Abstract
Amaranth (Amaranthus hypochondriacus Linn.) is an important pseudocereal crop having important nutrients along with the indispensable amino-acids. The present study was aimed to study the effect of plant growth promoting bacilli on proximate constituents of amaranth grains, including three of the essential amino acids (methionine, lysine and, tryptophan). The combination of Bacillus pumilus and Bacillus subtilis showed a significant increase in different proximate constituents, including crude protein (22.13%), dry matter (32.25%), fat (30.77%), and carbohydrate (49.08%) in amaranth grains. Similarly, a significant increase in essential amino-acids (methionine 47.68%, lysine 59.41% and, tryptophan 38.05%) was recorded. This study suggests that the combination of Bacillus pumilus BS-27 and Bacillus subtilis BS-58 provides the natural, persistent and durable potential to enhance the nutritive value of the crop. Therefore, present study was designed to explore the enhancement of most desirable amino acid synthesis in amaranth due to application of plant growth promoting Bacillus spp.
Keywords: Amaranth, Bacillus spp., Essential amino-acids, Proximate complete analysis
1. Introduction
Food security is an emerging issue all over the world and consequently pressure on major cereal crops is also increasing to accomplish the food requirement. But, the major crops such as rice, wheat, maize have insufficient amounts of important nutrients such as essential amino-acids, vitamins, minerals. This creates a fate towards nutrient scarcity especially in children and appears to be the foremost reason for malnutrition (Barrio and Anon, 2010). There are however certain crops which are nutritionally rich, but gained less attention and are therefore, considered as “Underutilized crops” including amaranth (Amaranthus hypochondriacus), barley (Hordeum vulgare), ragi (Elusine coracona), sorghum (Sorghum bicolor), etc. (Singh, 2017). Among these crops, grain amaranth has been reported as a very nutritive crop with high contents of nutrients such as protein (15.00–16.60%), amino-acids lysine (5.95 g/ 100 g of protein), tryptophan (1.80 g/100 g of protein), methionine (0.6 g/100 g of protein), fat (6.10–7.30%), carbohydrate (62.00–67.90%) and fiber (4.90–5.00%) (Mlakar et al., 2009). In addition, amaranth bears antipyretic, anti-inflammatory, immunomodulatory, antioxidant, anti allergic, antidiabetic properties, along with the ability to decrease the levels of plasma cholesterol (Mendonca et al., 2009, Mishra et al., 2012, Perales-Sanchez et al., 2014).
Poor soil fertility and crop losses due to plant diseases directly affect the production and nutritive quality of the crop. Though crop production and disease management can be done by the application of chemical fertilizers and pesticides, but their long-term application possess harmful effects on the plant ecology (Yadav et al., 2015). Therefore, use of plant growth promoting bacteria (PGPBs) to increase soil fertility, plant health, and disease management has become an inevitable strategy (Maheshwari et al., 2010, Chauhan et al., 2016). Use of these PGPBs being cost-effective and eco-friendly loom is getting popular especially among the marginal and small farmers (Negi et al., 2011). The application of PGPBs enhances the yield and nutritive value of the various crops especially beans, wheat, etc. (Al-Erwy et al., 2016).
Among all PGPBs, Bacillus spp. has been reported to have tolerance towards the adverse conditions and, therefore, the most potential candidate used for enhancing the soil fertility and crop health (Vivas et al., 2003). Bacillus spp. is also known to enhance of macro- and micronutrients in the soil and their uptake by host plant (Stefan et al., 2013). However, there is little knowledge on the role of bacilli in the enhancement of nutritional properties of amaranth in the harvested grain of plants raised due to the seeds dressed with PGP bacilli. It is therefore, explored to study the influence of most potential plant growth promoting bacilli in the enhancement of three important essential amino-acids, methionine, lysine and tryptophan in amaranth grains hawed in plants raised due to seeds dressed with Bacillus spp.
2. Material and methods
2.1. Bacterial strains
Three bacterial strains (BS-27, BS-41, and BS-58) having good plant growth promoting attributes were used in the present study. The strains were procured from the well-characterized repository of Microbiology lab, Dept. of Basic Science, College of Forestry, Ranichauri, Tehri Garhwal (Uttarakhand) India. Details of the PGP attributes of these three strains are given in Table 1. Talc formulation of these strains was made following the method of Negi et al. (2008).
Table 1.
PGP traits of selected Bacillus isolates.
| Isolates | P-solubilization | Siderophore production | IAA production | HCN production |
|---|---|---|---|---|
| BS-27 | ++ | + | ++ | + |
| BS-41 | + | ++ | ++ | ++ |
| BS-58 | ++ | ++ | ++ | − |
Abbreviation: +, ++ Presence, − Absence. All the assays were performed in triplicates.
