Abstract
The M5 muscarinic receptor is the most recent member of the muscarinic acetylcholine receptor family (M1-M5) to be cloned. At present, the physiological relevance of this receptor subtype remains unknown, primarily because of its low expression levels and the lack of M5 receptor-selective ligands. To circumvent these difficulties, we used gene targeting technology to generate M5 receptor-deficient mice (M5R−/− mice). M5R−/− mice did not differ from their wild-type littermates in various behavioral and pharmacologic tests. However, in vitro neurotransmitter release experiments showed that M5 receptors play a role in facilitating muscarinic agonist-induced dopamine release in the striatum. Because M5 receptor mRNA has been detected in several blood vessels, we also investigated whether the lack of M5 receptors led to changes in vascular tone by using several in vivo and in vitro vascular preparations. Strikingly, acetylcholine, a powerful dilator of most vascular beds, virtually lost the ability to dilate cerebral arteries and arterioles in M5R−/− mice. This effect was specific for cerebral blood vessels, because acetylcholine-mediated dilation of extra-cerebral arteries remained fully intact in M5R−/− mice. Our findings provide direct evidence that M5 muscarinic receptors are physiologically relevant. Because it has been suggested that impaired cholinergic dilation of cerebral blood vessels may play a role in the pathophysiology of Alzheimer's disease and focal cerebral ischemia, cerebrovascular M5 receptors may represent an attractive therapeutic target.
Molecular cloning studies have revealed the existence of five molecularly distinct muscarinic acetylcholine receptor subtypes (M1-M5) (1, 2). During the past decade, considerable progress has been made in delineating the physiological roles of the M1-M4 muscarinic receptors (3, 4). In contrast, the physiological relevance of the M5 receptor subtype, which is the most recent member of the muscarinic receptor family to be cloned (5, 6), remains unknown at present (7, 8). However, expression of the cloned M5 muscarinic receptor gene in cultured mammalian cells has shown that the encoded receptor protein is functional and efficiently couples to G proteins of the Gq family, similar to the M1 and M3 receptor subtypes (5–8).
Immunoprecipitation and in situ mRNA hybridization studies have demonstrated the presence of M5 receptor protein/mRNA in different areas of the brain including hippocampus, hypothalamus, and distinct midbrain regions (substantia nigra pars compacta and ventral tegmental area) (9–11). However, M5 receptors are expressed at very low levels, representing less than 2% of the total muscarinic receptor population (M1-M5) expressed in the brain (9). Interestingly, the use of highly sensitive reverse transcriptase–PCR (RT-PCR) techniques suggests that M5 receptors are expressed in all major brain regions (12). More recently, M5 receptors also have been detected in several peripheral tissues or cells including peripheral blood lymphocytes (13, 14), skin fibroblasts (15), smooth muscle of the iris sphincter (16) and the esophagus (17), and parotid gland tissue (18), where they are colocalized with several other muscarinic receptor subtypes (13–18).
Given the lack of M5 muscarinic receptor-selective ligands (2, 8), we decided to use gene targeting technology to generate M5 receptor-deficient mice (M5R−/− mice) to gain insight into the potential physiological roles of the M5 receptor. M5 receptor mRNA has been identified as the only muscarinic receptor mRNA in dopamine-containing neurons of the substantia nigra pars compacta (10, 11), and activation of striatal muscarinic receptors is known to facilitate the release of dopamine in the striatum (19, 20). It has therefore been proposed (10, 11) that striatal M5 receptors may play a role in the control of locomotor activity and coordination by regulating the release of striatal dopamine. Thus one major focus of the present study was to investigate whether the lack of M5 receptors was associated with changes in locomotor behavior and striatal dopamine release.
Interestingly, RT-PCR experiments recently showed that M5 receptor mRNA is also present in several vascular tissues (21, 22). Acetylcholine is known to be a powerful dilator of most blood vessels including cerebral arteries and microvessels (23–28). The cerebral circulation (both large arteries and microvessels) receives cholinergic innervation from extrinsic and intrinsic sources (29–33), and neuronally released acetylcholine is thought to play a role in the regulation of cerebral vascular resistance and regional blood flow (34–38). Moreover, impaired cholinergic dilation of cerebral blood vessels has been implicated in the pathophysiology of Alzheimer's disease (29, 34, 39) and focal cerebral ischemia (35, 40). Thus, another focus of the present study was to investigate whether the lack of M5 receptors affected acetylcholine-mediated vasodilation, using several in vivo and in vitro cerebral and noncerebral vascular preparations as model systems.
Materials and Methods
Generation of M5R−/− Mice.
A murine M5 muscarinic receptor clone was isolated from a 129SvJ mouse genomic library (Genome Systems, St. Louis) by hybridization with a PCR fragment coding for the central portion of the third intracellular loop of the mouse M5 receptor. The targeting vector contained two copies of the herpes simplex virus thymidine kinase gene (TK) and a phosphoglycerate kinase-neomycin resistance cassette (neo), which replaced an 0.8-kb MscI–BglII genomic fragment coding for the first 250 aa of the mouse M5 receptor including the translation start site (Fig. 1A). After linearization with NotI, the targeting vector was introduced into TC1(129SvEv) embryonic stem cells by electroporation (41). Clones resistant to G418 and gancyclovir were tested for the occurrence of homologous recombination via Southern hybridization. Properly targeted embryonic stem cell clones were microinjected into C57BL/6J blastocysts to generate male chimeric offspring, which in turn were mated with female CF-1 mice (Charles River Breeding Laboratories) to generate F1 offspring. F1 animals heterozygous for the M5 receptor mutation were then intermated to produce homozygous M5 receptor mutant mice (F2). All experiments were carried out with littermates of the F2 or F3 generation (CF-1 × 129SvEv hybrids). Similar functional responses were obtained with F2 and F3 mice.
