Abstract
Arbuscular mycorrhizal fungi (AMF) association increases plant stress tolerance. This study aimed to determine the mitigation effect of AMF on the growth and metabolic changes of cucumbers under adverse impact of salt stress. Salinity reduced the water content and synthesis of pigments. However, AMF inoculation ameliorated negative effects by enhancing the biomass, synthesis of pigments, activity of antioxidant enzymes, including superoxide dismutase, catalase, ascorbate peroxidase and glutathione reductase, and the content of ascorbic acid, which might be the result of lower level lipid peroxidation and electrolyte leakage. An accumulation of phenols and proline in AMF-inoculated plants also mediated the elimination of superoxide radicals. In addition, jasmonic acid, salicylic acid and several important mineral elements (K, Ca, Mg, Zn, Fe, Mn and Cu) were enhanced with significant reductions in the uptake of deleterious ions like Na+. These results suggested that AMF can protect cucumber growth from salt stress.
Keywords: AMF, Lipid peroxidation, Antioxidant enzymes, Proline, Growth hormones, NaCl
1. Introduction
Plants often encounter several environmental stresses that result in significant unfavourable changes in growth and metabolism, which ultimately affects the yield of plants. Among these stress factors, soil salinity is a crucial damaging factor for plant growth and development (Hashem et al., 2016a, Hashem et al., 2014). Particularly, high salinity in the arid and semiarid regions of the world is a major cause of crop damage and yield loss. Anthropogenic activities, including the excessive use of saline water for irrigation and low rainfall due to climatic changes, also convert fertile arable land into salt-affected waste lands, and nearly 7% of agricultural land area is affected by salinity (Ruiz-Lozano et al., 2012). An increasing rate of salinity creates osmotic and ionic stress in plants and hampers plant growth by affecting their physiological and biochemical homeostasis via negative impacts on photosynthesis, protein synthesis, enzyme activity and mineral nutrition (Iqbal et al., 2015, Hashem et al., 2016a), which retards both the growth and yield of many vegetables, including cucumbers (Tejera et al., 2004, Gamalero et al., 2010, Balliu et al., 2015). Additionally, it poses major threats to all metabolic pathways through excessive generation of toxic reactive oxygen species (ROS), which damage the structural and functional integrity of several key macromolecules, including proteins and nucleic acids. ROS (superoxide ions, hydrogen peroxide, hydroxyl and peroxide radicals) induce oxidative stress (Qun et al., 2007, Kohler et al., 2009), reduce membrane permeability by affecting the polyunsaturated lipid component and increase cellular electro-leakage (Alqarawi et al., 2014a, Alqarawi et al., 2014b).
To overcome the deleterious effect of salinity on growth, a series of tolerance mechanisms are initiated to maintain the growth and development of plants. The up-regulation of the antioxidant system, greater accumulation of compatible osmolytes and the efficient compartmentalization of excessive toxic ions into the vacuole are considered important tolerance strategies (Hashem et al., 2015, Hashem et al., 2014). The antioxidant defence system comprises both enzymatic and non-enzymatic components, which protect plants from salinity stress by eliminating excess accumulated ROS. Antioxidant enzymes include superoxide dismutase (SOD), catalase (CAT), peroxidase (POD) and glutathione reductase (GR), which are intricate electron donors during the enzymatic neutralization of ROS, while the non-enzymatic antioxidants include ascorbic acid and glutathione, which are involved in stress tolerance (Saqib et al., 2008, Velarde-Buendía et al., 2012, Abd_Allah et al., 2017). The greater synthesis and accumulation of compatible osmolytes like free proline, glycine betaine, soluble sugars and amino acids under stressed conditions stimulate osmoregulation to maintain the cellular tissue water content, thereby helping plants to maintain growth (Khan et al., 2014, Hashem et al., 2015, Mo et al., 2016). Plants exposed to salt stress usually take up ions like sodium, chloride and potassium from the growth media, and the excess sodium is compartmentalized into the vacuole, transported by the apoplast pathway or excluded from the tissues (Khan et al., 2014).
Arbuscular mycorrhiza fungi (AMF) are beneficial fungal organisms that share symbiotic association with many land plants. AMF have the potential to improve soil characteristics, thereby promoting plant growth in normal and stressful environments (Gamalero et al., 2010, Navarro et al., 2014, Alqarawi et al., 2014a, Alqarawi et al., 2014b). AMF colonization enhances plant growth and vigour (Tang et al., 2009, Yang et al., 2015, Mo et al., 2016) and changes the morphological, nutritional and physiological levels of plants to improve resistance against different abiotic stresses (Alqarawi et al., 2014a, Alqarawi et al., 2014b, Hashem et al., 2015). AMF inoculation protects Ocimum basilicum against salinity stress by improving mineral uptake, chlorophyll synthesis and water use efficiency (Shekoofeh et al., 2012). Tomato plants inoculated with AMF show an increase in the leaf area, nitrogen, potassium, calcium and phosphorous contents to enhance the plant growth rate compared to controls (Balliuet al., 2015).
Cucumber (Cucumis sativus L.) belongs to the family Cucurbitaceae, which is an important cash crop worldwide and is mostly used in salad. The cucumber is an energetic vegetable and is a rich source of vitamin K. Cucumber cultivation is affected by soil salinity (Kirnak, 2006). The inoculation of AMF in cucumber plants can enhance antioxidant metabolism, nutrient uptake and osmotic regulation under saline conditions. Thus, the present study was conducted to analyse the impact of AMF on the growth, physiological and biochemical attributes of cucumber plants and their mitigating role against the deleterious effects of salt stress.
2. Materials and methods
2.1. Arbuscular mycorrhizal inoculum
The AMF (Claroideoglomus etunicatum [syn. Glomus etunicatum]; Rhizophagus intraradices [syn. Glomus intraradices]; and Funneliformis mosseae [syn. Glomus mosseae]) used in the current experiment were isolated previously (Hashem et al., 2016a, Hashem et al., 2016b) from the rhizosphere of the Talh tree (Acacia gerrardii) grown in a salt marsh habitat in the Riyadh region of Saudi Arabia per the method of Daniels and Skipper, 1982, Utobo et al., 2011. The identification of AMF was carried out according to the description of subcellular asexual spore structures provided by the International Culture Collection of Vesicular and Arbuscular Mycorrhizal Fungi (INVAM, 2014). The morphological characterizations of the experimental AMF used in the current study were described in detail with the help of illustrative pictures in our previous study (Hashem et al., 2016a, Hashem et al., 2016b). The propagation of the AMF inoculum was carried out by the trap culture protocol using corn plants (Zea mays L.) as the host, and the infected roots, hyphae, spores, and substrates were collected. In this protocol, single spores of each AMF isolate were inoculated on autoclaved sand (121 °C for 3 separated times) as the culture bed in plastic pots (25 cm diameter), and sorghum seeds (0.5% [v/v] NaOCl used for seed surface sterilization) were sown in the pots (five seeds/pot). The pots were incubated in a plant growth chamber at 27 ± 1 °C with an 18 h photoperiod, 750 μmol m−2 s−1 photosynthetic photon flux density, and 70–75% relative humidity for 3 months. Half-strength Hoagland's solution was used to irrigate the pots. The trap culture was used as the mycorrhizal inoculum and was added to the experimental soil as 25 g of trap soil culture (approx. 150 spores/g trap soil)/pot. Soil that was not inoculated with mycorrhiza served as the control.
2.2. Plant growth with salt and AMF treatments
Certified cucumber seeds (Cucumis sativus, cv. Dasher II), which were the product of Seminis Co, USA (http://www.seminis-us.com/product/dasher-ii/73), were germinated on a wetted blotter in Petri dishes at 26 °C in the dark for three days. Healthy and uniformly sized seedlings were selected and transplanted into plastic pots (25 cm diameter, one seedling/pot) containing autoclaved peat, perlite and sand (1:1:1, v/v/v). Plants were maintained one week after transplantation in the growth chamber (16 h light/8 h dark) at 25 ± 1 °C The plants were irrigated daily (25 ml/pot) with Hoagland nutrient solution (Hoagland and Arnon 1950). The pots were divided into four groups: (A) plant control (no treatments); (B) plants sown in pots inoculated with AMF; (C) plants stressed with 200 mM NaCl in the absence of AMF; and (D) plants stressed with 200 mM NaCl in the presence of AMF. The salt concentration was increased gradually (25 mM NaCl/day) until 200 mM NaCl to adapt the plants before being salt treatment. The experiment was carried in three biological replicates with completely randomized design. The mycorrhizal inoculum was added to the experimental soil as 25 g of trap soil culture as described above (approx. 150 spores/g trap soil)/pot and without AMF used as the soil control. The plants were allowed to grow in the growth chamber for more two months, and root samples were collected to determine the mycorrhizal root colonization. Similarly, the third leaves of cucumber plants were collected and stored at −80 °C until further use for biochemical analysis.
2.3. Determination of arbuscular mycorrhizal colonization
The roots of the cucumber were gently separated and fixed in FAA (formalin/acetic acid/alcohol, v/v/v) solution until they were stained according to the protocol of Phillips and Hayman, 1970, Koske and Gemma, 1989 using trypan blue in lactophenol. To determine the arbuscular mycorrhizal colonization of the cucumber roots, the stained root segments (one cm in length, 50 segments used as replicate for each sample) were observed under a digital computerized microscope (model DP-72, Olympus) at 20× magnification. The presence of mycelia, vesicles and arbuscules was recorded and analysed to assess the structural colonization as described by Giovannetti and Mosse (1980) according to the following formula:
2.4. Determination of photosynthetic pigments and stomatal conductance
The photosynthetic pigments were estimated in the leaves in dimethyl sulfoxide (DMSO) as described by Hiscox and Israelstam (1979). Absorbance was determined at 480, 510, 645, and 663 nm in a UV/VIS spectrophotometer (T80, PG Instruments Ltd, USA). Stomatal conductance in each treatment was recorded in fully expanded leaves using an infra-red gas analyser (CID-340, Photosynthesis system, Bio-Science, USA).
2.5. Determination of leaf water content
Leaf discs were punched from each treated plant, and their fresh weight was determined. The same leaf discs were floated on water for 4 h, and turgid weight was recorded after the samples were dried in an oven at 85 °C (Smart and Bingham 1974). Calculation of the leaf water content was done using the following formula:
2.6. Determination of membrane stability index, lipid peroxidation and hydrogen peroxide
The method of Sairam et al. (1997) was employed for determining the membrane stability index (MSI). With this method, 0.1 g of fresh leaf tissue was taken in two separate sets of test tubes containing 10 ml of double distilled water. One set was kept in a water bath for half an hour at 40 °C, and the electric conductivity (EC) was recorded (C1), while another set was kept in a water bath at boiling (100 °C), and the EC was recorded (C2). The MSI was calculated per the formula:
The quantitative estimation of malondialdehyde (MDA) was carried out as the level of lipid peroxidation according to the protocol by Heath and Packer (1968). In this method, fresh leaves (0.5 g) were ground in 1% trichloroacetic acid (TCA), and the homogenate was centrifuged at 10,000 rpm for 5 min. The supernatant (1.0 ml) was mixed with 4.0 ml of 0.5% (w/v) thiobarbituric acid (TBA), and the mixture was heated at 95 °C for 30 min, cooled in an ice bath and centrifuged at 5000 rpm for 5 min. Absorbance of the supernatant was measured at 532 and 600 nm. The MDA content was calculated according to the following formula:
Fresh tissue was homogenized in 5 ml of trichloroacetic acid (0.1%, TCA) followed by centrifugation at 12,000 g for 15 min. The supernatant (0.5 ml) was mixed with an equal volume of potassium phosphate buffer (pH 7.0) and potassium iodide. Samples were vortexed, and the absorbance was read at 390 nm to measure the hydrogen peroxide (Velikova et al., 2000).