2.2. Amaranth seeds
Amaranth seeds of variety PRA-1 procured from Dept. of Crop Improvement, College of Forestry, Ranichauri, Tehri Garhwal, were used in this study.
2.3. Experimental detail
Healthy seeds of amaranth (PRA-1) were bacterized by talc formulation (@8.0 g kg−1 seeds) of three selected Bacillus strains. The experiment was conducted with factorial random block design (RBD) at crop improvement research block of the College of Forestry, Ranichauri (N30°18.647′ E78°24.454′), Uttarakhand, India during April to October in the years 2015 and 2016.
2.4. Proximate complete analysis
The grains were collected from each treatment after harvesting and were used for the proximate complete analysis and amino-acid analysis. Proximate complete analysis of amaranth was done for the assessment of moisture, dry matter, total fat, carbohydrates, crude proteins, total ash and the fiber contents in amaranth grains following the methods as described by Nielsen (2017).
2.5. Qualitative and quantitative analysis of amino acids
Presence of test amino-acids was detected by thin layer chromatography (TLC) using the standard amino-acids. Extraction of amino-acids was carried out by crushing the grains (2.0 g) in a mortar pestle with the addition of 10 ml of 80% ethanol. The filtrate was collected in Petri-dishes and ethanol present in the filtrate was evaporated. Dried material was re-dissolved in 10% isopropanol and used for TLC following the method described by Blau and Halket (1993). The specific amino-acids were identified on the basis of Rf values of test and standard amino-acids. Quantitative estimation of total free amino-acids was done spectrophotometrically by following the method of Moore and Stein (1948). Briefly, 500 mg grain powder was suspended in 5–10 ml of 80% ethanol for the extraction of free amino-acids. To this, 0.1 ml of the supernatant was taken and 1.0 ml of the ninhydrin solution (0.1%) was added. The total volume of the sample was made up to 2.0 ml of distilled water. The tubes were heat treated in a boiling water bath for 20 min and then 5.0 ml of the diluent solution was added in each test tube. The absorbance of the resultant purple color solution was recorded at 570 nm using a double beam UV/Vis spectrophotometer (Shimadzu UV-VIS 1600) and the free amino-acid content was calculated from the regression equation of the standard curve and the results were expressed as μg/ml.
2.6. Quantitative estimations
2.6.1. Methionine
Methionine content in grain samples was estimated following the method of Horn et al. (1946) and the standard graph was derived and calculated by using the following formula:
2.6.2. Tryptophan
Tryptophan content in amaranth grain sample was estimated following the method of Mertz et al. (1975). Tryptophan content from the standard graph was drawn and calculated by formula given below:
2.6.3. Lysine
Lysine was estimated following the method given by Mertz et al. (1975). Lysine content was estimated by comparing from the standard graph and calculated by using the following formula:
2.7. Statistical analysis
The values were presented as means with standard errors (±SEM). The data recorded was subjected to analysis of variance (ANOVA) to evaluate the significance of differences (p < 0.05) among the treatments as given by Gomez and Gomez (1984).
3. Results
3.1. Proximate complete analysis
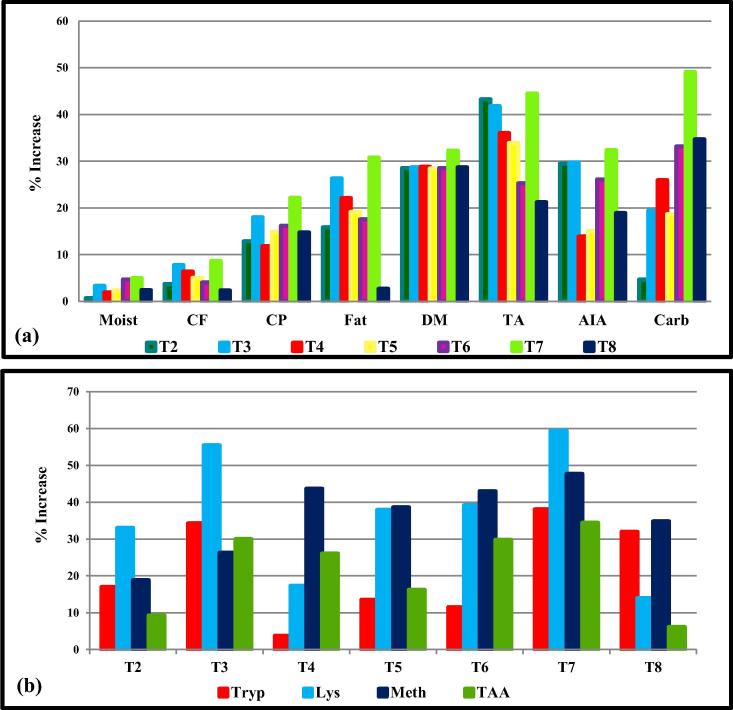
