Abstract
Mild hypothyroidism, also known as subclinical hypothyroidism (SH), is biochemically defined as serum TSH levels above the upper limit of the reference range, in the presence of normal serum concentrations of total T4 and free T4 (FT4). In the neonatal period, mild hypothyroidism can be defined by the presence of a TSH value between 6 and 20 mIU/L and normal FT4 levels. After the neonatal period, SH can be defined mild if TSH ranges between 4.5 and 10 mIU/L. The management of mild hypothyroidism in childhood is challenging. The major concern is to establish whether this condition should always be considered an expression of mild thyroid dysfunction. Indeed, the effects of untreated mild hypothyroidism are still not completely defined. In the neonatal period, concern exists about neurocognitive outcome; in children, although there is no clear evidence of alterations in growth or neurocognitive development, subtle cardiovascular abnormalities have been documented. Therefore, there is still uncertainty about the need of treatment across all ages, and the management should be based on the age of the child, the etiology, and the degree of TSH elevation, as well as on other patient factors. This review updates current evidences on diagnosis and management of mild hypothyroidism in childhood.
Keywords: mild hypothyroidism, subclinical hypothyroidism
This review updates current evidence on diagnosis and management of mild hypothyroidism in childhood.
Mild hypothyroidism, also known as subclinical hypothyroidism (SH), is biochemically defined as serum TSH levels above the upper limit of the reference range, in the presence of normal serum concentrations of total T4 and free T4 (FT4) [1]. In the neonatal period, mild hypothyroidism can be defined by the presence of a TSH value between 6 and 20 mIU/L and normal FT4 levels [2]. After the neonatal period, SH can be defined as mild (TSH 4.5 to 10 mIU/L) or severe (TSH >10 mIU/L) [3]. Due to the wide variability of TSH concentrations among healthy individuals [4] and among different biochemical methods [5], two independent TSH measurements above the upper limit of the reference range, in the presence of normal FT4 values, are needed to define persistent SH [3]. Recent data confirm systematic differences between the most common commercially available TSH immunoassays [5], and disagreement has been observed in particular for the normal upper range [6].
In adults, treatment with levothyroxine (L-T4) is recommended when serum TSH levels are >10 mIU/L for the increased risk of hypothyroid symptoms and cardiovascular events, whereas for patients who have TSH levels <10 mIU/L, the management is based on individual factors [7].
Mild hypothyroidism in children differs from that in adults in both the etiology and the natural history. Moreover, although in childhood, overt hypothyroidism is known to severely affect growth and neurocognitive development, the effects of mild hypothyroidism are still not completely defined. Therefore, the management of this condition is challenging and is strictly related to the age of the patients, differing between neonates and children.
This review provides an update on the diagnosis and management of mild SH in childhood.
1. Mild Hypothyroidism in Neonates
In the neonatal period, mild hypothyroidism can be defined by the presence of a TSH value between 6 and 20 mIU/L and normal FT4 levels [2]. Mild congenital hypothyroidism (CH) can be transient or permanent. Over the past years, the increased sensitivity in the TSH assay, the use of lowered TSH cutoff values, and an increased survival rate of a growing number of preterm babies have resulted in a progressively increased incidence of mild and potentially transient forms of CH [8–11].
CH and, in particular, mild CH are more common in some risk categories such as preterm or ill neonates [12], small for gestational age infants [13], children born after in vitro fertilization (IVF) [14], and in multiple pregnancies [15]. In these categories of neonates, the initial screening tests may be inappropriate or provide normal results, and therefore the European Society for Pediatric Endocrinologist (ESPE) guidelines [2] suggest a strategy of second screening, at about 2 weeks of age or 2 weeks after the first screening test. Other risk factors that should be taken into account in the decision to perform the second screening test are the presence of chromosopathies, malformations, steroid treatment during pregnancy or in the neonatal period, and maternal thyroid dysfunction.
The optimal management of neonates with a mild isolated increase in TSH levels is still debated and should be individualized. Moreover, due to the potential neurodevelopmental impairment, each case of mild CH should be carefully monitored.
In the next subheadings, the peculiarity of each category of at-risk neonates and the management of mild hypothyroidism in the neonatal period are discussed.
A. Maternal Autoimmune Thyroid Disease
Autoimmune maternal thyroiditis is the most frequent cause of hypothyroidism during pregnancy. It could be related either to the presence of thyroid peroxidase antibodies or, rarely, TSH receptor antibodies (rTSH-Abs). Moreover, newborns of mothers treated with thionamide during pregnancy could develop a transient form of mild hypothyroidism because these antithyroid drugs are able to cross the placenta [16].
In newborns of mothers affected by autoimmune thyroiditis, there is no consensus on whether to repeat thyroid function evaluation during the first month of life in addition to the first neonatal screening. Rovelli et al. [17] suggested to retest thyroid function between days 15 and 30 because they found a mild TSH increase in ~28% of a neonatal cohort. However, replacement therapy was not necessary in the majority of these patients (93.3%) [17], and they all presented a transient form of hypothyroidism (unpublished data). On the contrary, according to a more recent study from McGovern et al. [18], the first neonatal screening seems to be able to identify all cases of mild hypothyroidism, and further thyroid function tests are not necessary [18]. In conclusion, further studies are required to clarify whether a second screening is needed for all newborns of mothers affected by autoimmune thyroiditis.
In addition to thyroid peroxidase antibodies, rTSH-Abs should be tested in case of neonatal hypothyroidism with autoimmune maternal thyroid disease and/or other siblings affected with transient CH [2]. The transplacental passage of TSH receptor-blocking antibodies is responsible for 2% of cases of CH in North America, with an incidence of 1:180,000 healthy children [19]. The alterations in neonatal thyroid function are usually transient as rTSH-Abs are cleared from the neonatal circulation in 3 to 4 months [20]. Nevertheless, replacement treatment should be started in some cases [21]. Moreover, the presence of blocking antibodies could also interfere with the scintigraphic iodine uptake of a normal eutopic gland [22].
B. Prematurity and Low-Birthweight Infants
Preterm and low-birthweight (LBW) neonates are at risk for hypothalamic-pituitary immaturity, premature loss of the contribution of transplacental T4 and iodine, limited thyroid gland reserve, immaturity of the mechanism of thermogenesis mediated by brown adipose tissue, persistent fetal thyroid hormone metabolism, and a high morbidity predisposing to euthyroid sick syndrome [23]. Moreover, the administration of certain drugs (dopamine, caffeine, phenobarbital, etc) can cause alterations of thyroid function.
Therefore, premature and LBW babies may face variable degrees of thyroid dysfunction such as CH, SH, hypothyroxinemia, euthyroid sick syndrome, and delayed TSH rise. However, prematurity seems to be associated with transient rather than permanent thyroid failure [24].
Woo et al. [25] showed that CH with a delayed TSH elevation occurred in 1 in 58 extremely LBW and 1 in 95 very LBW (VLBW) infants, in comparison with only 1 in 30,329 infants weighing ≥1500 g (P < 0.0001). In the study by Cavarzere et al. [26], 57.5% of LBW newborns presented CH with delayed TSH elevation and required L-T4 treatment, whereas the remaining infants presented SH (21.25%) or normal thyroid function (21.25%).
Although the effect of transient hypothyroxinemia of prematurity on the neurologic outcome has been extensively studied [27–30], the impact of persistent SH on the neurologic development of preterm infants has not been adequately investigated. Woo et al. [25] showed that the developmental outcomes at 18-month corrected age in preterm infants with delayed TSH elevation were similar to control infants. However, this study was limited by the small sample size.
In conclusion, thyroid function should be carefully monitored in preterm and LBW infants. In a recent systematic review, Hashemipour et al. [12] recommend repeating the screening test in preterm, LBW, and VLBW infants at the age of 2, 6, and 10 weeks by measuring TSH and FT4 levels simultaneously and considering TSH of 10 mIU/L as the cutoff level for positive and suspicious cases.
According to ESPE guidelines [2], we suggest a strategy of second screening at ~2 weeks of age in all preterm or LBW neonates and in neonates admitted to neonatal intensive care units. A strategy of an additional screening at 4 to 6 weeks of age might be considered in case of severe prematurity, VLBW, and critically ill neonates.
C. Twins
Recent studies reported a high incidence of CH with eutopic thyroid in multiple pregnancies. The incidence is nearly double in twin births compared with singletons and even higher with multiple births [15, 31]. Data from the Italian National Registry of infants with CH have also shown a high frequency of twins that is threefold higher in the CH population (3.5%) than in the Italian general population (1.1%) [15]. A high prevalence of twins among infants affected by transient hypothyroidism has also been reported [15].