2.7. Determination of proline and total phenols
For estimation of proline, 0.5 g of plant tissue was extracted in 3% (w/v) sulfosalicylic acid, and the homogenate was subjected to centrifugation at 3,000 rpm for 20 min. The supernatant was reacted with acetic acid and ninhydrin, the mixture was boiled for 1 h, and the absorbance at 520 nm was read to quantify the proline content (Bates et al., 1973). Total phenol was extracted in ethanol (80% v/v) and estimated using Folin and Ciocalteau’s phenol reagent. Absorbance was read at 750 nm and measured from a standard curve of pyrogallol (Slinkard and Singleton, 1977).
2.8. Assay of antioxidant enzymes and ascorbic acid content
Fresh leaves (5 g) were homogenized in 50 mM sodium phosphate buffer (pH 7.0) containing 1% soluble polyvinyl pyrolidine. The homogenate was centrifuged at 15,000 rpm for 20 min at 4 °C, and the supernatant was used to assay the enzyme activity. Protein in the enzyme extract was estimated according to Lowry et al. (1951). Superoxide dismutase (SOD, EC 1.15.1.1) was estimated according to Bayer and Fridovich (1987) following the photoreduction of nitroblue tetrazolium (NBT). The activity of SOD was expressed as enzyme units (EU) mg−1 protein, and one unit of SOD was defined as the amount of protein causing a 50% decrease in the SOD-inhibitable NBT reduction. Catalase (CAT, EC 1.11.1.6) activity was assayed by the method described by Luck (1974). The CAT activity was calculated using an extinction co-efficient of 36 × 103 mM−l cm-l and expressed as EU mg−1 protein. For determination of the ascorbate peroxidase (APX, EC 1.11.1.11) activity, the method of Nakano and Asada (1981) was followed. The assay mixture contained 0.1 ml of enzyme extract, 0.1 mM EDTA, 0.5 mM ascorbate, 0.1 mM H2O2 and 1 ml of potassium phosphate buffer (pH 7.0). The decrease in the absorbance of ascorbate was taken at 290 nm, and the activity was expressed as EU mg-l protein. The glutathione reductase (GR, EC 1.6.4.2) activity was assayed according to Carlberg and Mannervik (1985). Decreases in the absorbance were read at 340 nm for 2 min. The activity of GR was calculated using an extinction coefficient of 0.12 mM NADPH of 6.2 mM−1 cm−1 and expressed as the EU mg−l protein. Ascorbate was extracted from fresh leaves (0.8 g) in 3 ml ice-cold meta-phosphoric acid (5%) containing 1 mM EDTA. The homogenate was centrifuged at 10,000 rpm for 20 min, and the supernatant was used for ascorbate analysis (Huang et al., 2005).
2.9. Extraction and quantification of plant growth regulators
The leaves were extracted in 80% aqueous acetone (4:1, v/v) containing butylated hydroxy toluene (10 mg/l) and were purified using EtOAc and NaHCO3 (Kusaba et al., 1998) to measure the endogenous plant growth regulators. Quantification of abscisic acid (ABA) was done as described by Kamboj et al. (1999) and calculated using a standard curve of ABA. For salicylic acid estimation, the extracts were vacuum dried at room temperature, and the concentration of SA was quantified per the method of Siegrist et al. (2000) using HPLC equipped with a fluorescence detector (LC-2010 AHT, SHIMADZU, Japan). The extraction and quantitative estimation of JA was carried out using an HPLC (Agilent 1100 HPLC system; Agilent Technologies, Böblingen, Germany) with a Dionex column as described by Kramell et al., 1999, Pellegrini et al., 2013. JA was extracted from frozen fresh leaf samples (250 mg) using ethyl acetate at 4 °C, and the extract was kept at 4 °C overnight. The samples were centrifugation for 10 min at 10,000 rpm at 4 °C. The organic extract phase was shocked with acidified water, and the aqueous phase was separated using a separation funnel and analysed immediately using HPLC with a Dionex column (Acclaim 120, C18, 5 µm particle size, 4.6 mm internal diameter × 150 mm length). The detection of JA was carried out at 210 nm. The endogenous JA was quantified using the peak area of the standard JA.
2.10. Estimation of ions
Oven dried (110 °C) leaf and root samples were acid digested, and Na+, K+, Mg2+ and Ca2+ were estimated according to the method of Wolf (1982) using a flame photometer (Jenway Flame Photometer, Bibby Scientific Ltd-Stone-Staffs-St15 0SA–UK). For estimation of Mn, Fe, Cu and Zn, 1 M hydrochloric acid was added, and the digested dried leaf powder and their elemental (Mn, Fe, Cu and Zn) concentration were determined by atomic absorption spectrophotometer.
2.11. Statistical analysis
The experiments were repeated three times and completely randomized. Statistical significance between the control and treatments were calculated by one-way ANOVA performed by Duncan's Multiple Range Test (SPSS-21 software), and the differences in the means were determined by the least significant differences (LSD) (p = 0.05) test.
3. Results
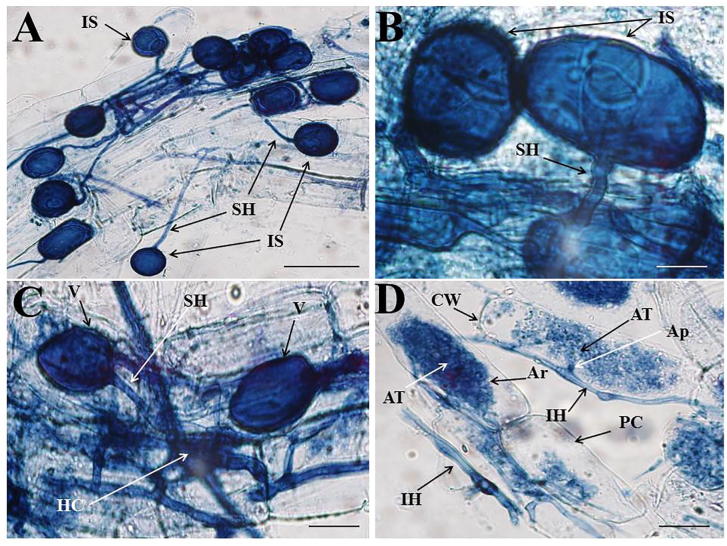
Root colonization by the arbuscular mycorrhizal fungi was observed with most different structural colonisations in roots of cucumber at control treatment in high rates (Fig. 1A–D). Soil salinity (200 mM NaCl) reduced the AMF colonization in cucumber roots (Table 1). The number of mycelia, vesicles and arbuscule formation were reduced to 55.01%, 61.06% and 71.69%, respectively, due to the effect of salinity. The intensity of the structural colonization of AMF in cucumber plants was categorized as poor (P), medium (M) and abundant to determine the toxic effect of salinity on AMF growth (Table 2). Mycelial colonization of 17.21% to 56.77% was observed in the roots, while salt stress reduced the AMF growth, which resulted in a maximum of 5.21% colonization. Similar trends were noted in arbuscule formation, but development of the vesicle was higher (28.34%) in salt stress than in the control.
Fig. 1.
A-D. AM fungal colonization structures within the roots of Cucumis sativus. A, B (Higher magnification): Root fragment densely colonized by intact spores (IS) with subtending hyphae (SH) showed by arrow. C: subtending hyphae (SH), vesicle (V) and hyphal coils (HC) showed by arrow. D: Plant cell (PC), cell wall (CW), intraradical hyphae (IH), arbuscules (Ar), arbuscular trunk (AT) and appressorium (Ap) showed by arrow. Bar: 50 μm.
Table 1.
Effect of salinity on mycorrhizal colonization (%) in Cucumis sativus L. roots. Data presented are the means ± SE (n = 5). Data followed by different letters are significantly different at P < 0.05.
| Treatment | Mycelium | Vesicles | Arbuscules |
|---|---|---|---|
| AMF | 82.61 ± 5.23a | 38.17 ± 2.72a | 83.71 ± 5.74a |
| Salinity + AMF | 37.16 ± 2.91b | 14.85 ± 3.13b | 23.69 ± 2.12b |
| LSD at: 0.05 | 16.622 | 11.530 | 17.012 |
Table 2.
Effect of salinity on the intensity of structural colonization (%) in Cucumis sativus L. roots. Data presented are the means ± SE (n = 5). Data followed by different letters are significantly different at P < 0.05.
| Treatment | Intensity of structural colonization (%) |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Mycelium |
Vesicles |
Arbuscules |
|||||||
| Poor | Medium | Abundant | Poor | Medium | Abundant | Poor | Medium | Abundant | |
| AMF | 17.21 ± 2.74a | 13.61 ± 1.13a | 56.77 ± 4.47a | 8.37 ± 0.98b | 11.15 ± 3.63b | 9.89 ± 1.84b | 5.16 ± 1.67b | 9.18 ± 1.93a | 66.56 ± 9.26a |
| Salinity + AMF | 24.73 ± 3.32b | 7.406 ± 1.13b | 5.21 ± 0.53b | 22.38 ± 1.51a | 25.30 ± 1.40a | 38.23 ± 1.88a | 20.53 ± 0.92a | 4.36 ± 0.32b | 10.32 ± 1.17b |
| LSD at: 0.05 | 5.026 | 3.772 | 34.816 | 11.463 | 8.438 | 12.834 | 8.093 | 2.735 | 23.961 |
Plant growth is directly and indirectly regulated by photosynthetic pigments. AMF colonization supported and increased the pigment synthesis of chlorophyll and carotenoids. Salinity affected the cucumber plants, which showed less chlorophyll a, chlorophyll b, total chlorophyll and carotenoids (Table 3). However, the inoculation of AMF mitigated the negative effect by enhancing chlorophyll a (27.40%), chlorophyll b (17.51%), total chlorophyll (26.74%) and carotenoid (42.32%) compared to the NaCl-stressed seedlings. AMF association in cucumber plants altered the stomatal function. Salt stress decreased the stomatal conductance, while AMF significantly enhanced the stomatal conductance in leaves in the salinity condition.
Table 3.
Effect of AMF and salinity on chlorophyll a, chlorophyll b, total chlorophyll, carotenoids and stomatal conductance in Cucumis sativus. Data presented are the means ± SE (n = 5). Data followed by different letters are significantly different at P < 0.05.
| Treatment | Chl a (mg/g FW) | Chl b (mg/g FW) | Total Chl (mg/g FW) | Carotenoids (mg/g FW) | Stomatal conductance (mmol m−2 s−1) |
|---|---|---|---|---|---|
| Control | 0.873 ± 0.02b | 0.438 ± 0.02b | 1.606 ± 0.03b | 0.2953 ± 0.01b | 22.17 ± 0.63b |
| AMF | 1.182 ± 0.02a | 0.512 ± 0.00a | 2.064 ± 0.01a | 0.3693 ± 0.01d | 39.08 ± 0.54a |
| Salinity | 0.556 ± 0.01c | 0.295 ± 0.00d | 0.956 ± 0.02d | 0.1046 ± 0.00d | 6.94 ± 0.11d |
| Salinity + AMF | 0.766 ± 0.02b | 0.358 ± 0.01c | 1.305 ± 0.01c | 0.1813 ± 0.01c | 16.92 ± 0.36c |
| LSD at: 0.05 | 0.0504 | 0.0352 | 0.0544 | 0.0244 | 8.9451 |
Osmolyte production during stress conditions is a plant protective mechanism against stress. The accumulation of proline and total phenol was increased in the individual or combined effect of AMF and salt stress (Table 4). Salinity enhanced proline synthesis in the control and AMF treatment. However, AMF inoculation increased the proline by 24.54% and 83.01% in cucumber plants in the control and salt stress condition, respectively. A similar trend was observed in total phenol synthesis in plants exposed to salt and AMF treatments. NaCl caused an increase in phenol (59.58%) in plants, and this content was further enhanced (19.23%) by AMF inoculation. The water content in plants during the salt stress condition was drastically reduced (50.03%), and AMF association was favourable in plants by enhancing the relative water content to mitigate the salt stress.
Table 4.