The results of the proximate complete analysis revealed that the treatment with the Bacillus species enhanced most of the chemical constituents of amaranth (Amaranthus hypochondriacus) grains. Among all the treatments, combinations of two bacilli (Bacillus pumilus BS-27 and Bacillus subtilis BS-58) were found best to enhance the maximum chemical constituents. Maximum increase (22.1%) in crude protein was recorded by the consortia of Bacillus pumilus and Bacillus subtilis followed by Bacillus pumilus (20.3%) over the control plant. However, combination of all three isolates was 6.5% lower in comparison to combination of two isolates and 18.6% higher to that of non-treated control plants. A significant increase in dry matter (32.2%) was recorded in the plants raised with combination of Bacillus pumilus and Bacillus subtilis followed by Bacillus subtilis which was 2.67% lower in comparison to combination of two bacilli and 28.8% higher in comparison to control. Consortia of Bacillus pumilus and Bacillus subtilis also exhibited maximum increase in fat content (30.7%) followed by Bacillus pumilus treated plants which was 5.4% lower in comparison to that of consortia of two bacilli and 30.36% higher to that of non-treated control plants. Carbohydrate content (49.0%) was increased by the combination of two bacilli followed by the consortia of three bacilli which was 34.6% over untreated plants while 10.6% lesser than that of combination of two bacilli. However, no significant effect of bacilli treatments was observed on total ash, acid insoluble ash, moisture, and fiber contents of amaranth grains (Fig. 1a, Table 2).
Fig. 1.
Effect of different bacilli treatments on various chemical constituents and amino acid compositions of amaranth grains. (a) Effect of different treatments on nutrients of amaranth grains [Moist: Moisture; CF: Crude Fiber; CP: Crude protein; CF: Crude fiber; DM: Dry matter; TA: Total Ash; AiA: Acid insoluble ash, Carb: Carbohydrate]. (b) Effect of different treatments on aminoacid composition of amaranth grains [Tryp: Tryptophan; Lys: Lysine; Meth: Methionine; TAA: Total amino-acid].
Table 2.
Effects of different treatments on chemical constituents of amaranth seeds.
| Treatments | Strain(s) used | Dry matter (%) | Moisture (%) | Crude protein (%) | Crude fiber (%) | Total fat (%) | Total ash (%) | Acid insoluble ash (%) | Carbohydrates (%) |
|---|---|---|---|---|---|---|---|---|---|
| T-1 | Control | 72.14 ± 1.23 | 6.92 ± 0.89 | 13.33 ± 0.68 | 2.97 ± 0.89 | 6.06 ± 0.12 | 2.09 ± 0.36 | 0.26 ± 0.02 | 46.06 ± 1.13 |
| T-2 | BS-41 | 92.72 ± 1.87 | 6.96 ± 0.77 | 15.04 ± 1.20 | 3.08 ± 1.02 | 7.02 ± 0.40 | 3.02 ± 1.13 | 0.33 ± 0.04 | 48.20 ± 0.72 |
| T-3 | BS-27 | 92.85 ± 1.51 | 7.15 ± 0.67 | 16.04 ± 0.28 | 3.20 ± 0.8 | 7.90 ± 0.57 | 3.34 ± 1.09 | 0.36 ± 0.03 | 55.00 ± 1.00 |
| T-4 | BS-58 | 92.92 ± 0.95 | 7.05 ± 0.54 | 15.23 ± 0.64 | 3.16 ± 0.60 | 7.80 ± 0.34 | 3.29 ± 0.63 | 0.31 ± 0.03 | 58.00 ± 1.56 |
| T-5 | BS-41+BS-27 | 92.64 ± 1.06 | 7.08 ± 0.11 | 15.59 ± 1.33 | 3.12 ± 0.40 | 7.55 ± 0.59 | 3.20 ± 0.29 | 0.30 ± 0.06 | 54.66 ± 0.57 |
| T-6 | BS-41+BS-58 | 92.72 ± 0.99 | 7.24 ± 0.43 | 15.85 ± 1.00 | 3.09 ± 0.43 | 7.38 ± 0.55 | 2.90 ± 0.27 | 0.34 ± 0.12 | 61.33 ± 1.15 |
| T-7 | BS-27+BS-58 | 95.41 ± 0.54 | 7.26 ± 1.01 | 16.84 ± 0.80 | 3.22 ± 0.48 | 8.33 ± 0.70 | 3.38 ± 0.74 | 0.37 ± 0.05 | 68.66 ± 1.52 |
| T-8 | BS-41+BS-27+BS-58 | 92.82 ± 2.43 | 7.08 ± 0.86 | 15.81 ± 1.06 | 3.04 ± 0.94 | 7.24 ± 0.35 | 2.80 ± 0.39 | 0.330.02 | 62.03 ± 0.90 |
| P-value (ANOVA) | P < 0.001 | P < 0.99 | P < 0.016 | P < 0.99 | P < 0.001 | P < 0.39 | P < 0.43 | P < 0.001 | |
Values are mean of three replicates. 2 g seed for each test (in triplicate) was used.