This increased CH risk in multiple pregnancies has important implications in terms of public health given the high number of induced pregnancies in Italy as well as in other Western countries [32].
Twins born monozygotic usually show a delayed TSH rise, and thus rescreening at 2 to 4 weeks should be recommended [2].
D. IVF
Onal et al. [14] showed a high prevalence of SH in IVF babies, being diagnosed in ~10% of the IVF neonates at postnatal ages of 2 weeks to 1 month. In addition, Sakka et al. [33] demonstrated a significantly higher prevalence of SH in children aged 4 to 14 years, conceived after IVF than in the control group, in the absence of detectable thyroid antibodies. A possible explanation provided by the authors was an epigenetic developmental abnormality in the set point of TSH sensitivity related to the preimplantation manipulation of the embryo [33].
Currently, IVF neonates are not considered a special risk category by the screening programs. However, in view of the documented increased risk to develop SH, these patients should be carefully monitored.
E. Genetic Mutations
Even though CH is more frequently a sporadic disease, evidence has been provided that the CH population is significantly enriched with rare/low-frequency alleles in the CH related genes (NKX2-1, FOXE1, PAX8, GLIS3, JAG1, TSHR, SLC26A4, DUOX2, DUOXA2, TPO, TG), and the frequency of multiple gene involvement is two- to fourfold higher than in the control population [34]. Congenital thyroid dysfunction may also arise in the context of other complex disorders, such as Alagille syndrome type 1 due to JAG1 mutations (ALGS1) [35] and hepatic or parotid massive hemangiomas, which may produce the thyroid hormone–inactivating enzyme type 3 iodothyronine deiodinase [36, 37].
So far, only a few studies have focused on the incidence and/or the evolution of SH in these genetic forms, except for those related to mutations of DUOX2 and TSH receptor (TSHR) genes.
Loss-of-function variants of the TSHR are the most frequent causes of TSH resistance (RTSH) (OMIM 275200), causing various clinical phenotypes depending on the degree of the impairment of the TSHR function [38, 39]. In case of biallelic variants, patients can have complete RTSH, resulting in severe hypothyroidism. Patients with monoallelic mutations have a partial RTSH, which results in nonautoimmune SH [40]. To date, at least 68 loss-of-function mutations of the TSHR have been described [39] with a variable prevalence (11% to 29%) depending on the population tested [38, 41–44]. Most carriers have a positive family history for thyroid diseases.
The long-term follow-up of pediatric patients with SH due to RTSH showed a favorable clinical outcome with regular growth, normal metabolic profile and bone density, and normal intellectual outcome [45].
The DUOX2 and DUOXA2 genes are the principal elements generating the hydrogen peroxide needed for TPO function [46]. Defects in DUOX2/DUOXA2 genes lead to partial dyshormonogenic defects. Monoallelic DUOX2 mutations are associated with transient CH, whereas biallelic DUOX2 mutations can lead to transient or permanent CH, with a highly variable intra- and interfamilial phenotype, suggesting a role of genetic/environmental modulators [46].
Patients with DUOX2 variants usually show borderline blood spot TSH levels at first neonatal screening and subsequently high serum TSH at confirmatory tests (TSH >100 mIU/L) with low FT4, higher thyroglobulin (Tg) levels, and hyperplastic thyroid gland at birth [47].
2. Management of Mild Hypothyroidism in the Neonatal Period
According to ESPE guidelines [2] L-T4 treatment is recommended if serum FT4 concentration is below the normal values for age, regardless of TSH concentration, and if venous TSH concentration is persistently >20 mIU/L, even if serum FT4 concentration is normal.
When venous TSH concentration is between 6 and 20 mIU/L in a healthy baby with FT4 concentration within the normal limits for age, the optimum management is debated. The decision to start treatment with L-T4 depends on multiple factors, such as the age of the patient, the duration of the thyroid dysfunction, the trend of TSH values, the etiology, the presence of syndromes and/or other pathologies, and the parents’ choice and reliability.
When venous TSH concentration is between 6 and 20 mU/L in a well baby for more than 3 to 4 weeks, ESPE guidelines suggest, after discussion with the family, either starting L-T4 supplementation immediately and retesting off treatment at a later stage or retesting 2 weeks later without treatment in particular if TSH value is <10 mIU/L.
If treatment is started, TSH should be maintained in the age-specific reference range and serum concentrations of FT4 in the upper half of the age-specific reference range. Thyroid function should be assessed 1 to 2 weeks after the start of L-T4 treatment, with frequent monitoring during the first year of life. In special risk categories (preterm, LBW, twins, infants from mothers affected by autoimmune thyroiditis, twins), mild alteration of TSH may regress, persist, or even worsen and should be carefully monitored. Reevaluation of the thyroid axis, off treatment, should normally take place after the age of 3 years, but earlier reevaluation may be indicated if transient increases in TSH concentration are highly probable [2].
In most children with a mildly elevated TSH level at neonatal screening, a progressive normalization of thyroid function is generally observed even though a mild TSH increase may persist in up to 32% of these patients [48]. Indeed, in a prospective study on 44 children with a mildly elevated TSH level at neonatal screening, thyroid function normalized in 56.8% of children at an average age of 5.3 years and in 68.8% of children at 7.2 to 9.5 years of age [48].
In cases with known genetic etiology, the management of SH is also controversial. In patients heterozygous for TSHR mutations, SH is generally considered a compensated thyroid dysfunction with an appropriately adjusted set point for pituitary-thyroid feedback and does not require treatment [49]. Nevertheless, if heterozygous mutations of TSHR are detected in patients belonging to special categories (preterm infants, small for gestational age neonates, infants born after multiple pregnancies and/or conceived by assisted reproduction techniques), L-T4 supplementation might be considered [44].
In contrast, in carriers of biallelic mutations, a trend toward an increase in TSH levels with a concomitant decline in FT4 concentrations has been observed, suggesting the need for long-term follow-up and/or L-T4 therapy [49].
Most patients with DUOX2 mutations are identified at neonatal screening for CH and start L-T4 treatment soon after birth for either mild or severe hypothyroidism. The wide clinical variability suggests that all patients with CH due to DUOX2 mutations should undergo reevaluation after the age of 3 years. Indeed, after reevaluation, most heterozygous patients show normal thyroid function; however, it remains to be investigated whether these patients are at risk for hypothyroidism in other periods of life characterized by high thyroid hormone requirements such as puberty or pregnancy [50].
3. Mild Hypothyroidism in Children
Mild hypothyroidism after the neonatal period can be defined as TSH 4.5 to 10 mIU/L in the presence of normal FT4.
Data on the epidemiology of SH in childhood are scarce; the prevalence of mild subclinical thyroid dysfunction in children and adolescents according to two large population studies ranges between 1.7% and 2.9% [51, 52].
Most studies indicate that SH in children frequently resolves spontaneously or may persist without progressing to overt hypothyroidism. In a large Israeli study, 73.6% of children with mild SH normalized their TSH over 5 years, whereas in ~25% of them, TSH remained stable [52]. Moreover, a 2-year prospective study on 92 children demonstrated that mild SH resolved spontaneously in 41.3% of participants, remained stable in 46.7%, and progressed to hypothyroidism (TSH >10 mIU/L) only in 12.0% [53].
However, the natural history of SH substantially depends on its etiology, as discussed in the next paragraphs.
A. Hashimoto Thyroiditis
Hashimoto thyroiditis (HT) is one of the most frequent causes of persistent SH in children and adolescents [54]. The underlying cause of HT is still unknown, even though several genetic and environmental factors have been associated with this disorder [55]. HT is particularly common in children with genetic conditions, such as Down syndrome (DS) or Turner syndrome [56], and in patients with other autoimmune diseases (i.e., celiac disease or type 1 diabetes mellitus) [56, 57].
The clinical course of HT is highly variable depending on the severity of the immunological damage [56]. Thyroid function at presentation may vary from euthyroidism to overt hypothyroidism or, occasionally, hyperthyroidism. In a retrospective multicenter study on 608 children and adolescents (age range 2.5 to 18.0 years), euthyroidism was observed in 52.1% of patients at presentation, whereas SH and overt hypothyroidism were detected in 19.2% and 22.2% of patients, respectively [58].
The risk of progression to overt hypothyroidism in children affected by autoimmune SH is higher with respect to those affected by nonautoimmune forms. A worsening of thyroid function was indeed observed after 3 years of follow-up among 21.4% of children with HT compared with 13.6% of patients with isolated hyperthyrotropinemia in a retrospective multicenter study [57]. These data were further confirmed in a 2-year prospective study that documented an increased risk of progression to overt hypothyroidism among children with mild SH associated with HT (53.1%) with respect to children with a mild nonautoimmune form (11.1%) [59].