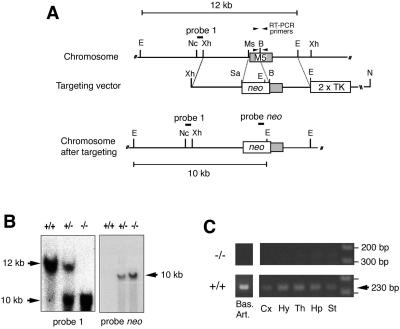
Figure 1.
Targeted disruption of the mouse M5 muscarinic receptor gene. (A) Schematic diagrams and partial restriction maps of wild-type receptor locus, targeting vector, and targeted allele. The M5 receptor coding region is indicated by a shaded box. The probes used for Southern analyses and the sizes of the restriction fragments detected with these probes are indicated. B, BglII; E, EcoRV; Ms, MscI; Nc, NcoI; N, NotI; Sa, SalI; Xh, XhoI. (B) Southern blot analysis of EcoRV-digested mouse tail DNA prepared from F2 pups generated by intermating of F1 heterozygotes. The 12- and 10-kb bands represent the wild-type and mutant M5 receptor alleles, respectively. (C) RT-PCR analysis demonstrating the absence of wild-type M5 receptor transcripts in tissues from M5R−/− mice. Total RNA (≈1 μg) prepared from the indicated brain tissues (Right) and basilar artery (Left) was subjected to RT-PCR analysis using the primers indicated in A. Note that the target sequence for the forward primer is absent after proper targeting of the M5 receptor locus. No bands were observed in control samples in which RT had been omitted (data not shown). The positions of the 200- and 300-bp marker bands are indicated. Bas. Art., basilar artery; Cx, cerebral cortex; Hy, hypothalamus; Th, thalamus; Hp, hippocampus; St, striatum.
RT-PCR.
Total RNA was extracted from tissues derived from wild-type and M5R−/− mice using Trizol reagent (GIBCO). After DNase treatment (2 units⋅μl−1; Ambion), total RNA (≈1 μg) was reverse-transcribed by using oligo(dT)16 primers and murine leukemia virus RT (Perkin–Elmer), followed by PCR amplification of a 230-bp DNA segment specific for the wild-type M5 receptor gene (PCR conditions: 95°C for 2 min; 35 cycles of 95°C for 15 sec and 60°C for 30 sec; 72°C for 7 min). The following primer pair was used: f5′-GTCTCCGTCATGACCATACTCTA-3′ [nucleotides 616–638 (coding sequence)] and r5′-CCCGTTGTTGAGGTGCTTCTAC-3′ (nucleotides 824–845).
Pharmacologic and Behavioral Studies.
All studies were carried out with adult mice (males) that were at least 3 months old. Oxotremorine-induced tremor, hypothermia, and salivation responses were quantitated as described (41, 42). To assess spontaneous locomotor activity, mice were placed in polypropylene cages [24 × 45 × 15 cm (height)], and the number of photocell beam interruptions were counted for 60 min to monitor horizontal activity by using a Photobeam Activity System (San Diego Instruments) as described (41). In amphetamine and apomorphine injection experiments, mice were placed into the locomotion boxes for a 30-min acclimatization period, followed by the s.c. administration of drugs and immediate monitoring of locomotor activity for the next 60 min (41). To assess locomotor coordination, mice were tested for their ability to stay on an accelerating rotor-rod (San Diego Instruments). Mice received four 2-min trials separated by a 2-min intertrial interval. Two separate experiments were conducted. In experiment 1, the speed of the rotor-rod accelerated over the 2-min trial with the rate of acceleration increasing from 0 to 25 rpm on trials 1 and 2. On trials 3 and 4, the rotor-rod reached a maximum of 50 rpm by the end of the 2-min trial. In experiment 2, the rotor-rod accelerated to 50 rpm in 2 min in trials 1 and 2. In trials 3 and 4, the 50-rpm rate was reached by the end of the first minute.
Dopamine Release Studies.
Striatal slices prepared from male mice were dispersed in oxygenated (95% O2, 5% CO2) Krebs-Ringer buffer (11.5 mM glucose/25 mM NaHCO3/1.2 mM MgCl2/1.2 mM NaH2PO4/118 mM NaCl/4.8 mM KCl/2.5 mM CaCl2/0.004 mM Na2EDTA, pH 7.4) and incubated with 0.2 μM [3H]dopamine (48.2 Ci mmol−1; NEN Life Sciences) for 30 min in presence of the antioxidant, ascorbate (5 mM); the monoamine oxidase inhibitor, pargyline (10 μM); the 5-hydroxytryptamine uptake inhibitor, citalopram (1 μM); and the norepinephrine uptake blocker, desipramine (5 μM). Slices were then transferred to a superfusion system (SF-12, Brandel, Bethesda, MD) and superfused at 33°C at a constant rate of 0.4 ml⋅min−1. Fractions were collected every 4 min beginning after a 60-min superfusion. Two 2-min periods of 20 mM KCl were applied after 72 (S1) and 104 (S2) min of superfusion. Drugs were added to the superfusion buffer 20 min before S2. The efflux of tritium collected was calculated as a percentage of the total tritium present in the slices at the start of the fraction considered. The results were expressed as % increase in [3H]dopamine release above control by using the following equation: {(S2/S1 [drug]) − (S2/S1 [no drug])}/(S2/S1 [no drug]) × 100.
Vascular Preparations.
Actylcholine-dependent vasodilation responses were studied in four different blood vessel preparations derived from wild-type and M5R−/− mice (females).
Cerebral arterioles (in vivo).
