Abstract
Recent research has confirmed that Panax ginseng (P. ginseng) has effect on cultured osteoblast of the mouse. In this study we aim to validate the usefulness of tibia quantification by correlating micro-computed tomographic (microCT) images with histology analysis in the aged male rats. A total of thirty – old male WISTAR rats were used and divided into ten 8 weeks rats and ten 112 weeks aged rats with vehicle and ten 112 weeks aged rats with P. ginseng (300 mg/kg/day). Daily oral administration of P. ginseng lasted for 8 weeks. Bone histomorphometric parameters and the trabecular bone microarchitectural properties of tibia were determined by microCT scan. MicroCT analysis showed significantly lower bone mineral density (BMD) and trabecular bone number in the aged group. Ginseng prevented total BMD decrease in the tibia induced by natural aging, which was accompanied by a significant decrease in skeletal remodeling. Furthermore, the aged group with ginseng was found to have a significantly higher osteoblast. In the blood biochemistry results, serum phosphorus, calcium, osteocalcin, T3, and T4 remained unchanged. The present study indicated that P. ginseng might be a potential alternative medicine for the prevention and treatment of natural aging-induced osteoporosis in human.
Keywords: Microcomputed tomography, Panax ginseng, Osteoporosis, Bone mineral density
1. Introduction
Aging and disease, which affect the bony changes such as osteoporosis (Comelekoglu et al., 2007), result in continued aggravation or alteration in bony quality and structure (Kanis et al., 1994). Osteoporosis, is a multifactorial skeletal disease characterized by a decrease in bone mass and disruption of bone microarchitecture, resulting in decreased bone strength and thus an increased risk of fracture (Kim et al., 2011). The most studies have focused on bone mass as the important evidence in finding the cause of disease and understanding the trabecular bone and its structure (Muller et al., 1996). The large changes observed in the biomechanical characteristics of trabecular bone should reveal the structural properties in the analysis (Burr et al., 1997). Before, determination of bone strength depended on the bone mineral density. 94% of the mechanical properties of trabecular bone can be showed using a combination of bone density and architectural measurements but 64% with bone density measurement alone (Hildebrand et al., 1999). Therefore, more consideration could be given in measuring the trabecular microstructure with bone mass measurements. The trabecular bone has plates, rods, and combination of these in their microstructure. Three-dimensional CT has been introduced that make high-resolution images in vivo (Rüegsegger, 1994). And it is sufficient to visualize the bone quality and trabecular bone structure (Müller et al., 1994). Lately, traditional herbal medicines using natural ingredients have acquired considerable attention as possible treatments for osteoporosis with fewer side effects (Putnam et al., 2007).
P. ginseng has a variety of potential effects, including immune effects, anticancer effects, sexual function-enhancing effects, and others. However, few studies have assessed its anti-osteoporosis effects (Kropotov et al., 2002). Many researchers have studied the anti-osteoporosis effects of P. ginseng in ovariectomized rats (Chen et al., 2012). In the present study, we conducted histomorphometric analyses of the old rat tibia using microCT scans and evaluated changes in trabecular bone after P. ginseng treatment. In the aspect of osteoporosis, Panaxnoto ginseng saponins have been studied for their effects on pathological bone remodeling (Shen et al., 2010). A ginseng mixture was reported to induce the deposition of bone mineral density (BMD), increase femoral trabecular width and estrogen levels, and decrease tartrate-resistant acid phosphatase (TRAP) activity in ovariectomized rats. However, ginseng extract has not been sufficiently studied in age-related osteoporosis. Therefore, we attempted to characterize the effects of ginseng in age-related osteoporosis by measuring the histologic structure, as part of an ongoing effort to provide scientific data on the effects of P. ginseng. MicroCT was introduced by Feldkamp in the 1980s (Feldkamp et al., 1989). It is highly accurate, non-destructive method that enables to produce a three dimensional image, where a larger number of slices and immediate analysis of small-sized calcified specimens in achievable (8). It is now frequently used for quantitative evaluation of the morphology and microarchitecture of trabecular bone in animal models (Müller et al., 1996). Therefore, the purposes of this study are to measure the trabecular microstructure of tibia using micro-CT and to provide fundamental basis for more accurate biomechanical analysis such as micro-finite element analysis of the tibia.