3.2. Qualitative and quantitative analysis of amino-acids
Results of TLC revealed the presence of all three test amino-acids namely, lysine, methionine, and tryptophan in different treatments as detected with their respective Rf values. In present study, maximum enhancement (34.4%) in the total free amino-acid contents of amaranth was recorded in the plants raised with combination of Bacillus pumilus BS-27 and Bacillus subtilis BS-58 followed by Bacillus pumilus (30%) over untreated control plant. Amino-acid content in Bacillus pumilus treated plants was 3.4% lesser than that of plants raised with the combination of Bacillus pumilus and Bacillus subtilis (Fig. 1b; Table 3). An increase of 29.8% in amino-acid contents of amaranth was observed by the combination of another Bacillus pumilus BS-41 and Bacillus subtilis BS-58 over the control plant but 3.5% lower than that of combination of Bacillus pumilus (BS-27) and Bacillus subtilis.
Table 3.
Effects of different treatments on amino-acid contents of amaranth seeds.
| Treatments | Strain(s) used | Amino-acid (μg/ml) | Methionine (g/16 gN) | Tryptophan (g/16 gN) | Lysine (g/16 gN) |
|---|---|---|---|---|---|
| T1 | Control | 23.80 ± 1.08 | 0.48 ± 0.01 | 1.37 ± 0.01 | 5.31 ± 0.39 |
| T2 | BS-41 | 26.00 ± 1.61 | 0.58 ± 0.03 | 1.60 ± 0.01 | 7.07 ± 0.11 |
| T3 | BS-27 | 30.94 ± 0.53 | 0.61 ± 0.009 | 1.84 ± 0.06 | 8.26 ± 0.65 |
| T4 | BS-58 | 30.00 ± 1.55 | 0.70 ± 0.007 | 1.42 ± 0.26 | 6.23 ± 0.44 |
| T5 | BS-41+BS-27 | 27.67 ± 1.09 | 0.67 ± 0.01 | 1.55 ± 0.12 | 7.32 ± 0.28 |
| T6 | BS-41+BS-58 | 30.90 ± 1.71 | 0.69 ± 0.04 | 1.53 ± 0.02 | 7.39 ± 0.24 |
| T7 | BS-27+BS-58 | 32.00 ± 1.47 | 0.72 ± 0.01 | 1.96 ± 0.11 | 8.47 ± 0.70 |
| T8 | BS-41+BS-27+BS-58 | 25.26 ± 1.00 | 0.66 ± 0.02 | 1.81 ± 0.02 | 6.05 ± 0.56 |
| P-value (ANOVA) | P < 0.001 | P < 0.001 | P < 0.001 | P < 0.001 | |
Values are mean of three replicates. 2 g seed for each test (in triplicate) was used.
3.3. Estimation of methionine, tryptophan and lysine
Among all the treatments, consortia of Bacillus pumilus and Bacillus subtilis was found best to enhance essential amino-acid contents in amaranth especially in reference to methionine (0.72 g/16 gN), tryptophan (1.96 g/16 gN) and lysine (8.47 g/16 gN). An increase of 50% in methionine content was observed by the consortia of Bacillus pumilus and Bacillus subtilis followed by Bacillus subtilis (45.83%) individually over plants raised without any treatment. However, consortia of two isolates were found significantly better those were 2.8% higher over the plants raised with Bacillus subtilis individually. The combination of three isolates was less effective than the consortia of two isolates; there was 37.5% increase in methionine content was observed over non-treated plant while combination of two isolates was 9.0% higher than the combination of three isolates.
Increase in tryptophan content by 43% was observed by the combination of two isolates followed by Bacillus pumilus BS-27 (34.3%) over the untreated plants. Tryptophan content in the plants treated with Bacillus pumilus was 6.5% less than the combination of two bacilli, however it was 1.5% higher than the combination of three bacilli. Further, 32.1% increase in tryptophan content was observed in plants treated with combination of three bacilli over the plant raised without any seed treatment and 8.28% lower content than the combination of two bacilli.