The long-term thyroid function was also evaluated in a recent 5-year prospective study on 127 girls with mild autoimmune and nonautoimmune SH. At the end of the study, 61.9% of girls with mild nonautoimmune SH normalized thyroid function, 26.2% maintained unchanged their TSH, and only 11.9% progressed to overt hypothyroidism. Conversely, in the group with autoimmune mild SH, a progressive deterioration of thyroid function was observed in 30.6% of girls, and only 10.6% normalized their TSH [60].
Levels of TSH and thyroid peroxidase antibodies at presentation and concomitant celiac disease were associated with an increased risk of developing overt hypothyroidism in children with HT [57]. Moreover, in girls with HT, the association with either Turner syndrome or DS further increased the risk of thyroid function deterioration [60].
B. Obesity
An isolated mild increase in TSH levels, associated with FT4 values and free T3 values that are within or slightly above the upper normal range, is a common finding in children who are overweight and obese, with a prevalence ranging between 7% and 23% [61, 62].
Nearly one-third of children with obesity and elevated TSH levels have a hypoechogenic thyroid gland pattern at ultrasound [63, 64], which could represent a feature of thyroid derangement due to obesity itself or an early marker of a seronegative autoimmune thyroiditis. However, autoimmune thyroiditis has seldom been reported as a cause of a mild TSH increase in childhood obesity, and a recent study on a large cohort of 938 children and adolescents with obesity reported that only 7% had autoimmune thyroiditis [65].
Further mechanisms that may lead to increased TSH levels in children with obesity are mutations in the TSHR gene, functional derangements in the hypothalamic-pituitary-thyroid axis, and thyroid hormone resistance. However, the most promising link between obesity and elevated TSH levels seems to be the increased leptin-mediated production of pro-TRH, because leptin is able to stimulate and thus regulate the hypothalamic-pituitary-thyroid axis function [62].
Despite the uncertainty regarding the underlying mechanism, the findings that abnormalities in thyroid function and TSH mostly normalize after weight loss support the hypothesis that the TSH increase in patients who are obese is reversible and seems to be a consequence rather than a cause of obesity [62, 65, 66].
Moreover, the mild increase in TSH levels in obesity might represent an adaptive response designed to reduce the availability of energy for conversion into fat [62]; therefore, treatment with thyroxine seems unnecessary in children who are obese.
C. Genetic Syndromes
It is well established that patients with DS are at increased risk for the development of thyroid hormone abnormalities, including either congenital or acquired hypothyroidism [67]. A high prevalence of SH has been reported among these children, ranging from 25.3% to 60.0% [68].
The underlying cause of the isolated elevations in TSH levels among children with DS has not been elucidated. In addition to thyroid autoimmunity, possible mechanisms include a central disorder causing inappropriate release of TSH, production of TSH with lowered activity, and some degree of TSH insensitivity in the thyroid gland [69].
The natural history has yet to be defined [70] even though mild hypothyroidism in DS seems to be frequently self-limiting [3]; therefore, regular monitoring of thyroid function is recommended.
The effectiveness of L-T4 administration among children with DS and subtle thyroid abnormalities is still controversial. A recent study [71] suggests that the treatment with L-T4 in the first 2 years of life may lead to an improvement in the hypothalamic-pituitary-thyroid axis set-point and to a reduction in the risk of developing thyroid autoimmunity. However, randomized, double-blind, controlled studies are needed to establish the effectiveness of L-T4 therapy in children with DS.
Another genetic syndrome frequently associated with mild hypothyroidism is pseudohypoparathyroidism type 1a, a rare genetic disorder caused by deficiency of Gsα, leading to multiple hormone resistance [72].
D. Iodine Deficiency and Excess
The relation between iodine intake and thyroid disorders is U-shaped because both deficient and excess iodine intake can impair thyroid function.
Although iodine deficiency is often thought to be a problem of developing countries, industrialized countries are not immune. In moderate to severe iodine deficiency, mean serum TSH concentration often slightly increases, whereas T4/FT4 remains normal. As iodine deficiency becomes more severe, TSH further rises, and goiter and overt hypothyroidism can develop [73].
On the other hand, increasing iodine intake also leads to a small increase in the incidence of mild SH, more often in individuals positive for thyroid antibodies [73].
Drugs that contain iodine, such as amiodarone and its main metabolite, desethylamiodarone, are known to cause an elevation in TSH levels, blocking the ability of the type 2 iodothyronine deiodinase to mediate conversion of T4 to T3 [74]. Moreover, recent attention has been paid to the role of iatrogenic iodine excess from radiographic contrast. In a recent study, children receiving iodinated radiographic contrast had an increased risk (2.6-fold) of developing hypothyroidism, although the duration and impact of such thyroid dysfunction remain unclear [75].
Alterations in thyroid function have been reported after treatment with 131I-metaiodobenzylguanidine in children with neuroblastoma despite protection with potassium iodide. The occurrence of thyroid dysfunction increases over time; therefore, continuous screening for thyroid alterations in these survivors is recommended [76].
Finally, cough suppressants and nutritional supplements containing iodine may also cause thyroid dysfunction [77].
E. Medications and Exposure to Ionizing Radiation
Treatment with interferon (IFN)–α has been associated with alterations of thyroid function [78]. An autoimmune mechanism has been hypothesized, but also nonautoimmune thyroid dysfunction can be related to IFN-α [78, 79]. In a recent study on 61 children with chronic hepatitis C receiving therapy with IFN-α and ribavirin, 27.94% developed SH and 6.6% developed autoimmune thyroiditis during treatment; SH was transient in most cases (93.4%), whereas autoimmune thyroiditis persisted in 75% of cases 24 weeks after the end of treatment [79].
Antiepileptic drugs (phenobarbital, phenytoin, carbamazepine, and valproic acid) can also impair thyroid function [80]. Even though the underlying mechanisms are not completely understood, accelerated clearance of TH or interference with the regulation of pituitary TSH secretion has been suggested [80, 81].
Lithium treatment has been associated with the development of thyroid dysfunction. The common clinical side effects of the drug are hypothyroidism and goiter. The prevalence of lithium-induced hypothyroidism varies between 6% and 52% according to several series, and it is usually subclinical, although severe hypothyroidism has been reported. Hypothyroidism may occur without thyroid enlargement as well as with goiter. Furthermore, lithium exacerbates preexisting autoimmune thyroid disease by accelerating the increase in thyroid antibody titer. Therefore, thyroid function should be tested and carefully monitored in patients receiving lithium therapy [82].
Therapeutic as well as environmental exposure to ionizing radiation during childhood can cause mild thyroid dysfunction. Although primary hypothyroidism in childhood cancer survivors is a well-known effect [83] the prevalence of SH compared with overt hypothyroidism is not yet well defined.
The incidence of SH has been reported ~26.5% in children who had received irradiation before bone marrow transplantation, with a tendency to resolve spontaneously over years in most cases [84].
Finally, a clear association has also been reported between iodine-131 exposure in Ukraine and Belarusian children and thyroid dysfunction, including SH [85, 86].
F. Endocrine Disruptors
Several endocrine disruptors, such as chemicals, food, and consumer products, can interfere with thyroid function by acting on different points of regulation of thyroid hormone [87]. Human studies investigating the relationship between chemical exposures and alterations in circulating levels of thyroid hormones led to variable results, possibly because an association between chemical exposures and circulating hormones is difficult to test directly in humans; however, there is evidence that perfluorinated chemicals, polychlorinated biphenyls and dioxins, bisphenol A, perchlorate, and phthalates may have thyroid-disrupting properties in humans [88].
In particular, dioxins and polychlorinated biphenyls may cross the placenta and be excreted in breast milk. In utero or perinatal exposure to these chemicals has been associated with increased TSH levels in infants in some but not all studies, with unclear long-lasting effects in older ages [88]. Further studies are needed to clarify the relationship between mild thyroid dysfunction and the exposure to endocrine disruptors in children.
G. Idiopathic SH
The term idiopathic SH refers to those patients with a persistent mild increase in TSH levels in whom no clear etiology has been identified.
4. Management of Mild SH in Childhood
Current guidelines from the American Thyroid Association and the European Thyroid Association suggest offering L-T4 treatment to adults with TSH levels >10 mIU/L, as well as to those with TSH levels of 4.5 to 10.0 mIU/L in the presence of symptoms or signs of hypothyroidism and/or antithyroid antibodies and/or evidence of atherosclerotic cardiovascular disease [89, 90].
