In a cohort of young black men who have sex with men, we observed high human immunodeficiency virus incidence despite education and access to preexposure prophylaxis (PrEP). Although PrEP has efficacy in clinical trials, we identify barriers to real-world effectiveness.
Keywords: health disparities, HIV, preexposure prophylaxis
Abstract
Human immunodeficiency virus (HIV) preexposure prophylaxis (PrEP) has high biomedical efficacy; however, awareness, access, uptake, and persistence on therapy remain low among black men who have sex with men (BMSM), who are at highest risk of HIV in the United States. To date, discussions of “PrEP failure” have focused on one typology: rare, documented HIV acquisitions among PrEP users with adequate serum drug levels (ie, biomedical failure). In our cohort of HIV-negative young BMSM in Atlanta, Georgia, we continue to observe a high HIV incidence (6.2% annually at interim analysis) despite access to free PrEP services. Among 14 seroconversions, all were offered PrEP before acquiring HIV. Among these participants, we identified 4 additional typologies of PrEP failure that expand beyond biomedical failure: low PrEP adherence, PrEP discontinuation, PrEP contemplation without initiation, and PrEP refusal. We describe the 5 typologies and suggest interventions to improve PrEP effectiveness among those at highest risk.
To date, most discussion of human immunodeficiency virus (HIV) preexposure prophylaxis (PrEP) failure has focused on a small number of well-documented seroconversions despite adequate medication adherence and detectable drug levels [1–3]. However, in the broader context of HIV acquisition, these are likely to be rare events, with many more seroconversions occurring among those with access to PrEP who either do not start or do not continue taking PrEP. Identifying and quantifying the contributors to these HIV seroconversions in an era when PrEP is available are crucial to realizing the benefits of PrEP in reducing HIV incidence at the population level.
Although overall use of PrEP is increasing, unacceptable disparities in PrEP uptake have emerged among minority groups in the United States in a pattern that parallels the disparities in HIV incidence [4–6]. Black men who have sex with men (BMSM), especially in the southeastern United States, have the highest HIV incidence of any race/risk-specific group [7] but also have less PrEP awareness, less self-reported PrEP use, and less persistence on therapy than white men who have sex with men (MSM) [4, 8–13]. Although black persons make up 44% of those with new HIV infections, they made up only 10% of persons taking PrEP in 2016 [12]. The gaps in PrEP use among young BMSM could exacerbate the existing disparities in HIV incidence [14, 15]. Thus, it is imperative that we address gaps in the PrEP continuum of care [16].
Marcus and colleagues called in 2017 [17] for redefining “PrEP failure” beyond the biomedical realm, and we seek to expand that concept to include any HIV seroconversion occurring along the PrEP care continuum that begins with awareness of and willingness to take PrEP and culminates in PrEP efficacy with full adherence. Here, we focus on barriers to PrEP efficacy in a cohort of young BMSM occurring in the setting of PrEP education and access provided by the study. By broadening the definition to include all seroconversions occurring when PrEP is available, we seek to bring attention, clinical investigation, and resources to the many obstacles preventing the presence of inhibitory PrEP drug levels at the time of HIV exposure.
STUDY OVERVIEW
In June 2017 we completed enrollment of a prospective cohort (the EleMENt study; NCT02503618) of 300 young BMSM in Atlanta, Georgia, aged 16–29 years, to understand the pathways by which substances influence HIV/sexually transmitted infection (STI) risk, with follow-up continuing through 2019 [18]. Participants include HIV-negative young BMSM who reported ≥1 male sexual partner in the past 3 months. At each study visit, participants receive comprehensive HIV/STI testing and risk-reduction counseling that includes the provision of condoms and lubricants. Participants complete questionnaires to assess demographics, HIV prevention behaviors, and HIV risk factors. HIV-negative participants are followed up prospectively, with visits at baseline and at 3, 6, 12, 18, and 24 months. Those taking PrEP through the study also undergo HIV/STI testing and creatinine monitoring at months 9, 15, and 21. Participants who test positive for HIV or other STIs are linked by study staff to treatment services.