As far as lysine content was concerned maximum increase (59.51%) in lysine content was observed by the combination of two bacilli Bacillus pumilus + Bacillus subtilis followed by Bacillus pumilus BS-27 over the plants raised without any treatment. Increase in lysine content to the extent of 14% in amaranth’s grains observed when treated with the consortium of three isolates Bacillus pumilus BS-27, Bacillus pumilus BS-41 and Bacillus subtilis BS-58 over the plants raised without any treatment however combination of three bacilli was not significantly effective on increase in lysine content of amaranth as it was 40% lower than that of combination of two bacilli (Fig. 1b; Table 3).
4. Discussion
Use of PGPBs to increase the nutrient content of Amaranthus hypochondriacus to accomplish the food security and to fulfill the demand of food, has been found as an essential approach to feed the gradually increasing population (Alemayehu et al., 2015). Enhancement in maximum proximate chemical constituents including carbohydrate, proteins, dry matter, etc. was recorded by the application of PGP bacilli. Stefan et al. (2013) reported enhanced (11.97%) protein and carbohydrate contents in the seeds of beans when treated with the plant growth promoting rhizobacteria (PGPR). In fact, such enhancement in the chemical constituents could be because of PGP activities of bacilli strains. Bacillus isolates have been found effective to enhance nitrogen, phosphorus and potassium contents in soil and their uptake by Amaranthus hypochondriacus (data not given). Increase in N, P, and K content of corn by the application of PGPR and AMF was earlier observed by Adesemoye et al. (2008). Increased protein content in present study might be the result of the enhanced nitrogen content by the Bacillus isolates which can also be utilized for protein synthesis in the plant as evidenced by Kumar et al. (2015). Along with that, enhanced nutrient accumulation boost the entire chemical constituents of Amaranthus hypochondriacus. Al-Erwy et al. (2016) reported a significant increase in total carbohydrates and mineral contents of wheat when treated with PGPR (Azotobacter and Rhizobium). Kang et al. (2012) reported increased protein and amino-acid content of cucumber due to the treatment of PGPRs. Since bacilli strains were K solubilizers therefore, effective solubilization of potassium by bacilli significantly influences the protein synthesis by activating the enzyme nitrate reductase which catalyzes protein synthesis (Ranade-Malvi, 2011). Ma and Shi (2011) suggested the influence of potassium treatment on carbohydrate content of Stevia rebaudiana by increasing photosynthesis rate. Li et al. (2017) suggested nitrogen and phosphorus application contributed in the photosynthesis and positively influenced protein, carbohydrate and essential amino-acid content. The increased carbohydrate content of Amaranthus hypochondriacus may be the effect of the PGP Bacillus species to solubilize nutrients in their vicinity which further get accumulated in the plants. The possibility of these nutrients could involve in the ATP production which further regulate the photosynthesis cannot be ruled out.
Presence of all three test amino-acids (lysine, methionine, and tryptophan) in Amaranthus hypochondriacus grains obtained by different bacilli treatments was evidenced by TLC. Similar study was carried out by Sen et al. (2012) who reported the presence of different amino-acids in the seeds of Amanita excelsa. Inoculation of PGPRs has been reported very effective to enhance the amino-acid contents of various crops such as maize (Hamdia et al., 2004). We have obtained enhancement in the total free amino-acid contents of Amaranthus hypochondriacus grains by consortia of Bacillus pumilus and Bacillus subtilis. Kalita et al. (2015) observed enhancement in carbohydrate, lipid, protein, and amino-acid contents in chilli, cauliflower and brinjal by using PGP bacterial consortia involved Bacillus cereus and, Pseudomonas rhodesiae. The enhanced nitrogen content by the Bacillus spp. could be accountable for the enhanced amino-acid contents in the Amaranthus hypochondriacus. The effect of PGPBs on nutrient enhancement is already published in various literatures which suggest their role in the increased bioavailability of nutrients (N, P, K) in the soil and their accumulation in the plant (Esitken et al., 2010, Lavakush et al., 2014). Since, amino-acids are essential for protein synthesis and other metabolic processes in humans, the enhanced amino-acid content in amaranth grains by the Bacillus spp. may augment the bioavailability in humans when consumed and facilitate in protein synthesis regulation, immune response besides growth and metabolism (Perianayagam et al., 2005, Perales-Sanchez et al., 2014).
The increase in methionine content was due to the influence of consortia of Bacillus pumilus and Bacillus subtilis to enhance the availability of soil nutrients to the host plant as supported by Vivas et al. (2003). Ekinci et al. (2014) reported higher methionine content in Brassica oleracea L. var. botrytis after the application of B. megaterium KBA-10. Nosheen et al. (2016) observed high methionine content of safflower seed when treated with NP fertilizer and PGPRs. Being an essential amino-acid, methionine is used in the production of sulfur, which is essential for the synthesis of haemoglobin and glutathione that fight against free radicals. Deficient intake of methionine not only impairs growth, but also affects the sulfur metabolic pathways (Mato et al., 2002) because, methionine structure contains sulfur contents which increases lecithin production in the liver by the reduction of cholesterol (Bentley, 2005). This might be possible that the Bacillus pumilus and Bacillus subtilis having soil nutrient solubilization ability could be responsible to enhance sulfur content and thereby enhance methionine content.