Effect of AMF and salinity on proline, total phenol and relative water content (RWC) in Cucumis sativus L. Data presented are the means ± SE (n = 5). Data followed by different letters are significantly different at P < 0.05.
| Treatment | Proline (μmol/g FW) |
Total phenols (mg/g FW) | RWC (%) |
|---|---|---|---|
| Control | 3.75 ± 0.25cd | 1.991 ± 0.06d | 92.99 ± 0.11a |
| AMF | 4.97 ± 0.10c | 2.304 ± 0.05c | 95.70 ± 0.25a |
| Salinity | 17.09 ± 0.15b | 4.926 ± 0.07b | 46.46 ± 0.34c |
| Salinity + AMF | 22.08 ± 0.40a | 6.099 ± 0.06a | 69.27 ± 0.61b |
| LSD at: 0.05 | 0.5909 | 0.1511 | 0.8721 |
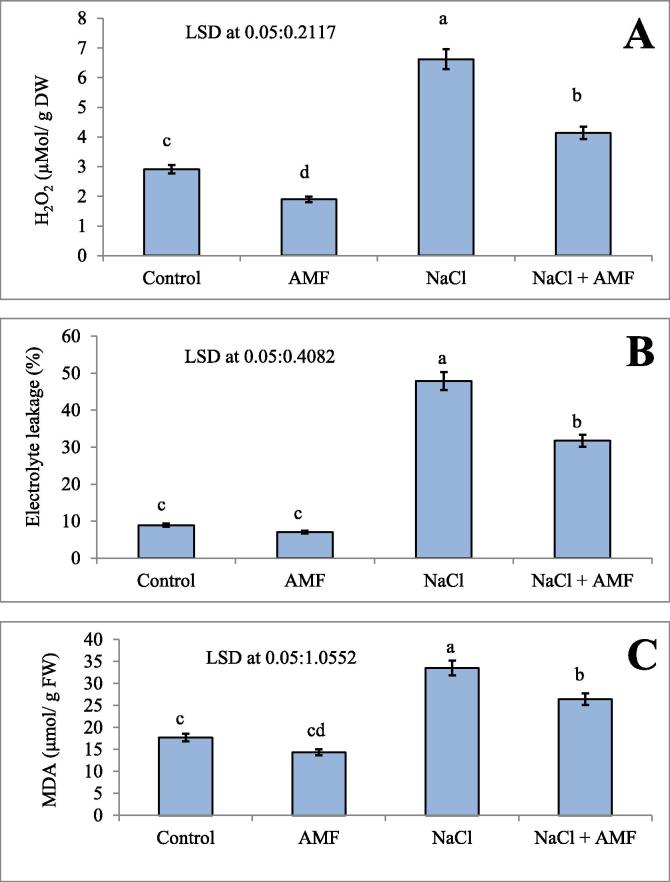
The over-synthesis of ROS during stress conditions is toxic to other metabolic processes in plants. Salt-induced oxidative stress in cucumbers was measured by hydrogen peroxide production, electrolytic leakage and lipid peroxidation (Fig. 2A–C). The results of our study showed that there was a higher accumulation of hydrogen peroxide (56.04%), electrolytic leakage (47.18%) and lipid peroxidation (81.41%) in salt-affected plants when compared to the control. AMF helped to ameliorate the salt stress effects in plants by reducing hydrogen peroxide (37.52%), lipid peroxidation (21.20%) and electrolyte leakage (33.71%) in cucumbers compared to NaCl-stressed plants. AMF helped to ameliorate the salt stress effects in plants by reducing AMF-acquired systemic resistance in C. sativus L. under salt stress, and the Pearson’s correlation coefficients between AMF treatment and oxidative stress metabolism are presented in Table 5. The mycelium had a negative correlation with the vesicles, H2O2, EL, proline, MDA and TPC, while a positive correlation was recorded in arbuscules, StCond, RWC and AsA. The vesicles showed a significant negative correlation with arbuscules, StCond, RWC, and AsA, while there was a positive correlation with H2O2, EL, proline, MDA and TPC. However, a positive correlation with StCond, RWC and AsA was observed in the arbuscules, but there was a negative correlation with H2O2, EL, proline, MDA and TPC. Hydrogen peroxide was recorded as having a positive correlation with EL, proline content, MDA and TPC, while there was a negative correlation recorded with StCond, RWC and AsA. StCond showed a negative correlation with EL, proline, MDA and TPC, while a positive correlation was recorded with RWC and AsA. Electrical conductivity (EC) was noted as having a positive and significant correlation with proline, MDA and TPC, while a negative effect on RWC and AsA was shown. However, a positive correlation of RWC was observed in AsA, while a negative correlation was recorded in the proline results, MDA and TPC. The proline content had a significant positive effect with MDA and TPC, while a negative correlation was recorded for AsA. MDA had a negative correlation with AsA, while a positive response was recorded with TPC. The AsA content showed a negative correlation with TPC.
Fig. 2.
A-C: Effect of salinity (200 mM NaCl) on (A) hydrogen peroxide (µMol/ g DW), (B) electrolyte leakage (%) and (C) lipid peroxidation (malondialdehyde [MDA], μmol/g FW) in Cucumis sativus with and without AMF inoculation. Data presented are the means ± SE (n = 5). Data followed by different letters are significantly different at P < 0.05.
Table 5.
Pearson’s correlation coefficients between AMF colonization and oxidative stress attributes in cucumber plants.
| M | V | A | H2O2 | StCond | EL | RWC | Proline | MDA | AsA | TPC | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| M | 1.00000 | −0.91030 0.0117 |
0.91448 0.0117 | −0.97579 0.0009 |
0.97116 0.0012 |
−0.96403 0.0019 |
0.96260 0.0021 |
−0.96500 0.0018 |
−0.96139 0.0022 |
0.95935 0.0024 |
−0.97674 0.0008 |
| V | 1.00000 | −0.93867 0.0055 |
0.94101 0.0051 |
−0.95663 0.0028 |
0.94558 0.0044 |
−0.93364 0.0065 |
0.92901 0.0074 |
0.91869 0.0096 |
−0.93369 0.0064 |
0.94502 0.0045 |
|
| A | 1.00000 | −0.97537 0.0009 |
0.97394 0.0010 |
−0.97924 0.0006 |
0.97882 0.0007 |
−0.97444 0.0010 |
−0.96603 0.0017 |
0.98080 0.0005 |
−0.97545 0.0009 |
||
| H2O2 | 1.00000 | −0.99519 <.0001 |
0.99604 <.0001 |
−0.99220 <.0001 |
0.99304 <.0001 |
0.98174 0.0005 |
−0.99707 <.0001 |
0.99813 <.0001 |
|||
| StCond | 1.00000 | −0.99857 < .0001 |
0.99608 <.0001 |
−0.99596 <.0001 |
−0.98961 <.0002 |
0.99395 <.0001 |
−0.99854 <.0001 |
||||
| EL | 1.00000 | −0.99806 <.0001 |
0.99828 <.0001 |
0.99056 <.0001 |
−0.99790 <.0001 |
0.99825 <.0001 |
|||||
| RWC | 1.00000 | −0.99955 <.0001 |
−0.99676 <.0001 |
0.99463 <.0001 |
−0.99691 <.0001 |
||||||
| Proline | 1.00000 | 0.99592 <.0001 |
−0.99563 <.0001 |
0.99700 <.0001 |
|||||||
| MDA | 1.00000 | −0.98365 <.0004 |
0.99060 <.0001 |
||||||||
| AsA | 1.00000 | −0.99575 <.0001 |
|||||||||
| TPC | 1.00000 |
M: Mycelium; V: Vesicles; A: Arbuscules; H2O2: Hydrogen peroxide; StCond: Stomatal conductance; EL: Electric conductivity; RWC: Relative water content; MDA: Malondialdehyde; AsA: Ascorbic acid; TPC: Total protein content.
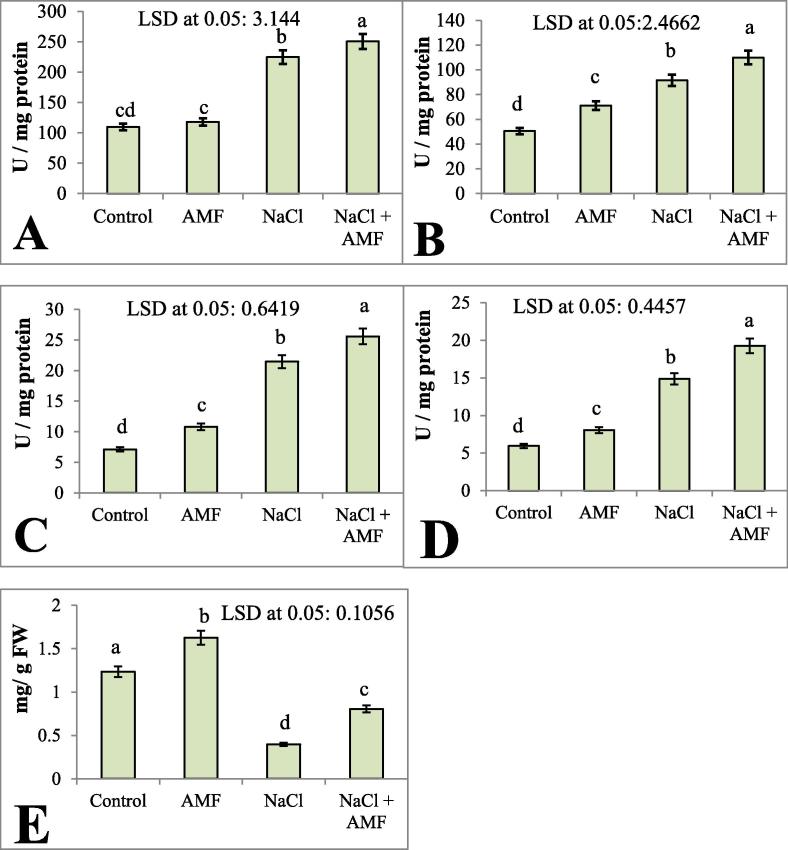
The expression of antioxidants in cucumber plants during the salt and AMF treatments are given in Fig. 3A–E. When compared to control plants, the AMF-treated plants showed higher SOD, CAT, APX and GR activity (6.87%, 28.83%, 34.10% and 26.17%, respectively). Salt stress also triggered these enzyme activities in plants. The combined effect of NaCl and AMF significantly accelerated the enzyme activities. AMF additionally induced an increase in SOD, CAT, APX and GR activities compared to salt-affected plants. AsA is a non-enzymatic antioxidant that significantly declined due to the effect of salt stress, and AMF helped to maintain the AsA content, which showed an increase of 32.27% over the control. The mitigation effect of AMF was expressed by enhancing the AsA content in salt-stressed plants.
Fig. 3.
A-E: Effect of salinity (200 mM NaCl) on the activity of (A) SOD, (B) CAT (C) APX, (D) GR (U/mg protein) and the (E) AsA content (mg/g FW) in Cucumis sativus with and without AMF inoculation. Data presented are the means ± SE (n = 5). Data followed by different letters are significantly different at P < 0.05.
The endogenous concentration of the plant stress hormones, such as abscisic acid (ABA), jasmonic acid (JA) and salicylic acid (SA), were altered during salt stress and AMF treatment (Table 6). Salt stress up-regulated the synthesis of ABA, JA and SA in plants, while AMF inoculation decreased the ABA and increased the JA and SA content in salt-affected plants.
Table 6.