Based on our previous observations of high HIV incidence among young BMSM in Atlanta [6] and the ethical obligation to ensure access to the most effective HIV prevention modalities, we offer nonincentivized PrEP as the standard of care to all HIV-negative participants [18]. All participants receive PrEP education and are offered the opportunity to meet with a study physician for PrEP initiation. Tenofovir disoproxil fumarate–emtricitabine (TDF/FTC) is not directly provided by the study, but a patient navigator assists with access to low- or no-cost medication via the participants’ health insurance plan and/or the manufacturer assistance programs. To offer PrEP directly within the cohort and without the need for referral or navigation to external PrEP services, we obtained supplemental funding for financial coverage of provider visits and laboratory costs. As of December 2017, 52.5%of men in the cohort (158 of 300) had attended a PrEP initiation visit and were given a prescription, or had already been taking PrEP outside the study (6.7%; 20 of 300).
TYPOLOGIES OF PREP FAILURES
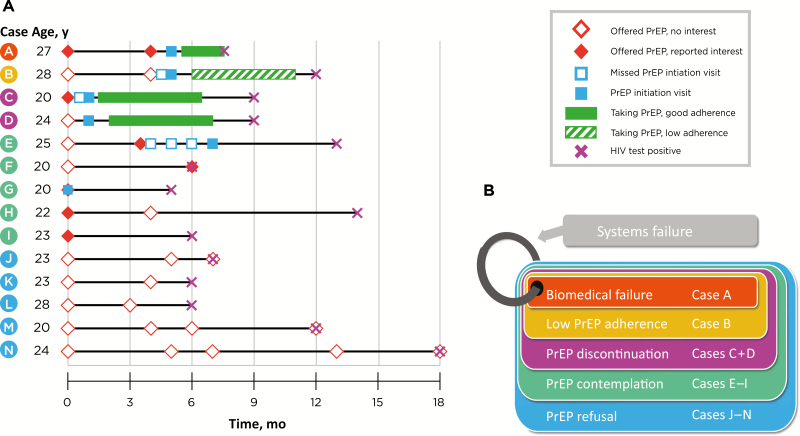
At interim analysis in December 2017, there were 14 incident HIV diagnoses (6.2% annually). Of these 14 participants, 5 reported no interest in PrEP initiation, 5 expressed interest but did not start taking PrEP before seroconversion, and 4 took PrEP at some time before seroconversion (Figure 1A). Of the 4 who took PrEP before seroconversion, we identified 1 with biomedical failure who may have had acute HIV infection at the time of PrEP initiation, 1 with low adherence, and 2 who discontinued therapy altogether. Using these 14 cases, we identified 5 typologies of HIV seroconversion occurring in the setting of PrEP access: biomedical failure, low PrEP adherence, PrEP discontinuation, PrEP contemplation, and PrEP refusal. A framework is presented (Figure 1B) to demonstrate the increasing number of seroconversions occurring outside traditional biomedical PrEP failure. We also identified systematic barriers that contribute to PrEP failure across all the typologies.
Figure 1.
A, Timelines in 14 participants (A–N) with human immunodeficiency virus (HIV) seroconversion occurring after preexposure prophylaxis (PrEP) was offered in the EleMENt cohort. B, Proposed framework of PrEP failure typologies, beginning with biomedical failures (case A) and expanding to include low PrEP adherence (case B), PrEP discontinuation (cases C and D), PrEP contemplation (cases E–I), and PrEP refusal (cases J–N). Systems failures are represented as a ring joining the other 4 typologies, given the cross-cutting nature of this barrier to PrEP effectiveness.
Biomedical Failure of PrEP
Since Food and Drug Administration approval of TDF/FTC, there have been 3 cases of well-documented PrEP failure in the setting of appropriate adherence and therapeutic drug levels at the time of HIV acquisition [1–3]. There are also 3 cases of HIV acquisition in patients taking TDF monotherapy for hepatitis B virus infection [19, 20]. In 3 of these 6 cases, infection occurred with drug-resistant viruses. Even without a virus harboring resistance to TDF and/or FTC, a very high-inoculum exposure with significant mucosal injury could potentially overcome therapeutic TDF/FTC levels and allow infection [3]. In patients who acquire HIV while taking PrEP, presentation can be atypical for acute HIV. Of the 6 patients noted above, only 2 had clinically evident acute retroviral syndrome. Development of HIV-1 antibody can be delayed, viral loads modest, and symptoms minor or absent compared with classic acute HIV infection [21].
