Abstract
The HB-19 pseudopeptide 5[Kψ(CH2N)PR]-TASP, ψ(CH2N) for reduced peptide bond, is a specific inhibitor of HIV infection in different CD4+ cell lines and in primary T-lymphocytes and macrophages. It blocks virus-particle attachment to permissive cells by binding and forming a stable complex with nucleolin expressed on the cell surface. Here, we have investigated the tissue distribution of the tritiated HB-19 by using β-radio imager whole-body mapping in rats. A rapid, selective, and stable distribution and accumulation of the systematically administered HB-19 was demonstrated within the spleen, liver, bone, and kidney as soon as 5 min following its administration. No apparent uptake of HB-19 occurred in the brain and the muscle tissue. Interestingly and despite its rapid clearance from the blood, at 24 h postexposure a significant proportion of HB-19 was still recovered from target organs, of which 16–37% could be acounted for intact pseudopeptide. The elimination of HB-19 mainly occurred by renal glomerular filtration and most of the excreted radioactivity appeared to be HB-19 metabolites. Finally, injection of the biotin-labeled HB-19 pseudopeptide but not its control counterpart allowed the recovery of the HB-19–nucleolin complex from the liver, spleen, thymus, and bone marrow, thus indicating that the in vivo molecular target of HB-19 is surface nucleolin. Our results demonstrate the preferential uptake and stability of HB-19 in lymphoid organs that are the site of HIV propagation.
The human immunodeficiency virus (HIV) is an enveloped virus that infects permissive cells by the fusion of viral and cellular membranes, a process initiated by the attachment of HIV particles to cells. Consequently, to complement the current anti-HIV protease and reverse transcriptase drugs, several novel anti-HIV molecules have been developed that target different components implicated in the mechanism of HIV attachment and entry into cells (1). In this respect, the pentameric HB-19 pseudopeptide, 5[Kψ(CH2N)PR]TASP, is an anti-HIV synthetic agent that inhibits HIV infection by binding to the cell-surface-expressed nucleolin and results in a block in the attachment of virus particles to cells (2–4). Nucleolin is one of the major RNA binding proteins of the nucleolus, which has also been found on the cell surface where it serves as a binding protein for different ligands including HIV (5–7). In view of its potent and specific mode of action against various isolates of HIV, HB-19 represents a potential anti-HIV drug.
In the present study, we have mapped potential peripheral targets for the HB-19 pseudopeptide by examining in vivo the gross tissue distribution of the radiolabeled pseudopeptide with whole rat body mapping (8). The visual imaging of the entire animal by β-radio imaging provided a convenient and efficient technique to examine and record simultaneously the uptake of HB-19 in a large number of tissues and compartments. Of particular interest in respect to pathogenesis of HIV infection (9), our results show a preferential uptake of HB-19 by lymphoid organs. Moreover, the chromatographic characteristics of the bloodstream labeled compound indicated that more than 90% of the label is associated with the intact HB-19. This latter, and the significant, proportion of HB-19 recovered from target organs as late as 24 h post-administration of the labeled pseudopeptide could account for the strong peptidase resistance of HB-19. Furthermore, in accord with our previous in vitro results in cell cultures, nucleolin was recovered from the target tissues of rats systematically injected with the biotin-labeled HB-19, by virtue of this pseudopeptide to form in vivo a stable and an irreversible complex with the surface-expressed nucleolin.
Materials and Methods
Peptide Constructs.
The synthesis of 5[Kψ(CH2N)PR]-TASP (herein referred to as HB-19), the control peptide 5[Rψ(CH2N)PN]-TASP (herein referred to as CP-51), and the corresponding biotin-labeled peptides were as described (2, 7). For the preparation of the tritium-labeled HB-19, a precursor molecule of HB-19 was synthesized in which the proline residue in the tripeptide Kψ(CH2N)PR moiety was replaced by a 3,4-dehydroproline residue before labeling with tritium [3H]. The tritiation of HB-19 was generously carried out by R. Genet (Department d'Ingénierie et d'étude des protéines, Commissariat à l'Energie Atomique/Saclay, Gif-sur-Yvette, France). The specific activity of the final purified product of [3H]-labeled HB-19 was 0.22 TBq/mmol (6 Ci/mmol; 1 Ci = 37 GBq). All of the peptides used in this study had a purity ≥90%. After lyophilization, the peptides were dissolved and/or diluted in PBS (Dulbecco's) and stored at −80°C.
Animals.
Wistar male rats (5 weeks old; 100 g body weight), purchased from Iffa Credo were kept two animals per cage under controlled lighting and temperature with free access to food and water until 5–7 days before injection of the [3H]-labeled HB-19, and the biotin-labeled HB-19 or CP-51.
Whole-Body Mapping of HB-19 Uptake by Living Tissues.
