Abstract
In this issue of Stem Cell Reports, Hastreiter et al. (2018) use continuous time-lapse imaging of mouse embryonic stem cells to investigate how the inhibition of GSK3b and MEK/ERK (2i) leads to homogeneous expression of the transcription factor Nanog. They show that both induction of Nanog expression and selection against cells expressing low levels of Nanog contribute to the homogeneous appearance of 2i cultures.
In this issue of Stem Cell Reports, Hastreiter et al. (2018) use continuous time-lapse imaging of mouse embryonic stem cells to investigate how the inhibition of GSK3b and MEK/ERK (2i) leads to homogeneous expression of the transcription factor Nanog. They show that both induction of Nanog expression and selection against cells expressing low levels of Nanog contribute to the homogeneous appearance of 2i cultures.
Main Text
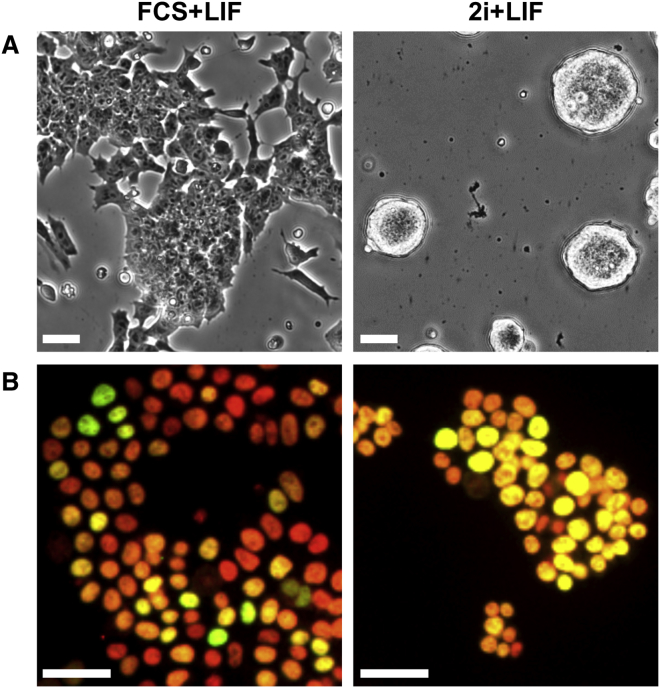
In one of the most famous declamations ever written, Hamlet asks himself whether he should remain alive and continue to exist in an unbearable situation or, on the contrary, whether he should end his own life. “To be, or not to be: that is the question” also asked by Hastreiter et al. (2018) in this issue, wherein the authors investigate the behavior of individual mouse embryonic stem cells (ESCs) placed under the so-called 2i medium. Several years ago, it was shown that dual inhibition of MEK/ERK and GSK3b enables ESCs to attain a state known as the ground state of pluripotency (Ying et al., 2008). In this state, ESCs are thought to resemble more closely the epiblast of preimplantation embryos (Boroviak et al., 2014); it is characterized by a number of hallmarks, including rather homogeneous expression levels of the transcription factor Nanog (Wray et al., 2010). Indeed, when ESCs are grown under traditional culture conditions employing serum and the leukemia inhibitory factor (LIF) cytokine, they show heterogeneous and dynamic expression patterns of several key regulators including Nanog (Chambers et al., 2007). While Nanog-HIGH cells exhibit robust self-renewal, a fraction of Nanog-LOW cells is prone to undergo differentiation (Filipczyk et al., 2015). Hence, it was not surprising that, upon 2i treatment, which leads to enforced self-renewal and a dramatic loss of spontaneously differentiating cells in the cultures (Figure 1A), Nanog appears to be more homogeneously expressed (Figure 1B). Yet, whether all individual ESCs cultured in serum/LIF respond equally to 2i treatment, notably by inducing higher and more constant levels of Nanog, had not been concretely addressed prior to this study. Hence, despite the general assumption that 2i induces high Nanog expression, it remained a possibility that 2i also triggers selective effects by eliminating or disadvantaging some subpopulations present in serum/LIF ESC cultures. This possibility was strongly implied by the observation that differentiated cells, and even other, more developmentally advanced pluripotent cell types, such as epiblast stem cells, cannot survive in 2i/LIF (Guo et al., 2009). Only those pluripotent cells generally referred to as naive are indeed capable of proliferating in 2i/LIF and transit easily to the ground state. Nanog-LOW cells naturally present in serum/LIF cultures express a number of differentiation markers, albeit at low levels (Abranches et al., 2014). Hence, while they are not yet committed to differentiate, they appear to be primed to do so, and whether they survive in 2i/LIF conditions was therefore an important question that remained unanswered. To address this, Hastreiter et al. (2018) used continuous time-lapse imaging of two independent and previously generated and validated Nanog reporter cell lines: one carrying a Nanog-GFP transgene randomly integrated (Schaniel et al., 2009) and another one expressing a Nanog:Katushka fusion protein from one endogenous allele (Filipczyk et al., 2013). They clearly show that both inductive and selective mechanisms underlie the homogeneous expression pattern of Nanog when ESCs reach the ground state of pluripotency.