Mice were anesthetized with pentobarbital sodium (75–90 mg/kg i.p.), supplemented regularly at ≈20 mg/kg per h. Animals were ventilated mechanically, and arterial blood pressure and blood gasses were monitored as described (27). A cranial window was made over the left parietal cortex, and a segment of a pial arteriole was exposed (27). The diameter of cerebral arterioles was measured by using a microscope equipped with a television camera coupled to a video monitor and an image shearing device (27). The diameter of one arteriole per animal was measured under control conditions and during topical application of drugs (cumulative administration; ref. 27).
Basilar and coronary artery (in vitro).
After anesthesia (pentobarbital, 100 mg/kg i.p.), the brain and heart were rapidly removed and placed in ice-cold Krebs buffer of the following composition: 118.3 mM NaCl, 4.7 mM KCl, 2.5 mM CaCl2, 1.2 mM MgSO4, 1.2 mM KH2PO4, 25 mM NaHCO3, and 11.1 mM glucose. The basilar and the left anterior descending or right coronary arteries were isolated by using a dissecting microscope, cannulated onto glass micropipettes (30–80 μm in diameter) filled with Krebs buffer in an organ chamber, and secured with nylon monofilament suture as described (43). Arteries were pressurized to 40 (coronary artery) or 60 (basilar artery) mm Hg. Using a microscope and a video camera, the vessel image was projected on a video monitor. An electronic dimension analyzer was used to measure lumen diameter. Isolated artery segments were submaximally contracted (50–60% of maximal KCl responses) with the thromboxane mimetic, U-46619 (0.05–0.1 μg/ml; Biomol, Plymouth Meeting, PA), to minimize possible variability in the degree of intrinsic vascular tone. After development of stable contractions, cumulative acetylcholine dose–response curves were obtained as described (43).
Carotid artery (in vitro).
Mice were anesthetized with pentobarbital (100 mg/kg i.p), and both carotid arteries were quickly removed and placed in Krebs buffer. Loose connective tissue was removed, and each artery was cut into two rings. Vascular rings were suspended in an organ bath containing Krebs solution maintained at 37°C. The rings were connected to a force transducer to measure isometric tension (contraction). Resting tension was increased stepwise to reach the final tension of 0.25 g (44). After submaximal preconstriction of the vascular preparations with U-46619 (0.4 μg/ml), relaxation responses to acetylcholine (10−9–10−5 M) were measured by using a cumulative concentration-response protocol, as described (44). Acetylcholine responses were expressed as percent decrease in tension from preconstriction values.
Results
Generation of M5R−/− Mice.
The M5 muscarinic receptor gene was inactivated in mouse TC1 (129SvEv) embryonic stem cells, by replacing a segment of the receptor coding sequence (encoding the first 250 aa of the M5 receptor including the first five transmembrane domains) with a neomycin-resistance cassette (Fig. 1A). By using standard transgenic and mouse breeding techniques (41, 42), we initially obtained chimeric mice that were used to generate F1 offspring heterozygous for the M5 receptor mutation. F1 mice were then intercrossed to obtain wild-type (M5R+/+) as well as heterozygous and homozygous (M5R−/−) M5 receptor mutant mice, as determined by Southern blotting analysis (Fig. 1B).
M5R−/− mice were obtained with the expected Mendelian frequency and did not differ from their wild-type littermates in body weight, overall health, fertility, and longevity. Also, M5R−/− mice showed no obvious morphological or behavioral abnormalities. Immunoprecipitation studies with receptor-subtype selective antisera demonstrated that inactivation of the M5 receptor gene had no significant effect on the expression levels of the M1-M4 muscarinic receptors in mouse brain (data not shown). In agreement with a previous report (12), RT-PCR studies showed that M5 receptor mRNA is present in all major brain regions in wild-type mice (Fig. 1C). As expected, no PCR product was obtained with RNA derived from tissues from M5R−/− mice, confirming the lack of intact M5 receptor transcripts in M5R−/− mice (Fig. 1C).
Tremor, Salivation, and Body Temperature Responses.
Administration of centrally active muscarinic agonists results in several striking behavioral and physiological responses, including whole body tremor, hypothermia, and profound salivation (41, 42). To investigate whether M5 receptors are involved in mediating these effects, M5R−/− mice and their wild-type littermates were injected with increasing doses of the centrally active, nonselective muscarinic agonist, oxotremorine (0.03, 0.1, and 0.3 mg/kg s.c.; n = 5 per dose and genotype). This analysis showed that oxotremorine-induced tremor, salivation, and hypothermia responses were not significantly affected by the lack of M5 receptors (data not shown).
Muscarinic Agonist-Induced Dopamine Release in Striatal Slices.
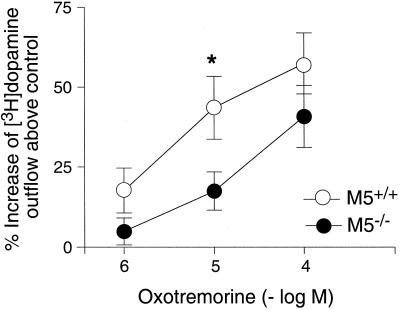
M5 receptor mRNA is expressed by dopamine-containing neurons of the substantia nigra pars compacta (10, 11), and low levels of M5 receptor protein have been detected in the striatum (9). To test the hypothesis that M5 receptors located on the terminals of nigrostriatal dopaminergic projection neurons may regulate dopamine release in the striatum, we carried out a series of in vitro dopamine release experiments using striatal slice preparations prelabeled with [3H]dopamine. Basal [3H]dopamine outflow did not differ between wild-type and M5R−/− mice (data not shown). In agreement with previous findings (19, 20), incubation of striatal slices prepared from wild-type mice with increasing concentrations of oxotremorine resulted in concentration-dependent increases in [3H]dopamine release (Fig. 2). This increase in [3H]dopamine output was completely abolished in the presence of atropine (10 μM; data not shown), indicating that it is mediated by stimulation of striatal muscarinic receptors. Although the magnitude of this response was not significantly affected by the lack of M5 receptors at the highest oxotremorine concentration used (100 μM), a >50% reduction (P < 0.05) in [3H]dopamine output was observed after incubation with 10 μM oxotremorine (Fig. 2). These data clearly indicate that M5 receptors play a role in facilitating dopamine release in the striatum.