Rats were killed while under halothane anesthesia after i.v. injection of 1.8 MBq (50 μCi) [3H]-labeled HB-19. At the selected time, the restrained animals were immediately immersed in a −80°C mixture of dry ice and isopentane to prevent artifactual peptide redistribution. After 48 h in self-sealing plastic stored at −30°C, the animal was then blocked in mounting medium. Whole-body sagittal sections (20 μm) of the frozen rat were made at −30°C with a cryostat (whole-body slicing microtome Leitz 400 with a chest mobile freezer Leitz OM from Leica, Deerfield, IL). Sections adhering to Scotch tape were left in a freezer (−30°C) for 4 days to ensure complete drying. The quantitative determination of the radioactivity in whole-body sections was carried out by β-imager 2200 (Biospace, Paris; refs. 8, 10, and 11). Data from whole-body sections or organ sections were collected for 8–48 h. The tritium activity was determined as counts per square millimeter. The linearity of this method of detection allowed measurement of the nonspecific binding [3H]-labeled HB-19 defined within each of the sections. The high-resolution images were obtained by using μ-imager (Biospace; refs. 8 and 11).
Pharmacokinetic Studies.
Anesthesized rats were given a bolus i.v. injection of 1.8 MBq (50 μCi; 8 nmol or 20 μg) [3H]-labeled HB-19 diluted in 100 μl of PBS-Dulbecco's medium. Blood was collected in ice-cold tubes containing a mixture of peptidase inhibitors [1 mM EDTA/1000 units/ml aprotinin/130 μM bestatin/1 μM leupeptin/0.4 mM Pefabloc (Boehringer Mannheim)/1 μM pepstatin; final concentrations] by means of a Silastic catheter implanted into the external jugular vein as described in ref. 8. At different time points after peptide administration (2–120 min), 250 μl of blood sample were withdrawn, and the entire urinary bladder content was collected at the last selected point. The blood and urine samples were centrifuged (2500 × g for 15 min at 4°C) and the supernatants stored at −80°C. Aliquots (100 μl) of the biological samples were acidified by addition of 10 μl of 1N HCl, kept at 4°C for 10 min, and then centrifuged (12,000 × g for 10 min at 4°C), before adsorption on Sep-Pak Plus tC2 cartridges (Waters). Peptide fractions were eluted from the Sep-Pak with 0.1% trifluoroacetic acid (TFA) in acetonitrile (Merck), and after evaporation of the solvent phase they were applied to a Kromasil column (150 × 4.6 mm; HPLC RP-C18 chromatography; AIT, St. Germain en Laye, France). Following a 15-min isocratic run of 0.1% TFA in water, the elution was performed with a linear gradient of 0.1% TFA in water–0.1% TFA in acetonitrile for 15 min at a flow rate of 1 ml/min. Fractions were collected every 60 s and analyzed for radioactivity by using a liquid scintillation counter.
SIPHAR (Simed, Créteil, France), a computer program designed for nonlinear regression of pharmacokinetic data, was used to fit plasma concentration. The data used correspond to the plasma or urine concentration of nonmetabolized HB-19. Pharmacokinetic parameters were evaluated as described (12), using a two-compartmental model. The area under the plasma concentration curve (AUC) was determined by using trapezoidal rule and extrapolated to infinity. Clearance was calculated from the dose divided by AUC. Elimination rate constants (kα and kβ) were evaluated from the decay of the plasma concentration curve after i.v. administration of HB-19. Volume of distribution was determined by using the ratio clearance:kβ.
Chromatographic Characterization of the Radioactive Pseudopeptide.
The chromatographic characteristics of the labeled pseudopeptide in the plasma, urine, and in different tissues were assessed by RP-HPLC (8). At selected times (4, 8, and 24 h) after i.v. injection of 20 μg (50 μCi) of [3H]-labeled HB-19, urine was collected and the rats were anesthetized and killed by cardiac blood puncture. Tissues were quickly removed weighed and homogenized mechanically at 4°C in 5–10 volumes of 0.1 M chlorhydric acid in a Polytron system (Polytron, type PT 10/35, Kinematica, Lucerne, Switzerland). Homogenates were centrifuged at 15,000 × g for 30 min at 4°C. To determine total radioactivity, aliquots of the supernatant solution were dried on filter and counted in a liquid scintillation counter. The biological samples were submitted to Sep-Pak Plus tC2 extraction conditions as described above. Chromatography using the HPLC procedure described above was then applied to each extracted sample. The radioactivity content of samples before and after chromatographic separations was determined by using a beta counter (MR300 from Kontron, Zurich).
Characterization of Tissue Nucleolin Complexed with the Biotin-Labeled HB-19.