Lysine is quite essential nutrient and is generally found low in concentrations in major crops such as wheat, rice, maize. Its deficiency limits the synthesis of proteins and the proliferation of lymphocytes (Konashi et al., 2000, Li et al., 2007). Previously, Pisarikova et al. (2005) reported lysine, and tryptophan content in amaranth. In our study, maximum increase of lysine and tryptophan contents was observed when treated with consortia of Bacillus pumilus and Bacillus subtilis. Ekinci et al. (2014) reported B. megaterium TV-3D as potential to increase the lysine content in Brassica oleracea L. var. botrytis while B. megaterium KBA-10 was effective to enhance tryptophan content. Increased lysine content of safflower seeds was recorded by PGPBs along with nitrogen and phosphorus fertilizers (Nosheen et al., 2016). In our study, significant increase in lysine and tryptophan indicates the potential of Bacillus pumilus and Bacillus subtilis to enhance the amino-acid synthesis in amaranth which could be effective to accomplish the demand of nutritionally rich food and to prevent various diseases in infants along with adults. Tryptophan is essentially required for infants and adults and its catabolic products serotonin, melatonin and N-acetylserotonin can enhance host neuroimmunity and mitochondrial functions (Keszthelyi and Troost, 2009). The essential amino acids have been reported to increase in various crops such as wheat and maize by genetic modification and breeding (Hash et al., 2002). However, the use of PGPBs is a cost effective, farmer’s friendly and easy to apply and up-regulate the nutrient synthesis and crop yield.
5. Conclusion
Present study concludes the significant role of Bacillus isolates in the enhancement of essential amino-acid contents and other nutritive chemical constituents in amaranth grains. The study suggests treatment (Bacillus pumilus BS-27 and Bacillus subtilis BS-58) to be the best for the enhancement of maximum parameters, thus can be promoted among the farmers to enhance the nutritive as well as the market value of this economically important crop. To the best of our knowledge, this study is the first in introducing enhancement of amino acid synthesis in amaranth with the application of plant growth promoting Bacillus spp. (PGPBs).
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
Yogesh Kumar Negi, Email: yknegi@rediffmail.com.
Irfan A. Rather, Email: rather@ynu.ac.kr.
D.K. Maheshwari, Email: maheshwaridk@gmail.com.
References
- Adesemoye A.O., Torbert H.A., Kloepper J.W. Enhanced plant nutrient use efficiency with PGPR and AMF in an integrated nutrient management system. Can. J. Microbiol. 2008;54:876–886. doi: 10.1139/w08-081. [DOI] [PubMed] [Google Scholar]
- Al-Erwy A.S., Al-Toukhy A., Bafeel S.O. Effect of chemical, organic and biofertilizers on photosynthetic pigments, carbohydrates and minerals of wheat (Triticum aestivum. L) irrigated with sea water. Int. J. Adv. Res. Biol. Sci. 2016;3:296–310. [Google Scholar]
- Alemayehu F., Bendevis M.A., Jacobsen S.E. The potential for utilizing the seed crop amaranth (Amaranthus spp.) in East Africa as an alternative crop to support food security and climate change mitigation. J. Agron. Crop Sci. 2015;201:321–329. [Google Scholar]
- Barrio D.A., Anon M.C. Potential antitumor properties of a protein isolate obtained from the seeds of Amaranthus mantegazzianus. Eur. L. Nutr. 2010;49:73–82. doi: 10.1007/s00394-009-0051-9. [DOI] [PubMed] [Google Scholar]
- Bentley R. Methionine and derivatives. Exploring chirality at sulphur. Int. Union Biochem. Mol. Biol. 2005;33:274–276. [Google Scholar]
- Blau K., Halket J., editors. Handbook of Derivatives for Chromatography. John Wiley; Chichester: 1993. [Google Scholar]
- Chauhan A.K., Maheshwari D.K., Kim K., Bajpai V.K. Termitarium inhabiting Bacillus endophyticus TSH42 and Bacillus cereus TSH77 colonizing Curcuma longa L.: isolation, characterization and evaluation of their biocontrol and plant growth promoting activities. Can. J. Microbiol. 2016;62:880–892. doi: 10.1139/cjm-2016-0249. [DOI] [PubMed] [Google Scholar]