Effect of AMF and salinity on endogenous abscisic acid (ABA), jasmonic acid (JA) and salicylic acid (SA) in Cucumis sativus L. Data presented are the means ± SE (n = 5). Data followed by different letters are significantly different at P < 0.05.
| Treatment | ABA (ng/g FW) | JA (ng/g FW) | SA (ng/g FW) |
|---|---|---|---|
| Control | 216.4 ± 2.99c | 1102.5 ± 7.73d | 136.1 ± 0.95d |
| AMF | 139.9 ± 0.703d | 1552.1 ± 11.9c | 156.2 ± 3.02c |
| Salinity | 779.7 ± 3.25a | 2494.0 ± 5.29b | 352.1 ± 3.24b |
| Salinity + AMF | 310.6 ± 1.06b | 2770.3 ± 33.6a | 470.2 ± 3.62a |
| LSD at: 0.05 | 5.3095 | 42.553 | 6.7013 |
Salt stress caused a significant reduction in the uptake of essential elements, such as potassium, calcium, magnesium, iron, zinc, manganese and copper and increased the sodium content in both leaf and root tissues (Table 7, Table 9). AMF inoculation increased the content of K, Ca, Mg, Fe, Zn, Mn and Cu by 23.09%, 36.92%, 23.11%, 5.85%, 18.96%, 6.77% and 25.32%, respectively, in leaves and by 29.77%, 48.93%, 30.97%, 7.9%, 28.10%, 14.98% and 30.99%, respectively, in root tissues. NaCl stressed plants showed declines in K, Ca, Mg, Fe, Zn, Mn and Cu compared to control plants. However, AMF ameliorated the stress on nutrition by improving the uptake of K (46.71%), Ca (49.50%), Mg (34.57%), Fe (19.29%), Zn (20.49%), Mn (33.28%) and Cu (24.71%) in salt-affected plants. A similar trend was observed in the nutritional uptake by roots under AMF and salt stress conditions. Specifically, AMF proved the mitigation effects by reducing the sodium (23%) content in leaves in NaCl-stressed plants.
Table 7.
Effect of AMF and salinity on sodium (Na+), potassium (K+), calcium (Ca+2), magnesium (Mg2+), iron (Fe2+), zinc (Zn2+), manganese (Mn2+) and copper (Mn2+) contents in the leaves of Cucumis sativus L. Data presented are the means ± SE (n = 5). Data followed by different letters are significantly different at P < 0.05.
| Treatment | Na+ (mg/g DW) | K+ (mg/g DW) | Ca2+ (mg/g DW) | Mg2+ (mg/g DW) | Fe2+ (mg/g DW) | Zn2+ (mg/g DW) | Mn2+ (mg/g DW) | Mn2+ (mg/g DW) |
|---|---|---|---|---|---|---|---|---|
| Control | 19.6 ± 0.75c | 38.5 ± 0.92b | 1.933 ± 0.04b | 1.533 ± 0.04b | 114.2 ± 0.97b | 39.4 ± 0.48b | 187.8 ± 1.27b | 8.52 ± 0.46b |
| AMF | 15.1 ± 0.43d | 50.1 ± 0.58a | 3.060 ± 0.09a | 2.003 ± 0.08a | 121.3 ± 0.43a | 48.6 ± 0.66a | 201.4 ± 2.08a | 11.41 ± 0.60a |
| Salinity | 44.3 ± 0.39a | 15.9 ± 0.34d | 0.903 ± 0.01d | 0.630 ± 0.02d | 73.8 ± 0.81d | 26.2 ± 0.55d | 107.1 ± 4.41d | 4.63 ± 0.27d |
| Salinity + AMF | 25.5 ± 0.33b | 29.8 ± 0.78c | 1.350 ± 0.07c | 0.963 ± 0.05c | 91.4 ± 0.36c | 33.0 ± 0.95c | 160.5 ± 1.44c | 6.15 ± 0.14c |
| LSD at: 0.05 | 1.1694 | 1.5997 | 0.1452 | 0.1356 | 1.6063 | 1.587 | 6.052 | 0.9478 |
Table 9.
Effect of AMF and salinity on the sodium (Na+), potassium (K+), calcium (Ca2+), magnesium (Mg2+), iron (Fe2+), zinc (Zn2+), manganese (Mn2+) and copper (Mn2+) contents in the roots of Cucumis sativus L. Data presented are the means ± SE (n = 5). Data followed by different letters are significantly different at P < 0.05.
| Treatment | Na+ (mg/g DW) | K+ (mg/g DW) | Ca2+ (mg/g DW) | Mg2+ (mg/g DW) | Fe2+ (mg/g DW) | Zn2+ (mg/g DW) | Mn2+ (mg/g DW) | Cu2+ (mg/g DW) |
|---|---|---|---|---|---|---|---|---|
| Control | 36.4 ± 0.37d | 51.8 ± 0.49b | 3.58 ± 0.19b | 2.786 ± 0.17b | 705.2 ± 2.67b | 21.23 ± 0.38b | 118.3 ± 1.09b | 6.01 ± 0.10b |
| AMF | 41.3 ± 0.36c | 73.8 ± 0.75a | 7.01 ± 0.07a | 4.036 ± 0.04a | 765.7 ± 2.44a | 29.53 ± 0.32a | 139.1 ± 1.10a | 8.71 ± 0.19a |
| Salinity | 68.7 ± 1.57b | 34.6 ± 1.24d | 1.78 ± 0.17d | 1.666 ± 0.07 cd | 113.8 ± 2.15d | 14.78 ± 0.46d | 61.2 ± 1.02d | 4.40 ± 0.32d |
| Salinity + AMF | 80.3 ± 0.74a | 41.5 ± 0.31c | 2.70 ± 0.08c | 1.996 ± 0.06c | 410.5 ± 0.89c | 17.93 ± 0.28c | 90.6 ± 1.38c | 5.01 ± 0.05c |
| LSD at: 0.05 | 2.0967 | 1.8099 | 0.325 | 0.2363 | 4.9614 | 0.8542 | 2.6741 | 0.4618 |
The Pearson correlation coefficient between AMF colonization and the accumulation of elements in leaves are shown in Table 8. The mycelium showed a negative correlation for vesicles and Na+ ion concentration due to the effect of the significant positive correlation between arbuscules, K+, Ca2+, Mg2+, Fe2+, Zn2+, Mn2+ and Cu2+ ions in cucumber leaves. The statistical analysis showed a positive and significant correlation between vesicles and Na+ (0.938), while there was a negative correlation for arbuscules with the K+, Ca2+, Mg2+, Fe2+, Zn2+, Mn2+ and Cu2+ contents. Arbuscules showed a negative correlation with Na+, while there was a positive and significant correlation for K+, Ca2+, Mg2+, Fe2+, Zn2+, Mn2+, and Cu2+. Similarly, Na+ had a negative correlation with other nutritional elements, including K+, Ca2+, Mg2+, Fe2+, Zn2+, Mn2+ and Cu2+. The quantity of K+ in leaves showed a positive correlation with Ca2+, Mg2+, Fe2+, Zn2+, Mn2+ and Cu2+. The Ca2+ content was expressed as a positive relationship with Mg2+, Fe2+, Zn2+, Mn2+ and Cu2+. However, the accumulation of Mg2+in leaves indicated a positive and significant correlation with Fe2+, Zn2+, Mn2+ and Cu2+. The ion concentration of Fe2+ had a positive correlation with Zn2+, Mn2+ and Cu2+. The presence of Zn2+in leaves showed a positive correlation with Mn2+ and Cu2+. In addition, the amount of Mn+2showed a positive correlation with the concentration of Cu2+.
Table 8.
Pearson’s correlation coefficients between AMF colonization and the accumulation of elements in cucumber leaves.
| M | V | A | Na+ | K+ | Ca2+ | Mg2+ | Fe2+ | Zn2+ | Mn2+ | Cu2+ | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| M | 1.00000 | −0.91030 0.0117 |
0.91448 0.0107 |
−0.97152 0.0012 |
0.94585 0.0043 |
0.97956 0.0006 |
0.98779 0.0002 |
0.96114 0.0022 |
0.94761 0.0040 |
0.93358 0.0065 |
0.0065 0.0069 |
| V | 1.00000 | −0.93867 0.0055 |
0.93819 0.0056 |
−0.93754 0.0057 |
−0.96640 0.0017 |
−0.95826 0.0026 |
−0.94892 0.0038 |
−0.97625 0.0008 |
−0.95245 0.0033 |
−0.96365 0.0020 |
|
| A | 1.00000 | −0.96773 0.0015 |
0.97552 0.0009 |
0.95856 0.0025 |
0.94161 0.94161 |
0.98535 0.0003 |
0.98561 0.0003 |
0.98949 0.0002 |
0.94907 0.0038 |
||
| Na+ | 1.00000 | −0.99209 <.0001 |
−0.98987 0.0002 |
−0.98696 0.0003 |
−0.99154 0.0001 |
−0.97906 0.0007 |
−0.97898 0.0007 |
−0.98201 0.0005 |
|||
| K+ | 1.00000 | 0.98282 0.0004 |
0.96894 0.0014 |
0.99442 <.0001 |
0.98309 0.0004 |
0.99217 <.0001 |
0.98325 0.0004 |
||||
| Ca2+ | 1.00000 | 0.99598 <.0001 |
0.98968 0.0002 |
0.98838 0.0002 |
0.97897 0.0007 |
0.97971 0.0006 |
|||||
| Mg2+ | 1.00000 | 0.97754 0.0008 |
0.97517 0.0009 |
0.95960 0.0024 |
0.97288 0.0011 |
||||||
| Fe2+ | 1.00000 | 0.99335 <.0001 |
0.99573 <.0001 |
0.97262 0.0011 |
|||||||
| Zn2+ | 1.00000 | 0.99401 <.0001 |
0.97320 0.0011 |
||||||||
| Mn2+ | 1.00000 | 0.96995 0.0013 |
|||||||||
| Cu2+ | 1.00000 |
M: Mycelium; V: Vesicles; A: Arbuscules.
The Pearson’s correlation coefficients between AMF colonization and the accumulation of elements in the roots are described in Table 10. The growth of the mycelium had a negative correlation with vesicles and Na+, while there was a positive correlation with the formation of arbuscules and the concentration of K+, Ca2+, Mg2+, Fe2+, Zn2+, Mn2+ and Cu2+. The vesicles showed a positive and significant correlation with the accumulation of Na+ and Cu2+, while a negative effect was recorded for arbuscule formation and the concentration of K+, Ca2+, Mg2+, Fe2+, Zn2+ and Mn2+. In the statistical analysis, arbuscules showed a positive and significant correlation with the K+, Ca2+, Mg2+, Fe2+, Zn2+ and Mn2+ contents, while there was a negative correlation with the accumulation of Na+. The presence of Na+ in roots showed a negative and significant correlation with the K+, Ca2+, Mg2+, Fe2+, Zn2+, Mn2+ and Cu2+ contents. The accumulation of K+ in cucumber roots had a positive correlation with the concentration of Ca2+, Mg2+, Fe2+, Zn2+, Mn2+ and Cu2+. The Ca2+ content in roots showed a significant positive correlation with Mg2+, Fe2+, Zn2+, Mn2+ and Cu2+. The presence of Mg2+ in the roots indicated a positive correlation with the concentration of Fe2+, Zn2+, Mn2+ and Cu2+. The Fe2+ ion concentration had a positive correlation with the accumulation of Zn2+, Mn2+ and Cu2+ in cucumber roots. However, the Zn2+content showed significant and positive correlation with Mn+2 and Cu2+ concentration. In addition, the quantity of Mn2+ was recorded as a positive and significant correlation with the amount of Cu2+ in roots.
Table 10.