Of more concern is the initiation of PrEP during the window between infection and positive results of fourth-generation HIV tests [22]. Participant A in our cohort study (Figure 1A) had negative rapid test and qualitative HIV nucleic acid test (NAT) results at his baseline visit. His PrEP initiation visit did not occur until 40 days later owing to difficulties in establishing communication. At that visit, a second rapid HIV test had negative results, and the participant reported no symptoms of acute retroviral syndrome. He started PrEP and reported near 100% adherence (corroborated by pharmacy refill records) at a 1-month follow-up phone call. While adherent to PrEP, he reported unprotected anal intercourse with an HIV-positive partner who was not receiving antiretroviral therapy. At his next study visit 3 months after starting PrEP, a third rapid HIV test result was negative, but the HIV NAT result was positive. Two weeks later, a fourth HIV rapid test result was negative, but a fourth-generation antigen-antibody test result was positive, the viral load was 494 copies/mL, and an HIV genotype showed an FTC resistance–associated mutation (M184V). At the time the participant tested positive, the dried blood spot tenofovir diphosphate level (2382 fmol per punch) was consistent with his taking ≥4 doses of TDF/FTC per week, which is protective against HIV acquisition [23].
We suspect that this participant either had acute HIV infection at the time of his PrEP initiation visit with a negative rapid HIV test result or was exposed to HIV before achieving protective drug levels; however, a true biomedical PrEP failure cannot be excluded. TDF/FTC may have kept his viral load low, selected for the M184V mutation, and delayed seroconversion despite detectable viremia. Alternatively, he may have been infected with a virus already harboring resistance to FTC, though transmitted M184V mutations are rare [24]. We noted unexpectedly long delays between regular study visits and PrEP initiation visits, primarily owing to difficulty contacting participants along with cancelled and missed appointments. Based on these delays, we updated our protocols to include qualitative HIV NAT at all PrEP initiation visits. Many rapid tests, even those including testing for p24 antigen, have unacceptably low sensitivity for detecting acute HIV infection in a high-incidence population [25–27]. This case demonstrates the importance of ruling out acute HIV infection at the time of PrEP initiation, either with a fourth-generation antigen-antibody test or with NAT.
Low PrEP Adherence
For optimal efficacy of daily PrEP, ≥4 doses of TDF/FTC must be taken per week [23, 28]. PrEP failure due to low adherence occurs when a participant seroconverts owing to subtherapeutic drug levels. In a review of data from Kaiser Permanente Northern California, overall adherence was 92%, as measured by timing of pharmacy refills [29]. Black patients were significantly more likely to be nonadherent (relative risk, 3.0; 95% confidence interval, 1.7–5.1; P < .001) and patients <30 years of age had a trend toward decreased adherence (1.6; .8–3.3). In a study of adolescent MSM aged 15–17 years receiving PrEP (29% African American), only 22% had inhibitory drug levels of TDF after 48 weeks of follow-up with provision of an adherence-promoting behavioral intervention [30].
In our cohort, seroconversion occurred in case B owing to low adherence when he traveled on a few occasions and did not bring his medication. All PrEP participants were given a keychain pill holder for “emergency doses” if they forgot medications at home and were offered enrollment in a text-message reminder service. More frequent assessment of adherence and intensive culturally competent counseling, such as client-centered care coordination, provided in HPTN 073, may lead to improved outcomes among BMSM [31]. Therapeutic drug monitoring to identify patients at higher risk of PrEP failure have been developed. In 1 model, investigators were able to direct targeted adherence counseling based on near real-time drug level data resulting in increased biomarker-validated adherence [32]. Other proven interventions to increase adherence to antiretroviral therapy in the HIV-infected population should be evaluated for efficacy in delivery of PrEP [33]. Development of novel adherence support mechanisms will be essential to improving efficacy of PrEP in those at highest risk.
PrEP Discontinuation
There is some overlap between PrEP nonadherence and PrEP discontinuation, but differences can be distinguished by whether users consider themselves to be “on PrEP.” Anecdotally, we have noted that some participants start and stop PrEP for periods of time, while others take PrEP initially and then stop indefinitely. In the Kaiser cohort referenced above, discontinuation occurred in 22.5% of patients by the end of follow-up [29], similar to the 17% discontinuation rate from a prior interim analysis of our cohort [18]. Reasons for discontinuation include adverse drug effects, changes in perceived HIV risk, and pill fatigue. More granular data on the timing and motivations of specific PrEP start/stop events would strengthen interventions to maintain adherence. In-depth qualitative interviews are ongoing in our cohort to better explain these factors.