2. Materials and methods
2.1. Preparation of P. Ginseng extract
Four-year-old ground roots of P. ginseng were purchased from the Korea Medicine Herbal Association in Seoul. The plants were identified by the Institute of Rural Development Administration, where the voucher specimens were deposited. P. ginseng roots were extracted with water at 80–85 °C, and concentrated through a vacuum evaporator. Then, it was dried using a freeze dryer to prepare P. ginseng extract.
2.2. Biochemical analysis
The serum sample were prepared by centrifugation of the blood (1.013 g for 15 min 4 °C), then stored at then stored at −80 °C for biochemical assay. The serum osteocalcin (OC) concentration was determined using a rat OC ELISA kit (San Clemente, CA, USA). The levels of serum calcium was measured on an automatic analyzer (Ciba-Corning 550, USA) using a diagnostic reagent kit for the in vitro determination.
2.3. Experimental animal
Male WISTAR rats were used in this experiment (ten were 8-week-old rats, ten were 112-week-old rats treated with vehicle, and ten were 112-week-old rats treated with P. ginseng at a daily dosage of 300 mg/kg body weight for 8 weeks). The rats were provided a standard pelleted diet and water ad libitum. The experimental animals were housed in an air-conditioned room at 23 °C ± 2 °C with 12 h/12 h light–dark illumination cycles at constant humidity (45–50%). The rats were housed in the Regional Innovation Center experimental animal facility at Kyung Hee University in accordance to the Institutional Animal Care and Use Committee guidelines. The appropriate amount of P. ginseng was determined and adjusted by weighing the rats weekly and measuring their daily dietary intake. The tibias were removed, fixed in 4% formaldehyde in phosphate-buffered saline (pH 7.4) for 24 h, and then stored (4 °C) in 80% ethanol for bone mass measurements.
2.4. MicroCT (micro-CT) imaging and histomorphometric analysis
micro-CT is an alternate approach to image and quantify trabecular bone in three dimensions. The previously reported methods was modified (Valverde-Franco et al., 2004). Each specimen was scanned and reconstructed into a three-dimensional structure with microCT (SkyScan 1172TM; SkyScan, Kontich, Belgium) with X-ray energy settings of 60 kV and 167 μA12. A two-dimensional X-ray CCD camera was connected to the frame-grabber and a dual Pentium III computer with CT-Analyzer software (SkyScan, Kontich, Belgium). For bone analysis, the tibia was selected as the region of interest. Bone volume (BV) is calculated by the marching cubes method to triangulate the surface of the trabecular bone 7. Total volume (TV) ix the volume of the whole examined sample and the normalized index, bone volume fraction (BV/TV) enables the comparison of samples of different size. The mean thickness of the trabecular, trabecular thickness (Tb, Th), is obtained by filling maximum size of spheres in the structure with the distance transformation (Hildebrand and Rüegsegger, 1997). Then the average thickness of al bone voxels is calculated to give Tb.Th. Trabecular separation (Tb.Sp) is the mean thickness of the marrow cavity. This value is calculated by using same method as the Tb. Th but the spheres are filled into non-bony part. Tb. Sp is thus the thickness of the marrow cavities. Trabecular number (Tb. N) is defined as the number of trabecular bone per unit length and taken as the inverse of the mean distance between the midaxes of the structure. Then the mean distance between the mid axes is determined in analogy to the Tb.Sp calculation, i.e., the separation between the midaxes is assessed (Carballido-Gamio et al., 2006). Structural Model Index (SMI), an estimation of the plate-rod like characteristic of the structure is calculated by a differential analysis of a triangulated surface of the structure and is defined as SMI = 6{(BV(dBS/dr))/BS2}. The dBS/dr is the surface area derivative with respect to al linear measure r, corresponding to the half thickness or the radius assumed constant over the entire structure. The SMI values of 0 and 3 are an ideal plate and rod value, respectively. If both the rod and plate thickness are existed in a structure, the SMI values can vary between 0 and 3. All DEXA measurements were performed with the Norland pDEXA Sabre (Fort Atkinson, WI, USA) equipped with Sabre Research software (v3.6). Right tibias were scanned with DEXA to determine BMC and BMD. Ex vivo measurements of the right proximaltibias were performed on excised bones (1-mm-thick) on a 3-mm-thick piece of cotton in a 10 cm diameter culture dish at a constant location on the scan table.
