 and 5.20–5.28 ppm, corresponding to the CONH and 4CH proton present in 1,4-dihydropyridine ring, respectively. The 13C NMR spectrum of 3a–e showed peaks range at δ 162.0–162.8, 174.0–174.9, 151.8–154.3, and 32.0–32.8 ppm, corresponding to the CO, 2C-NH and 4-CH carbon present in 1,4-dihydropyridine, respectively.
The important IR spectrum characteristics bonds of compounds 4a–e shows that the absorption bands at 3170–3175, 1641–1644, 708–732, 1470–1475, and 3054–3066 cm−1, corresponding to the -NH, -HNCO, -C-O-C, -C=N, and aromatic CH groups, respectively. The 1H NMR spectrum of compound 4a–e showed signals range at δ 13.20–13.23 and 5.20–5.25 ppm, corresponding to the CONH and 4CH protons, respectively. The 13C NMR spectrum of 4a–e showed peaks range at δ 162.0–163.2, and 32.1–32.4 ppm, corresponding to a C=O group, and the 4-CH carbon atom present in 1,4-dihydropyridine ring, respectively.
3.2. Biological activity
3.2.1. Anticoagulant activity
All of the synthesized compounds (2a–h), (3a–e), and (4a–e) were screened for their anticoagulant activity, and the data were compared with heparin. The anticoagulant evaluations data of the synthesized compounds were represented in Table 1. The in vitro anticoagulant activity of APTT and PT in human plasma was examined. All synthesized compounds (2a–h), (3a–e), and (4a–e) showed higher APTT and PT values than the vehicle control. Among the synthesized compounds 3d was highly active against APTT and PT assays for anticoagulant screening. The APTT coagulation assay was performed at 60 μg/mL concentration.
Table 1.
Anti-coagulant activity of compounds 2a–h, 3a–h, and 4a–h with standard heparin.
| Comp. No | Concentration (60 μg/mL) |
|||
|---|---|---|---|---|
| Clotting time(s) (APTT) | APTT Index | Clotting time(s) (PT) | PT index | |
| 2a | 424.42 | 11.65 | 112.58 | 5.66 |
| 2b | 316.81 | 8.69 | 110.20 | 5.54 |
| 2c | 519.65 | 14.26 | 132.62 | 6.67 |
| 2d | 662.76 | 18.19 | 123.21 | 6.20 |
| 2e | 622.41 | 17.08 | 141.05 | 7.10 |
| 2f | 514.03 | 14.11 | 108.52 | 5.46 |
| 2g | 458.75 | 12.59 | 128.87 | 6.48 |
| 2h | 659.92 | 18.11 | 142.67 | 7.18 |
| 3a | 834.21 | 22.90 | 104.30 | 5.26 |
| 3b | 578.48 | 15.88 | 107.61 | 5.41 |
| 3c | 768.95 | 21.11 | 131.53 | 6.62 |
| 3d | >1000a | 27.45 | 106.62 | 5.38 |
| 3e | 675.32 | 18.54 | 103.85 | 5.24 |
| 4a | 156.30 | 4.29 | 37.52 | 1.88 |
| 4b | 152.73 | 4.19 | 25.21 | 1.26 |
| 4c | 158.81 | 4.36 | 30.05 | 1.38 |
| 4d | 163.40 | 4.48 | 27.56 | 1.36 |
| 4e | 142.82 | 3.92 | 30.78 | 1.54 |
| Heparin | >1000a | 27.45 | – | – |
| Control | 36.42 | 1.0 | 19.86 | 1.0 |
Clotting time >1000 s considered as 1000 s to calculate the relative clotting potency.
Values are expressed as mean of five trails.
Highly significant index.
Compounds 4a–e was low active compared to 2a–h and 3a–3e against APTT and PT assays. Particularly, compound 2d shows low active (662.76 s) in APTT assays compared with compound 3d (>1000 s) where as compound 2d was (662.76 s) highly active compared with other compounds (2a, 2b, 2c, 2e, 2f, 2g, and 2h).
The heparin is caused by its complexing with antithrombin 111, which accelerates the formation of a stable 1:1 complex between antithrombin 111 and thrombin (Harpel and Rosenberg, 1976). Hirano et al., reported that sulphur derivatives shows high anticoagulant activities with respect to activated partial thromboplastin time (Hirano et al., 1985). This study was propose that the synthetic sulphur containing derivatives work as anticoagulants in a mechanism same from that of heparin.