Figure 2.
Muscarinic agonist-mediated increase in [3H]dopamine release in striatal slices from M5R−/− mice and their wild-type littermates. Striatal slices that had been preincubated with [3H]dopamine were depolarized with 20 mM KCl, and the resulting [3H]dopamine outflow was quantitated in the absence and the presence of the indicated concentrations of oxotremorine. Data are expressed as percent increase in [3H]dopamine release above control levels (no oxotremorine). Basal [3H]dopamine outflow did not differ between wild-type and M5R−/− mice. Each data point represents the mean ± SEM of 9–11 independent experiments (mice). *, P < 0.05 (vs. wild type; Student's t test).
Behavioral Studies Examining Locomotor Activity and Coordination.
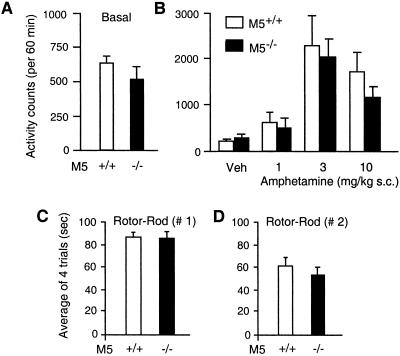
Because muscarinic and dopamine receptors interact in the striatum to control normal locomotor behavior (45–47), we next examined whether locomotor activity and coordination were changed in the M5R−/− mice. As shown in Fig. 3A, M5R−/− mice showed normal levels of spontaneous locomotor activity in an “open-field” test. Similarly, administration of the indirect dopamine receptor agonist, amphetamine (1, 3, and 10 mg/kg s.c.), resulted in comparable stimulatory locomotor responses in wild-type and M5R−/− mice (Fig. 3B). Similar results were obtained after administration of the direct dopamine receptor agonist, apomorphine (0.3, 1, 2, and 4 mg/kg s.c.; n = 10 per dose and genotype; data not shown). Likewise, M5R−/− mice performed equally well as their wild-type littermates on an accelerating rotor-rod (Fig. 3 C and D), a test widely used to assess proper locomotor coordination. Taken together, these findings suggest that M5 receptors do not play a major role in the striatal circuitry controlling locomotor activity and coordination.
Figure 3.
Locomotor activity and coordination of M5R−/− mice and their wild-type littermates. (A) Basal locomotor activity. Data are expressed as total number of photobeam breaks during a 60-min observation period (n = 10 per genotype). (B) Amphetamine-induced locomotor stimulation. Mice of the indicated genotypes were injected s.c. with vehicle (Veh) or increasing doses of amphetamine (n = 10 per dose and genotype). Locomotor activity measurements were carried out as described in Materials and Methods. Data are expressed as number of total photobeam breaks during a 60-min observation period. (C and D) Locomotor coordination determined in rotor-rod experiments. Mice were tested for their ability to stay on an accelerating rotor-rod, as described in Materials and Methods. Animals received four 2-min trials separated by a 2-min intertrial interval. Two types of experiments (C and D) were conducted that differed primarily in the rate of acceleration of the rotor-rod (experiment 2 was more stringent; for experimental details, see Materials and Methods; experiment 1, n = 16; experiment 2, n = 10). Data are given as means ± SEM.
Acetylcholine-Mediated Dilation of Cerebral Arterioles and Arteries.
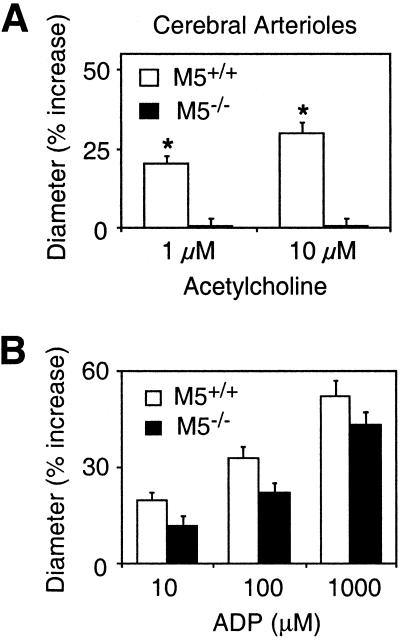
M5 receptor mRNA has been identified in various peripheral and cerebral blood vessels (21, 22). To examine whether cerebral M5 receptors play a role in mediating cholinergic vasomotor effects, we studied acetylcholine-dependent dilator responses in cerebral microvessels (pial arterioles) and a large cerebral artery (basilar artery), using vascular preparations from wild-type and M5R−/− mice. We first assessed acetylcholine-mediated increases in the diameter of pial arterioles by intravital microscopy through a cranial window (27). The lack of M5 receptors had no significant effect on the diameter of mouse cerebral arterioles under basal conditions (wild-type, 30 ± 1 μm; M5R−/−, 28 ± 2 μm), mean arterial pressure (wild-type, 76 ± 4 mm Hg; M5R−/−, 75 ± 5 mm Hg), or arterial pH and blood gasses (data not shown; n = 8 per genotype in each case). In wild-type mice, as reported previously (24–27), topical application of acetylcholine (1 and 10 μM) produced concentration-dependent vasodilator responses (Fig. 4A). Strikingly, this activity was totally abolished in M5R−/− mice (Fig. 4A). However, cerebral arterioles from M5R−/− mice retained pronounced dilator responses after application of another endothelium-dependent vasodilator agent, ADP (10–1,000 μM; Fig. 4B), indicating that the absence of functional M5 receptors does not interfere with the downstream signaling cascades that ultimately trigger vasorelaxation.