In children, the management of mild SH is still a matter of debate, and the need for therapy is questionable. Indeed, the potential effects of mild hypothyroidism on health outcomes are not clear, and current data do not suggest detrimental effects on neurocognitive and growth development.
A few studies have evaluated linear growth among children with untreated mild autoimmune [91, 92] and nonautoimmune SH [53, 64, 93, 94] and most of them reported normal height even after longstanding untreated SH [53, 92–94]. Moreover, no appreciable effects after 2 years of L-T4 treatment on linear growth were detected among children with mild idiopathic SH [95, 96].
Data on the effect of SH on neurocognitive outcome in children are conflicting. Even though subtle abnormalities in attention have been reported in two small studies on SH children [97, 98], data from a large survey reported normal cognitive performance in SH adolescents [51]. Furthermore, a recent prospective case-control study among 30 children with mild but long-lasting idiopathic SH reported normal verbal, performance and full-scale IQ, comparable to euthyroid controls [93].
No abnormalities in biochemical markers of bone metabolism, lumbar bone mineral density, and bone quality were detected in two studies evaluating the effects of untreated mild SH on bone health [99, 100].
Recently, concern has been raised on adverse cardiovascular (CV) outcome in patients with untreated SH. Coronary heart disease and heart failure seem to occur most frequently among adults with SH, particularly when the TSH levels exceed 10 mIU/L [1, 4].
Higher TSH levels have been associated with less favorable lipid levels, even in children [3]. Moreover, recent longitudinal case-control studies demonstrated that untreated mild SH in children can be associated with early CV risk factors such as mild dyslipidemia, increased visceral adiposity, increased levels of homocysteine, early markers of endothelial dysfunction, and preclinical alteration in left ventricular function [94, 96, 101]. L-T4 treatment was associated with beneficial effects on most biochemical markers of CV risk and cardiac and endothelial function [96, 101].
However, despite these subtle CV abnormalities, current data are not sufficient to recommend treatment of all children with mild SH [3, 96], and the decision to treat should be based on individual patient factors.
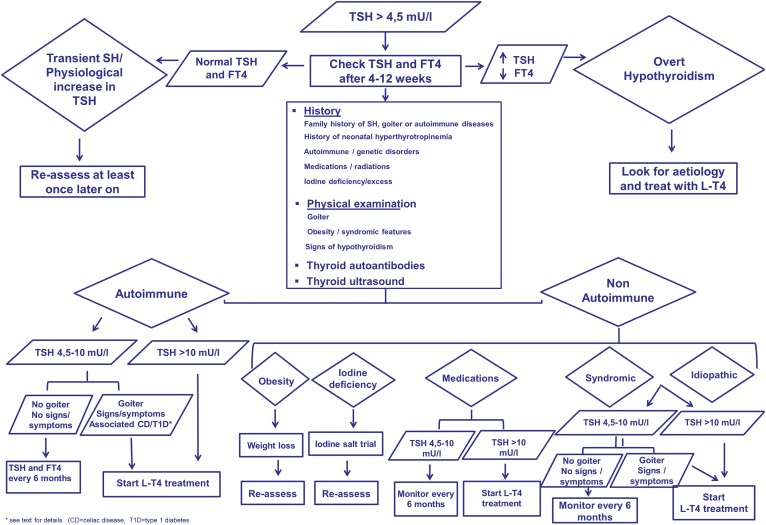
A schematic approach to the management of mild SH in children is summarized in Fig. 1.
Figure 1.
Schematic approach to diagnosis and management of SH in children. (Adapted with permission from Salerno M, Capalbo D, Cerbone M, De Luca F. Sublinical hypothyroidism in childhood: current knowledge and open issues. Nat Rev Endocrinol. 2016; 12(12):734-746 [3].)
The first step in managing a child with a mild increase in TSH levels should be to rule out abnormal values caused by laboratory problems, diurnal variation in TSH concentration, and transient causes of SH (recovery phase from nonthyroidal illness or subacute thyroiditis). Persistent SH should be confirmed by reevaluation of the TSH levels at 4 to 12 weeks after the first test.
In children with persistent elevated TSH level, a diagnostic workup is recommended. The child’s history should focus on the presence of neonatal hyperthyrotropinemia, autoimmune and/or genetic conditions, use of medications known to interfere with thyroid function, previous exposure to ionizing radiation, and endemic iodine deficiency. Attention should be given to the presence of SH, goiter, and endocrine, genetic, or autoimmune diseases in family members. Physical examination should focus on signs of hypothyroidism, goiter, weight gain, and clinical features suggestive of specific genetic conditions.
All patients should be screened for the presence of antithyroid antibodies. Thyroid ultrasound may provide additional information on gland morphology and structure. Further investigations can be considered on the basis of personal history, physical examination, and dysmorphic features. Urinary iodine excretion for those living in endemically deficient areas should be carried out. For cases arising in familial settings, genetic analysis can be considered. In patients with mild SH and risk factors such as a family history of hyperlipidemia, presence of acanthosis, or body mass index >90%, a screening lipid panel may be considered.
The subsequent management and the decision on treatment should depend on the etiology, the degree of TSH elevation, the risk of progression to overt hypothyroidism, and the presence of clinical symptoms or signs of mild thyroid impairment.
In the most common clinical scenario of autoimmune SH, treatment with L-T4 should be started for all children affected by severe forms (TSH level >10 mIU/L) or among those with mild SH in the presence of goiter or the signs or symptoms of hypothyroidism. In children with HT, a beneficial effect of L-T4 on goiter has been documented in several studies, even in patients with normal or subclinical thyroid dysfunction [102–104].
In children with untreated HT, thyroid function (FT4 and TSH) should be monitored every 6 months. Repeated measurements of thyroid antibodies during the follow-up do not contribute to the management of SH. The frequency of imaging should be personalized based on signs and symptoms. Children with Turner syndrome and DS should be carefully monitored for the greater risk of progressive thyroid dysfunction.
The management of SH associated with other autoimmune diseases such as celiac disease and type 1 diabetes represents a difficult issue. Even though a recent study suggests that the presence of celiac disease in patients with HT and SH is a predictive factor for thyroid failure [58] there are no data on the effects of early L-T4 treatment in these children.
Studies in children and adolescents with type 1 diabetes suggest that mild SH may be associated with an increased risk of dyslipidemia [105] and symptomatic hypoglycemia [106], whereas growth impairment has been documented only in children with severe overt thyroid dysfunction [91].
In the absence of longitudinal studies on the benefits of early treatment with L-T4, the decision of treating mild SH associated with diabetes or celiac disease remains controversial.
Currently, data are insufficient to establish a specific TSH cutoff to treat these patients, and L-T4 treatment should be personalized, taking into account not only the presence of subtle signs or symptoms of thyroid failure but also the control of the underlying disease.
TSH resistance should be considered in the differential diagnosis of all patients with nonautoimmune, nongoitrous hyperthyrotropinemia, but the need for therapy is questionable, and intervention should depend on the child’s age and the degree of TSH elevation. Therapy is recommended in case of overt hypothyroidism, whereas careful monitoring is suggested for mild and asymptomatic forms.
In children who are overweight or obese, diet and lifestyle changes should be recommended and thyroid function should be rechecked after weight loss. Iodine supplementation is recommended among children living in areas with endemic iodine deficiency and/or with documented reduced iodine excretion.
In children treated with medications that might interfere with thyroid function, treatment with L-T4 should be considered for children with a TSH level >10 mIU/L. A possible recovery of thyroid function should be evaluated at medication withdrawal.
Finally, in all forms of SH that resolve at any point during follow-up, a reevaluation of thyroid function should be considered later in life, particularly during adolescence and pregnancy.
5. Conclusions
The management of mild hypothyroidism in childhood is challenging. The major concern is to establish whether this condition should always be considered an expression of mild thyroid dysfunction. In the neonatal period, the decision to start treatment with L-T4 depends on multiple factors, such as the age of the patient, the duration of the thyroid dysfunction, the trend of TSH values, the etiology, the presence of syndromes and/or other pathologies, and the parents’ choice and reliability. In case of a persistent mild increase in TSH levels and in special risk categories (preterm, LBW, twins, infants from mothers affected by autoimmune thyroiditis, twins), it might be prudent to start treatment and to reevaluate the thyroid axis at a later stage.
The optimum management of children with SH depends on the etiology and degree of TSH elevation and should be individually tailored. Although mild untreated SH seems not to be associated with alterations in growth or neurocognitive development, subtle proatherogenic abnormalities have been detected, which are reversed with treatment with L-T4.