3.2.2. In vitro antimicrobial activity
Compound 2e was highly active against E. coli and compounds 3b and 4b exhibited moderately antibacterial activities against E. coli. The primary screening was performed using the agar disc diffusion method on Müller-Hinton medium. The majority of the synthesized compounds showed varying degrees of inhibition against the microorganisms tested. The in vitro antifungal activities of compounds 2a–h, 3a–e, and 4a–e were evaluated at 100 μg/mL against fungal spices. The primary screening was performed using the agar dilution method on Sabouraud’s dextrose agar (Hi-Media). Compound 2c was highly active against C. albicans (growth inhibition zones 26 mm) compared with the positive control and compounds 3b and 4b, which showed moderate activity against C. albicans.
Minimum inhibitory concentrations (MICs): The data in Table 2 shows that the antibacterial and antifungal activities of compounds 2c, 2e, 3b, and 4b were greater than those of the other 1,4-dihydropyridine derivatives and standards. The antibacterial activity compound 2e was highly activity MIC: (0.25 μg/mL) were comparable against E. coli. compared with positive control MIC: (0.5 μg/mL). The antifungal activity compound 2c was highly activity (MIC: 0.5 μg/mL) were comparable against C. albicans compared with positive control (MIC: 1 μg/mL).
Table 2.
Antimicrobial activity with minimum inhibitory concentrations (MIC, μg/mL).
| Comp. No. | Minimum Inhibitory Concentration (MIC, μg/mL) |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Antibacterial activity |
Antifungal activity |
||||||||
| E.c | P.a | S.a | S.e | K.p | A. n | C. a | C. n | M. a | |
| 2a | 64 | 1 | 32 | 32 | – | 64 | 64 | >100 | >100 |
| 2b | 32 | 32 | 32 | 32 | 8 | 32 | 32 | >100 | >100 |
| 2c | 2 | 0.5 | 8 | 32 | >100 | 32 | 0.5 | 64 | >100 |
| 2d | 64 | >100 | 32 | 64 | 64 | 8 | 64 | >100 | >100 |
| 2e | 0.25 | 64 | >100 | 32 | 32 | >100 | >100 | >100 | 64 |
| 2f | 32 | >100 | 32 | 8 | >100 | 8 | 32 | >100 | >100 |
| 2g | 32 | 64 | 64 | 64 | >100 | 4 | 64 | >100 | >100 |
| 2h | 64 | >100 | >100 | 64 | >100 | 32 | 32 | >100 | >100 |
| 3a | 64 | >100 | >100 | 32 | 64 | 4 | 64 | >100 | 16 |
| 3b | 1 | >100 | 32 | >100 | 8 | 32 | 2 | 8 | 64 |
| 3c | 32 | 32 | 16 | 32 | 4 | 32 | 32 | 16 | 8 |
| 3d | 64 | 32 | >100 | 64 | 64 | 8 | 32 | >100 | 16 |
| 3e | 64 | 32 | 32 | 64 | >100 | 32 | 16 | 64 | 8 |
| 4a | 32 | 8 | 32 | >100 | >100 | 32 | >100 | >100 | 8 |
| 4b | 4 | 64 | 64 | >100 | 8 | 64 | 1 | 16 | 8 |
| 4c | 32 | 32 | 64 | 16 | 32 | 8 | >100 | 16 | 16 |
| 4d | 32 | 64 | 16 | 16 | 8 | >100 | 8 | 32 | 64 |
| 4e | 32 | >100 | – | 32 | 8 | 8 | >100 | >100 | 32 |
| Ciprofloxacin | 0.5 | 1 | 0.5 | 4 | 2 | – | – | – | – |
| Clotrimazole | – | – | – | – | – | 2 | 1 | 0.5 | 0.5 |
3.2.3. Cytotoxicity screening
All compounds were also screened for anticancer activity against liver, cervical, and breast cancer cell lines. The GI50, tumor growth inhibition (TGI), and LC50 values were determined. Compound (2c) was highly active against HeLa (GI50: 0.02 μm), and MCF-7 cells (GI50:0.03 μm) compared with the activity of other compounds. Compound (2e) were equipotant active against MCF-7 cell (GI50: 0.03 μm) compared with Doxorubicin. The other compounds (2c, 2e, 3d, 4b) showed moderate activity against HepG2 and MCF-7 cell line compared to Doxorubicin. The cytotoxicity screening results are summarized in Table 3.
Table 3.