These experimental conditions were previously optimized for the recovery of the cell-surface-expressed nucleolin complexed with the biotin-labeled HB-19 in vitro (7, 13, 14). Anesthetized rats were injected intravenously with 1 mg of the biotin-labeled HB-19 or the biotin-labeled control peptide CP-51. After 45 min, the anesthetized rats were killed (cardiac blood puncture) and the tissues quickly removed and frozen at −80°C. Frozen tissues were homogenized mechanically (Polytron) in low-salt buffer (10 mM Hepes, pH 7.6/10 mM KCl/2 mM magnesium acetate/7 mM 2-mercaptoethanol and the protease inhibitor mixture) containing unlabeled HB-19 (50 μM). This suspension was left for 15 min at 4°C before addition of Nonidet P-40 at a final concentration of 0.5%. After 15 min, each suspension was sonicated for 10 s and centrifuged at 5000 × g for 20 min. The potential complexes formed between tissue proteins and the biotin-labeled HB-19 or the biotin-labeled CP-51 were isolated by purification of the extracts (50 mg), using 100 μl of avidin-agarose (ImmunoPure Immobilized Avidin from Pierce) in PBS containing EDTA (1 mM). After 2 h of incubation at 4°C, the samples were washed extensively with PBS-EDTA. The purified peptide-complexed proteins were denatured by heating in the electrophoresis sample buffer containing SDS and analyzed by SDS/PAGE electrophoresis (14). The presence of nucleolin was then revealed by immunoblotting using rabbit polyclonal antibody against hamster (CHO cells) nucleolin as described (14). Similarly purified peptide-complexed proteins were eluted by 1 M NaCl and dialyzed against PBS containing 1 mM EDTA and protease inhibitor mixture. The eluted samples were then analyzed by dot-immunoblotting (15) using rabbit polyclonal antibody against hamster nucleolin.
Results and Discussion
Lack of Apparent Toxicity of HB-19.
In the HeLa CD4+ experimental model, >95% of HIV infection is inhibited in cells pretreated at 0.5 μM of HB-19 in the absence of any apparent effect on cell proliferation (4). Indeed, cells passaged and cultured in the presence of 0.5–5 μM HB-19 grow and multiply as efficiently as control cells (data not shown). Furthermore, no apparent effect is observed on cell proliferation of HeLa cells after eight passages for a period of 1 month in culture medium continuously containing 1 μM HB-19. Similarly, HB-19 has no apparent effect on cell multiplication in cultures of CD4+ cells and in peripheral blood lymphocytes (data not shown). After binding to the cell-surface-expressed nucleolin, HB-19 becomes internalized by an active process similar to the internalization of an antibody specific to nucleolin (6). Internalized HB-19 accumulates in the cytoplasm of cells, but it does not enter the nuclei. Therefore, HB-19 does not cross the nuclear membrane, and consequently its effect is exerted differentially through the cell-surface-expressed nucleolin. Because nucleolar nucleolin could control cell growth through the biosynthesis of ribosomes (16), the lack of translocation of HB-19 to the nucleus and nucleolus might account for its lack of toxicity in cultured cells.
For a preliminary evaluation of a potential acute toxicity of HB-19 in animals, male rats (body weight of 100 g) were injected intravenously through the jugular vein with 0.03, 0.3, and 3 mg/kg of HB-19 (n = 2 rats per dose). No apparent change was noted in the behavior and physiological characteristics of rats injected with HB-19 during the 48 h period of observation, at which time the animals were killed for gross anatomic examination.
Target Organs for HB-19 in Rats.
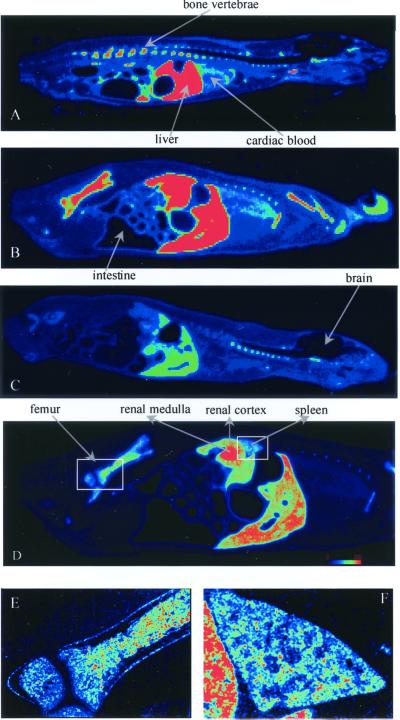
The gross anatomic mapping and time course of tissue distribution of HB-19 was investigated by β-radio imager in whole-body sagittal sections of rats 5 min, 1 h, and 24 h postinjection of 20 μg of [3H]-labeled HB-19 (Fig. 1 A–C). The dynamic profile of HB-19 uptake in rats revealed that the radioactivity is rapidly distributed and differentially retained in the liver, spleen, kidney, and bones including vertebrae as early as 5 min postinjection and remained stably accumulated at least 1 h after systemic injection. Interestingly, the radioactivity persisted within different organs as late as 24 h postinjection of [3H]-labeled HB-19. In a less exposed sagittal section at 1 h postinjection (Fig. 1D), it was possible to clearly distinguish the significant radioactive signal (in red) within the kidney concentrated toward the inner medullar collecting ducts, which is consistent with the fact that the clearance of the bloodstream HB-19 occurred through the urine (see below). The μ-imager magnification of the spleen (Fig. 1F) and a section from the long bone femur (Fig. 1E) illustrated the uptake and accumulation of [3H]-labeled HB-19 at 1 h postinjection within distinct structure of the spleen and the inner bone, respectively. No significant signal was found within the brain, hearth, muscles, and intestinal content.