- Ekinci M., Turan M., Yildirim E., Gunes A., Kotan R., Dursun A. Effect of plant growth promoting rhizobacteria on growth, nutrient, organic acid, amino acid and hormone content of cauliflower (Brassica oleracea L. var. botrytis) transplants. Acta Sci. Pol. Hortorum Cultus. 2014;13:71–85. [Google Scholar]
- Esitken A., Yildiz H.E., Ercisli S., Donmez M.F., Turan M., Gunes A. Effects of plant growth promoting bacteria (PGPB) on yield, growth and nutrient contents of organically grown strawberry. Scientia Horticulturae. 2010;124:62–66. [Google Scholar]
- Gomez K.A., Gomez A.A. Statistical Procedures for Agricultural Research. second ed. John Wiley & Sons; New York: 1984. p. 680. [Google Scholar]
- Hamdia M.A.E., Shaddad M.A.K., Doaa M.M. Mechanisms of salt tolerance and interactive effects of Azospirillum brasilense inoculation on maize cultivars grown under salt stress conditions. Plant Growth Regul. 2004;44:165–174. [Google Scholar]
- Hash C.T., Schaffert R.E., Peacock J.M. Food Security in Nutrient-stressed Environments: Exploiting Plants’ Genetic Capabilities. Springer; Netherlands: 2002. Prospects for using conventional techniques and molecular biological tools to enhance performance of ‘orphan’ crop plants on soils low in available phosphorus; pp. 25–36. [Google Scholar]
- Horn M.J., Jones D.B., Blum A.E. Colorimetric determination of methionine in proteins and foods. J. Biol. Chem. 1946;166:313–320. [PubMed] [Google Scholar]
- Kalita M., Bharadwaz M., Dey T., Gogoi K., Dowarah P., Unni B.G., Ozah D., Saikia I. Developing novel bacterial based formulation having PGPR properties for enhanced production of agricultural crops. Indian J. Exp. Biol. 2015;53:56–60. [PubMed] [Google Scholar]
- Kang S.M., Khan A.L., Hamayun M., Shinwari Z.K., Kim Y.H., Joo G.J., Lee I.J. Acinetobacter calcoaceticus ameliorated plant growth and influenced gibberellins and functional biochemicals. Pak. J. Bot. 2012;44:365–372. [Google Scholar]
- Keszthelyi D., Troost F.J. Understanding the role of tryptophan and serotonin metabolism in gastrointestinal function. Neurogastroenterol. Motil. 2009;21:1239–1249. doi: 10.1111/j.1365-2982.2009.01370.x. [DOI] [PubMed] [Google Scholar]
- Konashi S., Takahashi K., Akiba Y. Effects of dietary essential amino acid deficiencies on immunological variables in broiler chickens. Br. J. Nutr. 2000;83:449–456. [PubMed] [Google Scholar]
- Kumar A., Rathi B., Kumar S. Effects of PGPR, sulphur, and some micronutrients on protein, carbohydrate and fat contents in lentil (Lens culinaris) Legume Res. 2015;38:707–709. [Google Scholar]
- Lavakush, Yadav J., Verma J.P., Jaiswal D.K., Kumar A. Evaluation of PGPR and different concentration of phosphorus level on plant growth, yield and nutrient content of rice (Oryza sativa) Ecol. Eng. 2014;62:123–128. [Google Scholar]
- Li P., Yin Y.L., Li D., Kim S.W., Wu G. Amino acids and immune function. Br. J. Nutrit. 2007;98:237–252. doi: 10.1017/S000711450769936X. [DOI] [PubMed] [Google Scholar]
- Li X., He P., Xu J., Fu G., Chen Y. Effect of nitrogen and phosphorus on growth and amino-acid contents of Porphyra yezoensis. Aqua. Res. 2017;48:2798–2802. [Google Scholar]
- Ma L., Shi Y. Effects of potassium fertilizer on physiological and biochemical index of Stevia rebaudiana Bertoni. Energy Proc. 2011;5:581–586. [Google Scholar]
- Maheshwari D.K., Kumar S., Kumar B., Pandey P. Co-inoculation of Urea and DAP tolerant Sinorhizobium meliloti and Pseudomonas aeruginosa as integrated approach for growth enhancement of Brassica juncea. Indian J. Microbiol. 2010;50:425–431. doi: 10.1007/s12088-011-0085-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mato J.M., Corrales F.J., Lu S.C., Avila M.A. S-Adenosylmethionine: a control switch that regulates liver function. FASEBJ. 2002;16:15–26. doi: 10.1096/fj.01-0401rev. [DOI] [PubMed] [Google Scholar]
- Mendonca S., Saldiva P.H., Cruz R.J., Areas J.A.G. Amaranth protein presents cholesterol-lowering effect. Food Chem. 2009;116:738–742. [Google Scholar]
- Mertz, E.T., Jambunathan, R., Misra, P.S., 1975. In: Protein Quality, Stn. Bull No. 70, Agric. Exp. Stn. Purdue University, USA, p. 14.