Cytotoxic activity of compounds (2a–h), (3a–e), and (4a–e).
| Compounds | HepG2 |
MCF-7 |
HeLa |
||||||
|---|---|---|---|---|---|---|---|---|---|
| GI50 (μM) | TGI (μM) | LC50 (μM) | GI50 (μM) | TGI (μM) | LC50 (μM) | GI50 (μM) | TGI (μM) | LC50 (μM) | |
| 2a | 16.2 | 29.1 | >100 | 22.9 | 46.8 | 57.2 | 11.6 | 29.4 | 41.2 |
| 2b | 13.3 | 24.8 | 81.2 | 20.1 | 45.1 | 56.4 | 11.0 | 27.2 | 47.3 |
| 2c | 08.2 | 18.1 | 25.1 | 0.03 | 0.64 | 0.81 | 0.02 | 0.48 | 0.89 |
| 2d | 12.9 | 21.6 | 42.7 | 25.9 | 57.4 | 60.8 | 12.9 | 22.5 | 47.9 |
| 2e | 03.9 | 23.9 | 47.9 | 0.03 | 47.6 | 87.0 | 09.8 | 16.0 | 34.6 |
| 2f | 16.3 | 25.3 | 48.2 | 15.2 | 20.1 | 38.2 | 18.1 | 27.1 | 45.3 |
| 2g | 15.4 | 22.5 | 42.5 | 13.5 | 26.9 | 43.5 | 16.8 | 24.7 | 42.8 |
| 2h | 11.7 | 22.1 | 40.9 | 12.6 | 22.5 | 48.4 | 19.8 | 22.6 | 41.0 |
| 3a | 17.9 | 27.9 | 42.9 | 10.7 | 27.9 | 41.2 | 19.7 | 23.2 | 45.4 |
| 3b | 14.8 | 25.8 | 47.1 | 16.2 | 26.4 | 42.3 | 13.8 | 22.4 | 46.3 |
| 3c | 11.0 | 22.1 | 42.1 | 14.9 | 24.9 | 43.1 | 11.7 | 28.9 | 56.2 |
| 3d | 01.6 | 14.8 | 22.4 | 01.6 | 15.0 | 3.8 | 12.8 | 20.4 | 41.2 |
| 3e | 18.6 | 20.3 | 42.2 | 11.7 | 24.8 | 64.9 | 17.9 | 31.0 | 47.7 |
| 4a | 17.9 | 24.8 | 43.2 | 11.8 | 23.8 | 40.3 | 12.8 | 24.9 | 39.5 |
| 4b | 05.9 | 16.9 | 28.3 | 07.6 | 18.9 | 32.1 | 18.9 | 21.9 | 35.6 |
| 4c | 16.7 | 21.0 | 40.4 | 18.3 | 20.2 | 42.4 | 13.3 | 26.0 | 48.3 |
| 4d | 14.2 | 26.8 | 42.4 | 15.6 | 29.3 | 92.3 | 13.2 | 24.0 | 42.3 |
| 4e | 16.0 | 28.6 | 42.0 | 19.3 | 25.2 | 39.0 | 15.3 | 23.2 | 44.3 |
| Doxorubicin | 0.01 | 0.13 | 0.58 | 0.02 | 0.21 | 0.74 | 0.05 | 0.41 | 0.88 |
3.2.4. Structure activity relationship
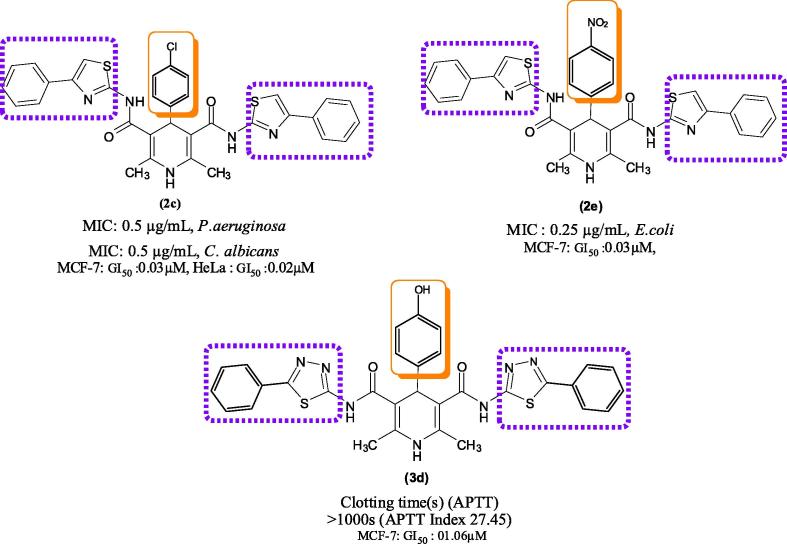
The relationship between the chemical structures and biological activities were validates several assumptions. Fig. 2 shows that the 4-substituted phenyl ring acts as a lipophilic domain, while the C=O and NH groups act as electron donors and hydrogen bonding domains, respectively. Therefore, the 1,4-dihydropyridine moiety with thiazole moiety was an essential pharmacophoric requirement of biological activity.