2.5. Statistical analysis
Statistical analysis was performed by one-way analysis of variance (ANOVA) followed by Student’s t-test. Results are expressed as means ± standard deviations (SD). Ap value of <0.05 was considered to indicate statistical significance.
3. Results and discussion
In this study, we successfully correlated imaging and histological analyses of the trabecular bone relationship in the aging skeleton. This study is the first to show the beneficial effects of P. ginseng on the decreases in bone mass and strength induced by aging and its ability to improve the cancellous bone structure in aging rats. Our findings show that at a concentration of 300 mg/kg, P. ginseng attenuates bone loss in aging rats. The mechanical characteristics of bone are influenced by bone mass as well as bone size, bone quality, and cancellous bone architecture (Kinney et al., 2000).
3.1. Biochemical parameters of serum
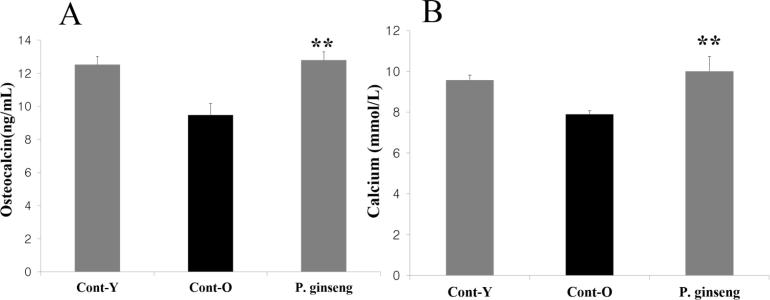
Plasma osteocalcin activity, bone formation markers, was significantly increased in P. ginseng treated group compared with young rats group (Quin et al., 2005, Taku et al., 2010). In this study, compared with old rats group, 8 weeks of treatment with 300 mg/kg P. ginseng significantly increased in serum osteocalcin level (p < 0.01); P. ginseng at 300 mg/kg significantly increased serum calcium level (p < 0.01) (Fig. 1). As osteocalcin is produced by osteoblasts (Lee et al., 2007), it is often used as a marker for the bone formation process. It has been observed that higher serum-osteocalcin levels are relatively well correlated with increases in bone mineral density (BMD) during treatment with anabolic bone formation drugs (Bharadwaj et al., 2009). And acts on Leydig cells of the testis to stimulate testosterone biosynthesis and therefore affect male fertility (Karsenty and Oury, 2014).
Fig. 1.
Effects of 8-week treatment with ginseng on biochemical parameters in serum osteocalcin and calcium of old rats. Values are means ± SD (n = 17). osteocalcin, serum calcium, compared with the Cont-O group **P < 0.01 compared with the Cont-O group. Cont-Y: 8-week-young rats, Cont-O: 112-week-old rats, Ginseng: 112-week-old rats treated with P. ginseng.
3.2. Total bone and density of the tibia
Osteoporosis is a bone disease characterized by mass density reduction and a deterioration of the trabecular bone micro architecture thus increasing the risk of fracture is not sufficiently comprehensive for complete characterization of trabecular bone deterioration (Carballido-Gamio et al., 2006).
BMD measurement is widely used for detecting osteoporosis. Bone mineral components, such as Ca, decreased in the tibia of the aging rats, indicating that Ca deposition decreased in aging rats. P. ginseng treatment also improved the loss of Ca content in aging rats. These factors indicated that P. ginseng not only inhibited bone resorption, but also increased the mineral concentration in the bones of aging rats. In the present study, bone loss was manifested by reductions in the tibial BMC and BMD in aging rats. Treatment with P. ginseng significantly reduced this bone loss in the aging rats. Although low bone mass is a major risk factor for fracture (borah) the preservation of trabecular bone architecture significantly contributes to bone strength and may reduce fracture risk beyond.