Figure 1.
Changes in Morphology and Nanog Expression between Serum/LIF and Serum-free 2i/LIF ESCs
(A) Bright-field microscopic image of mouse ESCs cultured in serum/LIF (left) and in 2i/LIF (right).
(B) Immunostaining of Oct4 (red) and Nanog (green) in mouse ESCs cultured in serum/LIF (left) and in 2i/LIF (right).
Scale bars represent 30 μm.
The experimental setup used by the authors is elegant and simple: by imaging ESCs during 2 days after adding 2i to serum/LIF cultures, they assess Nanog levels and death events in continuous single-cell branches. They first observe that, after 2 days in 2i, both reporters already express Nanog homogenously and at high levels. However, the dynamics of the two reporters are somewhat different. On the one hand, the Nanog-GFP transgene, which is a better proxy of transcriptional activity than of protein levels, upregulates GFP expression rapidly upon 2i addition: Nanog-GFP-LOW cells activate transcription almost immediately and the others within 6 hr of treatment. On the other hand, Nanog:Katushka cells where protein levels can be directly monitored display different behaviors: Nanog-LOW cells upregulate Nanog rapidly, Nanog-MID cells within 24 hr, and Nanog-HIGH cells initially display a slight downregulation in a 2i-independent fashion but reacquire higher expression after 36 hr in the presence of 2i. The basis of these differences remains unclear, but they may depend on post-translational regulation of Nanog or on other regulatory parameters, such as Nanog autorepression (Navarro et al., 2012). This latter phenomenon may be particularly important to understand the delayed activation of Nanog-MID&HIGH cells and the initial downregulation observed in Nanog:Katushka-HIGH cells, until the global effects of 2i on the gene regulatory network become dominant. Irrespective of these differences, however, these analyses clearly demonstrate that 2i does indeed induce Nanog expression, most likely via transcriptional mechanisms that remain to be molecularly understood.
In addition, the authors also observe that Nanog-LOW cells have a stronger tendency to die prematurely, before a first cell division. This occurs in both the presence and the absence of 2i, suggesting that Nanog expression is somehow beneficial for ESC survival. In contrast, cell death at later generations, after 1 to 3 cell divisions, is significantly higher in the progeny of Nanog-LOW cells specifically in 2i. Therefore, not all ESCs have an equal capacity of growing in the presence of 2i: they seem to require Nanog to do so efficiently. Hence, treatment of ESCs with 2i leads to homogeneous Nanog expression levels not only because Nanog is induced but also because Nanog-LOW cells are eliminated. Strikingly, the death of the progeny of Nanog-LOW cells often occurs despite Nanog being reexpressed. This represents an interesting observation that indicates that the protective effect of Nanog is not immediately established upon Nanog expression but most likely after a prolonged exposure of the cells to Nanog activity.
The observation that Nanog-LOW cells have a higher propensity to undergo cell death in 2i has two important implications. First, as 2i treatment (and more specifically the inhibition of MEK/ERK activity) is compatible with cell survival and proliferation only in the context of naive pluripotency, this result adds weight to the notion that not all undifferentiated and pluripotent cells in serum/LIF conditions are naive. Second, it calls into question the nature of Nanog null cells. Indeed, Nanog can be homozygously deleted without altering pluripotency; Nanog null ESCs remain undifferentiated and contribute to all tissues upon blastocyst injection (Chambers et al., 2007). The question, therefore, is to what extent Nanog null cells can proliferate in 2i. While the results presented above suggest that Nanog null cells should die in 2i, several studies have used 2i/LIF to culture Nanog null ESCs without commenting on overt proliferation deficits. To address this important question, the authors of the present study cultured Nanog null cells in serum/LIF supplemented with 2i and observed dramatic cell loss, eventually leading to a general collapse of the cultures. In contrast, adding a Nanog-expressing transgene to Nanog null cells (Chambers et al., 2007) rescues their ability to proliferate in 2i. How such an important observation has stayed so far unnoticed remains unclear, but the authors themselves provide additional interesting observations on this matter. In fact, 2i was originally conceived as a minimal medium that would lack other constituents generally used for ESC culture, such as feeders, serum, and LIF. In this case, however, the authors were culturing the cells in serum/LIF supplemented with 2i. Other cell culture conditions, which are imposed by the requirements of time-lapse microscopy in some experiments, are also relatively infrequent, such as the use of E-cadherin as a substrate or low oxygen tension. The authors carefully controlled that the increased rates of death of Nanog-LOW cells placed under 2i also apply to more standard conditions (serum-free 2i/LIF and gelatin instead of E-cadherin). Hence, their results may be extended to other recipes used under the generic 2i denomination. Nevertheless, and in contrast to their observations in serum/LIF/2i, when Nanog null cells were placed in serum-free 2i/LIF medium, the cultures were not completely lost: they did exhibit a lower rate of proliferation, but after 10 days in culture, they apparently adapted to serum-free 2i/LIF and the colonies adopted the typical morphology of 2i cultures. Therefore, an additional important conclusion from this study is that every parameter has potential to have important consequences for the behavior of ESCs. Special attention should be placed by the ESC community into how 2i is used and described; otherwise, inconsistencies among independent studies could lead to important observations being missed and inappropriate conclusions being drawn. In this regard, Hastreiter et al. (2018) present a compelling example of the impact of serum in ESCs, even in the presence of 2i.