Figure 4.
Dilation of cerebral (pial) arterioles (in vivo) from M5R−/− mice and their wild-type littermates. (A) Acetylcholine-induced dilation responses of cerebral arterioles were abolished in M5R−/− mice. (B) In contrast, ADP-dependent vasodilator effects were largely retained in M5R−/− mice. Values are expressed as means ± SEM (n = 8 per dose and genotype). *, P < 0.05 (vs. wild-type; ANOVA followed by posthoc Bonferroni's t test).
To investigate whether M5 receptors play a similar role in cerebral arteries, we next studied acetylcholine-induced vasomotor responses in mouse basilar artery in vitro. As shown in Fig. 5A, acetylcholine (10−9–10−4 M) caused concentration-dependent dilator responses in basilar artery preparations from wild-type mice. Strikingly, this activity was almost completely abolished in arterial preparations derived from M5R−/− mice (Fig. 5A). Consistent with this observation, RT-PCR studies revealed the presence of M5 receptor transcripts in the basilar artery (Fig. 1C). On the other hand, papaverine (100 μM), an endothelium-independent vasodilating agent, caused similar relaxation responses in basilar artery preparations from wild-type and M5R−/− mice (Fig. 5B). Taken together, these results convincingly demonstrate that acetylcholine-dependent dilator responses of cerebral arterioles and arteries are mediated by the M5 muscarinic receptor subtype.
Figure 5.
Dilation of basilar artery preparations (in vitro) from M5R−/− mice and their wild-type littermates. (A) Acetylcholine-mediated vasodilation was almost completely abolished in basilar arteries from M5R−/− mice. (B) In contrast, papaverine-induced vasodilator effects were not significantly affected by the lack of M5 receptors. Basilar artery preparations were precontracted submaximally with the thromboxane mimetic, U-46619. Values are expressed as means ± SEM (n = 7 per concentration and genotype). *, P < 0.05 (vs. wild type; ANOVA followed by posthoc Bonferroni's t test).
Acetylcholine-Mediated Dilation of Extra-Cerebral Arteries.
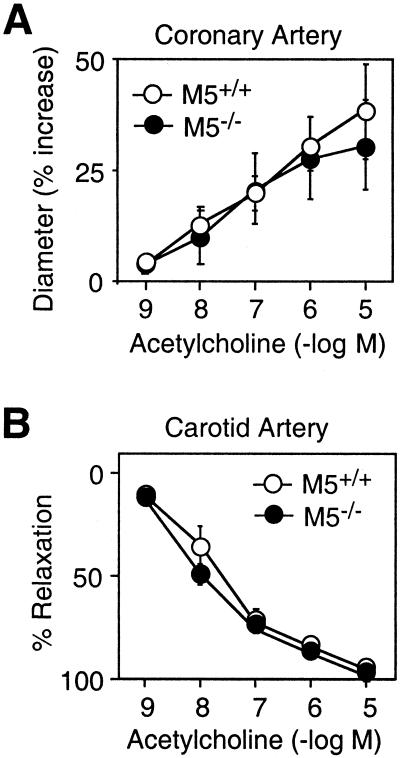
We next wanted to explore whether M5 receptors are also required for acetylcholine-dependent dilation of extra-cerebral arteries. To address this question, we carried out a series of in vitro studies with coronary (43) and carotid (44) artery preparations, two acetylcholine-sensitive peripheral blood vessels widely used in vascular biology research. In coronary and carotid artery preparations from wild-type mice, acetylcholine (10−9–10−5 M) caused concentration-dependent dilator/relaxation effects (Fig. 6). The magnitude of these responses did not differ significantly between preparations from wild-type and M5R−/− mice (Fig. 6). Consistent with this observation, we were unable to detect M5 mRNA in either of the two peripheral vascular preparations by using an RT-PCR strategy (data not shown).
Figure 6.
Acetylcholine-mediated relaxation of extra-cerebral arteries from M5R−/− mice and their wild-type littermates. (A) Coronary artery (in vitro). (B) Carotid artery (in vitro). Coronary artery preparations and carotid artery rings were precontracted submaximally with the thromboxane mimetic, U-46619, before the addition of acetylcholine. Data are expressed as percent increase in vascular diameter (A) or as percent relaxation (B). Acetylcholine-mediated vascular responses were not significantly affected by the lack of M5 receptors. Values are given as means ± SEM (n = 4–8 per concentration and genotype).