3.3. Trabecular microstructural changes in the proximal tibial metaphysis
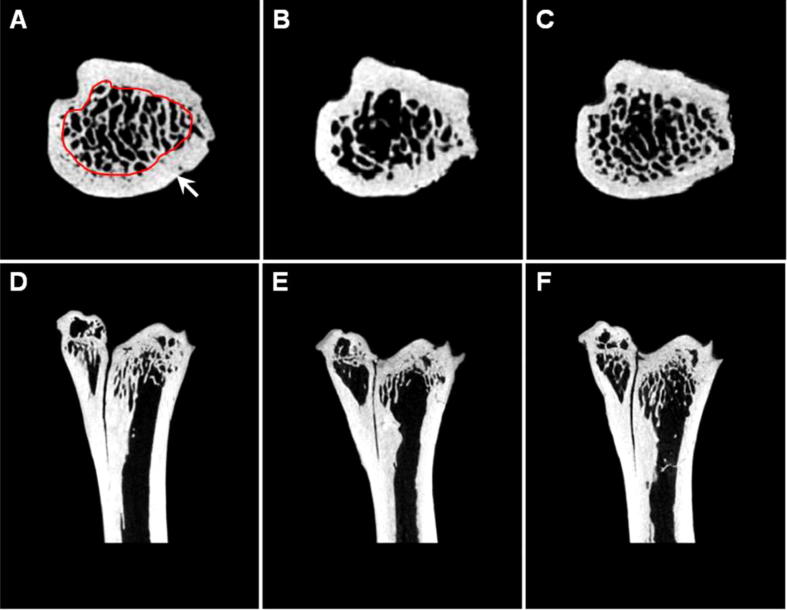
Table 1 shows that old rats induced significant changes in all trabecular microstructural parameters in the proximal tibial metaphysis, as measured by micro-CT. Micro-CT revealed that proximal tibialmetaphysis from old rats had lower trabecular bone compared to P. ginseng treated group. The most relevant morphologic values in the characterization of trabecular bone are bone volume to total volume ratio, trabecular thickness, number and separation (Alberich-Bayarri et al., 2008). In addition, an evaluation of the bone strength helps in the characterization of some bone properties (Comelekoglu et al., 2007, Bassett and Williams, 2016). The basic morphometric indices include bone volume (BV) and the total volume of interest (TV). The ratio of these two measurements is termed the bone volume fraction (BV/TV). The 112-week-old rats treated with P. ginseng showed a significant 33% increase in BMD and a significant 45% increase in the trabecular BV/TV (18.78% ± 1.04% vs. 13.68% ± 0.33%, respectively). In addition, the 112-week-old rats showed a significant decrease in Tb.N. (0.22 ± 0.03/mm2vs. 0.33 ± 0.01/mm2, respectively) and a significant increase in Tb.Sp. (2.20 ± 0.18 mm vs. 1.59 ± 0.02 mm, respectively)compared with the 112-week-old rats treated with P. ginseng. Tb. Th. in the 112-week-old rats was slightly different from that in the 112-week-old rats treated P. ginseng (0.63 ± 0.06 in the 112-week-old rats and 0.59 ± 0.63 in the 112-week-old rats treated with P. ginseng). An SMI of 0 and 3 indicate bones that consist purely of plate or rod-like structures, respectively. P. ginseng treatment prevented the increase in SMI induced by aging (Table 1 and Fig. 2). MicroCT is an ideal strategy for tracking bone change and provides high resolution while allowing for longitudinal research of bone morphology. The microCT images of the tibia with the region of interest shown in white were reconstructed in three dimensions to see the structural details of the trabecular bone. Loss of trabecular bone was apparent in the 112-week-old rats compared with the 8-week-old rats, with associated signs of other changes in trabecular bone microarchitecture. The 112-week-old rats had fewer trabecular bone structures than did the 112-week-old rats treated with P. ginseng (Fig. 2). In the 112-week-old rats, the plate-like structures mostly resolved into rod-like structures with many missing connecting rods, whereas in the 112-week-old rats treated with P. ginseng, this loss of trabecular bone mass and connectivity was prevented (Fig. 2). BMD measurement is widely used for detecting osteoporosis. In the present study, bone loss was manifested by reductions in the tibial BMD in age related rats. Trabecular BMD was increased by 33% in P. ginseng treated group. BMD, suggesting that it can prevent the loss of bone mass associated with aging (Table 2). One of the structural parameters, BMD analysis was conducted on samples formed by both cortical and cancellous bone, whereas microCT analysis was only performed on cancellous bone in this study. The results suggest that P. ginseng might increase bone strength by increasing BMD and trabecular architecture. Through this study, it was found that a relatively low dose of P. ginseng (300 mg/kg) showed higher efficacy preventing bone loss.