Current recommendations support L-T4 therapy for children with severe SH, goiter, or symptoms suggestive of hypothyroidism, whereas there is not enough evidence to recommend treatment of all children with mild asymptomatic forms of SH.
In the absence of therapeutic intervention, clinical examination and thyroid function tests should be regularly performed to ensure early identification of children who might benefit from treatment.
Further data are necessary prior to fully implement recommendation in the management of children with mild SH.
6. Search Methods
We conducted a PubMed searches for articles published between 2007 and 2017 using the following terms or combinations of terms: congenital hypothyroidism (CH), mild hypothyroidism, mild thyroid dysfunction, and subclinical hypothyroidism. This search allowed us to identify several publications that were selected on the basis of research methodology and scientific relevance.
Acknowledgments
Disclosure Summary: The authors have nothing to disclose.
Glossary
Abbreviations:
- CH
congenital hypothyroidism
- CV
cardiovascular
- DS
Down syndrome
- ESPE
European Society for Pediatric Endocrinologist
- FT4
free T4
- HT
Hashimoto thyroiditis
- IFN
interferon
- IVF
in vitro fertilization
- LBW
low birthweight
- L-T4
levothyroxine
- rTSH-Ab
TSH receptor antibody
- SH
subclinical hypothyroidism
- VLBW
very low birthweight
References and Notes
- 1. Biondi B, Cooper DS. The clinical significance of subclinical thyroid dysfunction. Endocr Rev. 2008;29(1):76–131. [DOI] [PubMed] [Google Scholar]
- 2. Léger J, Olivieri A, Donaldson M, Torresani T, Krude H, van Vliet G, Polak M, Butler G; ESPE-PES-SLEP-JSPE-APEG-APPES-ISPAE Congenital Hypothyroidism Consensus Conference Group . European Society for Paediatric Endocrinology consensus guidelines on screening, diagnosis, and management of congenital hypothyroidism. J Clin Endocrinol Metab. 2014;99(2):363–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Salerno M, Capalbo D, Cerbone M, De Luca F. Subclinical hypothyroidism in childhood—current knowledge and open issues. Nat Rev Endocrinol. 2016;12(12):734–746. [DOI] [PubMed] [Google Scholar]
- 4. Karmisholt J, Andersen S, Laurberg P. Variation in thyroid function in subclinical hypothyroidism: importance of clinical follow-up and therapy. Eur J Endocrinol. 2011;164(3):317–323. [DOI] [PubMed] [Google Scholar]
- 5. Clerico A, Ripoli A, Fortunato A, Alfano A, Carrozza C, Correale M, Dittadi R, Gessoni G, Migliardi M, Rizzardi S, Prontera C, Masotti S, Zucchelli G, Guiotto C, Iacovazzi PA, Iervasi G; Italian Section of the European Ligand Assay Society (ELAS) . Harmonization protocols for TSH immunoassays: a multicenter study in Italy. Clin Chem Lab Med. 2017;55(11):1722–1733. [DOI] [PubMed] [Google Scholar]
- 6. da Silva VA, de Almeida RJ, Cavalcante MP, Pereira Junior LA, Reis FM, Pereira MF, Kasamatsu TS, Camacho CP. Two thyroid stimulating hormone assays correlated in clinical practice show disagreement in subclinical hypothyroidism patients. Clin Biochem. 2018;53:13–18. [DOI] [PubMed] [Google Scholar]
- 7. Peeters RP. Subclinical hypothyroidism. N Engl J Med. 2017;376(26):2556–2565. [DOI] [PubMed] [Google Scholar]
- 8. Deladoëy J, Ruel J, Giguère Y, Van Vliet G. Is the incidence of congenital hypothyroidism really increasing? A 20-year retrospective population-based study in Québec. J Clin Endocrinol Metab. 2011;96(8):2422–2429. [DOI] [PubMed] [Google Scholar]
- 9. Olivieri A, Fazzini C, Medda E; Italian Study Group for Congenital Hypothyroidism . Multiple factors influencing the incidence of congenital hypothyroidism detected by neonatal screening. Horm Res Paediatr. 2015;83(2):86–93. [DOI] [PubMed] [Google Scholar]
- 10. Rabbiosi S, Vigone MC, Cortinovis F, Zamproni I, Fugazzola L, Persani L, Corbetta C, Chiumello G, Weber G. Congenital hypothyroidism with eutopic thyroid gland: analysis of clinical and biochemical features at diagnosis and after re-evaluation. J Clin Endocrinol Metab. 2013;98(4):1395–1402. [DOI] [PubMed] [Google Scholar]
- 11. Corbetta C, Weber G, Cortinovis F, Calebiro D, Passoni A, Vigone MC, Beck-Peccoz P, Chiumello G, Persani L. A 7-year experience with low blood TSH cutoff levels for neonatal screening reveals an unsuspected frequency of congenital hypothyroidism (CH). Clin Endocrinol (Oxf). 2009;71(5):739–745. [DOI] [PubMed] [Google Scholar]
- 12. Hashemipour M, Hovsepian S, Ansari A, Keikha M, Khalighinejad P, Niknam N. Screening of congenital hypothyroidism in preterm, low birth weight and very low birth weight neonates: a systematic review. Pediatr Neonatol. 2018;59(1):3–14. [DOI] [PubMed] [Google Scholar]
- 13. Thorpe-Beeston JG, Nicolaides KH, Snijders RJ, Felton CV, McGregor AM. Thyroid function in small for gestational age fetuses. Obstet Gynecol. 1991;77(5):701–706. [PubMed] [Google Scholar]
- 14. Onal H, Ercan O, Adal E, Ersen A, Onal Z. Subclinical hypothyroidism in in vitro fertilization babies. Acta Paediatr. 2012;101(6):e248–e252. [DOI] [PubMed] [Google Scholar]
- 15. Olivieri A, Medda E, De Angelis S, Valensise H, De Felice M, Fazzini C, Cascino I, Cordeddu V, Sorcini M, Stazi MA; Study Group for Congenital Hypothyroidism . High risk of congenital hypothyroidism in multiple pregnancies. J Clin Endocrinol Metab. 2007;92(8):3141–3147. [DOI] [PubMed] [Google Scholar]
- 16. Léger J. Management of fetal and neonatal Graves’ disease. Horm Res Paediatr. 2016;87(1):1–6. [DOI] [PubMed] [Google Scholar]
- 17. Rovelli R, Vigone MC, Giovanettoni C, Passoni A, Maina L, Corrias A, Corbetta C, Mosca F, Chiumello G, Weber G. Newborn of mothers affected by autoimmune thyroiditis: the importance of thyroid function monitoring in the first months of life. Ital J Pediatr. 2010;36:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. McGovern M, Reyani Z, O’Connor P, White M, Miletin J. Thyroid function testing in neonates born to women with hypothyroidism. Eur J Pediatr. 2016;175(12):2015–2018. [DOI] [PubMed] [Google Scholar]
- 19. Brown RS, Bellisario RL, Botero D, Fournier L, Abrams CA, Cowger ML, David R, Fort P, Richman RA. Incidence of transient congenital hypothyroidism due to maternal thyrotropin receptor-blocking antibodies in over one million babies. J Clin Endocrinol Metab. 1996;81(3):1147–1151. [DOI] [PubMed] [Google Scholar]
- 20. Hallett A, Evans C, Moat S, Barton J, Warner J, Gregory JW. Hypothyroidism in preterm infants following normal screening. Ann Clin Biochem. 2011;48(6):572–574. [DOI] [PubMed] [Google Scholar]
- 21. Bona G, De Luca F, Monzani A, eds. Thyroid Diseases in Childhood: Recent Advances From Basic Science to Clinical Practice. Springer Switzerland: Springer International; 2015:75–83. [Google Scholar]
- 22. Brown RS, Alter CA, Sadeghi-Nejad A. Severe unsuspected maternal hypothyroidism discovered after the diagnosis of thyrotropin receptor-blocking antibody-induced congenital hypothyroidism in the neonate: failure to recognize and implications to the fetus. Horm Res Paediatr. 2014;83(2):132–135. [DOI] [PubMed] [Google Scholar]
- 23. Fisher DA. Thyroid system immaturities in very low birth weight premature infants. Semin Perinatol. 2008;32(6):387–397. [DOI] [PubMed] [Google Scholar]