Intermittent or temporary PrEP discontinuation for personal or medical reasons poses logistical problems when trying to safely help patients reinitiate in a timely manner. Case C discontinued PrEP for an extended period of time due to an acute medical illness. During the illness and recovery, he reported no sexual activity. However, on feeling better, he immediately reentered a period of high risk. At the time of his recovery, his patient assistance program (PAP) enrollment had expired. When he ultimately presented for repeated HIV testing and reinitiation of PrEP, his HIV test result was positive. In our experience, participants who stop PrEP during periods of abstinence or low risk often wish to reinitiate it once they have already reengaged in high-risk behavior, whereas PrEP should ideally be reinitiated before high-risk behavior. PrEP programs should focus on making it easier to access necessary laboratory testing and obtain new prescriptions, to decrease barriers to PrEP reinitiation.
PrEP Contemplation
Those contemplating PrEP express interest in initiation but fail to start PrEP before HIV acquisition. Case F initially reported no interest in PrEP at baseline and tested HIV negative. However, he reported interest in PrEP 6 months later, on the day he tested HIV positive. Parsons and colleagues [34] used a “motivational PrEP cascade” framework based on the transtheoretical model of change to evaluate willingness to initiate PrEP. Among 995 HIV-negative MSM, the largest dropoffs in the willingness cascade were in the precontemplative, contemplative, and “PrEParation” (mentally preparing to start PrEP) stages. Movement from contemplation to PrEParation was numerically lowest among BMSM, although the difference was not statistically significant. Further research is needed to identify effective methods to shepherd persons from contemplation to initiation when PrEP is available.
We have noted that participants’ interest in PrEP waxes and wanes throughout the study; thus, it is important to continually reoffer PrEP over time. There is a tension between respect for patient autonomy—allowing them to “think things over”—and expedient initiation of PrEP for eligible and willing at-risk persons. These concerns should also be balanced against the effectiveness of medications in someone who initiates PrEP without being fully invested [35]. For those who passively display some interest, motivational interviewing could be could be a way to help persons analyze their reasons for and against PrEP uptake [36].
PrEP Refusal
The most proximal barriers to PrEP implementation are lack of awareness, willingness, and access to healthcare. By enrolling in the study, participants receive PrEP education (awareness) and have access to PrEP care and patient navigation, yet many are not willing to start therapy. In addition, other effective interventions for protection from HIV exist, including condoms and treatment as prevention for partners known to be living with HIV [37]. Although patient autonomy should be respected and validated, it remains important to identify and understand reasons for refusal to improve messaging and education about this proven intervention.
Reasons for refusal of PrEP include low risk perception, concerns about medication adverse effects (case J), medical mistrust, pill aversion (case K), fear of sexual disinhibition, and stigma [38–40]. The PrEP education video presented in our study may not have been engaging for all participants and may not have specifically addressed participants’ concerns. Motivations to use PrEP and perceived barriers can differ by age, race, and income, and messaging should be population-specific whenever possible [41]. Focused and culturally relevant peer education about PrEP could improve interest and mitigate barriers of awareness, adverse effects, stigma, and medical mistrust among minority communities [42].
Low self-perceived HIV risk has been universally cited as a reason not to use PrEP across a broad range of demographics. Self-perceived risk is inaccurate among some MSM in the United States, with those at highest risk being more likely to underestimate their risk [43, 44]. Evidence-based positive-framed approaches to PrEP education for those at risk of HIV are needed. Golub and colleagues [45] found that a “health promotion” message led to better PrEP comprehension than a “risk reduction” message among young BMSM in New York City. Focus on health-promotion messaging rather than a risk-mitigation approach to PrEP use may assist in normalizing PrEP and decrease potential stigma associated with labeling persons as “high risk” [46].
SYSTEMS FAILURES
We defined “systems failures” as structural contributors to PrEP failure that are not explicitly related to the individual patient or provider. Rather than define an independent typology of PrEP failure, systems failures contribute to seroconversion in each of the typologies we identified. Systems failures develop at the intersection of both discrete policy-related problems (lack of health insurance, PAP requirements) and more abstract societal barriers to health (stigma, low self-efficacy, low health literacy) [47, 48]. Although not a barrier for participants in the current study, limited access to affordable healthcare [16], provider unfamiliarity with PrEP, and medication cost also contribute to systems-related seroconversion.