Discussion
To reveal the potential physiological roles of the M5 muscarinic receptor, we generated and analyzed M5R−/− mice. The M5 receptor, like the remaining four muscarinic receptor subtypes (M1-M4; refs. 48 and 49), is known to be expressed in the striatum (9), a region that is critically involved in the control of extrapyramidal locomotor activity. In agreement with previous studies (19, 20), we found that agonist-mediated activation of striatal muscarinic receptors led to a significant increase in the release of dopamine in striatal slice preparations from wild-type mice (Fig. 2). Interestingly, M5 receptor RNA has been identified in dopamine-containing neurons of the substantia nigra pars compacta (10, 11), the source of striatal dopamine, raising the possibility that M5 receptors may play a role in regulating dopamine release in the striatum. Consistent with this hypothesis, we observed that the lack of M5 receptors resulted in impairments in muscarinic agonist-induced dopamine release at an intermediate agonist (oxotremorine) dose (Fig. 2). However, maximum dopamine release was unaltered in M5R−/− mice, indicating that other muscarinic receptor subtypes also contribute to this response. Despite these neurochemical deficits, M5R−/− mice showed normal locomotor coordination and did not differ from their wild-type littermates in spontaneous locomotor activity and in the magnitude of the stimulatory locomotor response caused by administration of direct and indirect dopamine receptor agonists (Fig. 3). In contrast, recent studies have shown that the lack of either M1 (50) or M4 (41) muscarinic receptors results in significant increases in basal locomotor activity, probably caused by changes in the activity of striatal projection neurons that express both receptor subtypes at high levels (48, 51).
M5 receptor mRNA also has been detected in dopamine-containing neurons of the ventral tegmental area (10, 11), a region known to mediate important cognitive and affective functions and thought to be involved in the etiology of several neuropsychiatric disorders including schizophrenia and drug addiction (52, 53). Because stimulation of mesolimbic muscarinic receptors leads to the activation of dopaminergic ventral tegmental area neurons (54, 55), M5 receptors present on ventral tegmental area neurons may play a role in the pathophysiology of schizophrenia or addictive behavior (52).
It is well known that acetylcholine is a powerful dilator of most vascular beds and that this activity is mediated by endothelial muscarinic receptors triggering the release of the actual vasorelaxing agent, NO (23–28). Recently, RT-PCR studies have demonstrated the presence of M5 receptor mRNA, along with transcripts coding for other muscarinic receptor subtypes, in various peripheral and cerebral blood vessels (21) including human brain microvessels (22). In the present study, we made the striking observation that acetylcholine-mediated dilation of cerebral arteries and microvessels is virtually abolished in M5R−/− mice (Figs. 4 and 5). It is very unlikely that this striking phenotype was caused by the mixed genetic background (CF-1 × 129SvEv) of the mice used in this study, because acetylcholine-mediated dilation of cerebral blood vessels has been demonstrated in all mouse strains examined so far, including ICR (24, 27), SV-129 (26, 57), and C57BL/6 mice (57, 58). These data therefore support a model in which activation of endothelial M5 receptors represents a first step in the cholinergic relaxation response of cerebral blood vessels. In subsequent steps, M5 receptor-mediated activation of G proteins of the Gq family is predicted to trigger increases in intracellular calcium and inositol 1,4,5-trisphosphate levels (5–8), which eventually lead to the activation of endothelial NO synthase and the production of NO (56).
The cerebral circulation (both large arteries and microvessels) receives cholinergic innervation from extrinsic and intrinsic sources (29–33), and neuronally released acetylcholine is known to be involved in the regulation of cerebral vascular resistance and regional blood flow (34–38). For example, cortical microvessels are known to be innervated by cholinergic neurons of the basal forebrain (29, 33), and stimulation of basal forebrain cholinergic nuclei leads to considerable increases in cortical blood flow (34, 37, 38). It has also been reported that cortical microvessels are deficient in cholinergic neurogenic control in Alzheimer's disease (29) and that central cholinergic stimulation with the cholinesterase inhibitor, physostigmine, can reverse the decrease in cortical blood flow observed in Alzheimer's disease (39), implicating deficits in cortical cholinergic vasodilation in the pathophysiology of this disorder. In addition, several studies suggest that cholinergic vasodilator fibers may play a protective role during focal cerebral ischemia (35, 40). M5R−/− mice therefore represent excellent tools for more detailed investigations into the physiological and pathophysiological roles of the central cholinergic vasodilator system.
In contrast to the results obtained with the cerebral vascular preparations, the lack of M5 receptors had no significant effect on acetylcholine-mediated relaxation of extra-cerebral arteries (carotid and coronary arteries; Fig. 6). It is likely, that another Gq-coupled muscarinic receptor, such as the M3 receptor subtype that is widely expressed in peripheral tissues (59), mediates the vasodilator effects of acetylcholine in peripheral vascular beds.
In conclusion, we provide direct evidence that M5 muscarinic receptors are not physiologically “silent” but are intimately involved in regulating the diameter of cerebral arterioles and arteries. From a clinical point of view, selective M5 muscarinic receptor agonists may become therapeutically useful to increase cerebral blood flow in certain pathophysiological conditions including Alzheimer's disease and cerebral ischemia.
Acknowledgments
We thank A. S. Basile and T. Miyakawa for advice and helpful discussions, J. Gan, C. Lynch, D. Nuno, D. Kinzenbaw, and C. Li for expert technical assistance, and A. M. Spiegel and I. W. Levin for generous support of this work. This work was supported by the Japan Society for the Promotion of Science Research Fellowship Program (M.Y.) and National Institutes of Health Grants HL-38901, HL-39050, HL-62984, and NS-24621 (K.G.L. and F.M.F.).