- Mishra S.B., Verma A., Mukerjee A., Vijaykumar M. Amaranthus spinosus L. (Amaranthaceae) leaf extract attenuates streptozotocin-nicotinamide induced diabetes and oxidative stress in albino-rats: a histopathological analysis. Asian Pac. J. Trop. Biomed. 2012;2:S1647–S1652. [Google Scholar]
- Mlakar S.G., Turinek M., Jakop M., Bavec M., Bavec F. Nutrition value and use of grain amaranth: potential future application in bread making. Agricultura. 2009;6:43–53. [Google Scholar]
- Moore S., Stein W.H. Photometric ninhydrin method for use in the chromatography of amino acids. J. Biol. Chem. 1948;176:367–388. [PubMed] [Google Scholar]
- Negi Y.K., Garg S.K., Kumar J. Plant growth promoting and biocontrol activities of cold-tolerant Pseudomonas fluorescens isolates against root rot in pea. Indian Phytopath. 2008;61:461–470. [Google Scholar]
- Negi Y.K., Prabha D., Garg S.K., Kumar J. Genetic diversity among cold-tolerant fluorescent Pseudomonas isolates from Indian Himalayas and their characterization for biocontrol and plant growth promoting activities. J. Plant Growth Regul. 2011;30:128–143. [Google Scholar]
- Nielsen S.S. Springer; New York: 2017. Food Analysis; pp. 257–359. [Google Scholar]
- Nosheen A., Bano A., Yasmin H., Keyani R., Habib R., Shah S.T.A., Naz R. Protein quantity and quality of safflower seed improved by NP fertilizer and rhizobacteria (Azospirillum and Azotobacter spp.) Front Plant Sci. 2016;7:104. doi: 10.3389/fpls.2016.00104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perales-Sanchez J.X., Reyes-Moreno C., Gomez-Favela M.A., Milan-Carrillo J., Cuevas-Rodriguez E.O., Valdez-Ortiz A., Gutierrez-Dorado R. Increasing the antioxidant activity, total phenolic and flavonoid contents by optimizing the germination conditions of amaranth seeds. Plant Food Hum. Nutr. 2014;69:196–202. doi: 10.1007/s11130-014-0430-0. [DOI] [PubMed] [Google Scholar]
- Perianayagam M.C., Oxenkrug G.F., Jaber B.L. Immune-modulating effects of melatonin, N-acetylserotonin, and N-acetyldopamine. Ann. N. Y. Acad. Sci. 2005;1053:386–393. doi: 10.1111/j.1749-6632.2005.tb00046.x. [DOI] [PubMed] [Google Scholar]
- Pisarikova B., Kracmar S., Herzig I. Amino-acid contents and biological value of protein in various amaranth species. Czech J. Anim. Sci. 2005;50:169–174. [Google Scholar]
- Ranade-Malvi U. Interaction of micronutrients with major nutrients with special reference to potassium. Karnataka J. Agric. Sci. 2011;24:106–109. [Google Scholar]
- Sen S., Sarkar S., Kundu P., Laskar S. Separation of amino-acids based on thin-layer chromatography by a novel quinazoline based antimicrobial agent. Am. J. Anal. Chem. 2012;3:669–674. [Google Scholar]
- Singh A.N. Ethnoecological survey of underutilized plant diversity of Hamirpur district, Himachal Pradesh, India: an edibility assessment. Environ. Ecol. Res. 2017;5:12–28. [Google Scholar]
- Stefan M., Munteanu N., Mihasan M. Application of plant growth promoting rhizobacteria to runner bean increases seed carbohydrate and protein yield. Analele Stiintifice ale Universitatii“ Al. I. Cuza” Din Iasi. (SerieNoua). Sectiunea 2. a. Geneticasi Biologie Moleculara. 2013;14:29–36. [Google Scholar]
- Vivas A., Marulanda A., Ruiz-Lozano J.M., Barea J.M., Azcon R. Influence of a Bacillus sp. on physiological activities of two arbuscular mycorrhizal fungi and on plant responses to PEG-induced drought stress. Mycorrhiza. 2003;13:249–256. doi: 10.1007/s00572-003-0223-z. [DOI] [PubMed] [Google Scholar]
- Yadav I.C., Devi N.L., Syed J.H., Cheng Z., Li J., Zhang G., Jones K.C. Current status of persistent organic pesticides residues in air, water, and soil and their possible effect on neighboring countries: a comprehensive review of India. Sci. Total Environ. 2015;511:123–137. doi: 10.1016/j.scitotenv.2014.12.041. [DOI] [PubMed] [Google Scholar]