We were unable to provide all participants with same-day PrEP starts or to provide walk-in appointments to initiate PrEP; however, PrEP initiation appointments were available on a weekly basis for participants to schedule. Free transportation was provided by the study through smartphone-based rideshare services and frequent telephone/text/e-mail reminders were sent; nonetheless, there was a 57% no-show rate at PrEP initiation visits. Case E missed 3 PrEP initiation visits before attending a fourth but never successfully filled the PrEP prescription. Increased capacity for same-day PrEP starts could be helpful to increase PrEP uptake; however, this also may lead to higher PrEP discontinuation rates.
Manufacturer assistance programs have been an indispensable component of PrEP provision for study participants. Many participants were uninsured, marginally housed, and/or unstably employed. Obtaining proper documentation of income and residence required by assistance programs was a hardship for our participants and delayed PrEP initiation. All study participants taking PrEP used either the PAP or a copay card; without these programs, PrEP would not be accessible for many. As generic versions of TDF/FTC become available, it is possible that these vital pharmaceutical company-sponsored programs could become less generous without a significant decrease in drug price, which may decrease access to medications [49].
Participants also experienced delay and difficulty obtaining medications with the use of PAPs (case E) and copay cards. Some pharmacies charged the entire price of TDF/FTC to the copay card, rather than first applying participants’ private insurance. For participants with private insurance, prior authorization requests were often required, which also delayed PrEP initiation. Although these delays are not unique to PrEP, a difference of a few days can result in a missed prevention opportunity in persons at high risk of HIV infection.
CONCLUSIONS
Despite its demonstrated efficacy, tolerability, and increasing availability, PrEP implementation remains in its infancy. Along with treatment as prevention, PrEP will be an integral tool in ending the HIV epidemic, but only if its use can be successfully scaled up and provided to those at highest risk [50]. We have conducted a population-level approach to identifying barriers to PrEP effectiveness, with special attention to factors beyond the biomedical realm. Although we believe PrEP should be available to all who are at risk of acquiring HIV without medical contraindication, it is clear that increasing awareness and access to PrEP alone are inadequate. By identifying and addressing specific contributors to low PrEP adherence, PrEP discontinuation, PrEP contemplation, and PrEP refusal, we can ensure that all who desire PrEP can achieve optimal efficacy and realize reductions in population HIV incidence.
Notes
Acknowledgments. We thank the EleMENt staff for their contributions to the study. We are especially appreciative of our study participants, who help us learn how to provide optimal human immunodeficiency virus prevention services. We also thank Zabrina Quidiello for her help with graphic design of the figure.
Financial support. This work was supported by the National Institutes of Health (NIH): (grants R01DA038196 [principal investigators (PIs): E. S. R. and P. S. S.] and K23AI108335 [PI: C. F. K.]), the Georgia Clinical and Translational Science Alliance (grants UL1TR002378 and TL1TR002382 to D. P. S.), a supplement grant to the Emory Center for AIDS Research from NIH (P30 AI050409; PI: C. F. K.), and Gilead Sciences (grant IN-US-276–4369; PI: C. F. K.).