Abbreviations
- M5R−/− mice
M5 receptor-deficient mice
- RT-PCR
reverse transcriptase–PCR
References
- 1.Wess J. Crit Rev Neurobiol. 1996;10:69–99. doi: 10.1615/critrevneurobiol.v10.i1.40. [DOI] [PubMed] [Google Scholar]
- 2.Caulfield M P, Birdsall N J M. Pharmacol Rev. 1998;50:279–290. [PubMed] [Google Scholar]
- 3.Levine R R, Birdsall N J M, Nathanson N M, editors. Life Sci. 1999;64:355–593. [Google Scholar]
- 4.Levine R R, Birdsall N J M, Nathanson N M, editors. Life Sci. 2001;68:2449–2642. [Google Scholar]
- 5.Bonner T I, Young A C, Brann M R, Buckley N J. Neuron. 1988;1:403–410. doi: 10.1016/0896-6273(88)90190-0. [DOI] [PubMed] [Google Scholar]
- 6.Liao C F, Themmen A P, Joho R, Barberis C, Birnbaumer M, Birnbaumer L. J Biol Chem. 1989;264:7328–7337. [PubMed] [Google Scholar]
- 7.Reever C M, Ferrari-DiLeo G, Flynn D D. Life Sci. 1997;60:1105–1112. doi: 10.1016/s0024-3205(97)00054-4. [DOI] [PubMed] [Google Scholar]
- 8.Eglen R M, Nahorski S R. Br J Pharmacol. 2000;130:13–21. doi: 10.1038/sj.bjp.0703276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yasuda R P, Ciesla W, Flores L R, Wall S J, Li M, Satkus S A, Weisstein J S, Spagnola B V, Wolfe B B. Mol Pharmacol. 1993;43:149–157. [PubMed] [Google Scholar]
- 10.Vilaro M T, Palacios J M, Mengod G. Neurosci Lett. 1990;114:154–159. doi: 10.1016/0304-3940(90)90064-g. [DOI] [PubMed] [Google Scholar]
- 11.Weiner D M, Levey A I, Brann M R. Proc Natl Acad Sci USA. 1990;87:7050–7054. doi: 10.1073/pnas.87.18.7050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wei J, Walton E A, Milici A, Buccafusco J J. J Neurochem. 1994;63:815–821. doi: 10.1046/j.1471-4159.1994.63030815.x. [DOI] [PubMed] [Google Scholar]
- 13.Costa P, Auger C B, Traver D J, Costa L G. J Neuroimmunol. 1995;60:45–51. doi: 10.1016/0165-5728(95)00051-3. [DOI] [PubMed] [Google Scholar]
- 14.Tayebati S K, Codini M, Gallai V, Mannino F, Parnetti L, Ricci A, Sarchielli P, Amenta F. J Neuroimmunol. 1999;99:224–229. doi: 10.1016/s0165-5728(99)00119-8. [DOI] [PubMed] [Google Scholar]
- 15.Buchli R, Ndoye A, Rodriguez J G, Zia S, Webber R J, Grando S A. J Cell Biochem. 1999;74:264–277. [PubMed] [Google Scholar]
- 16.Gil D W, Krauss H A, Bogardus A M, WoldeMussie E. Invest Ophthalmol Visual Sci. 1997;38:1434–1442. [PubMed] [Google Scholar]
- 17.Preiksaitis H G, Krysiak P S, Chrones T, Rajgopal V, Laurier L G. J Pharmacol Exp Ther. 2000;295:879–888. [PubMed] [Google Scholar]
- 18.Bockman C S, Bradley M E, Dang H K, Zeng W, Scofield M A, Dowd F J. J Pharmacol Exp Ther. 2001;297:718–726. [PubMed] [Google Scholar]
- 19.Lehmann J, Langer S Z. Brain Res. 1982;248:61–69. doi: 10.1016/0006-8993(82)91147-7. [DOI] [PubMed] [Google Scholar]
- 20.Raiteri M, Leardi R, Marchi M. J Pharmacol Exp Ther. 1984;228:209–214. [PubMed] [Google Scholar]
- 21.Phillips J K, Vidovic M, Hill C E. J Auton Nerv Syst. 1997;62:85–93. doi: 10.1016/s0165-1838(96)00114-2. [DOI] [PubMed] [Google Scholar]
- 22.Elhusseiny A, Cohen Z, Olivier A, Stanimirovic D B, Hamel E. J Cereb Blood Flow Metab. 1999;19:794–802. doi: 10.1097/00004647-199907000-00010. [DOI] [PubMed] [Google Scholar]
- 23.Furchgott R F, Zawadzki J V. Nature (London) 1980;288:373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- 24.Rosenblum W I. Stroke. 1986;17:494–497. doi: 10.1161/01.str.17.3.494. [DOI] [PubMed] [Google Scholar]
- 25.Huang P L, Huang Z, Mashimo H, Bloch K D, Moskowitz M A, Bevan J A, Fishman M C. Nature (London) 1995;377:239–242. doi: 10.1038/377239a0. [DOI] [PubMed] [Google Scholar]
- 26.Meng W, Ma J, Ayata C, Hara H, Huang P L, Fishman M C, Moskowitz M A. Am J Physiol. 1996;271:H1145–H1150. doi: 10.1152/ajpheart.1996.271.3.H1145. [DOI] [PubMed] [Google Scholar]
- 27.Sobey C G, Faraci F M. Stroke. 1997;28:837–843. doi: 10.1161/01.str.28.4.837. [DOI] [PubMed] [Google Scholar]
- 28.Faraci F M, Sigmund C D. Circ Res. 1999;85:1214–1225. doi: 10.1161/01.res.85.12.1214. [DOI] [PubMed] [Google Scholar]
- 29.Tong X K, Hamel E. Neuroscience. 1999;92:163–175. doi: 10.1016/s0306-4522(98)00750-7. [DOI] [PubMed] [Google Scholar]
- 30.Iadecola C. In: Stimulated Cerebral Blood Flow. Schmiedek P, Einhaupl K, Kirsch C-M, editors. Berlin: Springer; 1992. pp. 19–36. [Google Scholar]