- 24. Vigone MC, Caiulo S, Di Frenna M, Ghirardello S, Corbetta C, Mosca F, Weber G. Evolution of thyroid function in preterm infants detected by screening for congenital hypothyroidism. J Pediatr. 2014;164(6):1296–1302. [DOI] [PubMed] [Google Scholar]
- 25. Woo HC, Lizarda A, Tucker R, Mitchell ML, Vohr B, Oh W, Phornphutkul C. Congenital hypothyroidism with a delayed thyroid-stimulating hormone elevation in very premature infants: incidence and growth and developmental outcomes. J Pediatr. 2011;158(4):538–542. [DOI] [PubMed] [Google Scholar]
- 26. Cavarzere P, Camilot M, Popa FI, Lauriola S, Teofoli F, Gaudino R, Vincenzi M, Antoniazzi F. Congenital hypothyroidism with delayed TSH elevation in low-birth-weight infants: incidence, diagnosis and management. Eur J Endocrinol. 2016;175(5):395–402. [DOI] [PubMed] [Google Scholar]
- 27. Delahunty C, Falconer S, Hume R, Jackson L, Midgley P, Mirfield M, Ogston S, Perra O, Simpson J, Watson J, Willatts P, Williams F; Scottish Preterm Thyroid Group . Levels of neonatal thyroid hormone in preterm infants and neurodevelopmental outcome at 5 1/2 years: millennium cohort study. J Clin Endocrinol Metab. 2010;95(11):4898–4908. [DOI] [PubMed] [Google Scholar]
- 28. Uchiyama A, Kushima R, Watanabe T, Kusuda S. Effect of L-thyroxine supplementation on very low birth weight infants with transient hypothyroxinemia of prematurity at 3 years of age. J Perinatol. 2017;37(5):602–605. [DOI] [PubMed] [Google Scholar]
- 29. La Gamma EF, van Wassenaer AG, Ares S, Golombek SG, Kok JH, Quero J, Hong T, Rahbar MH, de Escobar GM, Fisher DA, Paneth N. Phase 1 trial of 4 thyroid hormone regimens for transient hypothyroxinemia in neonates of <28 weeks’ gestation. Pediatrics. 2009;124(2):e258–e268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Osborn DA, Hunt RW. Postnatal thyroid hormones for preterm infants with transient hypothyroxinaemia. Cochrane Database Syst Rev. 2007;1(1):CD005945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Fagard R, Brguljan J, Staessen J, Thijs L, Derom C, Thomis M, Vlietinck R. Heritability of conventional and ambulatory blood pressures: a study in twins. Hypertension. 1995;26(6 Pt 1):919–924. [DOI] [PubMed] [Google Scholar]
- 32. Umstad MP, Gronow MJ. Multiple pregnancy: a modern epidemic? Med J Aust. 2003;178(12):613–615. [DOI] [PubMed] [Google Scholar]
- 33. Sakka SD, Malamitsi-Puchner A, Loutradis D, Chrousos GP, Kanaka-Gantenbein C. Euthyroid hyperthyrotropinemia in children born after in vitro fertilization. J Clin Endocrinol Metab. 2009;94(4):1338–1341. [DOI] [PubMed] [Google Scholar]
- 34. de Filippis T, Gelmini G, Paraboschi E, Vigone MC, Di Frenna M, Marelli F, Bonomi M, Cassio A, Larizza D, Moro M, Radetti G, Salerno M, Ardissino D, Weber G, Gentilini D, Guizzardi F, Duga S, Persani L. A frequent oligogenic involvement in congenital hypothyroidism. Hum Mol Genet. 2017;26(13):2507–2514. [DOI] [PubMed] [Google Scholar]
- 35. de Filippis T, Marelli F, Nebbia G, Porazzi P, Corbetta S, Fugazzola L, Gastaldi R, Vigone MC, Biffanti R, Frizziero D, Mandarà L, Prontera P, Salerno M, Maghnie M, Tiso N, Radetti G, Weber G, Persani L. JAG1 loss-of-function variations as a novel predisposing event in the pathogenesis of congenital thyroid defects. J Clin Endocrinol Metab. 2016;101(3):861–870. [DOI] [PubMed] [Google Scholar]
- 36. Vigone MC, Cortinovis F, Rabbiosi S, Di Frenna M, Passoni A, Persani L, Chiumello G, Gelmetti C, Weber G. Difficult treatment of consumptive hypothyroidism in a child with massive parotid hemangioma. J Pediatr Endocrinol Metab. 2012;25(1–2):153–155. [DOI] [PubMed] [Google Scholar]
- 37. Jassam N, Visser TJ, Brisco T, Bathia D, McClean P, Barth JH. Consumptive hypothyroidism: a case report and review of the literature. Ann Clin Biochem. 2011;48(Pt 2):186–189. [DOI] [PubMed] [Google Scholar]
- 38. Persani L, Calebiro D, Cordella D, Weber G, Gelmini G, Libri D, de Filippis T, Bonomi M. Genetics and phenomics of hypothyroidism due to TSH resistance. Mol Cell Endocrinol. 2010;322(1–2):72–82. [DOI] [PubMed] [Google Scholar]
- 39. Grasberger H, Refetoff S. Resistance to thyrotropin. Best Pract Res Clin Endocrinol Metab. 2017;31(2):183–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Narumi S, Hasegawa T. TSH resistance revisited. Endocr J. 2015;62(5):393–398. [DOI] [PubMed] [Google Scholar]
- 41. Alberti L, Proverbio MC, Costagliola S, Romoli R, Boldrighini B, Vigone MC, Weber G, Chiumello G, Beck-Peccoz P, Persani L. Germline mutations of TSH receptor gene as cause of nonautoimmune subclinical hypothyroidism. J Clin Endocrinol Metab. 2002;87(6):2549–2555. [DOI] [PubMed] [Google Scholar]
- 42. Nicoletti A, Bal M, De Marco G, Baldazzi L, Agretti P, Menabò S, Ballarini E, Cicognani A, Tonacchera M, Cassio A. Thyrotropin-stimulating hormone receptor gene analysis in pediatric patients with non-autoimmune subclinical hypothyroidism. J Clin Endocrinol Metab. 2009;94(11):4187–4194. [DOI] [PubMed] [Google Scholar]
- 43. Cassio A, Nicoletti A, Rizzello A, Zazzetta E, Bal M, Baldazzi L. Current loss-of-function mutations in the thyrotropin receptor gene: when to investigate, clinical effects, and treatment. J Clin Res Pediatr Endocrinol. 2013;5(Suppl 1):29–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Vigone MC, Di Frenna M, Guizzardi F, Gelmini G, de Filippis T, Mora S, Caiulo S, Sonnino M, Bonomi M, Persani L, Weber G. Mild TSH resistance: clinical and hormonal features in childhood and adulthood. Clin Endocrinol (Oxf). 2017;87(5):587–596. [DOI] [PubMed] [Google Scholar]
- 45. Mizuno H, Kanda K, Sugiyama Y, Imamine H, Ito T, Kato I, Togari H, Kamoda T, Onigata K. Longitudinal evaluation of patients with a homozygous R450H mutation of the TSH receptor gene. Horm Res. 2009;71(6):318–323. [DOI] [PubMed] [Google Scholar]
- 46. Fugazzola L, Muzza M, Weber G, Beck-Peccoz P, Persani L. DUOXS defects: genotype-phenotype correlations. Ann Endocrinol (Paris). 2011;72(2):82–86. [DOI] [PubMed] [Google Scholar]
- 47. Muzza M, Rabbiosi S, Vigone MC, Zamproni I, Cirello V, Maffini MA, Maruca K, Schoenmakers N, Beccaria L, Gallo F, Park SM, Beck-Peccoz P, Persani L, Weber G, Fugazzola L. The clinical and molecular characterization of patients with dyshormonogenic congenital hypothyroidism reveals specific diagnostic clues for DUOX2 defects. J Clin Endocrinol Metab. 2014;99(3):E544–E553. [DOI] [PubMed] [Google Scholar]
- 48. Leonardi D, Polizzotti N, Carta A, Gelsomino R, Sava L, Vigneri R, Calaciura F. Longitudinal study of thyroid function in children with mild hyperthyrotropinemia at neonatal screening for congenital hypothyroidism. J Clin Endocrinol Metab. 2008;93(7):2679–2685. [DOI] [PubMed] [Google Scholar]
- 49. Tenenbaum-Rakover Y, Almashanu S, Hess O, Admoni O, Hag-Dahood Mahameed A, Schwartz N, Allon-Shalev S, Bercovich D, Refetoff S. Long-term outcome of loss-of-function mutations in thyrotropin receptor gene. Thyroid. 2015;25(3):292–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Weber G, Rabbiosi S, Zamproni I, Fugazzola L. Genetic defects of hydrogen peroxide generation in the thyroid gland. J Endocrinol Invest. 2013;36(4):261–266. [DOI] [PubMed] [Google Scholar]