Potential conflicts of interest. D. P. S. reports grants from the National Center for Advancing Translational Sciences during the conduct of the study. E. S. R. reports grants from the NIH during the conduct of the study, grants from the Centers for Disease Control and Prevention, personal fees from Medidata, and personal fees from Cengage Learning outside the submitted work. N. L. reports grants from the NIH during the conduct of the study. C. d. R. reports grants from the National Institute on Drug Abuse, NIH, during the conduct of the study. A. J. S. reports grants from the National Institute of Mental Health and the National Institute of Allergy and Infectious Diseases during the conduct of the study. T. H. S. reports grants from the NIH during the conduct of the study. P. S. S. reports grants from Gilead and the NIH during the conduct of the study. C. F. K. reports grants from Gilead Sciences and grants from the NIH during the conduct of the study. All other authors report no potential conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Knox DC, Anderson PL, Harrigan PR, Tan DH. Multidrug-resistant HIV-1 infection despite preexposure prophylaxis. N Engl J Med 2017; 376:501–2. [DOI] [PubMed] [Google Scholar]
- 2. Markowitz M, Grossman H, Anderson PL, et al. . Newly acquired infection with multidrug-resistant HIV-1 in a patient adherent to preexposure prophylaxis. J Acquir Immune Defic Syndr 2017; 76:e104–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hoornenborg E, Prins M, Achterbergh RCA, et al. ; Amsterdam PrEP project team in the HIV Transmission Elimination AMsterdam Consortium (H-TEAM) Acquisition of wild-type HIV-1 infection in a patient on pre-exposure prophylaxis with high intracellular concentrations of tenofovir diphosphate: a case report. Lancet HIV 2017; 4:e522–8. [DOI] [PubMed] [Google Scholar]
- 4. Hoots BE, Finlayson T, Nerlander L, Paz-Bailey G; National HIV Behavioral Surveillance Study Group Willingness to take, use of, and indications for pre-exposure prophylaxis among men who have sex with men-20 US cities, 2014. Clin Infect Dis 2016; 63:672–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hess K, Hu X, Lansky A, et al. . Estimating the lifetime risk of a diagnosis of HIV infection in the United States. Presented at: Conference on Retroviruses and Opportunistic Infections; 22–25 February2016; Boston, Massachusetts. [Google Scholar]
- 6. Sullivan PS, Rosenberg ES, Sanchez TH, et al. . Explaining racial disparities in HIV incidence in black and white men who have sex with men in Atlanta, GA: a prospective observational cohort study. Ann Epidemiol 2015; 25:445–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. HIV surveillance report, 2016 Atlanta, Georgia: Centers for Disease Control and Prevention, 2017; Available at: https://www.cdc.gov/hiv/pdf/library/reports/ surveillance/cdc-hiv-surveillance-report-2016-vol-28.pdf. Accessed 6 March 2018. [Google Scholar]
- 8. Delaney KP, Sanchez T, Bowles K, et al. . Awareness and use of PrEP appear to be increasing among Internet samples of US MSM. Presented at: Conference on Retroviruses and Opportunistic Infections;13–16 February2017; Seattle, WA. [Google Scholar]
- 9. Scott HM, Nordell M, Hirozawa A, et al. . Disparities in PrEP uptake among primary care patients screened for HIV and STIs in San Francisco. Presented at: Conference on Retroviruses and Opportunistic Infections; 4–7 March2018;Boston, MA. [Google Scholar]
- 10. Mayer KH, Biello K, Novak DS, et al. . PrEP uptake disparities in a diverse on-line sample of us men who have sex with men. Presented at: Conference on Retroviruses and Opportunistic Infections; 4–7 March2018; Seattle, WA. [Google Scholar]
- 11. Scott H, Nordell M, Hirozawa A, et al. . Racial/ethnic disparities in persistence among PrEP users in San Francisco Department of Public Health Primary Care Clinics. Presented at: Conference on Retroviruses and Opportunistic Infections;13-16 February2017;Seattle, WA. [Google Scholar]
- 12. Mera Giler R, Magnuson D, Trevor H, et al. . Changes in Truvada (TVD) for HIV pre-exposure prophylaxis (PrEP) utilization in the United States: (2012–2016). Presented at: International AIDS Society Meeting; 23–26 July 2017; Paris, France. [Google Scholar]