Figure 1.
Representative mapping of target organs for [3H]-labeled HB-19 by using the high resolution β-radio imager. Rats were injected with 1.8 MBq (50 μCi; 8 nmol or 20 μg) of the [3H]-labeled HB-19 at 5 min (A), 1 h (B), and 24 h (C). Midsagittal sections (A–C) and lateral sagittal section at 1 h postinjection (D) of rat whole body are shown. The spleen (E) and a section of the bone (F) regions were magnified by μ-imager. The highest concentration of radioactivity is seen in the renal inner medulla, liver, and spleen, as well as visible bone tissues (vertebra and limb). The colored scale from dark (no signal) to red (intense radioactive signal) is shown in D.
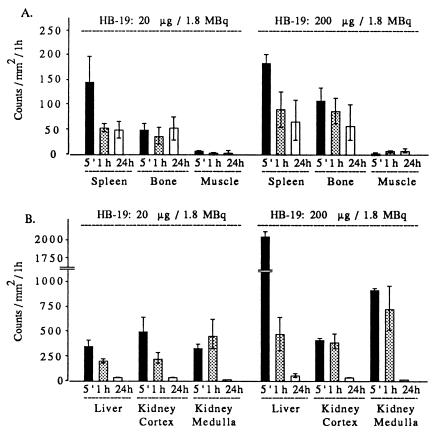
Assessment of tissue [3H]-labeled HB-19 targeting was accomplished by β-radio imager quantification. The number of β-particles emitted per unit area was collected directly from whole-body sections. The linearity and selectivity of this method of detection allowed a relevant measurement of labeled structures compared with blackening defined within a selected anatomic area of the same section. The concentration of radioactivity expressed as counts per square millimeter for 1 h counting was analyzed in the spleen, bone, muscle, and kidney 5 min, 1 h, and 24 h after i.v. injection of 20 and 200 μg of 1.8 MBq (50 μCi) of the tritiated peptide. The radioactivity associated with the muscle tissue at each postinjection period represented the background values (Fig. 2A). Compared with this background anatomic space, the relative high tissue accumulation of radioactivity in the spleen at 5 min postdose decreased 2–3 times by 1 h, but remained stably retained as late as 24 h after injection of 20 μg of HB-19. In addition, under these experimental conditions, the level of radioactivity was also rapidly and stably accumulated within the inner bone. On the other hand, an almost comparable level of labeling was observed in the spleen and bone of rats injected with 200 μg of HB-19, thus suggesting that saturation of the peptide uptake sites was not achieved in both tissues under these experimental conditions. In contrast, the 5-fold-higher radioactivity in the liver of rats injected with 200 μg of HB-19 compared with that injected with 20 μg at 5 min postinjection could be accounted for by the suggestion that the liver is an efficient organ of metabolism for the HB-19 pseudopeptide. Similarly, within the kidney of rats injected with 200 μg of HB-19, the level of the radioactivity was higher in the medullar area compared with the cortical area, which is consistent with an accelerated process of the peptide excretion. At both concentrations of HB-19, the labeling in the kidney at 24 h postinjection was almost completely excluded, thus indicating that most of the peptide had been eliminated (Fig. 2B).
Figure 2.
Quantitative profile of radioactivity in the spleen, bone, and muscle (A) and liver, renal cortex, and renal medulla (B) of rats at 5 min (histograms 5′), 1 h, and 24 h after i.v. injection of 20 and 200 μg [3H]-labeled HB-19 (1.8 MBq or 50 μCi each). Direct quantification of sagittal whole-body sections was assessed by using the β-radio imager. The number of β-particles emitted per area was counted for 50 h and expressed as counts per mm2 per 1 h. Assessment of quantitative regional differences was performed with computer-assisted image analysis using the β-VISION program. Bars represent means ± SD of triplicate determinations from the same structure and two whole-body sections.
The Stability of HB-19 in the Bloodstream, Urine, and Target Organs.