Pearson’s correlation coefficients between AMF colonization and the accumulation of elements in cucumber roots.
| M | V | A | Na+ | K+ | Ca2+ | Mg2+ | Fe2+ | Zn2+ | Mn2+ | Cu2+ | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| M | 1.00000 | −0.91030 0.0117 |
0.91448 0.0107 |
−0.96965 0.0014 |
0.95941 0.0024 |
0.95374 0.0032 |
0.95090 0.0036 |
0.96451 0.0019 |
0.97121 0.0012 |
0.95496 0.0030 |
0.96583 0.0017 |
| V | 1.00000 | −0.93867 0.0055 |
0.93036 0.0071 |
−0.94598 0.0043 |
−0.93758 0.0057 |
−0.92289 0.0087 |
−0.94339 0.0047 |
−0.94316 0.0048 |
−0.91868 0.0097 |
0.0097 0.0024 |
|
| A | 1.00000 | −0.97505 0.0009 |
0.98117 0.0005 |
0.98223 0.0005 |
0.97656 0.0008 | 0.98102 0.0005 |
0.97463 0.0010 |
0.97924 0.0006 |
0.96486 0.0018 |
||
| Na+ | 1.00000 | −0.99799 <.0001 |
−0.99755 <.0001 |
−0.99630 <.0001 |
−0.99897 <.0001 |
−0.99794 <.0001 |
−0.99793 <.0001 |
−0.99243 <.0001 |
|||
| K+ | 1.00000 | 0.99860 <.0001 |
0.99540 <.0001 |
0.99934 <.0001 |
0.99815 <.0001 |
0.99605 <.0001 |
0.99581 <.0001 |
||||
| Ca2+ | 1.00000 | 0.99885 <.0001 |
0.99903 <.0001 |
0.99448 <.0001 |
0.99848 <.0001 |
0.99182 <.0001 |
|||||
| Mg2+ | 1.00000 | 0.99672 <.0001 |
0.99037 0.0001 |
0.99906 <.0001 |
0.98707 0.0002 |
||||||
| Fe2+ | 1.00000 | 0.99761 <.0001 |
0.99732 <.0001 |
0.99439 <.0001 |
|||||||
| Zn2+ | 1.00000 | 0.99284 <.0001 |
0.99566 <.0001 |
||||||||
| Mn2+ | 1.00000 | 0.98604 0.0003 |
|||||||||
| Cu2+ | 1.00000 |
M: Mycelium; V: Vesicles; A: Arbuscules.
4. Discussion
The unfavourable climate changes in the contemporary era are a major challenge to improving crop growth and yield despite environmental stresses including soil salinity. The exploitation of the desirable genetic potential of plant species for crop cultivation without proper management techniques is difficult under unfavourable conditions (Hosseini et al., 2017, Egamberdieva et al., 2017). In the present study, AMF was tested for its salt stress amelioration potential in cucumber plants. Although salt stress inhibited the mycelial growth and formation of arbuscules in cucumber roots, it stimulated vesicle formation. Shekoofeh et al., 2012, Alqarawi et al., 2014a reported that AMF colonization and spore populations declined due to the salinity in soil and suggested that the greater accumulation of toxic sodium ions in AMF reduced their growth and survival. The uptake of excess toxic ion transport into roots resulted in a decreased rate of cell division and cell elongation (Hasanuzzaman et al., 2013). Reduced cell growth under stress probably occurs due to the reduced uptake of water, and AMF colonization significantly increased water uptake by the roots, improving root attributes. The plant growth-promoting effect of AMF and their subsequent mitigation effects against salinity stress have been studied by others (Tang et al., 2009, Gamalero et al., 2010, Alqarawi et al., 2014a). Aroca et al. (2013) demonstrated the amelioration effect of AMF against the deleterious effects of salinity in lettuce. The evidence for enhanced nutrient uptake in plants through the actions of AMF confirms the resilience of plants to abiotic stress conditions (Kohler et al., 2009, Tang et al., 2009). AMF triggered the growth promotion of C. sativus, which was correlated with the increased synthesis of photosynthetic pigments and a possible impact of AMF in protecting the structure and function of the pigment-protein complex (Hajiboland et al., 2010, Porcel et al., 2015, Yang et al., 2015) which is, however, drastically affected by salinity (Chaves et al., 2009, Aroca et al., 2013). High salinity hampers the de novo synthesis of proteins and chlorophyll components (El-Tayeb 2005). Several studies have documented reduced chlorophyll pigments due to salinity (Sudhir et al., 2005, Neelam and Subramanyam, 2013, Khan et al., 2014, Hashem et al., 2015, Yang et al., 2015). In the present study, AMF inoculation mitigated the effects on photosynthetic pigments in salt-stressed C. sativus. Our results corroborate with other crop plants, such as Solanum lycopersicum L., lettuce and Panicum turgidum subjected to salt and AMF treatments (Hajiboland et al., 2010, Aroca et al., 2013, Hashem et al., 2015). Many are of the opinion that the AMF-triggered synthesis of chlorophyll is due to greater uptake of Mg, which forms the central component of the chlorophyll pigment (Sheng et al., 2008). The higher concentration of pigments in AMF-inoculated plants might be due to the up-regulation of the enzymes involved in the synthesis of chlorophylls concomitant with the reduction of chlorophyllase activity. AMF increases the activity of Rubisco and the associated carbon-metabolizing enzymes leading to the positive impact on the growth under normal as well as salinity stress conditions (Hashem et al., 2015). The positive influence of AMF on the stomatal conductance indicates their contribution to growth regulation in C. sativus under stress conditions. Recently, Yang et al., (2015) observed that AMF colonization of the black locust (Robinia pseudoacacia L.) enhanced root growth, morphology, hydraulic conductivity, photosynthetic attributes like stomatal conductance and net photosynthesis, water use efficiency and nutrient uptake. AMF can protect the PSII activity in chloroplasts and thus improve the water use efficiency under metal stress (Lu and Vonshak, 2002, Allakhverdiev and Murata, 2008, Chaves et al., 2009, Yang et al., 2015).
It was interesting to observe that C. sativus seedlings colonized with AMF exhibited an apparent decline in the accumulation of ROS and prevented oxidative damage to cellular structures and their functional integrity (Kohler et al., 2009, Velarde-Buendía et al., 2012, Hashem et al., 2015). The results of NaCl-induced damage to the structure and stability of cellular membranes was described by Ruiz-Lozano et al., 2012, He et al., 2017, Yang et al., 2015 who observed that Oryza sativa, Lycium barbarum and Robinia pseudoacacia, respectively, exposed to increased salinity showed membrane leakage resulting from damage to their plasma membranes; however, greater maintenance of water balance in tissues due to AMF colonization may have protected their membrane structures. The stress-induced reduction in the structural and functional stability of the membranes mainly results from the rapid increase in the lipid peroxidation (Fouad et al., 2014, Abd_Allah et al., 2015a, Nath et al., 2016). Salinity stress reduces membrane permeability by affecting the polyunsaturated lipids of the membranes, resulting in considerable leakage of the cellular components (Sánchez-Rodríguez et al., 2010, Alqarawi et al., 2014b). Earlier, we reported greater protection of the membrane lipids and functional stability of the membranes due to AMF inoculation (Abd_Allah et al., 2015a, Mo et al., 2016). Additionally, the reduced production of hydrogen peroxide in AMF-colonized plants prevents membrane dysfunction (Andrio et al., 2013, Puppo et al., 2013). ROS primarily affects membrane lipids and proteins leading to the loss of membrane integrity, thereby causing the leakage of electrolytes. However, AMF inoculation mitigated stress effects via substantial increases in the antioxidant activity and the accumulation of osmolytes (Qun et al., 2007). The association of AMF with Solanum lycopersicum significantly improved the lipid and fatty acid content in cadmium-affected plants to reduce membrane damage and leakage (Fouad et al., 2014, Hashem et al., 2016b, Nath et al., 2016).
C. sativus exposed to salinity stress exhibited a significant decline in the leaf relative water content, and our results concur with the results of Arulbalachandran et al., 2009, Hashem et al., 2015, Yang et al., 2015. AMF application regulates root phenotypic characteristics causing greater uptake of water during stress (Augé, 2001). However, the inoculation of AMF enhanced the uptake of water in root cells under normal conditions, as well as during salinity stress. The increase of water levels in stressed plants maintains the cell turgor, photosynthesis, enzyme activity, cellular integrity and growth, all of which ameliorate the deleterious effect of salinity (Sade et al., 2010). Proline is one of the key osmoprotectants preventing cellular oxidative damage by maintaining tissue water potential and protein integrity and functioning (Mansour and Ali 2017). The current study showed that AMF inoculation improved the synthesis of proline, and it has been demonstrated that proline synthesis is up-regulated in stressful environments with a significant decline in its catabolism (Mo et al., 2016, Iqbal et al., 2015). This result supported the findings of Yooyongwech et al. (2013), who reported that proline was enhanced in response to AM treatment. Shekoofeh et al., 2012, Wu et al., 2016 also reported that AMF triggered the accumulation of proline, which leads to improved water transport that improves the metabolic efficiency of plants.
The enhancement of proline accumulation caused by AMF symbiosis might be due to the up-regulation of the delta1-pyrroline-5-carboxylate synthetase gene (the rate-limiting enzyme in proline biosynthesis, MeP5CS) as described by Huang et al. (2013).
To neutralize ROS and prevent oxidative damage to cells, plants up-regulate the antioxidant defence system, which is an intriguing protective system. SOD is a key enzyme mediating the scavenging of toxic superoxide radicals for protection of electron transport. SOD activity was increased in response to salinity and has been reported in cowpea, sorghum, cotton and Populus cathayana (Freitas et al., 2011, Wu et al., 2016). SOD modulates the Haber-Weiss reaction substrates, superoxide and H2O2, thereby reducing the formation of more toxic hydroxyl (OH−) radicals. Greater SOD activity in AMF-inoculated plants might be due to the increased uptake of Zn, Cu, Mn and Fe, which form co-factors for its isozymes (Ghorbanli et al., 2004, Qun et al., 2007, Kohler et al., 2009). In stressed plants, the generation of hydrogen peroxide after SOD-mediated neutralization of superoxide is eliminated by either CAT or APX via the ascorbate-glutathione cycle, in which GR, AsA and GSH are the key components controlling ROS neutralization (Velarde-Buendía et al., 2012). Greater APX and CAT activity due to AMF inoculation in C. sativus correlates with the results of Ghorbanli et al., 2004, Hashem et al., 2015, Wu et al., 2016. It revealed that AMF inoculation improves the ROS efflux activity of tap and lateral root results, with considerable reductions in their accumulation, and prevents cellular oxidative damage (Huang et al., 2017). An increase in CAT and APX activity due to AMF inoculation-mediated fast breakdown of H2O2 (Foyer et al., 1994, Abd_Allah et al., 2015a, Abd_Allah et al., 2015b) protects against cellular damage. Non-enzymatic antioxidants are also involved in the declining accumulation of ROS (Huang et al., 2017). APX and CAT play an indispensable role in stress amelioration, and the activity of APX is coupled to GR, AsA and GSH. The ascorbate–glutathione cycle, which contains a series of redox reactions, maintains the NADP/NADPH ratio and the cellular redox state to prevent the formation of ROS and thereby protect photosynthetic electron transport. The activity of APX and GR and the content of AsA and GSH increased due to AMF in salt-affected C. sativus (Hashem et al., 2016a, Abd_Allah et al., 2015a, Abd_Allah et al., 2015b). Similarly, Cekic et al. (2012) observed enhanced GR activity in AMF-inoculated Capsicum annuum under salt stress to maintain plant growth. The strengthening of the antioxidant system in AMF plants further boosted the synthesis of polyphenols. In our previous study, we demonstrated that greater accumulation of phenols in AMF-inoculated plants confers stress tolerance (Abd_Allah et al., 2015a, Abd_Allah et al., 2015b). Polyphenols regulate cellular functioning by modulating nitric oxide and the functioning of voltage-gated ion channels for integrating different signalling pathways (Upadhyay and Dixit 2015). AMF inoculation promoted the accumulation of several important polyphenols, contributing to the growth of marjoram, lemon balm and marigold (Engel et al., 2016). In addition, the priming of seeds with AMF can enhance the expression of enzymes involved in phenol biosynthetic pathways (Song et al., 2015).