- 13. Chan PA, Mena L, Patel R, et al. . Retention in care outcomes for HIV pre-exposure prophylaxis implementation programmes among men who have sex with men in three US cities. J Int AIDS Soc 2016; 19:20903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Calabrese SK, Krakower DS, Mayer KH. Integrating HIV preexposure prophylaxis (PrEP) into routine preventive health care to avoid exacerbating disparities. Am J Public Health 2017; 107:1883–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jenness SM, et al. . The PrEP care continuum and HIV racial disparities among men who have sex with men. Presented at: Conference on Retroviruses and Opportunistic Infections; 2018; Boston, MA. [Google Scholar]
- 16. Kelley CF, Kahle E, Siegler A, et al. . Applying a PrEP continuum of care for men who have sex with men in Atlanta, Georgia. Clin Infect Dis 2015; 61:1590–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Marcus JL, Hurley LB, Nguyen DP, Silverberg MJ, Volk JE. Redefining human immunodeficiency virus (HIV) preexposure prophylaxis failures. Clin Infect Dis 2017; 65:1768–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rolle CP, Rosenberg ES, Siegler AJ, et al. . Challenges in translating PrEP interest into uptake in an observational study of young black MSM. J Acquir Immune Defic Syndr 2017; 76:250–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fox J, Brady M, Alexander H, et al. . Tenofovir disoproxil fumarate fails to prevent HIV acquisition or the establishment of a viral reservoir: two case reports. Infect Dis Ther 2016; 5:65–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Streeck H, Verheyen J, Storim J, et al. . Pre-exposure prophylaxis failure with tenofovir disoproxil. AIDS 2017; 31:176–7. [DOI] [PubMed] [Google Scholar]
- 21. Donnell D, Ramos E, Celum C, et al. ; Partners PrEP Study Team The effect of oral preexposure prophylaxis on the progression of HIV-1 seroconversion. AIDS 2017; 31:2007–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Delaney KP, Hanson DL, Masciotra S, Ethridge SF, Wesolowski L, Owen SM. Time until emergence of HIV test reactivity following infection with HIV-1: implications for interpreting test results and retesting after exposure. Clin Infect Dis 2017; 64:53–9. [DOI] [PubMed] [Google Scholar]
- 23. Grant RM, Anderson PL, McMahan V, et al. ; iPrEx Study Team Uptake of pre-exposure prophylaxis, sexual practices, and HIV incidence in men and transgender women who have sex with men: a cohort study. Lancet Infect Dis 2014; 14:820–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Margot NA, Wong P, Kulkarni R, et al. . Commonly transmitted HIV-1 drug resistance mutations in reverse-transcriptase and protease in antiretroviral treatment-naive patients and response to regimens containing tenofovir disoproxil fumarate or tenofovir alafenamide. J Infect Dis 2017; 215:920–7. [DOI] [PubMed] [Google Scholar]
- 25. Delaugerre C, Antoni G, Mahjoub N, et al. ; IPERGAY Study Group Assessment of HIV screening tests for use in preexposure prophylaxis programs. J Infect Dis 2017; 216:382–6. [DOI] [PubMed] [Google Scholar]
- 26. Rosenberg NE, Kamanga G, Phiri S, et al. . Detection of acute HIV infection: a field evaluation of the Determine® HIV-1/2 Ag/Ab Combo test. J Infect Dis 2012; 205:528–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Fitzgerald N, Cross M, O’Shea S, Fox J. Diagnosing acute HIV infection at point of care: a retrospective analysis of the sensitivity and specificity of a fourth-generation point-of-care test for detection of HIV core protein p24. Sex Transm Infect 2017; 93:100–1. [DOI] [PubMed] [Google Scholar]
- 28. Anderson PL, Glidden DV, Liu A, et al. ; iPrEx Study Team Emtricitabine-tenofovir concentrations and pre-exposure prophylaxis efficacy in men who have sex with men. Sci Transl Med 2012; 4:151ra125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Marcus JL, Hurley LB, Hare CB, et al. . Preexposure prophylaxis for HIV prevention in a large integrated health care system: adherence, renal safety, and discontinuation. J Acquir Immune Defic Syndr 2016; 73:540–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hosek SG, Rudy B, Landovitz R, et al. ; Adolescent Trials Network (ATN) for HIVAIDS Interventions An HIV preexposure prophylaxis demonstration project and safety study for young MSM. J Acquir Immune Defic Syndr 2017; 74:21–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wheeler D, Fields SD, Wilton L, et al. . HPTN 073: PrEP uptake and use by black men who have sex with men in 3 U.S. cities. Presented at: Conference on Retroviruses and Opportunistic Infections; 22–25 February2016;Boston, MA. [Google Scholar]