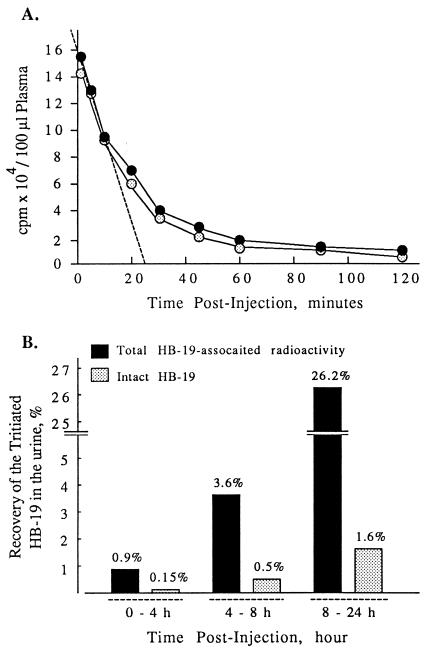
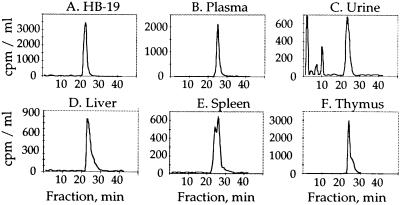
Plasma HB-19 and its metabolites were measured with time after a single injection of 20 μg of [3H]-labeled HB-19 (1.8 MBq in 100 μl PBS) into the circulation of two male rats. The plasma pseudopeptide concentration decreased rapidly, to return close to basal level from 60 min postinjection. A typical profile of the HB-19 distribution in male rat bloodstream is presented in Fig. 3A; the circulating tritiated HB-19 distribution phase could be estimated at 25 min (Fig. 3A). Sep-Pak and RP-HPLC fractionation of plasma peptide extracts revealed that in the bloodstream at least 90% of the radioactivity was recovered in a peak corresponding to the intact native form of HB-19 at any time after injection of [3H]-labeled HB-19 (Figs. 3A and 4 A and B). All these results indicated that the intact HB-19 disappears from the blood compartment with an apparent distribution half-life of about 15 min because of its biodistribution in other compartments, including target tissues. Hence, despite the fact that at 60 min 90% of HB-19 was cleared from circulation, a significant amount of radiolabeled HB-19 is accumulated within the lymphoid tissues even at 24 h postinjection (Fig. 2A). The HB-19 urinary elimination was investigated by measuring radioactivity excreted in two rats injected with 20 μg of [3H]-labeled HB-19. The radioactivity collected in the urine at 0–4, 4–8, and 8–24 h postinjection represented 0.9, 3.6, and 26.2%, respectively, of the administered material (Fig. 3B). Interestingly in the urine, the intact HB-19 represented only a small proportion of the total radioactivity recovered at the different time intervals. Indeed, more than 98% of the urine radioactivity appeared to be recovered as HB-19 metabolites that are eliminated through Sep-Pak extraction step and/or intermediate peptide products as revealed by the RP-HPLC modified elution profile (Fig. 4C). In contrast to the urine, significant levels of the intact HB-19 were still recovered from the target tissues (liver, spleen, kidney, and thymus) even at 24 h postinjection, which is consistent with its long half-life in the target tissues (Table 1 and Fig. 4 D–F). Our results show that HB-19 is stable in the rat bloodstream. This and the observation that at least 30% of HB-19 persists undegraded in the target lymphoid organs at 24 h postinjection suggest that HB-19 may exert a long-term effect on HIV infection in vivo. Such a resistance is consistent with our previous reports on the stability of HB-19 in vitro, in fetal calf or human sera (13).
Figure 3.
The pharmacokinetic properties of HB-19 in the blood and urine. Rats were injected intravenously with 20 μg (1.8 MBq or 50 μCi) of the [3H]-labeled HB-19. (A) For the plasma distribution profile of [3H]-labeled HB-19, blood was collected at selected times from 2–120 min and the total plasma radioactivity (closed circles) and the amount of the intact HB-19 were determined (open circles). The intact peptide was measured after RP-HPLC analysis as described in Materials and Methods. (B) The in vivo elimination of the 3H-labeled HB-19 was investigated by measuring radioactivity excreted in the urine collected at 0–4 h, 4–8 h, and 8–24 h. The % of total HB-19-associated radioactivity (black histograms) and the % of the intact [3H]-labeled HB-19 (gray histograms) corresponding to the RP-HPLC peak of HB-19 in the urine at each point were calculated in respect to the radioactivity injected.
Figure 4.