- 31.Edvinsson L, MacKenzie E T. Pharmacol Rev. 1976;28:275–348. [PubMed] [Google Scholar]
- 32.Dauphin F, MacKenzie E T. Pharmacol Ther. 1995;67:385–417. doi: 10.1016/0163-7258(95)00022-4. [DOI] [PubMed] [Google Scholar]
- 33.Vaucher E, Hamel E. J Neurosci. 1995;15:7427–7441. doi: 10.1523/JNEUROSCI.15-11-07427.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sato A, Sato Y. Alzheimer Dis Assoc Disord. 1995;9:28–38. doi: 10.1097/00002093-199505000-00007. [DOI] [PubMed] [Google Scholar]
- 35.Scremin O U, Jenden D J. Life Sci. 1996;5:2011–2018. doi: 10.1016/0024-3205(96)00192-0. [DOI] [PubMed] [Google Scholar]
- 36.Van Riper D A, Bevan J A. Circ Res. 1992;70:1104–1112. doi: 10.1161/01.res.70.6.1104. [DOI] [PubMed] [Google Scholar]
- 37.Zhang F, Xu S, Iadecola C. Neuroscience. 1995;69:1195–1204. doi: 10.1016/0306-4522(95)00302-y. [DOI] [PubMed] [Google Scholar]
- 38.Lacombe P, Sercombe R, Verrecchia C, Philipson V, MacKenzie E T, Seylaz J. Brain Res. 1989;491:1–14. doi: 10.1016/0006-8993(89)90083-8. [DOI] [PubMed] [Google Scholar]
- 39.Geaney D, Soper N, Shepstone B J, Cowen P J. Lancet. 1990;335:1484–1487. doi: 10.1016/0140-6736(90)93028-n. [DOI] [PubMed] [Google Scholar]
- 40.Kano M, Moskowitz M A, Yokota M. J Cereb Blood Flow Metab. 1991;11:628–637. doi: 10.1038/jcbfm.1991.114. [DOI] [PubMed] [Google Scholar]
- 41.Gomeza J, Zhang L, Kostenis E, Felder C, Bymaster F, Brodkin J, Shannon H, Xia B, Deng C-x, Wess J. Proc Natl Acad Sci USA. 1999;96:10483–10488. doi: 10.1073/pnas.96.18.10483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gomeza J, Shannon H, Kostenis E, Felder C, Zhang L, Brodkin J, Grinberg A, Sheng H, Wess J. Proc Natl Acad Sci USA. 1999;96:1692–1697. doi: 10.1073/pnas.96.4.1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lamping K G, Nuno D W, Chappell D A, Faraci F M. Am J Physiol. 1999;276:R1023–R1029. doi: 10.1152/ajpregu.1999.276.4.R1023. [DOI] [PubMed] [Google Scholar]
- 44.Faraci F M, Sigmund C D, Shesely E G, Maeda N, Heistad D D. Am J Physiol. 1998;274:H564–H570. doi: 10.1152/ajpheart.1998.274.2.H564. [DOI] [PubMed] [Google Scholar]
- 45.Hornykiewicz O. In: Movement Disorders. Marsden D C, Fahn S, editors. Boston: Butterworth; 1981. pp. 41–58. [Google Scholar]
- 46.Di Chiara G, Morelli M, Consolo S. Trends Neurosci. 1994;17:228–233. doi: 10.1016/0166-2236(94)90005-1. [DOI] [PubMed] [Google Scholar]
- 47.Kaneko S, Hikida T, Watanabe D, Ichinose H, Nagatsu T, Kreitman R J, Pastan I, Nakanishi S. Science. 2000;289:633–637. doi: 10.1126/science.289.5479.633. [DOI] [PubMed] [Google Scholar]
- 48.Hersch S M, Gutekunst C-A, Rees H D, Heilman C J, Levey A I. J Neurosci. 1994;14:3351–3363. doi: 10.1523/JNEUROSCI.14-05-03351.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vilaro M T, Mengod G, Palacios J M. Prog Brain Res. 1993;98:95–101. doi: 10.1016/s0079-6123(08)62385-7. [DOI] [PubMed] [Google Scholar]
- 50.Miyakawa T, Yamada M, Duttaroy A, Wess J. J Neurosci. 2001;21:5239–5250. doi: 10.1523/JNEUROSCI.21-14-05239.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bernard V, Normand E, Bloch B. J Neurosci. 1992;12:3591–3600. doi: 10.1523/JNEUROSCI.12-09-03591.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yeomans J S. Neuropsychopharmacology. 1995;12:3–16. doi: 10.1038/sj.npp.1380235. [DOI] [PubMed] [Google Scholar]
- 53.Wise R A. Curr Opin Neurobiol. 1996;6:243–251. doi: 10.1016/s0959-4388(96)80079-1. [DOI] [PubMed] [Google Scholar]
- 54.Gronier B, Perry K W, Rasmussen K. Psychopharmacology. 2000;147:347–355. doi: 10.1007/s002130050002. [DOI] [PubMed] [Google Scholar]
- 55.Forster G L, Blaha C D. Eur J Neurosci. 2000;12:3596–3604. doi: 10.1046/j.1460-9568.2000.00250.x. [DOI] [PubMed] [Google Scholar]
- 56.Wang S Z, Zhu S Z, El-Fakahany E E. J Pharmacol Exp Ther. 1994;268:552–557. [PubMed] [Google Scholar]
- 57.Fujii M, Hara H, Meng W, Vonsattel J P, Huang Z, Moskowitz M A. Stroke. 1997;28:1805–1811. doi: 10.1161/01.str.28.9.1805. [DOI] [PubMed] [Google Scholar]
- 58.Niwa K, Haensel C, Ross M E, Iadecola C. Circ Res. 2001;88:600–608. doi: 10.1161/01.res.88.6.600. [DOI] [PubMed] [Google Scholar]
- 59.Levey A I. Life Sci. 1993;52:441–448. doi: 10.1016/0024-3205(93)90300-r. [DOI] [PubMed] [Google Scholar]