AMF association with plant roots regulates stress hormone signalling during unfavourable environmental conditions. The inoculation of AMF stimulated the endogenous concentration of JA and SA. The synthesis and transport of SA and JA involve several cellular processes for plant growth development. In the present study, AMF inoculation reprogrammed their concentrations to enhance stress tolerance. Plant growth-promoting fungi cause an increase in the endogenous levels of phytohormones (Waqas et al., 2012, Waqas et al., 2014, Abd_Allah et al., 2015a, Abd_Allah et al., 2015b). ABA acts as an anti-transpirant leading to a reduction in water loss through the modification of stomatal functioning. However, AMF significantly reduced ABA production in cucumber plants and thereby regulating the rate of transpiration. JA and its subsequent derivatives are an important group in oxylipin signalling molecules, which regulate several key physiological processes and metabolite synthesis under stress conditions. JA signalling in plants is an important process that integrates different mechanisms against several biotic and abiotic stresses, while SA signalling is widely accepted as a biotroph immunity indicator (Wasternack and Hause, 2013, De-Vleesschauwer et al., 2014). The up-regulation of JA and SA in AMF-inoculated plants confers their potential role in the amelioration of salinity via improvement in the synthesis of secondary metabolites (Qun et al., 2007, Arulbalachandran et al., 2009, Yuan et al., 2010). The accumulation of ABA in plant cells has negative effects on stomatal conductance under salinity stress. The biosynthesis of ABA in guard cells in stressed plants triggers the redistribution and accumulation of ABA within the cells and induces the release of water and ions leading guard cells to turn flaccid and stomata to close (Bray, 1997). However, AMF reduced the synthesis of ABA to regulate stomatal functioning. Previous reports also showed an increase in ABA in soybean cultivars subjected to salt stress (Hamayun et al., 2010). ABA acts as a signal for regulating stomatal closure, plant growth and development under stressed conditions (Wasilewska et al., 2008). The enhancement of ABA in plants during stress conditions is an early response mechanism against stress (Xiong et al., 2002). AMF inoculation stimulated greater uptake of nutrients, including potassium, calcium, and magnesium to promote the synthesis of various important metabolites and enzymes (Yuan et al., 2010). In the current study, AMF triggered the production of SA, thereby reducing oxidative damage (Conrath, 2006) through the induction of systemic acquired resistance, which depends on the endogenous concentrations of SA and ROS and the expression of specific resistance genes (Durrant and Dong, 2004).
The results of the present study indicate that high salinity treatment restricted the uptake of key essential macronutrients, including potassium, calcium, and magnesium, as well as other microelements like zinc, iron and cupper. Sodium shows an antagonistic relationship with important mineral ions such as potassium and calcium. AMF protected plants by inhibiting sodium uptake and regulating the ionic balance in cells during soil salinity. Several reports have revealed that salinity affects mineral uptake by plant roots, including studies by Kohler et al., 2009, Hashem et al., 2015. For example, the growth of Acacia gerrardii was increased by the uptake of Mg, K, Ca and N due to AMF colonization (Hashem et al., 2016a). The inoculation of AMF ameliorated the negative effects of salinity due to a decrease in the partitioning of excess sodium to the upper parts of the plant, thereby restricting its toxicity. AMF-induced enhancement of mineral nutrition in plants has been reported by several researchers (Balliu et al., 2015, Abd_Allah et al., 2015a, Abd_Allah et al., 2015b). Wang et al. (2008) noted the greater uptake of mineral ions in cucumbers due to the inoculation of different AMF species. The accumulation of Mg content in AMF-inoculated plants contributes to chlorophyll production, while K and Ca are involved in the energy metabolism of cells by improving the activity of enzymes (Parre et al., 2007, Yousuf et al., 2015). In addition, Ca serves as an important cellular messenger for plant growth signalling (Talukdar, 2012, El-Beltagi and Mohamed, 2013). Accumulation of Ca in plants favours the colonization of AMF for better plant growth and adaptation (Jarstfer et al., 1998). AMF up-regulates the expression of K transport proteins in plant roots for mediating the increase in K uptake (Garcia and Zimmermann, 2014). Accumulation of K in AMF-associated plants contributed to increased protein synthesis as a result of its requirement for binding tRNA with ribosomes (Blaha et al., 2000). AMF inoculation restricts the uptake and accumulation of Na by regulating the expression levels of AKT2, SOS1 and SKOR genes in roots to maintain the homeostasis of K+ and Na+ (Estrada et al., 2013). The application of AMF in saline soil proved to be an affective stress mitigation agent to restrict the uptake of toxic Na and to increase the uptake of K, Ca, Zn, Fe, Cu and Mn in cucumber plants.
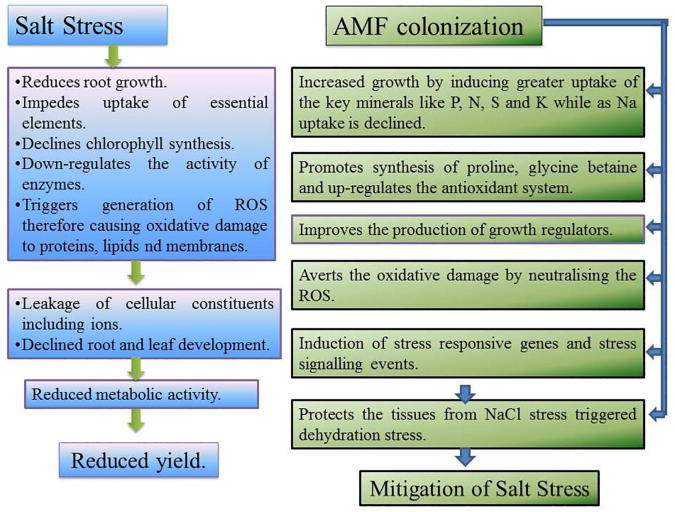
5. Conclusion
Cucumber subjected to salinity stress exhibited a considerable decline in the physiological and biochemical parameters. Salinity (200 mM NaCl) induced oxidative damage resulting in the loss of membrane functioning due to lipid peroxidation. However, the present results showed that the AM symbiosis could enhance the growth, biomass, root activity, nutrient content and gas exchange parameters in AMF-inoculated plants. AMF inoculation significantly favoured cucumber cultivation by reducing the negative impacts of salinity stress. AMF protected plants by up-regulating the activity of antioxidant enzymes and osmolytes and by regulating the synthesis of phytohormones, which might possibly interconnect the various tolerance mechanisms for cumulative stress response (Fig. 4). The prominent effect of AMF against salinity was proven to be due to a restriction in Na uptake by roots and to the homeostasis of nutrient uptake. The results of the present study strongly suggest and recommend that a consortium of AMF (Claroideoglomus etunicatum, Rhizophagus intraradices and Funneliformis mosseae) can be an effective biofertilizer to improve cucumber cultivation on saline agricultural lands.
Fig. 4.
Diagrammatic representation of main mycorrhizal functions to regulate salt stress in cucumber plant.
Acknowledgement
The authors would like to extend their sincere appreciation to the Deanship of Scientific Research at King Saud University for its funding to the Research Group number (RGP-271).
Footnotes
Peer review under responsibility of King Saud University.
References
- Abd_Allah E.F., Hashem A., Alqarawi A.A., Alwhibi Mona S. Alleviation of adverse impact of salt in Phaseolus vulgaris L. by arbuscular mycorrhizal fungi. Pak. J. Bot. 2015;47(3):1167–1176. https://www.pakbs.org/pjbot/PDFs/47(3)/45.pdf [Google Scholar]
- Abd_Allah E.F., Hashem A., Alqarawi A.A., Bahkali A.H., Alwhibi M.S. Enhancing growth performance and systemic acquired resistance of medicinal plant Sesbania sesban (L.) Merr using arbuscular mycorrhizal fungi under salt stress. Saudi. J. Bio. Sci. 2015;22:274–283. doi: 10.1016/j.sjbs.2015.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abd_Allah E.F., Hashem A., Alqarawi A.A., Wirth S., Egamberdieva D. Calcium application enhances growth and alleviates the damaging effects induced by Cd stress in sesame (Sesamum indicum L.) J. Plant. Interact. 2017;12(1):237–243. [Google Scholar]
- Allakhverdiev S.I., Murata N. Salt stress inhibits photosystems II and I in cyanobacteria. Photosynth. Res. 2008;98:529–539. doi: 10.1007/s11120-008-9334-x. [DOI] [PubMed] [Google Scholar]
- Alqarawi A.A., Hashem A., Abd_Allah E.F., Alshahrani T.S., Huqail A.A. Effect of salinity on moisture content, pigment system, and lipid composition in Ephedra alata Decne. Acta Biol. Hung. 2014;65(1):61–71. doi: 10.1556/ABiol.65.2014.1.6. [DOI] [PubMed] [Google Scholar]
- Alqarawi A.A., Abd_Allah E.F., Hashem A. Alleviation of salt-induced adverse impact via mycorrhizal fungi in Ephedra aphylla Forssk. J. Plant Interact. 2014;9(1):802–810. [Google Scholar]
- Andrio E., Marino D., Marmeys A., de Segonzac M.D., Damiani I. Hydrogen peroxide-regulated genes in the M. truncatula–Sinorhizobium meliloti symbiosis. New Phytol. 2013;198:190–202. doi: 10.1111/nph.12120. [DOI] [PubMed] [Google Scholar]
- Aroca R., Ruiz-Lozano J.M., Zamarreno A., Paz J.A., Garcia-Mina J.M., Pozo M.J., Lopez-Raez J.A. Arbuscular mycorrhizal symbiosis influences strigolactone production under salinity and alleviates salt stress in lettuce plants. J. Plant Physiol. 2013;170:47–55. doi: 10.1016/j.jplph.2012.08.020. [DOI] [PubMed] [Google Scholar]
- Arulbalachandran D., Ganesh K.S., Subramani A. Changes in metabolites and antioxidant enzyme activity of three Vigna Species induced by NaCl stress. Amer. Eur. J. Agron. 2009;2(2):109–116. http://citeseerx.ist.psu.edu/viewdoc/download?doi=10.1.1.600.6767&rep=rep1&type=pdf [Google Scholar]
- Augé R.M. Water relations, drought and VA mycorrhizal symbiosis. Mycorrhiza. 2001;11:3–42. [Google Scholar]
- Balliu A., Sallaku G., Rewald B. AMF Inoculation enhances growth and improves the nutrient uptake rates of transplanted, salt-stressed tomato seedlings. Sustainability. 2015;7:15967–15981. [Google Scholar]
- Bates L.S., Waldren R.P., Teare I.D. Rapid determination of free proline for water stress studied. Plant Soil. 1973;39:205–207. [Google Scholar]
- Bayer W.F., Fridovich J.L. Assaying for superoxide dismutase activity: some large consequences of minor changes in conditions. Anal. Biochem. 1987;161:559–566. doi: 10.1016/0003-2697(87)90489-1. [DOI] [PubMed] [Google Scholar]
- Blaha G., Stelzl U., Spahn C.M.T., Agrawal R.K., Frank J., Nierhaus K.H. Preparation of functional ribosomal complexes and effect of buffer conditions on tRNA positions observed by cryoelectron microscopy. Methods Enzymol. 2000;317:292–309. doi: 10.1016/s0076-6879(00)17021-1. [DOI] [PubMed] [Google Scholar]
- Bray E.A. Plant responses to water deficit. Trends Plant Sci. 1997;2:48–54. [Google Scholar]
- Carlberg, I., Mannervik, B., 1985. Glutathione reductase, Methods in Enzymology, vol. 113, Meister, A., Ed., New York, Academic, pp. 484–490. [DOI] [PubMed]
- Cekic F.O., Unyayar S., Ortas I. Effects of arbuscular mycorrhizal inoculation on biochemical parameters in Capsicum annuum grown under long term salt stress. Turk. J. Bot. 2012;36:63–72. [Google Scholar]