HPLC radiochromatogram profile of various fluid and HB-19 target organs after a single injection of 20 μg (1.8 MBq) of [3H]-labeled HB-19—i.e., the plasma at 60 min (B), urine at 8–24 h (C), liver, thymus, and spleen (D–F; at 24 h postinjection). The HPLC profile of the native [3H]-labeled HB-19 is shown in A. [3H]-labeled peptide metabolites were revealed in the Sep-Pak extracted urine collected between 8 and 24 h postinjection. The slight stretching of the HB-19 peak in the liver, thymus, and spleen samples could be due to the partial association of HB-19 with its molecular partner in these tissues (see Fig. 5).
Table 1.
The distribution and stability of HB-19 in target organs from rats injected with [3H]-labeled HB-19
| Organ | Total HB-19 counts, %/gm tissue | Intact HB-19 (of the % retained) |
|---|---|---|
| Spleen | 1.2%/1.0% | 37.7%/36.2% |
| Thymus | 0.6%/0.5% | 31.7%/32.0% |
| Liver | 24.4%/23.2% | 21.5%/22.9% |
| Kidney | 3.1%/3.4% | 16.0%/17.7% |
The two values in each column correspond to rat 1 and rat 2, respectively. Two rats were injected intravenously with [3H]-labeled HB-19 (20 μg; 1.8 MBq) and killed at 24 h. In order to estimate the presence of intact HB-19, the tissue extracts were assessed for RP-HPLC analysis (Materials and Methods). The total tritium activity was determined as counts per minute per gram of tissue. The % of total HB-19-associated tritium radioactivity within target organs represented the amount radioactivity quantified per organ in respect to the total radioactivity injected. The % of intact (undegraded) HB-19 in different tissues represented the amount of radioactivity quantified in the HB-19 HPLC peak with respect to the total radioactivity in each organ.
Pharmacokinetics.
A two-compartmental analysis of the time course of the plasma concentration of HB-19 indicates a first component with a half-life near 8 min and a second component that exhibits a half-life of 55 min (mean residence time: 51 min). The systemic clearance (5.29 ml/min/kg) is close to the filtration clearance in rat, suggesting that other elimination processes do not occur significantly in the fate of HB-19. This is in agreement with the chromatographic experiments on blood. Volume of distribution is 410 ml/kg, indicating that HB-19 is concentrated in a moderate manner in peripheral tissues.
The urinary excretion of HB-19 is rather slow because only 0.15% of the drug is eliminated during the first 4 h and that elimination still occurs between 8 and 24 h after administration. It seems that a very deep compartment could exist. By comparing plasma AUC (area under the plasma concentration curve) and renal elimination, the renal clearance could be estimated. The calculated value (0.1 ml/min/kg) is very low, suggesting that HB-19 could be subjected to tubular reabsorption or metabolism into the urine. This effect is consistent with the high ratio of metabolites:HB-19 calculated from the urine data. Such a case has already been shown for a 4-aa peptide, NacSDKP (17).
Recovery of the Molecular Target for HB-19 from Different Tissues in Vivo.
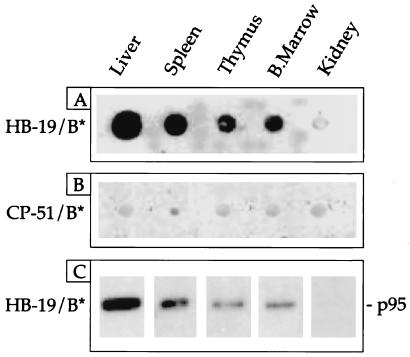
Rats were injected with the biotin-labeled HB-19 or its corresponding control pseudopeptide CP-51. Tissue extracts were then prepared and the biotin-labeled peptide complexes were purified by affinity chromatography using avidin-agarose. The presence of nucleolin copurified with the biotin-labeled HB-19 was revealed by immunoblotting with the antinucleolin antibody in dot-blot analysis of crude tissue extracts or after PAGE (Fig. 5). The results revealed that nucleolin was recovered from the liver, spleen, thymus, and bone marrow, but not from the kidney of rats injected with the biotin-labeled HB-19. We had previously demonstrated that in different types of cell cultures, HB-19 binds specifically to the cell-surface-expressed nucleolin, forming a stable complex that constitutes a specific event for the HB-19 action. Similarly, the recovery of the HB-19-complexed nucleolin from different rat tissues should also be the consequence of a specific event because nucleolin was not recovered when rats were injected with the biotin-labeled control pseudopeptide CP-51. It is of interest to note that the β-imager mapping of sagittal sections of rats injected with [3H]-labeled HB-19 showed that the peptide is highly accumulated in renal inner medulla (Fig. 1), whereas no nucleolin was recovered from the kidney tissue extracts of the rat injected with the biotin-labeled HB-19 (Fig. 5). Therefore, the presence of [3H]-labeled HB-19 within kidney reflects predominantly the clearance process of the pseudopeptide from the bloodstream. In contrast to the kidney, the liver that functions as an organ of metabolism for various drugs appears as an important target tissue for HB-19 action and/or metabolism through nucleolin binding sites.