Fig. 2.
Structural activity relationship of compound 2c, 2e, and 3b.
Compounds 2e and 2c, both of which bear a 1,4-dihydropyridine moiety with a 4′-substituted group (4′-NO2, 4-Cl-Ph), shows significant antibacterial and antifungal activities. Compound 2e was highly active against E. coli due to the presence of a NO2 containing 1,4-dihydropyridine moiety. Compound 2c was highly active against C. albicans compared with clotrimazole. In general, compounds with electron withdrawing groups (4′-Cl) exhibited significantly higher activity than those of compounds containing an electron withdrawing group (4′-NO2). These observations indicate that 4′-NO2 and 4-Cl-Ph groups are well tolerate in this series. The cyctotoxicity activity of compound (2c) showed highly active against HeLa cells, whereas the compound 2e had moderate activity against HeLa cells compared with that of doxorubicin. The compound 2c, 2e were highly activity against MCF-7 and also modarate active against HepG2.
Compound 3b, which bears a 1,4-dihydropyridine moiety with 1,3,4-thiadiazol-2-yl group showed significant antibacterial activity against E. coli. Compound 4b, which contains 1,4-dihydropyridine moiety with 1,3,4-oxadiazol-2-yl groups exhibited equipotent activity against C. albicans and the other fungal strains.
The compounds 2d, 3d, and 4d containing para position OH-atoms of phenyl ring shows high potent inhibitors against APTT assay (clotting time values 662.76 s, >1000 s, 163.40 s) and PT assays (clotting time values 123.21 s, 106.62, and 27.56 s).
This emphasizes that the hydrophobic and lipophilic domains in the molecules are responsible for the potent anticoagulant activity. In addition, the effect of electron donating groups on the substituted benzene with 1,4-dihydropyridine moiety.
As a result, synthesized 1,4-dihydropyridine-3,5-dicarboxamide derivatives shows highly anticoagulant activity due to the presence of sulphur group (2a–h, 3a–e) compared with compounds 4a–e in APTT and PT assays. Compound 3d containing OH group with 1,3,4-thiadiazole shows highly active among the synthesized compounds.
4. Conclusion
A new series of 1,4-dihydropyridine derivatives, 2a–h, 3a–e, and 4a–e were synthesized via ultrasound irradiation and evaluated for antimicrobial and anticoagulant activities. The compound 2e (1,4-dihydropyridine with thiadiazole) was highly active against Escherichia coli and compound 2c was also highly active against Pseudomonas aeruginosa compared with ciprofloxacin. The compound 2c was highly active against Candida albicans compared with clotrimazole. Compound 3d was also a strong anticoagulant activity compared with heparin. Compound (2c) was highly active against HeLa (GI50: 0.02 μm), and MCF-7 (GI50: 0.03 μm) cell lines compared with other compounds, the compound (2e) was equipotant active against MCF-7 (GI50: 0.03 μm) cell line compared with that of doxorubicin. Therefore, these new 1,4-dihydropyridine derivatives may serve as lead molecules in the development of a clinically useful and novel class of antimicrobial, and anticoagulant agents.
Conflict of interest
None.
Acknowledgement
The project was supported by King Saud University, Deanship of Scientific Research Chair. We are very grateful to Prince Sultan Research Chair for Environment and Wildlife & Saudi Biological Society. We thank the Department of Botany & Microbiology, College of Sciences, King Saud University (KSU), Riyadh, Saudi Arabia for encouragement and support for funding this work.
Footnotes
Peer review under responsibility of King Saud University.