- Chaves M.M., Flexas J., Pinheiro C. Photosynthesis under drought and salt stress: regulation mechanisms from whole plant to cell. Ann. Bot. 2009;103:551–560. doi: 10.1093/aob/mcn125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrath U. Systemic acquired resistance. Plant Sig. Beh. 2006;1(4):179–184. doi: 10.4161/psb.1.4.3221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels B.A., Skipper H.D. Methods for the recovery and quantitative estimation of propagules from soil. In: Schenck N.C., editor. Methods and Principles of Mycorrhizal Research. The American Phytopathological Society; 1982. pp. 29–36. [Google Scholar]
- De-Vleesschauwer D., Xu J., Hofte M. Making sense of hormone-mediated defense networking: From rice to Arabidopsis. Front. Plant Sci. 2014;5:1–15. doi: 10.3389/fpls.2014.00611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durrant W.E., Dong X. Systemic acquired resistance. Annu. Rev. Phytopathol. 2004;42:185–209. doi: 10.1146/annurev.phyto.42.040803.140421. [DOI] [PubMed] [Google Scholar]
- Egamberdieva D., Wirth S.J., Shurigin V.V., Hashem A., Abd_Allah E.F. Endophytic bacteria improve plant growth, symbiotic performance of chickpea (Cicer arietinum L.) and induce suppression of root rot caused by Fusarium solani under salt stress. Front. Microbiol. 2017;8:1887. doi: 10.3389/fmicb.2017.01887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Beltagi H.S., Mohamed H.I. Alleviation of cadmium toxicity in Pisum sativum L. seedlings by calcium chloride. Not. Bot. Horti. Agrobo. 2013;41:157–168. [Google Scholar]
- El-Tayeb M.A. Response of barley grains to the interactive effect of salinity and salicylic acid. Plant Growth Regul. 2005;45:215–224. [Google Scholar]
- Engel R., Szabo K., Abranko L., Rendes K., FuzyA Takacs T. Effect of arbuscular mycorrhizal fungi on the growth and polyphenol profile of marjoram, lemon balm, and marigold. J. Agric. Food Chem. 2016;64:3733–3742. doi: 10.1021/acs.jafc.6b00408. [DOI] [PubMed] [Google Scholar]
- Estrada B., Aroca R., Maathuis F.J.M., Barea J.M., Ruiz-Lozano J.M. Arbuscular mycorrhizal fungi native from a Mediterranean saline area enhance maize tolerance to salinity through improved ion homeostasis. Plant, Cell Environ. 2013;36:1771–1782. doi: 10.1111/pce.12082. [DOI] [PubMed] [Google Scholar]
- Fouad O.M., Essahibi A., Benhiba L., Qaddoury A. Effectiveness of arbuscular mycorrhizal fungi in the protection of olive plants against oxidative stress induced by drought. Spanish J. Agric. Res. 2014;12:763–771. [Google Scholar]
- Foyer C.H., Descourvieres P., Kunert K.J. Protection against oxygen radicals: an important defense mechanism studied in transgenic plants. Plant, Cell Environ. 1994;17:507–523. [Google Scholar]
- Freitas V.S., Alencar N.D.M., Lacerda C.F., Prisco J.Y., Gomes-Filho E. Changes in physiological and biochemical indicators associated with salt tolerance in cotton, sorghum and cowpea. Afri. J. Bio. Res. 2011;5(8):264–271. [Google Scholar]
- Gamalero E., Berta G., Massa N., Glick B.R., Lingua G. Interactions between Pseudomonas putida UW4 and Gigaspora rosea BEG9 and their consequences for the growth of cucumber under salt-stress conditions. J. Appl. Microbiol. 2010;108(1):236–245. doi: 10.1111/j.1365-2672.2009.04414.x. [DOI] [PubMed] [Google Scholar]
- Garcia K., Zimmermann S.D. The role of mycorrhizal associations in plant potassium nutrition. Front. Plant Sci. 2014;5:337. doi: 10.3389/fpls.2014.00337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghorbanli M., Ebrahimzadeh H., Sharifi M. Effects of NaCl and mycorrhizal fungi on antioxidative enzymes in soybean. Bio Plant. 2004;48(4):575–581. [Google Scholar]
- Giovannetti M., Mosse B. An evaluation of techniques for measuring vesicular arbuscular mycorrhizal infection in roots. New Phytol. 1980;84:489–500. [Google Scholar]
- Hajiboland R., Aliasgharzadeh A., Laiegh S.F., Poschenrieder C. Colonization with arbuscular mycorrhizal fungi improves salinity tolerance of tomato (Solanum lycopersicum L.) plants. Plant Soil. 2010;331:313–327. [Google Scholar]
- Hamayun M., Khan S.A., Khan A.L., Shinwari Z.K., Hussain J., Sohn E.Y., Kang S.M., Kim Y.H., Khan M.A., Lee I.J. Effect of salt stress on growth attributes and endogenous growth hormones of soybean cultivar Hwangkeumkong. Pak. J. Bot. 2010;42(5):3103–3112. [Google Scholar]
- Hasanuzzaman, M., Nahar, K., Fujita, M., 2013. Plant response to salt stress and role of exogenous protectants to mitigate salt-induced damages. In: Ahmad, P. et al. (Eds.), Ecophysiology and Responses of Plants under Salt Stress, doi 10.1007/978-1-4614-4747-4_2, © Springer Science+Business Media, LLC 2013.
- Hashem A., Abd_Allah E.F., Alqarawi A.A., El-Didamony G., Alwhibi Mona S., Egamberdieva D., Ahmad P. Alleviation of adverse impact of salinity on faba bean (Vicia faba L.) by arbuscular mycorrhizal fungi. Pak. J. Bot. 2014;46(6):2003–2013. [Google Scholar]
- Hashem A., Abd_Allah E.F., Alqarawi A.A., Aldubise A., Egamberdieva D. Arbuscular mycorrhizal fungi enhances salinity tolerance of Panicum turgidum Forssk by altering photosynthetic and antioxidant pathways. J. Plant Interact. 2015;10(1):230–242. [Google Scholar]
- Hashem A., Abd_Allah E.F., Alqarawi A.A., Al-Huqail A.A., Wirth S., Egamberdieva D. The interaction between arbuscular mycorrhizal fungi and endophytic bacteria enhances plant growth of Acacia gerrardii under salt stress. Front. Microbiol. 2016;7:1089. doi: 10.3389/fmicb.2016.01089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashem A., Abd_Allah E.F., Alqarawi A.A., Al-Huqail A.A., Egamberdieva D., Wirth S. Alleviation of cadmium stress in Solanum lycopersicum L. by arbuscular mycorrhizal fungi via induction of acquired systemic tolerance. Saudi J. Biol. Sci. 2016;23:272–281. doi: 10.1016/j.sjbs.2015.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He F., Sheng M., Tang M. Effects of Rhizophagus irregularis on photosynthesis and antioxidative enzymatic system in Robinia pseudoacacia L. under drought stress. Front. Plant Sci. 2017;8:183. doi: 10.3389/fpls.2017.00183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath R.L., Packer L. Photoperoxidation in isolated chloroplasts. I. Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys. 1968;125:189–198. doi: 10.1016/0003-9861(68)90654-1. [DOI] [PubMed] [Google Scholar]
- Hiscox J.D., Israelstam G.F. A method for the extraction of chlorophyll from leaf tissue without maceration. Can. J. Bot. 1979;57:1332–1334. [Google Scholar]
- Hoagland D.R., Arnon D.I. The water-culture method for growing plants without soil. California Agric. Exp. Station Circ. 347:1–32. Huang, C., He, W., Guo, J., Chang, X., Su, P., Zhang, L., 2005. Increased sensitivity to salt stress in ascorbate-deficient arabidopsis mutant. J. Exp. Bot. 1950;56:3041–3049. doi: 10.1093/jxb/eri301. [DOI] [PubMed] [Google Scholar]
- Hosseini S.A., Maillard A., Hajirezaei M.R., Ali N., Schwarzenberg A., Jamois F., Yvin J.C. Induction of barley silicon transporter HvLsi1 and HvLsi2, increased silicon concentration in the shoot and regulated starch and ABA homeostasis under osmotic stress and concomitant potassium deficiency. Front. Plant Sci. 2017;8:1359. doi: 10.3389/fpls.2017.01359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang D., Ou B., Prior R.L. The chemistry behind antioxidant capacity assays. J. Agric. Food Chem. 2005;53:1841–1856. doi: 10.1021/jf030723c. [DOI] [PubMed] [Google Scholar]
- Huang Z., Zhao L., Chen D., Liang M., Liu Z., Shao H., Long X. Salt stress encourages proline accumulation by regulating proline biosynthesis and degradation in Jerusalem artichoke plantlets. PloS ONE. 2013;8:e62085. doi: 10.1371/journal.pone.0062085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y.M., Zou Y.N., Wu Q.S. Alleviation of drought stress by mycorrhizas is related to increased root H2O2 efflux in trifoliate orange. Sci. Rep. 2017;7:42335. doi: 10.1038/srep42335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- INVAM, 2014. International Culture Collection of (Vesicular) Arbuscular Mycorrhizal Fungi. West Virginia University, Morgantown, West Virginia. URL: http://invam.wvu.edu/the-fungi/species-descriptions (accessed March 26).
- Iqbal N., Umar S., Khan N.A. Nitrogen availability regulates proline and ethylene production and alleviates salinity stress in mustard (Brassica juncea) J. Plant Physiol. 2015;178:84–91. doi: 10.1016/j.jplph.2015.02.006. [DOI] [PubMed] [Google Scholar]
- Jarstfer A.G., Farmer-Koppenol P., Sylvia D.M. Tissue magnesium and calcium affect mycorrhiza development and fungal reproduction. Mycorrhiza. 1998;7:237–242. doi: 10.1007/s005720050186. [DOI] [PubMed] [Google Scholar]
- Kamboj J.S., Blake P.S., Quinlan J.D., Baker D.A. Identification and quantitation by GC-MS of zeatin and zeatinriboside in xylem sap from rootstock and scion of grafted apple trees. Plant Growth Reg. 1999;28:199–205. [Google Scholar]
- Khan M.I.R., Asgher M., Khan N.A. Alleviation of salt-induced photosynthesis and growth inhibition by salicylic acid involves glycine betaine and ethylene in mungbean (Vigna radiata L) Plant Physiol. Biochem. 2014;80:67–74. doi: 10.1016/j.plaphy.2014.03.026. [DOI] [PubMed] [Google Scholar]
- Kirnak H. Effects of irrigation water salinity on yield and evapotranspiration of drip irrigated cucumber in a semiarid environment. In: Ozturk M., Waisel Y., Khan M.A., Görk G., editors. Biosaline Agriculture and Salinity Tolerance in Plants. Birkhäuser Basel; 2006. pp. 155–162. [Google Scholar]
- Kohler J., Hernandez J.A., Caravaca F., Roldan A. Induction of antioxidant enzymes is involved in the greater effectiveness of a PGPR versus AM fungi with respect to increasing the tolerance of lettuce to severe salt stress. Environ. Exp. Bot. 2009;65:245–252. [Google Scholar]
- Koske R.E., Gemma J.N. A modified procedure for staining roots to detect VA mycorrhizas. Mycol. Res. 1989;92:486–488. [Google Scholar]
- Kramell R., Miersch O., Schneider G., Wasternack C. Liquid chromatography of jasmonic acid amine conjugates. Chromatographia. 1999;49:42–46. [Google Scholar]
- Kusaba S., Kano-MurakamiY Matsuoka M, Tamaoki T., Sakamoto I., Yamaguchi I., Fukumoto M. Alteration of hormone levels in transgenic tobacco plants over expressing a rice homeobox gene OSH1. Plant Physiol. 1998;116:471–476. doi: 10.1104/pp.116.2.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowry O.H., Rosebrough N.S., Farrand A.L., Randall R.J. Protein measurement with folin phenol reagent. J. Bio. Chem. 1951;193:263–275. [PubMed] [Google Scholar]
- Lu C., Vonshak A. Effects of salinity stress on photosystem II function in cyanobacterial Spirulina platensis cells. Physiol. Plant. 2002;114:405–413. doi: 10.1034/j.1399-3054.2002.1140310.x. [DOI] [PubMed] [Google Scholar]
- Luck, H., 1974. Catalases, Methods of Enzymatic Analysis, vol. 2, Bregmeyer, H.U., (Ed.), New York, Academic.