- 32. Landovitz RJ, Beymer M, Kofron R, et al. . Plasma tenofovir levels to support adherence to TDF/FTC preexposure prophylaxis for HIV prevention in MSM in Los Angeles, California. J Acquir Immune Defic Syndr 2017; 76:501–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Chaiyachati KH, Ogbuoji O, Price M, Suthar AB, Negussie EK, Bärnighausen T. Interventions to improve adherence to antiretroviral therapy: a rapid systematic review. AIDS 2014; 28(suppl 2):S187–204. [DOI] [PubMed] [Google Scholar]
- 34. Parsons JT, Rendina HJ, Lassiter JM, Whitfield TH, Starks TJ, Grov C. Uptake of HIV pre-exposure prophylaxis (PrEP) in a national cohort of gay and bisexual men in the United States. J Acquir Immune Defic Syndr 2017; 74:285–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sabate E. Adherence to long-term therapies: evidence for action. Geneva, Switzerland:World Health Organization, 2003:19–25. [Google Scholar]
- 36. Crosby R. Motivational interviewing for PrEP uptake. 2016. Available at: https://clinicaltrials.gov/ct2/show/NCT03226496. Accessed 6 March 2018. [Google Scholar]
- 37. Cohen MS, Chen YQ, McCauley M, et al. ; HPTN 052 Study Team Antiretroviral therapy for the prevention of HIV-1 transmission. N Engl J Med 2016; 375:830–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kwakwa HA, Bessias S, Sturgis D, et al. . Attitudes toward HIV pre-exposure prophylaxis in a United States urban clinic population. AIDS Behav 2016; 20:1443–50. [DOI] [PubMed] [Google Scholar]
- 39. Kesler MA, Kaul R, Myers T, et al. . Perceived HIV risk, actual sexual HIV risk and willingness to take pre-exposure prophylaxis among men who have sex with men in Toronto, Canada. AIDS Care 2016; 28:1378–85. [DOI] [PubMed] [Google Scholar]
- 40. Philbin MM, Parker CM, Parker RG, Wilson PA, Garcia J, Hirsch JS. The promise of pre-exposure prophylaxis for black men who have sex with men: an ecological approach to attitudes, beliefs, and barriers. AIDS Patient Care STDS 2016; 30:282–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Golub SA, Gamarel KE, Surace A. Demographic differences in PrEP-related stereotypes: implications for implementation. AIDS Behav 2017; 21:1229–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Young LE, Schumm P, Alon L, et al. . PrEP Chicago: a randomized controlled peer change agent intervention to promote the adoption of pre-exposure prophylaxis for HIV prevention among young black men who have sex with men. Clin Trials 2018; 15:44–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Hall GC, Koenig LJ, Gray SC, et al. . Accuracy of HIV risk perceptions among episodic substance-using men who have sex with men. AIDS Behav 2017. [E-pub ahead of print]. doi: 10.1007/s10461-017-1935-y. [DOI] [PubMed] [Google Scholar]
- 44. Gallagher T, Link L, Ramos M, Bottger E, Aberg J, Daskalakis D. Self-perception of HIV risk and candidacy for pre-exposure prophylaxis among men who have sex with men testing for HIV at commercial sex venues in New York City. LGBT Health 2014; 1:218–24. [DOI] [PubMed] [Google Scholar]
- 45. Golub SA, Gamarel KE, Lelutiu-Weinberger C. The importance of sexual history taking for PrEP comprehension among young people of color. AIDS Behav 2017; 21:1315–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Golub SA, Pena S, Hilley A, et al. . Brief Behavioral intervention increases PrEP drug levels in a real-world setting. Presented at: Conference on Retroviruses and Opportunistic Infections; 13–16 February2017; Boston, MA. [Google Scholar]
- 47. Pingel ES, Rolle CP, Kelley C, et al. . “It’s just not for me”: exploring low PrEP uptake among young black men who have sex with men in the southern United States. In: STI & HIV World Congress; 9–12 July2017; Rio de Janeiro, Brazil. [Google Scholar]
- 48. Eaton LA, Kalichman SC, Price D, Finneran S, Allen A, Maksut J. Stigma and conspiracy beliefs related to pre-exposure prophylaxis (PrEP) and interest in Using PrEP among black and white men and transgender women who have sex with men. AIDS Behav 2017; 21:1236–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Landman K. The FDA has approved generic PrEP—but access may remain difficult June 15 June 2017. Available at: https://www.vice.com/en_us/article/xw875w/the-fda-has-approved-generic-prepbut-access-may-remain-difficult. Accessed 6 March 2018.
- 50. Yaylali EY, Farnham P, Jacobson E, et al. . Impact of improving HIV care and treatment and initiating PrEP in the United States, 2015–2020. In: Conference on Retroviruses and Opportunistic Infections;22–25 February2016; Boston, MA. [Google Scholar]