Table 1.
Microstructural properties of the distal tibia by microCT analysis.
| Cont-Y | Cont-O | P. ginseng | |
|---|---|---|---|
| BV/TV (%) | 17.56 ± 3.41 | 13.68 ± 0.33 | 18.78 ± 1.04b |
| Tb.N (/mm) | 0.29 ± 0.05 | 0.22 ± 0.03 | 0.33 ± 0.01b |
| Tb.Th (mm) | 0.61 ± 0.01 | 0.63 ± 0.06 | 0.59 ± 0.03 |
| Tb.Sp (mm) | 1.84 ± 0.22 | 2.20 ± 0.18a | 1.59 ± 0.02b |
| SMI | 2.83 ± 0.55 | 1.93 ± 0.84 | 2.19 ± 0.64 |
Values are expressed at mean ± SD.
Cont-Y: control 8-week-y rats, Cont-O: control 112-week-old rats, Ginseng: 112-week-old rats treated with P. ginseng, BV/TV: ratio of segmented bone volume to total volume of the region of interest, Tb.N: measure of the average trabecular number, Tb.Th: mean trabecular thickness assessed using direct 3D methods, Tb.Sp: mean trabecular distance assessed using direct 3D methods, SMI: structure model index, an indicator of the trabecular structure; SMI will be 0 for parallel plates and 3 for cylindrical rods.
p < 0.05 vs. Cont-Y group.
p < 0.05 vs. Cont-O.
Fig. 2.
Three approaches to identifying the trabecular bone region of interest.
Table 2.
BMD of the distal tibial trabecular bone.
| Cont-Y | Cont-O | P. ginseng | |
|---|---|---|---|
| BMD (mg/cm3) | 440.57 ± 76.39 | 351.84 ± 40.19 | 466.31 ± 29.62a |
Values are expressed as mean ± SD.
Cont-Y: 8-week-young rats, Cont-O: 112-week-old rats, Ginseng: 112-week-old rats treated with P. ginseng.
p < 0.05 vs. Cont-O.
This study was originally aimed at documenting the antiosteoporosis effect of P. ginseng. However, our study has some limitations. The one is the presence of a well-formed and active growth plate affected the action of P. ginseng on bone. Another limitation is that the experiment was not performed using a different dose for the prevention of osteoporosis, more specific doses of ginseng especially doses lower than 300 mg/kg (100, 200 mg/kg) by micro CT.
4. Conclusion
In summary, this study provides evidence that daily oral administration of P. ginseng contributes significantly to the prevention or treatment of the bone loss and deterioration of trabecular microarchitecture induced by aging in rats. Further studies are required to determine whether a dose higher than 300 mg/kg would provide better protection to bone microarchitecture. We believe that P. ginseng has potential for further development as a natural alternative for the management of senile osteoporosis.
Acknowledgments
This work was carried out with financial support from the Cooperative Research Program for Agriculture Science & Technology Development (Project No. PJ009559), granted by the Rural Development Administration of Republic of Korea.
Conflict of interest
There is no conflict of interest associated with this piece of work.
Footnotes
Peer review under responsibility of King Saud University.