Overall, Hastreiter et al. convincingly show that 2i has complex effects in mouse ESCs: it rapidly activates Nanog expression and, subsequently, leads to the death of many cells that were in the Nanog-LOW state when they were first exposed to GSK3b and MEK/ERK inhibition. Determining the underlying mechanisms and teasing apart the respective contribution of GSK3b and MEK/ERK are clear objectives for the future. This study also reveals numerous questions on how the intrinsic cellular gene regulatory network states should be paired with extrinsic cues to appropriately channel cell fate decisions in ESCs and in other developmental contexts.
Acknowledgments
The author is grateful to Agnès Dubois and Alexandra Tachtsidi for providing the images in Figure 1 and to Nick Owens for comments. Research in P.N.’s laboratory is supported by recurrent funding from the Institut Pasteur, the CNRS, and Revive (Investissement d’Avenir; ANR-10-LABX-73). P.N. acknowledges financial support from the Fondation Schlumberger (FRM FSER 2017), the Fondation ARC pour la recherche sur le cancer (ARC NAVARRO CA 14/12/2016), the Agence Nationale de la Recherche (ANR 16 CE12 0004 01 MITMAT), and the Ligue contre le Cancer (LNCC EL2018 NAVARRO).
References
- Abranches E., Guedes A.M., Moravec M., Maamar H., Svoboda P., Raj A., Henrique D. Stochastic NANOG fluctuations allow mouse embryonic stem cells to explore pluripotency. Development. 2014;141:2770–2779. doi: 10.1242/dev.108910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boroviak T., Loos R., Bertone P., Smith A., Nichols J. The ability of inner-cell-mass cells to self-renew as embryonic stem cells is acquired following epiblast specification. Nat. Cell Biol. 2014;16:516–528. doi: 10.1038/ncb2965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers I., Silva J., Colby D., Nichols J., Nijmeijer B., Robertson M., Vrana J., Jones K., Grotewold L., Smith A. Nanog safeguards pluripotency and mediates germline development. Nature. 2007;450:1230–1234. doi: 10.1038/nature06403. [DOI] [PubMed] [Google Scholar]
- Filipczyk A., Gkatzis K., Fu J., Hoppe P.S., Lickert H., Anastassiadis K., Schroeder T. Biallelic expression of nanog protein in mouse embryonic stem cells. Cell Stem Cell. 2013;13:12–13. doi: 10.1016/j.stem.2013.04.025. [DOI] [PubMed] [Google Scholar]
- Filipczyk A., Marr C., Hastreiter S., Feigelman J., Schwarzfischer M., Hoppe P.S., Loeffler D., Kokkaliaris K.D., Endele M., Schauberger B. Network plasticity of pluripotency transcription factors in embryonic stem cells. Nat. Cell Biol. 2015;17:1235–1246. doi: 10.1038/ncb3237. [DOI] [PubMed] [Google Scholar]
- Guo G., Yang J., Nichols J., Hall J.S., Eyres I., Mansfield W., Smith A. Klf4 reverts developmentally programmed restriction of ground state pluripotency. Development. 2009;136:1063–1069. doi: 10.1242/dev.030957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hastreiter S., Skylaki S., Loeffler D., Reimann A., Hilsenbeck O., Hoppe P.S., Coutu D.L., Kokkaliaris K.D., Schwarzfischer M., Anastassiadis K. Inductive and selective effects of GSK3 and MEK inhibition on Nanog heterogeneity in embryonic stem cells. Stem Cell Reports. 2018;11:58–69. doi: 10.1016/j.stemcr.2018.04.019. this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro P., Festuccia N., Colby D., Gagliardi A., Mullin N.P., Zhang W., Karwacki-Neisius V., Osorno R., Kelly D., Robertson M., Chambers I. OCT4/SOX2-independent Nanog autorepression modulates heterogeneous Nanog gene expression in mouse ES cells. EMBO J. 2012;31:4547–4562. doi: 10.1038/emboj.2012.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaniel C., Ang Y.S., Ratnakumar K., Cormier C., James T., Bernstein E., Lemischka I.R., Paddison P.J. Smarcc1/Baf155 couples self-renewal gene repression with changes in chromatin structure in mouse embryonic stem cells. Stem Cells. 2009;27:2979–2991. doi: 10.1002/stem.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wray J., Kalkan T., Smith A.G. The ground state of pluripotency. Biochem. Soc. Trans. 2010;38:1027–1032. doi: 10.1042/BST0381027. [DOI] [PubMed] [Google Scholar]
- Ying Q.L., Wray J., Nichols J., Batlle-Morera L., Doble B., Woodgett J., Cohen P., Smith A. The ground state of embryonic stem cell self-renewal. Nature. 2008;453:519–523. doi: 10.1038/nature06968. [DOI] [PMC free article] [PubMed] [Google Scholar]