- 51. Wu T, Flowers JW, Tudiver F, Wilson JL, Punyasavatsut N. Subclinical thyroid disorders and cognitive performance among adolescents in the United States. BMC Pediatr. 2006;6(1):12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Lazar L, Frumkin RB, Battat E, Lebenthal Y, Phillip M, Meyerovitch J. Natural history of thyroid function tests over 5 years in a large pediatric cohort. J Clin Endocrinol Metab. 2009;94(5):1678–1682. [DOI] [PubMed] [Google Scholar]
- 53. Wasniewska M, Salerno M, Cassio A, Corrias A, Aversa T, Zirilli G, Capalbo D, Bal M, Mussa A, De Luca F. Prospective evaluation of the natural course of idiopathic subclinical hypothyroidism in childhood and adolescence. Eur J Endocrinol. 2008;160(3):417–421. [DOI] [PubMed] [Google Scholar]
- 54. Gopalakrishnan S, Chugh PK, Chhillar M, Ambardar VK, Sahoo M, Sankar R. Goitrous autoimmune thyroiditis in a pediatric population: a longitudinal study. Pediatrics. 2008;122(3):e670–e674. [DOI] [PubMed] [Google Scholar]
- 55. Effraimidis G, Wiersinga WM. Mechanisms in endocrinology: autoimmune thyroid disease: old and new players. Eur J Endocrinol. 2014;170(6):R241–R252. [DOI] [PubMed] [Google Scholar]
- 56. Brown RS. Autoimmune thyroiditis in childhood. J Clin Res Pediatr Endocrinol. 2013;5(Suppl 1):45–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Radetti G, Maselli M, Buzi F, Corrias A, Mussa A, Cambiaso P, Salerno M, Cappa M, Baiocchi M, Gastaldi R, Minerba L, Loche S. The natural history of the normal/mild elevated TSH serum levels in children and adolescents with Hashimoto’s thyroiditis and isolated hyperthyrotropinaemia: a 3-year follow-up. Clin Endocrinol (Oxf). 2012;76(3):394–398. [DOI] [PubMed] [Google Scholar]
- 58. Wasniewska M, Corrias A, Salerno M, Mussa A, Capalbo D, Messina MF, Aversa T, Bombaci S, De Luca F, Valenzise M. Thyroid function patterns at Hashimoto’s thyroiditis presentation in childhood and adolescence are mainly conditioned by patients’ age. Horm Res Paediatr. 2012;78(4):232–236. [DOI] [PubMed] [Google Scholar]
- 59. Aversa T, Valenzise M, Corrias A, Salerno M, De Luca F, Mussa A, Rezzuto M, Lombardo F, Wasniewska M. Underlying Hashimoto’s thyroiditis negatively affects the evolution of subclinical hypothyroidism in children irrespective of other concomitant risk factors. Thyroid. 2015;25(2):183–187. [DOI] [PubMed] [Google Scholar]
- 60. Wasniewska M, Aversa T, Salerno M, Corrias A, Messina MF, Mussa A, Capalbo D, De Luca F, Valenzise M. Five-year prospective evaluation of thyroid function in girls with subclinical mild hypothyroidism of different etiology. Eur J Endocrinol. 2015;173(6):801–808. [DOI] [PubMed] [Google Scholar]
- 61. Niranjan U, Wright NP. Should we treat subclinical hypothyroidism in obese children? BMJ. 2016;352:i941. [DOI] [PubMed] [Google Scholar]
- 62. Reinehr T. Thyroid function in the nutritionally obese child and adolescent. Curr Opin Pediatr. 2011;23(4):415–420. [DOI] [PubMed] [Google Scholar]
- 63. Radetti G, Kleon W, Buzi F, Crivellaro C, Pappalardo L, di Iorgi N, Maghnie M. Thyroid function and structure are affected in childhood obesity. J Clin Endocrinol Metab. 2008;93(12):4749–4754. [DOI] [PubMed] [Google Scholar]
- 64. Rapa A, Monzani A, Moia S, Vivenza D, Bellone S, Petri A, Teofoli F, Cassio A, Cesaretti G, Corrias A, de Sanctis V, Di Maio S, Volta C, Wasniewska M, Tatò L, Bona G. Subclinical hypothyroidism in children and adolescents: a wide range of clinical, biochemical, and genetic factors involved. J Clin Endocrinol Metab. 2009;94(7):2414–2420. [DOI] [PubMed] [Google Scholar]
- 65. Grandone A, Santoro N, Coppola F, Calabrò P, Perrone L, Del Giudice EM. Thyroid function derangement and childhood obesity: an Italian experience. BMC Endocr Disord. 2010;10(1):8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Wolters B, Lass N, Reinehr T. TSH and free triiodothyronine concentrations are associated with weight loss in a lifestyle intervention and weight regain afterwards in obese children. Eur J Endocrinol. 2012;168(3):323–329. [DOI] [PubMed] [Google Scholar]
- 67. Pierce MJ, LaFranchi SH, Pinter JD. Characterization of thyroid abnormalities in a large cohort of children with Down syndrome. Horm Res Paediatr. 2017;87(3):170–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. King K, O’Gorman C, Gallagher S. Thyroid dysfunction in children with Down syndrome: a literature review. Ir J Med Sci. 2013;183(1):1–6. [DOI] [PubMed] [Google Scholar]
- 69. Gibson PA, Newton RW, Selby K, Price DA, Leyland K, Addison GM. Longitudinal study of thyroid function in Down’s syndrome in the first two decades. Arch Dis Child. 2005;90(6):574–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Van Vliet G. How often should we screen children with Down’s syndrome for hypothyroidism? Arch Dis Child. 2005;90(6):557–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Zwaveling-Soonawala N, Witteveen ME, Marchal JP, Klouwer FC, Ikelaar NA, Smets AM, van Rijn RR, Endert E, Fliers E, van Trotsenburg AS. Early thyroxine treatment in Down syndrome and thyroid function later in life. Eur J Endocrinol. 2017;176(5):505–513. [DOI] [PubMed] [Google Scholar]
- 72. Mantovani G, Spada A, Elli FM. Pseudohypoparathyroidism and Gsα-cAMP-linked disorders: current view and open issues. Nat Rev Endocrinol. 2016;12(6):347–356. [DOI] [PubMed] [Google Scholar]
- 73. Zimmermann MB, Boelaert K. Iodine deficiency and thyroid disorders. Lancet Diabetes Endocrinol. 2015;3(4):286–295. [DOI] [PubMed] [Google Scholar]
- 74. Rosene ML, Wittmann G, Arrojo e Drigo R, Singru PS, Lechan RM, Bianco AC. Inhibition of the type 2 iodothyronine deiodinase underlies the elevated plasma TSH associated with amiodarone treatment. Endocrinology. 2010;151(12):5961–5970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Barr ML, Chiu HK, Li N, Yeh MW, Rhee CM, Casillas J, Iskander PJ, Leung AM. Thyroid dysfunction in children exposed to iodinated contrast media. J Clin Endocrinol Metab. 2016;101(6):2366–2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Clement SC, van Eck-Smit BLF, van Trotsenburg AS, Kremer LCM, Tytgat GA, van Santen HM. Long-term follow-up of the thyroid gland after treatment with 131I-metaiodobenzylguanidine in children with neuroblastoma: importance of continuous surveillance. Pediatr Blood Cancer. 2013;60(11):1833–1838. [DOI] [PubMed] [Google Scholar]
- 77. Wassner AJ. Pediatric hypothyroidism: diagnosis and treatment. Paediatr Drugs. 2017;19(4):291–301. [DOI] [PubMed] [Google Scholar]
- 78. Tomer Y, Menconi F. Interferon induced thyroiditis. Best Pract Res Clin Endocrinol Metab. 2009;23(6):703–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Serranti D, Indolfi G, Nebbia G, Cananzi M, D’Antiga L, Ricci S, Stagi S, Azzari C, Resti M; Italian Study Group for Treatment of Chronic Hepatitis C in Children . Transient hypothyroidism and autoimmune thyroiditis in children with chronic hepatitis C treated with pegylated-interferon-α-2b and ribavirin. Pediatr Infect Dis J. 2018;37(4):287–291. [DOI] [PubMed] [Google Scholar]
- 80. Benedetti MS, Whomsley R, Baltes E, Tonner F. Alteration of thyroid hormone homeostasis by antiepileptic drugs in humans: involvement of glucuronosyltransferase induction. Eur J Clin Pharmacol. 2005;61(12):863–872. [DOI] [PubMed] [Google Scholar]