References
- Alberich-Bayarri A., Marti-Bonmati L., Sanz-Requena R., Belloch E., Moratal D. In vivo trabecular bone morphologic and mechanical relationship using high-resolution 3-T MRI. AJR Am. J. Roentgenol. 2008;191(3):721–726. doi: 10.2214/AJR.07.3528. [DOI] [PubMed] [Google Scholar]
- Bassett J.H.D., Williams G.R. Role of thyroid hormones in skeletal development and bone maintenance. Endocr. Rev. 2016;37(2):135–187. doi: 10.1210/er.2015-1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bharadwaj S., Naidu A.G., Betageri G.V., Prasadarao N.V., Naidu A.S. Milk ribonuclease-enriched lactoferrin induces positive effects on bone turnover markers in postmenopausal women. Osteoporosis Int. 2009;20(9):1603–1611. doi: 10.1007/s00198-009-0839-8. [DOI] [PubMed] [Google Scholar]
- Burr D.B., Forwood M.R., Fyhrie D.P., Martin R.B., Schaffler M.B., Turner C.H. Trabecular bone volume and microdamage accumulation in the femoral heads of women with and without femoral neck fractures. J. Bone Miner. Res. 1997;12(1):6–15. doi: 10.1016/s8756-3282(97)00200-7. [DOI] [PubMed] [Google Scholar]
- Carballido-Gamio J., Phan C., Link T.M., Majumdar S. Characterization of trabecular bone structure from high-resolution magnetic resonance images using fuzzy logic. Magn. Reson. Imaging. 2006;24(8):1023–1029. doi: 10.1016/j.mri.2006.04.010. Epub 2006 May 30. [DOI] [PubMed] [Google Scholar]
- Chen H., Wu M., Kubo K.Y. Combined treatment with a traditional Chinese medicine, Hachimi-jio-gan (Ba-Wei-Di-Huang-Wan) and alendronate improves bone microstructure in ovariectomized rats. J. Ethnopharmacol. 2012;142:80–85. doi: 10.1016/j.jep.2012.04.017. [DOI] [PubMed] [Google Scholar]
- Comelekoglu U., Bagis S., Yalin S., Ogenler O., Yildiz A., Sahin N.O., Oguz I., Hatungil R. Biomechanical evaluation in osteoporosis: ovariectomized rat model. Clin. Rheumatol. 2007;26(3):380–384. doi: 10.1007/s10067-006-0367-2. [DOI] [PubMed] [Google Scholar]
- Feldkamp L.A., Goldstein S.A., Parfitt M.A., Jesion G., Kleerekoper M. The direct examination of three-dimensional bone architecture in vitro by computed tomography. J. Ethnopharmacol. 1989;4:3–11. doi: 10.1002/jbmr.5650040103. [DOI] [PubMed] [Google Scholar]
- Hildebrand T., Laib A., Müller R., Dequeker J., Rüegsegger P. Direct three-dimensional morphometric analysis of human cancellous bone: microstructural data from spine, femur, iliac crest, and calcaneus. J. Bone Miner. Res. 1999;14:1167–1174. doi: 10.1359/jbmr.1999.14.7.1167. [DOI] [PubMed] [Google Scholar]
- Hildebrand T., Rüegsegger P. Quantification of bone microarchitecture with the structure model index. Comp. Meth. Biomech. Biomed. Eng. 1997;1:15–23. doi: 10.1080/01495739708936692. [DOI] [PubMed] [Google Scholar]
- Kanis J.A., Melton L.J., Christiansen C. The diagnosis of osteoporosis. J. Bone. Miner. Res. 1994;9(8):1137–1139. doi: 10.1002/jbmr.5650090802. [DOI] [PubMed] [Google Scholar]
- Karsenty G., Oury F. Regulation of male fertility by the bone-derived hormone osteocalcin. Mol. Cell. Endocrinol. 2014;382(1):521–526. doi: 10.1016/j.mce.2013.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim T.H., Jung J.W., Ha B.G. The effects of luteolin on osteoclast differentiation, function in vitro and ovariectomy-induced bone loss. J. NutrBiochem. 2011;22:8–15. doi: 10.1016/j.jnutbio.2009.11.002. [DOI] [PubMed] [Google Scholar]