Figure 5.
Recovery of the HB-19 complexed with nucleolin from lymphoid organs. Rats were intravenously injected with 1 mg of either the biotin-labeled HB-19 or its corresponding control peptide, the biotin-labeled CP-51. At 45 min postinjection, tissue extracts were prepared as described in Materials and Methods. The HB-19 (A) or CP-51 (B) bound proteins were assayed by dot-immunoblotting using the antinucleolin polyclonal antibody. The HB-19 bound proteins were also analyzed by immunoblotting after SDS/PAGE, using mAb D3 (C). A single band was identified by the antinucleolin antibody. C shows a section of the immunoblot (10% gel) at the region of the nucleolin migration (95 kDa).
It is of interest to note that the Kd value of HB-19 to bind partially purified preparations of nucleolin is in the order of 9.6 × 109 M−1 (14). Furthermore, under experimental conditions required for the inhibition of HIV particle attachment to cells, HB-19 forms a stable and irreversible complex in vitro with the cell-surface-expressed nucleolin in various types of cells (6, 7, 13, 14). This complex is then internalized by an active process into the cytoplasm where it becomes accumulated without entering the nuclei (A.G.H., B.K., and J.P.B., unpublished results). The recovery of the HB-19–nucleolin complex from lymphoid tissues of the rat injected with the biotin-labeled HB-19 suggests that surface nucleolin is the molecular target of HB-19 in vivo. The preferential uptake of HB-19 in lymphoid organs could then be accounted for by the presence of actively proliferating cells including lymphocytes in such organs, because surface expression of nucleolin is highly regulated by growth stimulation of cells. Indeed, surface-nucleolin expression is drastically down-regulated in growth-arrested cells, whereas in growth-activated cells its expression is markedly induced, both at the level of mRNA and protein expression at the cell surface (6, 18). The increased levels of surface nucleolin in growth-activated cells (6) by binding to HB-19 could contribute for the preferential uptake of HB-19 in lymphoid organs.
The Significance of the Preferential Uptake of the Anti-HIV Pseudopeptide in Lymphoid Organs.
This study shows the pharmacokinetic and biodistribution properties of the anti-HIV pseudopeptide HB-19 in vivo. The clear uptake of HB-19 in specific organs as soon as 5 min after injection of [3H]-labeled HB-19 is in close relationship to its rapid distribution from the bloodstream compartment. Interestingly, HB-19 is preferentially taken up within lymphoid organs, which are the sites of HIV replication. Indeed, primary HIV infection is associated with a burst of viremia and wide dissemination and seeding of the virus to various organs, predominantly the lymphoid tissues that present a favorable environment for HIV replication (9, 19). This acute phase of HIV infection is followed by a phase of “clinical latency” characterized by lack of symptoms that lasts for several years before the outburst of virus along the establishment of the acquired immunodeficiency syndrome. The fact that lymphoid tissues contain actively virus producing cells along latently infected lymphocytes suggests that they represent HIV-replicating organs referred to as “reservoirs.” Despite the “clinical latency,” the viral latency does not exist at any time during the course of HIV disease. Consequently, HIV sequestered in the lymphoid organs persistently replicate during the asymptomatic phase of the disease when the plasma viremia is declined compared with that of the acute phase. In HIV-infected patients who are receiving highly active antiretroviral therapy (HAART), although plasma viremia often becomes undetectable, a small level of virus infection persists for considerable periods of time in lymphoid tissues (9, 19). Our results showing the differential uptake of HB-19 in lymphoid organs is consistent with its potential anti-HIV action in tissues where HIV replicates preferentially. Furthermore, the uptake of HB-19 in the bone marrow favors the suggestion that HB-19 can even protect precursor cells against HIV infection.
The pseudopeptide HB-19 that we have developed is active against both T-lymphocyte- and macrophage-tropic HIV-1 isolates in various cell cultures. HB-19's reproducible synthesis, lack of apparent toxicity, rapid preferential uptake in lymphoid organs, and biochemical stability in vitro and in vivo in the blood and in target organs makes it an ideal anti-HIV drug candidate. An additional advantage over traditional anti-HIV drugs is the capacity of HB-19 to form an irreversible complex with the cell-surface-expressed nucleolin, a property that should enable the persistence of its anti-HIV action even after the clearance of the drug from circulation.
Acknowledgments
Rabbit antiserum raised against a purified preparation of hamster nucleolin was generously provided by Dr. M. Erard (CNRS, Toulouse, France). We thank Magalie Herrant for excellent technical assistance. This work was supported by grants from Ensemble Contre le Sida, SIDACTION; Institut Pasteur; and Centre National de la Recherche Scientifique.