References
- Agudoawu S.A., Yiu S.H., Wallace J.L., Knaus E.E. Synthesis and analgesic activity of 2-methyl-2-[1-(3-benzoyl-4-substituted-1,4-dihydropyridyl)]acetic acid methyl esters, acetic acids, and acetamides. Arch. Pharm. Chem. Life. Sci. 1999;332:213–218. doi: 10.1002/(sici)1521-4184(19996)332:6<213::aid-ardp213>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Almasirad A., Mousavi Z., Tajik M., Assarzadeh M.J., Shafiee A. Synthesis, analgesic and anti-inflammatory activities of new methyl-imidazolyl-1,3,4-oxadiazoles and 1,2,4-triazoles. Daru J. Pharm. Sci. 2014;22:22. doi: 10.1186/2008-2231-22-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argyropoulou I., Geronikaki A., Vicini P., Zanib F. Synthesis and biological evaluation of sulfonamide thiazole and benzothiazole derivatives as antimicrobial agents (HP-3516MP) Arkivoc. 2009;vi:89–102. [Google Scholar]
- Balaji G.L., Rajesh K., Venkatesh M., Sarveswari S., Vijayakumar V. Ultrasound- promoted synthesis of bi-, tri- and tetrapodal polyhydroquinolines, 1,4-dihydropyridines and the corresponding pyridines. RSC Adv. 2014;4:39–46. [Google Scholar]
- Boer R., Gekeler V. Chemosensitizer in tumor therapy: new compounds promise better efficacy. Drugs Future. 1995;20:499–509. [Google Scholar]
- De-Zoysa M., Nikapitiya C.H., Jeon Y.J., Jee Y., Lee J. Anticoagulant activity of sulfated polysaccharide isolated from fermented brown seaweed Sargassum fulvellum. J. Appl. Phycol. 2008;20:67–74. [Google Scholar]
- Geronikaki A., Theophilidis G. Synthesis of 2-(aminoacetylamino)thiazole derivatives and comparison of their local anaesthetic activity by the method of action potential. E. J. Med. Chem. 1992;27:709–716. [Google Scholar]
- Gillespie S.H. Butterworth Heinemann; London: 1994. Medical Microbiology-Illustrated. [Google Scholar]
- Giridhar T., Reddy R.B., Prasanna B., Chandra Mouli G.V.P. Aminothiazoles: Part 1 – Syntheses and pharmacological evaluation of 4-[isobutylphenyl]-2-substituted-aminothiazoles. Indian J. Chem. 2001;40B:1279–1281. [Google Scholar]
- Hadizadeh F., Shaficee A., Kazemi R., Mohammad M. Synthesis of 4-(1-phenylmethyl-5-imidazolyl)-1,4-dihydropyrimidines as calcium channel antagonists. Indian J. Chem. 2002;41B:2679–2682. [Google Scholar]
- Harpel P.C., Rosenberg R.D. Grune and Stratton Inc.; New York, USA: 1976. 2-Macroglobulin and antithrombin-heparin cofactor: modulators of hemostatic and inflammatory reactions; p. 145. [PubMed] [Google Scholar]
- He J.Y., Jia H.Z., Yao Q.G., Liu S.J., Yue H.K., Yu H.W., Hu R.S. Ultrasound-mediated synthesis of 4-substituted 1,4-dihydropyridine-3,5-dicarboxylates catalyzed by 1-carboxymethyl-3-methylimidazolium tetrafluoroborate under solvent free condition. Ultrason. Sonochem. 2015;22:144–148. doi: 10.1016/j.ultsonch.2014.05.026. [DOI] [PubMed] [Google Scholar]
- Hirano S., Tanaka Y., Hasegawa M., Tobetto K., Nishioka A. Effect of sulfated derivatives of chitosan on some blood coagulant factors. Carbohydr. Res. 1985;29:205–215. doi: 10.1016/0008-6215(85)85161-2. [DOI] [PubMed] [Google Scholar]
- Liu F., Luo X.Q., Song B.A., Bhadury P.S., Yang S., Jin L.H., Xue W., Hu D.Y. Synthesis and antifungal activity of novel sulfoxide derivatives containing trimethoxyphenyl substituted 1,3,4-thiadiazole and 1,3,4-oxadiazole moiety. Bioorg. Med. Chem. 2008;16:3632–3640. doi: 10.1016/j.bmc.2008.02.006. [DOI] [PubMed] [Google Scholar]
- Miri R., Javidnia K., Amirghofran Z., Salimi S.H., Sabetghadam Z., Meili S., Mehdipour A.R. Cytotoxic effect of some 1,4-dihydropyridine derivatives containing nitroimidazole moiety. Iran. J. Pharma. Res. 2011;10(3):497–503. [PMC free article] [PubMed] [Google Scholar]