- Kinney J.H., Haupt D.L., Balooch M., Ladd A.J., Ryaby J.T., Lane N.E. Three-dimensional morphometry of the L6 vertebra in the ovariectomized rat model of osteoporosis: biomechanical implications. J. Bone Miner. Res. 2000;15(10):1981–1991. doi: 10.1359/jbmr.2000.15.10.1981. [DOI] [PubMed] [Google Scholar]
- Kropotov A.V., Kolodnyak O.L., Koldaev V.M. Effects of Siberian ginseng extract and ipriflavone on the development of glucocorticoid-induced osteoporosis. Bull. ExpBiol. Med. 2002;133:252–254. doi: 10.1023/a:1015834717178. [DOI] [PubMed] [Google Scholar]
- Lee N.K., Sowa H., Hinoi E., Ferron M., Ahn J.D., Confavreux C., Dacquin R., Mee P.J., McKee M.D., Jung D.Y., Zhang Z., Kim J.K., Mauvais-Jarvis F., Ducy P., Karsenty G. Endocrine regulation of energy metabolism by the skeleton. Cell. 2007;130(3):456–469. doi: 10.1016/j.cell.2007.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller R., Hahn M., Vogel M., Delling G., Rüegsegger P. Morphometric analysis of noninvasively assessed bone biopsies: comparison of high-resolution computed tomography and histologic sections. Bone. 1996;18:215–220. doi: 10.1016/8756-3282(95)00489-0. [DOI] [PubMed] [Google Scholar]
- Muller R., Hahn M., Vogel M., Delling G., Ruegsegger P. Morphometric analysis of noninvasively assessed bone biopsies: comparison of high resolution computed tomography and histologic section. Bone. 1996;18(3):215–220. doi: 10.1016/8756-3282(95)00489-0. [DOI] [PubMed] [Google Scholar]
- Müller R., Hildebrand T., Ruüegsegger P. Non-invasive bone biopsy: a new method to analyse and display the three-dimensional structure of trabecular bone. Phys. Med. Biol. 1994;39(1994):145–164. doi: 10.1088/0031-9155/39/1/009. [DOI] [PubMed] [Google Scholar]
- Putnam S.E., Scutt A.M., Bicknell K., Priestley C.M., Williamson E.M. Natural products as alternative treatments for metabolic bone disorders and for maintenance of bone health. Phytother. Res. 2007;21(2):99–112. doi: 10.1002/ptr.2030. [DOI] [PubMed] [Google Scholar]
- Quin L., Zhang G., Shi YY., Lee KM., Leung PC., 2005., Prevention and treatment of osteoporosis with traditional herbal medicine. In: Deung, H.W. (Ed.), Current Topics of Osteoporosis. World Scientific Publisher. 513–531
- Rüegsegger P. The use of peripheral QCT in the evaluation of bone remodeling. Endocrinologist. 1994;4:167–176. [Google Scholar]
- Shen Y., Li Y.Q., Li S.P., Ma L., Ding L.J., Ji H. Alleviation of ovariectomy-induced osteoporosis in rats by Panaxnoto ginseng saponins. J. Nat. Med. 2010;64(3):336–345. doi: 10.1007/s11418-010-0416-7. [DOI] [PubMed] [Google Scholar]
- Taku K., Melby M.K., Kurzer M.S., Mizuno S., Watanabe S., Ishimi Y. Effects of soy isoflavone supplements on bone turnover markers in menopausal women; systematic review and meta-analysis of randomized controlled trial. Bone. 2010;47:417–423. doi: 10.1016/j.bone.2010.05.001. [DOI] [PubMed] [Google Scholar]
- Valverde-Franco G., Binette J.S., Li W., Wang H., Chai S., Laflamme F., Tran-Khanh N., Quenneville E., Meijers T., Poole A.R., Mort J.S., Buschmann M.D., Henderson J.E. Defects in articular cartilage metabolism and early arthritis in fibroblast growth factor receptor 3 deficient mice. Hum. Mol. Genet. 2004;15:1783–1792. doi: 10.1093/hmg/ddl100. [DOI] [PubMed] [Google Scholar]