Abbreviations
- HB-19
5[Kψ(CH2N)PR]-TASP
- CP-51
5[Rψ(CH2N)PN]-TASP
References
- 1.Chan D C, Kim P S. Cell. 1998;93:681–684. doi: 10.1016/s0092-8674(00)81430-0. [DOI] [PubMed] [Google Scholar]
- 2.Callebaut C, Jacotot E, Guichard G, Krust B, Rey-Cuille M, Cointe D, Benkirane N, Blanco J, Muller S, Briand J, Hovanessian A G. Virology. 1996;218:181–192. doi: 10.1006/viro.1996.0178. [DOI] [PubMed] [Google Scholar]
- 3.Seddiki N, Nisole S, Krust B, Callebaut C, Guichard G, Muller S, Briand J P, Hovanessian A G. AIDS Res Hum Retroviruses. 1999;15:381–390. doi: 10.1089/088922299311358. [DOI] [PubMed] [Google Scholar]
- 4.Nisole S, Krust B, Dam E, Bianco A, Seddiki N, Loaec S, Callebaut C, Guichard G, Muller S, Briand J P, Hovanessian A G. AIDS Res Hum Retroviruses. 2000;16:237–249. doi: 10.1089/088922200309331. [DOI] [PubMed] [Google Scholar]
- 5.Srivastava M, Pollard H B. FASEB J. 1999;13:1911–1922. [PubMed] [Google Scholar]
- 6.Hovanessian A G, Puvion-Dutilleul D, Nisole S, Svab J, Perret E, Deng J S, Krust B. Exp Cell Res. 2000;261:312–328. doi: 10.1006/excr.2000.5071. [DOI] [PubMed] [Google Scholar]
- 7.Nisole S, Krust B, Callebaut C, Guichard G, Muller S, Briand J P, Hovanessian A G. J Biol Chem. 1999;274:27875–27884. doi: 10.1074/jbc.274.39.27875. [DOI] [PubMed] [Google Scholar]
- 8.Rougeot C, Vienet R, Cardona A, Le Doledec L, Grongnet J M, Rougeon F. Am J Physiol. 1997;273:R1309–R1320. doi: 10.1152/ajpregu.1997.273.4.R1309. [DOI] [PubMed] [Google Scholar]
- 9.Chun T W, Engel D, Mizell S B, Hallahan C W, Fischette M, Park S, Davey R T, Jr, Dybul M, Kovacs J A, Metcalf J A, et al. Nat Med. 1999;5:651–655. doi: 10.1038/9498. [DOI] [PubMed] [Google Scholar]
- 10.Charpak G, Dominik W, Zaganidis N. Proc Natl Acad Sci USA. 1989;86:1741–1745. doi: 10.1073/pnas.86.6.1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jacotot E, Cardona A, Rebouillat D, Terradillos O, Marianneau P, Thoulouze M I, Lafon M, Deubel V, Edelman L. Apoptosis. 1999;4:169–178. doi: 10.1023/a:1009658522328. [DOI] [PubMed] [Google Scholar]
- 12.Gibaldi M, Perrier D. Pharmacokinetics. Vol. 15. New York: Dekker; 1982. [Google Scholar]
- 13.Callebaut C, Jacotot E, Krust B, Guichar G, Blanco J, Svab J, Muller S, Briand J P, Hovanessian A G. J Biol Chem. 1997;272:7159–7166. doi: 10.1074/jbc.272.11.7159. [DOI] [PubMed] [Google Scholar]
- 14.Callebaut C, Blanco J, Benkirane N, Krust B, Jacotot E, Guichard G, Seddiki N, Svab J, Dam E, Muller S, et al. J Biol Chem. 1998;273:21988–21992. doi: 10.1074/jbc.273.34.21988. [DOI] [PubMed] [Google Scholar]
- 15.Krust B, Laurent A G, Montagnier L, Hovanessian A G. Ann Inst Pasteur Virol. 1987;138:245–252. [Google Scholar]
- 16.Ginisty H, Sicard H, Roger B, Bouvet P. J Cell Sci. 1999;112:761–772. doi: 10.1242/jcs.112.6.761. [DOI] [PubMed] [Google Scholar]
- 17.Junot C, Menard J, Gonzales M F, Michaud A, Corvol P, Ezan E. J Pharmacol Exp Ther. 1999;289:1257–1261. [PubMed] [Google Scholar]
- 18.Callebaut C, Nisole S, Briand J-P, Krust B, Hovanessian A G. Virology. 2001;281:248–264. doi: 10.1006/viro.2000.0767. [DOI] [PubMed] [Google Scholar]
- 19.Levy J A. Microbiol Rev. 1993;57:183–289. doi: 10.1128/mr.57.1.183-289.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]