- Palakshi Reddy B., Sarveswari S., Vijayakumar V. Res. Chem. Intermed. 2015;41:6877–6883. [Google Scholar]
- Rogers M.J., Cundliffe E., Mccutchan T.F. The antibiotic micrococcin is a potent inhibitor of growth and protein synthesis in the malaria parasite. Antimicrob. Agents Chemother. 1966;42:715. doi: 10.1128/aac.42.3.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safari J., Zarnegar Z., Ahmadi M., Seyyedi S., audi J.S. An investigation of the catalytic potential of potassium cyanide and imidazolium salts for ultrasound-assisted synthesis of benzoin derivatives. J. Saudi Chem. Soc. 2015;19:628–633. [Google Scholar]
- Surendra Kumar R., Idhayadhulla A., Abdul Nasser A.J., Selvin J. Synthesis and anticoagulant activity of a new series of 1,4-dihydropyridine derivatives. Eur. J. Med. Chem. 2011;46:804–808. doi: 10.1016/j.ejmech.2010.12.006. [DOI] [PubMed] [Google Scholar]
- Surendra Kumar R., Idhayadhulla A., Nasser A.J.A., Selvin J. Synthesis and antimicrobial activity of a new series of 1,4-dihydropyridine derivatives. J. Serb. Chem. Soc. 2011;76:1–11. [Google Scholar]
- Surendra kumar R., Idhayadhulla A., Nasser A.J., Kavimani S., Indumathy S. Synthesis and anticonvulsant activity of a new series of 1,4-dihydropyridine derivatives. Indian J. Pharm. Sci. 2010;72:719–725. doi: 10.4103/0250-474X.84580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surendra Kumar R., Moydeen M., Al-Deyab S.S., Manilal A., Idhayadhulla A. Synthesis of new morpholine-connected pyrazolidine derivatives and their antimicrobial, antioxidant and cytotoxic activities. Bioorg. Med. Chem. Lett. 2017;27:66–71. doi: 10.1016/j.bmcl.2016.11.032. [DOI] [PubMed] [Google Scholar]
- Tawfeeq F.R., Al-Auqbi N.T., Al-Khalidy T. Inhibitory effect of oxadiazooles and thiadiazoles in vitro on serum alkaline phosphates' enzyme of pregnant woman. Am. J. Plant. Sci. 2012;11:1–8. [Google Scholar]
- Tsuruoka A., Kaku Y., Kakinuma H., Tsukada I., Yanagisawa M., Nara K., Naito T. Synthesis and antifungal activity of novel thiazole-containing triazole antifungals. II. Optically active ER-30346 and its derivatives. Chem. Pharm. Bull. 1998;46:623–630. doi: 10.1248/cpb.46.623. [DOI] [PubMed] [Google Scholar]
- Tsuruo T., Iida H., Nojiri M., Tsukagoshi S., Sakurai Y. Circumvention of vincristine and Adriamycin resistance in vitro and in vivo by calcium influx blockers. Cancer Res. 1983;43:2905–2910. [PubMed] [Google Scholar]
- Ulloora S., Kumar S., Shabaraya R., Adhikari A.V. Synthesis, anticonvulsant and anti-inflammatory studies of new 1,4-dihydropyridin-4-yl- phenoxyacetohydrazones. Med. Chem. Res. 2013;22:1549–1562. doi: 10.1016/j.ejmech.2013.10.010. [DOI] [PubMed] [Google Scholar]
- Wan J.P., Gan S.F., Sun G.L., Pan Y.J. Novel regioselectivity: three-component cascade synthesis of unsymmetrical 1,4- and 1,2-dihydropyridines. J. Org. Chem. 2009;74:2862–2865. doi: 10.1021/jo900068z. [DOI] [PubMed] [Google Scholar]
- Warren B.K., Knaus E.E. Some reactions of 1,4-dihydropyridines with organic azides. Synthesis of 2,7-diazabicyclo[4.1.0]hept-3-enes with analgesic and antiprotozoal activity. J. Med. Chem. 1981;24:462–464. doi: 10.1021/jm00136a017. [DOI] [PubMed] [Google Scholar]
- Wayne P.A. Clinical and Laboratory Standard Institute; USA: 2003. National Committee for Clinical Laboratory Standards, Performance Standards for Antimicrobial Disc Susceptibility Tests Approved Standard M2–A8. [Google Scholar]
- Wenzel R.R., Bruck H., Noll G., Schäfers R.F., Daul A.E., Philipp T. Antihypertensive drugs and the sympathetic nervous system. J. Cardiovasc. Pharmacol. 2000;35:S43–S52. doi: 10.1097/00005344-200000004-00006. [DOI] [PubMed] [Google Scholar]