- Mansour M.M.F., Ali E.F. Evaluation of proline functions in saline conditions. Phytochem. 2017;140:52–68. doi: 10.1016/j.phytochem.2017.04.016. [DOI] [PubMed] [Google Scholar]
- Mo Y., Wang Y., Yang R., Zheng J., Liu C., Li H., Ma J., Zhang Y., Wei C., Zhang X. Regulation of plant prowth, photosynthesis, antioxidation and osmosis by an arbuscular mycorrhizal fungus in watermelon seedlings under well-watered and drought conditions. Front. Plant Sci. 2016;7:644. doi: 10.3389/fpls.2016.00644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano Y., Asada K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach-chloroplasts. Plant Cell Physiol. 1981;22:867–880. [Google Scholar]
- Nath M., Bhatt D., Prasad R., Gill S.S., Anjum N.A., Tuteja T. Reactive oxygen species generation-scavenging and signaling during plant-arbuscular mycorrhizal and Piriformospora indica interaction under stress condition. Front. Plant Sci. 2016;7:1574. doi: 10.3389/fpls.2016.01574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro J.M., Perez-Tornero O., Morte A. Alleviation of salt stress in citrus seedlings inoculated with arbuscularmycorrhizal fungi depends on the rootstock salt tolerance. J. Plant Physiol. 2014;171(1):76–85. doi: 10.1016/j.jplph.2013.06.006. [DOI] [PubMed] [Google Scholar]
- Neelam S., Subramanyam R. Alteration of photochemistry and protein degradation of photosystem II from Chlamydomonas reinhardtii under high salt grown cells. J. Photochem. Photobiol., B. 2013;124:63–70. doi: 10.1016/j.jphotobiol.2013.04.007. [DOI] [PubMed] [Google Scholar]
- Parre E., Ghars M.A., Leprince A.S., Thiery L., Lefebvre D., Bordenave M., Richard L., Mazars C., Abdelly C., Savoure A. Calcium signaling via phospholipase C is essential for proline accumulation upon ionic but not nonionic hyperosmotic stresses in Arabidopsis. Plant Physiol. 2007;144:503–512. doi: 10.1104/pp.106.095281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellegrini E., Trivellini A., Campanella A., Francini A., Lorenzini G., Nali C., Vernieri P. Signaling molecules and cell death in Melissa officinalis plants exposed to ozone. Plant Cell Rep. 2013;32:1965–1980. doi: 10.1007/s00299-013-1508-0. [DOI] [PubMed] [Google Scholar]
- Phillips J.M., Hayman D.S. Improved procedures for clearing roots and staining parasitic and vesicular-arbuscularmycorrhizal fungi for rapid assessment of infection. Trans. Brit. Mycol. Soc. 1970;55:158–161. [Google Scholar]
- Porcel R., Redondo-Gómez S., Mateos-Naranjo E., Aroca R., Garcia R., Ruiz-Lozano J.M. Arbuscular mycorrhizal symbiosis ameliorates the optimum quantum yield of photosystem II and reduces non-photochemical quenching in rice plants subjected to salt stress. J. Plant Physiol. 2015;185:75–83. doi: 10.1016/j.jplph.2015.07.006. [DOI] [PubMed] [Google Scholar]
- Puppo A., Pauly N., Boscari A., Mandon K., Brouquisse R. Hydrogen peroxide and nitric oxide: key regulators of the legume Rhizobium and mycorrhizal symbioses. Antioxid. Redox Signal. 2013;18:2202–2219. doi: 10.1089/ars.2012.5136. [DOI] [PubMed] [Google Scholar]
- Qun H.Z., Xing H.C., Bin Z.Z., Rong Z.Z., Song W.H. Changes of antioxidative enzymes and cell membrane osmosis in tomato colonized by arbuscular mycorrhizae under NaCl stress. Coll. Surf. B: Bioint. 2007;59:128–133. doi: 10.1016/j.colsurfb.2007.04.023. [DOI] [PubMed] [Google Scholar]
- Ruiz-Lozano J.M., Porcel R., Azcon R., Aroca R. Regulation by arbuscularmycorrhizae of the integrated physiological response to salinity in plants. New challenges in physiological and molecular studies. J. Exp. Bot. 2012;63:4033–4044. doi: 10.1093/jxb/ers126. [DOI] [PubMed] [Google Scholar]
- Sade N., Gebretsadik M., Seligmann R., Schwartz A., Wallach R., Moshelion M. The role of tobacco aquaporin1 in improving water use efficiency, hydraulic conductivity, and yield production under salt stress. Plant Physiol. 2010;152:245–254. doi: 10.1104/pp.109.145854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sairam R.K., Deshmukh P.S., Shukla D.S. Tolerance of drought and temperature stress in relation to increased antioxidant enzyme activity in wheat. J. Agron. Crop Sci. 1997;178:171–178. [Google Scholar]
- Sánchez-Rodríguez E., Rubio-Wilhelmi M., Cervilla L.M., Blasco B., Rios J., Rosales M.A., Romero L., Ruiz J.M. Genotypic differences in some physiological parameters symptomatic for oxidative stress under moderate drought in tomato plants. Plant Sci. 2010;178:30–40. [Google Scholar]
- Saqib M., Zoerb C., Schubert S. Silicon-mediated improvement in the salt resistance of wheat (Triticum aestivum) results from increased sodium exclusion and resistance to oxidative stress. Funct. Plant Biol. 2008;35:633–639. doi: 10.1071/FP08100. [DOI] [PubMed] [Google Scholar]
- Shekoofeh E., Sepideh H., Roya R. Role of mycorrhizal fungi and salicylic acid in salinity tolerance of Ocimum basilicum resistance to salinity. J. Biotech. 2012;11(9):2223–2235. [Google Scholar]
- Sheng M., Tang M., Chan H., Yang B., Zhang F., Huang Y. Influence of arbuscular mycorrhizae on photosynthesis and water status of maize plants under salt stress. Mycorrhiza. 2008;18:287–296. doi: 10.1007/s00572-008-0180-7. [DOI] [PubMed] [Google Scholar]
- Siegrist J., Orober M., Buchenauer H. β-aminobutyric acid mediated enhancement of resistance in tobacco to tobacco mosaic virus depends on the accumulation of salicylic acid. Physiol. Mol. Plant Pathol. 2000;56:95–106. DOI: 10.1006}pmpp.1999.0255. [Google Scholar]
- Slinkard K., Singleton V.L. Total phenol analyses: automation and comparison with manual methods. Am. J. Enol. Viticult. 1977;28:49–55. [Google Scholar]
- Smart R.E., Bihgham G.E. Rapid estimates of relative water content. Plant Physiol. 1974;53:258–260. doi: 10.1104/pp.53.2.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Y., Chen D., Lu K., Sun Z., Zeng R. Enhanced tomato disease resistance primed by arbuscular mycorrhizal fungus. Front. Plant Sci. 2015;6:786. doi: 10.3389/fpls.2015.00786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudhir P.R., Pogoryelov D., Kovács L., Garab G., Murthy S.D.S. The effects of salt stress on photosynthetic electron transport and thylakoid membrane proteins in the cyanobacterium Spirulina platensis. J. Biochem. Mol. Biol. 2005;38:481–485. doi: 10.5483/bmbrep.2005.38.4.481. [DOI] [PubMed] [Google Scholar]
- Talukdar D. Exogenous calcium alleviates the impact of cadmium-induced oxidative stress in Lens culinaris Medic. seedlings through modulation of antioxidant enzyme activities. J. Crop Sci. Biotechnol. 2012;15:325–334. [Google Scholar]
- Tang M., Chen H., Huang J.C., Tian Z.Q. AM fungi effects on the growth and physiology of Zea mays seedlings under diesel stress. Soil Biol. Biochem. 2009;41:936–940. [Google Scholar]
- Tejera N.A., Campos R., Sanjuan J., Lluch C. Nitrogenase and antioxidant enzyme activities in Phaseolus vulgaris nodules formed by Rhizobium tropici isogenic strains with varying tolerance to salt stress. J. Plant Physiol. 2004;161:329–338. doi: 10.1078/0176-1617-01050. [DOI] [PubMed] [Google Scholar]
- Upadhyay S., Dixit M. Role of polyphenols and other phytochemicals on molecular signaling. Oxid. Med. Cell Longev. 2015;2015(504253):15. doi: 10.1155/2015/504253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utobo E.B., Ogbodo E.N., Nwogbaga A.C. Techniques for extraction and quantification of arbuscularr mycorrhizal fungi. Libyan Agric. Res. Center Int. 2011;2:68–78. [Google Scholar]
- Velarde-Buendía A.M., Shabala S., Cvikrova M., Dobrovinskaya O., Pottosin I. Salt-sensitive and salt-tolerant barley varieties differ in the extent of potentiation of the ROS-induced K+ efflux by polyamines. Plant Physiol. Biochem. 2012;61:18–23. doi: 10.1016/j.plaphy.2012.09.002. [DOI] [PubMed] [Google Scholar]
- Velikova V., Yordanov I., Edreva A. Oxidative stress and some antioxidant systems in acid rain treated bean plants, protective role of exogenous polyamines. Plant Sci. 2000;151:59–66. [Google Scholar]
- Wang C., Li X., Zhou J., Wang G., Dong Y. Effects of arbuscularmycorrhizal fungi on growth and yield of cucumber plants. Comm. Soil Sci. Plant Anal. 2008;39(3–4):499–509. [Google Scholar]
- Waqas M., Khan A.L., Kamran M., Hamayun M., Kang S.M., Kim Y.H., Lee I.J. Endophytic fungi produce gibberellins and indoleacetic acid and promotes host-plant growth during stress. Mol. 2012;17:10754–10773. doi: 10.3390/molecules170910754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waqas M., Khan M.L., Lee I.J. Bioactive chemical constituents produced by endophytes and effects on rice plant growth. J. Plant Interact. 2014;9(1):478–487. [Google Scholar]
- Wasilewska A., Vlad F., Sirichandra C., Redko Y., Jammes F., Valon C., Freidit Frey N., Leung J. An update on abscisic acid signaling in plants and more. Mol Plant. 2008;1:198–217. doi: 10.1093/mp/ssm022. [DOI] [PubMed] [Google Scholar]
- Wasternack C., Hause B. Jasmonates: Biosynthesis, perception, signal transduction and action in plant stress response, growth and development. Ann. Bot. 2013;111(6):1021–1058. doi: 10.1093/aob/mct067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf B. A comprehensive system of leaf analysis and its use for diagnosing crop nutrient status. Comm. Soil Sci. Plant Anal. 1982;13:1035–1059. [Google Scholar]
- Wu N., Li Z., Wu F., Tang M. Comparative photochemistry activity and antioxidant responses in male and female Populuscathayanacuttings inoculated with arbuscularmycorrhizal fungi under salt. SciRep. 2016;6:37663. doi: 10.1038/srep37663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong L., Schumaker K.S., Zhu J. Cell signaling during cold, drought and salt stresses. Plant Cell. 2002;14:163–183. doi: 10.1105/tpc.000596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y., Han X., Liang Y., Ghosh A., Chen J., Tang M. The Combined Effects of Arbuscular Mycorrhizal fungi (AMF) and lead (Pb) stress on Pb accumulation, plant growth parameters, photosynthesis, and antioxidant enzymes in Robinia pseudoacacia L. PLoS One. 2015;10(12):e0145726. doi: 10.1371/journal.pone.0145726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yooyongwech S., Phaukinsang N., Cha-Um S., Supaibulwatana K. Arbuscular mycorrhiza improved growth performance in Macadamia tetraphylla L. Grown under water deficit stress involves soluble sugar and proline accumulation. Plant Growth Regul. 2013;69:285–293. [Google Scholar]
- Yousuf P.Y., Ahmad A., Hemant Ganie A.H., Aref I.M., Iqbal M. Potassium and calcium application ameliorates growth and oxidative homeostasis in salt-stressed Indian mustard (Brassica juncea) Plants. Pak. J. Bot. 2015;47:1629–1639. [Google Scholar]
- Yuan Z.L., Zhang C.L., Lin F.C. Role of diverse non-systemic fungal endophytes in plant performance and response to stress: progress and approaches. J. Plant Growth Regul. 2010;29:116–126. [Google Scholar]