- 81. Verrotti A, Scardapane A, Manco R, Chiarelli F. Antiepileptic drugs and thyroid function. J Pediatr Endocrinol Metab. 2008;21(5):401–408. [DOI] [PubMed] [Google Scholar]
- 82. Lazarus JH. Lithium and thyroid. Best Pract Res Clin Endocrinol Metab. 2009;23(6):723–733. [DOI] [PubMed] [Google Scholar]
- 83. Chemaitilly W, Cohen LE. Diagnosis of endocrine disease: endocrine late-effects of childhood cancer and its treatments. Eur J Endocrinol. 2017;176(4):R183–R203. [DOI] [PubMed] [Google Scholar]
- 84. Ishiguro H, Yasuda Y, Tomita Y, Shinagawa T, Shimizu T, Morimoto T, Hattori K, Matsumoto M, Inoue H, Yabe H, Yabe M, Shinohara O, Kato S. Long-term follow-up of thyroid function in patients who received bone marrow transplantation during childhood and adolescence. J Clin Endocrinol Metab. 2004;89(12):5981–5986. [DOI] [PubMed] [Google Scholar]
- 85. Ostroumova E, Brenner A, Oliynyk V, McConnell R, Robbins J, Terekhova G, Zablotska L, Likhtarev I, Bouville A, Shpak V, Markov V, Masnyk I, Ron E, Tronko M, Hatch M. Subclinical hypothyroidism after radioiodine exposure: Ukrainian-American cohort study of thyroid cancer and other thyroid diseases after the Chornobyl accident (1998–2000). Environ Health Perspect. 2008;117(5):745–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Ostroumova E, Rozhko A, Hatch M, Furukawa K, Polyanskaya O, McConnell RJ, Nadyrov E, Petrenko S, Romanov G, Yauseyenka V, Drozdovitch V, Minenko V, Prokopovich A, Savasteeva I, Zablotska LB, Mabuchi K, Brenner AV. Measures of thyroid function among Belarusian children and adolescents exposed to iodine-131 from the accident at the Chernobyl nuclear plant. Environ Health Perspect. 2013;121(7):865–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Diamanti-Kandarakis E, Bourguignon JP, Giudice LC, Hauser R, Prins GS, Soto AM, Zoeller RT, Gore AC. Endocrine-disrupting chemicals: an Endocrine Society scientific statement. Endocr Rev. 2009;30(4):293–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Boas M, Feldt-Rasmussen U, Main KM. Thyroid effects of endocrine disrupting chemicals. Mol Cell Endocrinol. 2012;355(2):240–248. [DOI] [PubMed] [Google Scholar]
- 89. Garber JR, Cobin RH, Gharib H, Hennessey JV, Klein I, Mechanick JI, Pessah-Pollack R, Singer PA, Woeber KA; American Association of Clinical Endocrinologists and American Thyroid Association Taskforce on Hypothyroidism in Adults . Clinical practice guidelines for hypothyroidism in adults: cosponsored by the American Association of Clinical Endocrinologists and the American Thyroid Association. Thyroid. 2012;22(12):1200–1235. [DOI] [PubMed] [Google Scholar]
- 90. Pearce SH, Brabant G, Duntas LH, Monzani F, Peeters RP, Razvi S, Wemeau JL. 2013 ETA Guideline: management of subclinical hypothyroidism. Eur Thyroid J. 2013;2(4):215–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Chase HP, Garg SK, Cockerham RS, Wilcox WD, Walravens PA. Thyroid hormone replacement and growth of children with subclinical hypothyroidism and diabetes. Diabet Med. 1990;7(4):299–303. [DOI] [PubMed] [Google Scholar]
- 92. Radetti G, Gottardi E, Bona G, Corrias A, Salardi S, Loche S; Study Group for Thyroid Diseases of the Italian Society for Pediatric Endocrinology and Diabetes (SIEDP/ISPED) . The natural history of euthyroid Hashimoto’s thyroiditis in children. J Pediatr. 2006;149(6):827–832. [DOI] [PubMed] [Google Scholar]
- 93. Cerbone M, Bravaccio C, Capalbo D, Polizzi M, Wasniewska M, Cioffi D, Improda N, Valenzise M, Bruzzese D, De Luca F, Salerno M. Linear growth and intellectual outcome in children with long-term idiopathic subclinical hypothyroidism. Eur J Endocrinol. 2011;164(4):591–597. [DOI] [PubMed] [Google Scholar]
- 94. Cerbone M, Capalbo D, Wasniewska M, Mattace Raso G, Alfano S, Meli R, De Luca F, Salerno M. Cardiovascular risk factors in children with long-standing untreated idiopathic subclinical hypothyroidism. J Clin Endocrinol Metab. 2014;99(8):2697–2703. [DOI] [PubMed] [Google Scholar]
- 95. Wasniewska M, Corrias A, Aversa T, Valenzise M, Mussa A, De Martino L, Lombardo F, De Luca F, Salerno M. Comparative evaluation of therapy with L-thyroxine versus no treatment in children with idiopathic and mild subclinical hypothyroidism. Horm Res Paediatr. 2012;77(6):376–381. [DOI] [PubMed] [Google Scholar]
- 96. Cerbone M, Capalbo D, Wasniewska M, Alfano S, Mattace Raso G, Oliviero U, Cittadini A, De Luca F, Salerno M. Effects of L-thyroxine treatment on early markers of atherosclerotic disease in children with subclinical hypothyroidism. Eur J Endocrinol. 2016;175(1):11–19. [DOI] [PubMed] [Google Scholar]
- 97. Aijaz NJ, Flaherty EM, Preston T, Bracken SS, Lane AH, Wilson TA. Neurocognitive function in children with compensated hypothyroidism: lack of short term effects on or off thyroxin. BMC Endocr Disord. 2006;6(1):2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Ergür AT, Taner Y, Ata E, Melek E, Bakar EE, Sancak T. Neurocognitive functions in children and adolescents with subclinical hypothyroidism. J Clin Res Pediatr Endocrinol. 2012;4(1):21–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Saggese G, Bertelloni S, Baroncelli GI, Costa S, Ceccarelli C. Bone mineral density in adolescent females treated with L-thyroxine: a longitudinal study. Eur J Pediatr. 1996;155(6):452–457. [DOI] [PubMed] [Google Scholar]
- 100. Di Mase R, Cerbone M, Improda N, Esposito A, Capalbo D, Mainolfi C, Santamaria F, Pignata C, Salerno M. Bone health in children with long-term idiopathic subclinical hypothyroidism. Ital J Pediatr. 2012;38(1):56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Çatlı G, Kir M, Anik A, Yilmaz N, Böber E, Abaci A. The effect of L-thyroxine treatment on left ventricular functions in children with subclinical hypothyroidism. Arch Dis Child. 2014;100(2):130–137. [DOI] [PubMed] [Google Scholar]
- 102. Svensson J, Ericsson UB, Nilsson P, Olsson C, Jonsson B, Lindberg B, Ivarsson SA. Levothyroxine treatment reduces thyroid size in children and adolescents with chronic autoimmune thyroiditis. J Clin Endocrinol Metab. 2006;91(5):1729–1734. [DOI] [PubMed] [Google Scholar]
- 103. Scarpa V, Kousta E, Tertipi A, Vakaki M, Fotinou A, Petrou V, Hadjiathanasiou C, Papathanasiou A. Treatment with thyroxine reduces thyroid volume in euthyroid children and adolescents with chronic autoimmune thyroiditis. Horm Res Paediatr. 2010;73(1):61–67. [DOI] [PubMed] [Google Scholar]
- 104. Dörr HG, Bettendorf M, Binder G, Karges B, Kneppo C, Schmidt H, Voss E, Wabitsch M, Dötsch J. Levothyroxine treatment of euthyroid children with autoimmune hashimoto thyroiditis: results of a multicenter, randomized, controlled trial. Horm Res Paediatr. 2015;84(4):266–274. [DOI] [PubMed] [Google Scholar]
- 105. Denzer C, Karges B, Näke A, Rosenbauer J, Schober E, Schwab KO, Holl RW; DPV Initiative and the BMBF-Competence Network Diabetes Mellitus . Subclinical hypothyroidism and dyslipidemia in children and adolescents with type 1 diabetes mellitus. Eur J Endocrinol. 2013;168(4):601–608. [DOI] [PubMed] [Google Scholar]
- 106. Mohn A, Di Michele S, Di Luzio R, Tumini S, Chiarelli F. The effect of subclinical hypothyroidism on metabolic control in children and adolescents with type 1 diabetes mellitus. Diabet Med. 2002;19(1):70–73. [DOI] [PubMed] [Google Scholar]